Abstract
Background
There is no consensus regarding what volume of local anesthetic should be used to achieve successful supraclavicular block while minimizing hemidiaphragmatic paresis (HDP). This study investigated the dose-response relationship between local anesthetic volume and HDP after ultrasound-guided supraclavicular brachial plexus block.
Methods
A dose escalation design was used to define the dose response curve for local anesthetic volume and incidence of HDP in subjects undergoing upper extremity surgery with supraclavicular block as the primary anesthetic. Dosing levels of 5, 10, 15, 20, 25, 30, 35, and 40 mL of local anesthetic were administered in cohorts of three subjects per dose. Diaphragm function was assessed with M-mode ultrasound before and after block. Secondary objectives included assessment of negative inspiratory force (NIF), oxygen saturation, subjective dyspnea, and extent of sensory and motor blockade.
Results
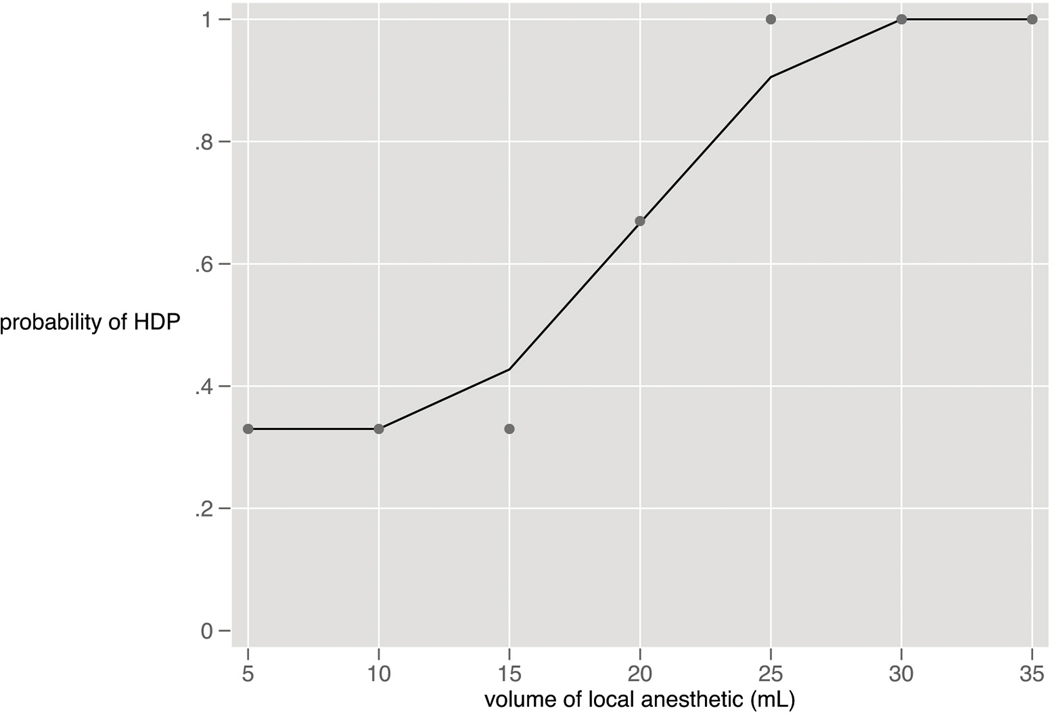
Twenty-one subjects completed the study. HDP was present at all doses, with an incidence of 33% at 5 mL to 100% at 30–35 mL. There was a significant decrease in NIF (7.5 cmH2O, IQR [22, 0]; P = 0.01) and oxygen saturation on room air (1%, IQR [2,0]; P = 0.01) 30 minutes post-block in subjects experiencing HDP but not in those without HDP. There was no increase in dyspnea in subjects with or without HDP. No subject required respiratory intervention. Motor and sensory block improved with increasing dose, and subjects with HDP exhibited denser blocks than those without (P < 0.01).
Conclusions
There is no clinically relevant volume of local anesthetic at which HDP can be avoided when performing a supraclavicular block. In our subject population free of respiratory disease, HDP was well-tolerated.
INTRODUCTION
Supraclavicular block can provide dense surgical anesthesia for upper extremity procedures but is associated with a variable incidence of phrenic nerve blockade.1 The phrenic nerve lies on the surface of the anterior scalene muscle in close proximity to the brachial plexus within the prevertebral layer of the deep cervical fascia,2; 3 and is often inadvertently blocked during supraclavicular block resulting in ipsilateral hemidiaphragmatic paresis (HDP). Although this is usually well tolerated in most individuals, respiratory insufficiency may arise in those with pre-existing pulmonary impairments or dysfunction of the contralateral hemidiaphragm.4
There is no established consensus regarding what volume of local anesthetic should be used to achieve a successful supraclavicular block while also minimizing HDP. Studies have shown the ED95 for supraclavicular block to be 17–27 mL of local anesthetic.5; 6 With traditional landmark-based or nerve-stimulator techniques, the incidence of HDP following supraclavicular blocks has been reported to be 50–67%.7; 8 Ultrasound-guided techniques are thought to facilitate more precise deposition of local anesthetic and enable operators to use less volume to achieve a successful block.1 Renes et al9 demonstrated a 0% incidence of HDP after ultrasound-guided supraclavicular block with 20 mL of local anesthetic; however, other studies have suggested that HDP is a more consistent side effect of supraclavicular block, even with ultrasound visualization.10 In practice, typical volumes of local anesthetic used for anesthetic supraclavicular blocks range from 20–40 mL. There have been no studies to date, however, that have attempted to determine the dose-response relationship between local anesthetic volume and the degree of HDP.
Motion-mode (M-mode) ultrasonography in the anterior subcostal position has been shown to be a reliable and reproducible modality for detecting anatomical or functional diaphragmatic abnormalities by measuring diaphragmatic excursion.11–13 A supine position is preferred, as diaphragmatic excursion is greater in the supine position compared to sitting or standing and is associated with less variability and greater reproducibility.12 Motion of the right hemidiaphragm is more reliably imaged than the left due to the smaller acoustic window of the spleen compared to the liver. The voluntary sniff test, in conjunction with M-mode ultrasonography, has been shown to be of value for the diagnosis of HDP, and is reported to potentiate findings that might not be obvious during normal, quite breathing.11; 13
Trial designs utilizing up-and-down methodology have been used in recent years in dose-finding studies for regional anesthesia, including to determine the ED95 dose for the supraclavicular block.5; 6 Up-and-down escalation methods are traditionally used in Phase I dose-finding trials in order to avoid unnecessary exposure of subjects to subtherapeutic doses of an agent while limiting toxicity.14 We utilized a modified “3+3” dose escalation design to define the primary objective, which was the dose-response relationship between local anesthetic volume and ipsilateral HDP in subjects undergoing ultrasound-guided supraclavicular brachial plexus blocks for surgeries of the right upper extremity in an observer-blinded, prospective trial. Our secondary objectives were to assess respiratory function (subjective dyspnea, oxygen saturation, negative inspiratory force (NIF)), and extent of sensory and motor blockade.
METHODS
Recruitment
Following approval from the institutional human research ethics board (Weill Cornell Medicine, New York City, New York) and written informed consent, subjects were enrolled between August 2018 and July 2019. Adult subjects (≥ 18 years of age) with American Society of Anesthesiologists (ASA) physical status classification I – III undergoing right upper extremity surgery with supraclavicular block as the primary anesthetic were recruited at NewYork- Presbyterian/Weill Cornell Medical Center. Only subjects having surgery on the right upper extremity were enrolled due to the more reliable imaging of the right hemidiaphragm on ultrasonography compared to the left.11 Exclusion criteria included allergy to local anesthetics, existing hemidiaphragmatic dysfunction, obstructive or restrictive pulmonary disease, suspected or known peripheral neuropathies of the right upper extremity, neuromuscular disease, pregnancy, inability to give informed consent, or subject refusal. This trial was registered with Clinicaltrials.gov (NCT03138577) on May 3, 2017; https://clinicaltrials.gov/ct2/show/NCT03138577.
Dose Determination
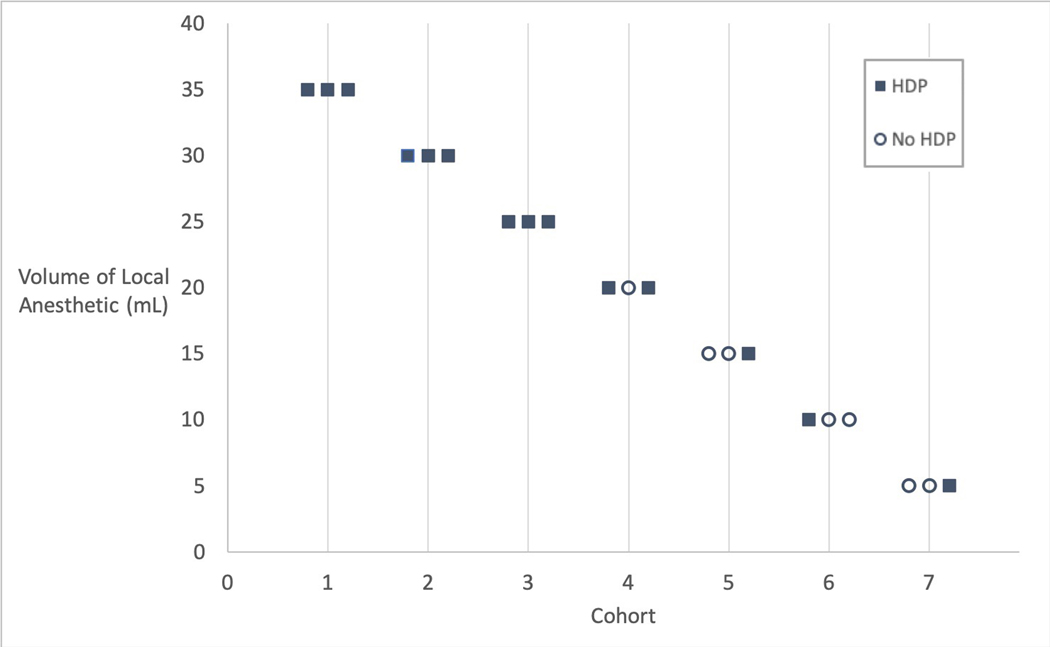
Dose escalation trials typically enroll 1–3 subjects at each dose. Eight possible dose levels of 5, 10, 15, 20, 25, 30, 35 and 40 mL were chosen for this trial. Three consecutive subjects were treated at a given dose level. The trial began with a total dose of 35 mL of local anesthetic. If no subjects had HDP at a given dose, the next cohort of three subjects would receive the next highest dose. If any of the three subjects exhibited HDP, the subsequent cohort would receive the next lowest dose. If or when the dose escalated or de-escalated to a previously tested level, and a total of six consecutive subjects at that dose exhibited no HDP, the trial would halt. This dose would represent the maximum tolerated dose that could be administered in a supraclavicular block without causing HDP. Other stopping rules included de-escalating to the lowest dose (5mL) and observing HDP, or enrollment of a total of 30 subjects.
Block Protocol
After application of standard ASA monitors, and at the discretion of the attending anesthesiologist, up to 0.03 mg/kg of midazolam was administered for sedation prior to the supraclavicular block. In cases of extreme subject anxiety, additional midazolam was given up to a total dose of 0.05 mg/kg. The subject was positioned supine with the head turned to the contralateral side, and the injection site disinfected with a 2% chlorhexidine and alcohol solution. Aseptic technique was used throughout block placement. A Sonosite® (Bothell, WA) HFL38xi ultrasound probe (13 – 6 MHz) was placed above and parallel to the clavicle to obtain a transverse cross-sectional view of the subclavian artery and brachial plexus. The skin and subcutaneous tissues were infiltrated lateral to the probe with 2% lidocaine. Using an in-plane technique, a 51 mm, 18G Pajunk® (Geisingen, Germany) E-Cath catheter-over-needle was inserted through the skin lateral to the probe and directed medially toward the “corner pocket”15 between the subclavian artery and first rib. Local anesthetic was administered initially at this location. The needle was redirected at the discretion of the anesthesiologist to perform additional injections for complete coverage of the brachial plexus. The local anesthetic mixture was a 2:1 mixture of 1.5% mepivacaine and 0.5% bupivacaine, and the total volume administered was pre-determined based on the above described protocol. All injections were made in 3–5 mL increments using frequent aspiration. After injection, the needle was removed to leave a catheter for further dosing of local anesthetic if supplemental nerve blockade was needed to provide surgical anesthesia. Following performance of the supraclavicular block, an additional block of the ipsilateral intercostobrachial nerve was completed: The subject’s arm was abducted and externally rotated and a 1.5-inch, 25-gauge needle was inserted from the deltoid prominence to the most inferior aspect of the medial arm to perform a subcutaneous field block using 10 mL of 0.5% bupivacaine.
Evaluation of Hemidiaphragmatic Paresis via M-Mode Ultrasonography
M-mode tracings of right diaphragm motion were made and recorded by the attending anesthesiologist at baseline (prior to sedation and block), and at 15 and 30 minutes after block placement. Subjects were examined in the supine position and a GE Logiq™ e (Arlington Heights, IL) 3s probe (1.5 – 3.6 MHz) was positioned below the right costal margin between the midclavicular and anterior axillary line. The liver was used as an acoustic window.12 In B mode, the dome position (highest point) of the diaphragm could be identified by finding the maximal distance from the top of the screen along a craniocaudal path. Once the dome was identified, M-mode was used to record diaphragm motion during three voluntary sniffs (VS), for which the subjects were instructed to forcefully inhale through the nose in a sniffing position. The M-mode recordings were reviewed by two blinded anesthesiologists. Diaphragmatic excursion from baseline to the point of maximum height of inspiration was measured in centimeters. At each time interval, three measurements were determined and averaged. The normal values for right diaphragmatic motion during voluntary sniffing are 2.9 ± 0.6 cm in men and 2.6 ± 0.5 cm in women.11 The standard deviation of these values represent a 20% change in excursion, therefore we defined a significant paresis to be a reduction of 60% (the equivalent of 3 standard deviations) or paradoxical movement during VS. If the two reviewers arrived at different conclusions regarding the diagnosis of paresis, a third blinded anesthesiologist was available to review the images and serve as a tie-breaker.
Evaluation of Respiratory Function
A bedside NIF meter was used prior to and 30 minutes after sedation and block to measure negative inspiratory force in cmH2O. Subjects were asked to exhale fully and then inhale forcefully through a flanged mouthpiece. Three attempts were made at each timepoint and the most negative value was used in analysis. Oxygen saturation was measured on room air before any sedatives were given for the block and 30 minutes after the block. A 0–10 point verbal rating scale was used to assess subjective dyspnea at 30 minutes (0 = no difficulty breathing, 10 = extreme difficulty breathing).16
Evaluation of Blockade
At 15 and 30 minutes, sensory blockade was assessed in the axillary (lateral upper arm), musculocutaneous (lateral forearm), radial (dorsal hand), median (thenar eminence), and ulnar (hypothenar eminence) distributions with the use of ice and a 3-point scale (0 = normal sensation of cold, 1 = perception of touch/pressure but not cold, 2 = no perception of touch/pressure, or cold). Motor function was evaluated at 15 and 30 minutes in the distribution of the axillary nerve (abduction at shoulder), musculocutaneous nerve (flexion at the elbow), radial nerve (extension at the elbow), median nerve (flexion at the wrist), and ulnar nerve (finger separation) using a 3 point scale (0 = normal strength, 1 = weakness, or 2 = no movement).16; 17 A total block score was determined out of a maximum of 20 (complete block in all sensory and motor distributions), and any score less than 10 was deemed an ineffective block. Supplementary dosing through the catheter after the 30-minute evaluation was allowed at the anesthesiologist’s discretion.
Statistical Analysis
The skewness and kurtosis tests were used to test for normality. Subject characteristics, such as age, sex, ASA physical status classification, and type of surgery were characterized by N and median [interquartile ranges (IQR)]. Demographics were compared between subjects with HDP and those without HDP using the Mann-Whitney U test and Fisher’s exact test. We used a lowess (locally weighted regression) smoothing technique to describe the dose-response relationship. Lowess smoothing is a non-parametric technique which uses prediction values obtained from multiple local regression models (one for each dose level) to produce a lowess curve, and is an accepted approach for describing nonlinear dose response relationships at a univariate level.18 Changes in NIF and oxygen saturation were analyzed using the Wilcoxon signed-rank test, dyspnea scores were compared with the Mann-Whitney U test, and data presented as median [inter-quartile range (IQR)]. Correlation between midazolam dose and change in NIF was examined using the Spearman correlation test. Data was analyzed using Stata 16 Software (StataCorp, College Station, TX).
RESULTS
Fifty-nine subjects were assessed for eligibility. Fourteen subjects were excluded or refused to participate, and 21 were eligible but not approached due to the anticipation of insufficient time to complete assessments. Twenty-four subjects consented to enrollment. Three subjects were excluded after enrollment for abnormal diaphragm motion or difficulty visualizing the diaphragm during the baseline scan. After exclusions, 21 subjects completed the study and were included in the analysis. Enrollment was halted as per the criteria of observing HDP at the lowest dose of 5 mL. All subjects underwent right upper extremity surgery as indicated (Table 1). A majority of enrolled subjects were of female gender.
Table 1.
Patient Demographics
| All (N=21) | With HDP (N=14) | Without HDP (N=7) | P Value | |
|---|---|---|---|---|
| Age in years: median (IQR) | 64 (56, 71) | 64.5 (61,73) | 59 (38,69) | 0.20 |
| Gender: M/F | 1/20 | 1/13 | 0/7 | 1.00 |
| BMI (kg/m2): median (IQR) | 25 (24, 28) | 24 (21,28) | 26 (24,31) | 0.15 |
| ASA: I/II/III | 5/15/1 | 2/11/1 | 3/4/0 | 0.40 |
| Surgery: proximal/mid/distal | 9/9/3 | 6/5/3 | 3/4/0 | 0.58 |
Primary Endpoint
Dosing began with 3 consecutive subjects receiving 35 mL, and followed the up-down methodology described above (Figure 1). HDP was present 30 minutes after the block at all dose levels from 35 mL (100% incidence) to 5 mL (33% incidence). (Figure 2). Subjects were not enrolled at the 40mL dose due to the 100% incidence of HDP at the starting dose of 35 mL. With the exception of one subject in the 25 mL cohort, all subjects with HDP at 30 minutes also demonstrated HDP at 15 minutes.
Figure 1:
Up-Down Methodology by Cohort and Anesthetic Volume
Figure 2:
Dose Response Curve: Hemidiaphragmatic Paresis and Volume of Local Anesthetic 30 Minutes after Block (curve generated with lowess smoothing technique)
Secondary Respiratory Endpoints
Subjects with hemidiaphragmatic paresis had a significant decrease in NIF 30 minutes after the block (7.5 cmH2O, IQR [22, 0]; P = 0.01). This decrease amounted to a NIF that was 81% (IQR [50, 100%]) of baseline. One subject exhibited an apparent increase in NIF even with an ultrasound scan consistent with diaphragmatic paresis. This subject’s results were included in the data analysis. There was no significant change in NIF at 30 minutes in the subjects without diaphragmatic paresis (0 cmH2O, IQR [10,0]; P = 0.32), amounting to 100% (IQR [83,100]) of baseline. There was no correlation between midazolam dose and change in NIF (P = 0.98).
There was a statistically significant decrease in oxygen saturation on room air in the subjects with HDP (1%, IQR [2,0]; P = 0.01), but not in the subjects without HDP (0%, IQR [0,0]; P = 1.00). There was no significant increase in subjective dyspnea in either group. No respiratory interventions were required in any of the subjects.
Secondary Block Effectiveness Endpoints
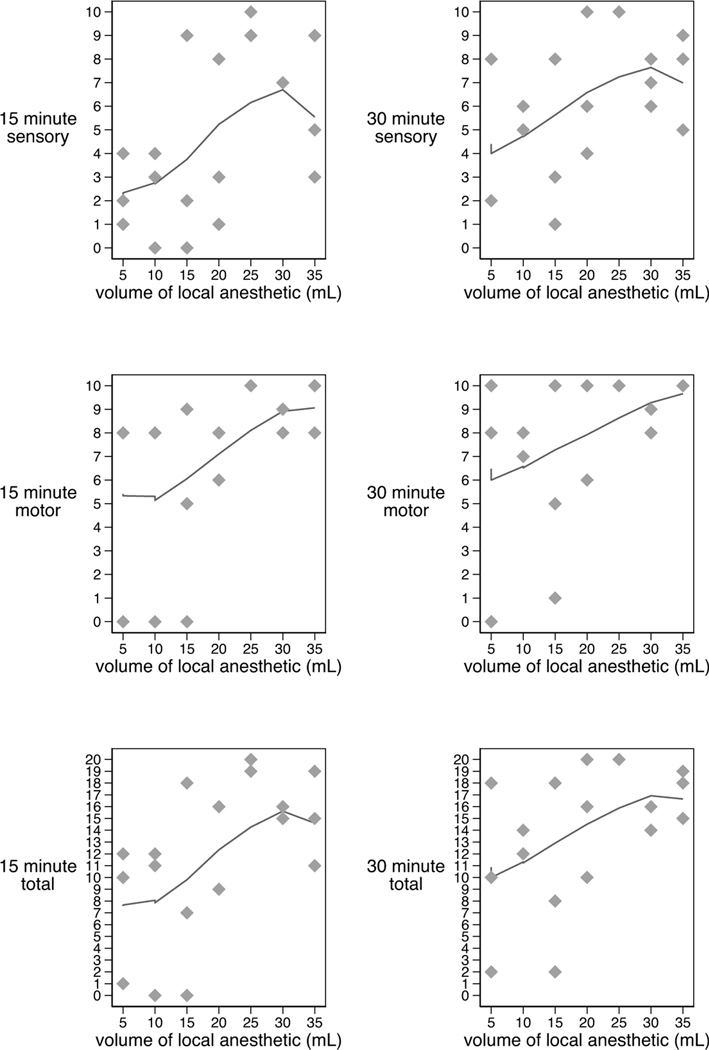
The total motor/sensory block score increased with doses up to 30 mL (Figure 3). Subjects with HDP exhibited significantly higher (denser block) scores than those without HDP (Table 2). A total of 3 out of 21 subjects achieved a total motor/sensory block score of less than 10 at 30 minutes (2 subjects at the 15mL dose and 1 subject at the 5 mL dose). These subjects received supplemental local anesthetic through the supraclavicular catheter. Four additional subjects with block scores of 12, 14, 14 and 18 also received additional local anesthetic through the catheter prior to surgical incision at the discretion of the attending anesthesiologist. No subjects required dosing after incision or conversion to general anesthesia due to inadequate block.
Figure 3:
Dose Response Curve: Sensory/Motor Block and Local Anesthetic Volume 15 and 30 Minutes after Block (curves generated with lowess smoothing technique)
Table 2.
Motor and Sensory Score Data
| Scorea | All (N=21) | With HDP (N=14) | Without HDP (N=7) | P value |
|---|---|---|---|---|
| 15-minute Sensory | 4 (2,7) | 7 (3,9) | 2 (0,4) | < 0.01 |
| 30-minute Sensory | 6 (5,8) | 8 (6,10) | 4 (2,6) | 0.01 |
| 15-minute Motor | 8 (6,9) | 8 (8,10) | 5 (0,8) | < 0.01 |
| 30-minute Motor | 8 (7,10) | 10 (8,10) | 6 (1,8) | < 0.01 |
| 15-minute Total | 12 (9,16) | 15 (11,18) | 7 (0,12) | < 0.01 |
| 30-minute Total | 16 (12,18) | 16 (15,19) | 10 (2,14) | < 0.01 |
Maximum Score 10 for Motor and Sensory; Maximum Score 20 for Total (motor + sensory)
Higher score = denser block
Results reported as Median (IQR)
DISCUSSION
The primary result of this study was that HDP occurred to some extent at all dose levels administered, suggesting that there is no clinically relevant dose at which HDP can be avoided. Subjects exhibiting HDP achieved better sensory and motor block scores than those without HDP. In this study, HDP was associated with a significant reduction in NIF and oxygen saturation. These respiratory effects were well-tolerated in our subject population free from respiratory disease.
The deep cervical fascia of the neck is divided into the investing (external), pretracheal (middle), and prevertebral (deep) layers. The prevertebral layer extends laterally from the ligamentum nuchae to encircle the vertebrae, scalene muscles, brachial plexus, phrenic nerve, vertebral vessels, and anteriorly attaches to the cervical transverse processes. The brachial plexus is contained within this prevertebral fascial layer from its point of origin at the level of the intervertebral foramen until its continuation as the axillary sheath.2; 3 This fascial sheath is the primary anatomical reason why it is likely impossible to reliably avoid phrenic nerve paresis with brachial plexus blocks performed in the neck. Studner et al.19 elegantly demonstrated this phenomenon with the interscalene block by injecting ropivacaine mixed with contrast dye, after which MRI studies demonstrated spread to both the epidural space and phrenic nerve at volumes of 5 and 20 mL.
Previously published literature on dosing in ultrasound-guided supraclavicular blocks demonstrates a variable incidence of HDP. Studies report an incidence of HDP between 0 and 66.7%.9; 10; 16; 20; 21 Of these, those that confined injections to the “corner pocket”15 (intersection of the subclavian artery and first rib), or posterolateral to the plexus, had the lowest incidence (0 – 27.8%).9; 20; 21 None of these studies examined the relationship between phrenic nerve paresis and block effectiveness. The current study demonstrates improved block scores in the setting of HDP, which is explained by the anatomy of the fascial planes of the neck: A local anesthetic volume, no matter how small, deposited within the prevertebral fascial layer that is sufficient to block the brachial plexus will likely also reach the phrenic nerve contained within the same fascial layer. Some posterolateral or “corner pocket” injections may potentially occur outside this fascial layer and result in brachial plexus blockade via diffusion, with less local anesthetic spread to the phrenic nerve. Spread to the phrenic nerve might also theoretically be avoided by injecting one layer inward from the prevertebral fascial plane (analogous to injection within the circumneural (paraneural) sheath of peripheral nerves).22; 23 With the current state of ultrasound technology, it is impossible to reliably confine injections to either outside the prevertebral fascial layer or within the equivalent of the circumneurium of the brachial plexus.
This study utilized NIF to assess changes in respiratory function. We found a significant decrease in NIF in subjects experiencing HDP after supraclavicular block. NIF reflects the strength of the diaphragm and other inspiratory muscles, and has been established as a useful clinical endpoint when evaluating respiratory muscle strength, especially of the diaphragm.24; 25 In addition, NIF is easy to perform and well-tolerated.24 Unilateral diaphragmatic paralysis decreases NIF to approximately 60% of predicted value.24 This study demonstrated a decrease in NIF to 81% of the pre-block baseline. NIF measurement is effort-dependent,24 and this may explain why one subject demonstrated an increase in NIF 30 minutes after the block despite demonstrating HDP on ultrasound exam.
The subjects with HDP experienced a statistically significant decrease in oxygen saturation, although the degree of decrease is arguably clinically insignificant. No difference in subjective dyspnea was seen in those subjects with HDP compared to those without.
No respiratory interventions were required in subjects with HDP. This is consistent with other studies that have demonstrated tolerance of unilateral diaphragmatic paresis associated with significant changes in pulmonary function tests in subjects free from baseline respiratory disease, 26; 27 and is likely attributable to compensation from the contralateral diaphragm and accessory muscles of respiration. Likewise, studies in healthy subjects demonstrate minimal to no change in oxygen saturation in the setting of block-induced unilateral paresis.28; 29 In subjects with obesity or baseline respiratory pathology, unilateral diaphragmatic paresis may result in dyspnea and hypoxia.4
Weaknesses
Algorithm-based up-and-down dose escalation designs are commonly used in Phase I dose-finding trials to avoid unnecessary exposure of subjects to subtherapeutic doses of an agent while limiting toxicity.14 This methodology was useful in this trial to minimize subject exposure to potentially sub-anesthetic volumes of local anesthetic while determining a dose-response curve for HDP. Algorithm-based methods use a set of pre-specified rules for escalation and de-escalation, without assuming any model on the dose toxicity curve. Although these are the most commonly utilized designs in Phase I dose-finding trials, algorithm-based designs have several weaknesses including a high variability in the estimate of the maximum-tolerated dose (MTD), a failure to incorporate an explicit targeted dose limiting toxicity rate, and small sample size.30 Due to this lack of precision, it should be recognized that the dose response curve presented here may not definitively represent the incidence of HDP at various volumes of local anesthetic. What the curve does accurately demonstrate, however, is that it is impossible to avoid HDP at doses as low as 5 mL when administering a supraclavicular block.
This study exhibited a gender asymmetry among subjects, with only 1 male among 21 subjects. This was purely coincidental. It has been established that the diaphragm excursion during VS is greater in males than females, most likely related to differences in height and weight11. This is irrelevant in terms of the NIF endpoint, as each subject was compared against him/herself. It is unlikely, but theoretically possible, that the susceptibility to supraclavicular block-induced HDP differs between males and females. There is no established relationship between gender and the effect of local anesthetic volume on motor or sensory block.
Conclusions
In conclusion, this study defined a dose-response relationship between local anesthetic volume and hemidiaphragmatic paresis after supraclavicular block. There is no clinically-relevant dose at which HDP can be reliably avoided, most likely due to the investment of the phrenic nerve and brachial plexus within the same prevertebral fascial sheath. In our subject population free from baseline respiratory disease, HDP and its subsequent effect on respiratory function as measured by NIF and oxygen saturation were well-tolerated.
ACKNOWLEDGEMENTS
We would like to thank Mariya Redko, Stephen Marcott, and Jenna Yousif for their invaluable help in the execution of this study. We would also like to thank Drs. William Ricci, Joseph Lane, and John Lyden for their assistance as co-investigators. Finally, we would like to thank Dr. William Urmey for his review of an initial draft of the manuscript.
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
REFERENCES
- 1.Tran DQ, Layera S, Bravo D, Cristi-Sanchez I, Bermudez L, Aliste J. Diaphragm-sparing nerve blocks for shoulder surgery, revisited. Reg Anesth Pain Med 2019. [DOI] [PubMed] [Google Scholar]
- 2.Sutcliffe P, Lasrado S. Anatomy, head and neck, deep cervical neck fascia. In: Statpearls. Treasure Island (FL): StatPearls Publishing; [PubMed] [Google Scholar]
- 3.Gervasio A, Mujahed I, Biasio A, Alessi S. Ultrasound anatomy of the neck: The infrahyoid region. J Ultrasound 2010;13:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Boghdadly K, Chin KJ, Chan VWS. Phrenic nerve palsy and regional anesthesia for shoulder surgery: Anatomical, physiologic, and clinical considerations. Anesthesiology 2017;127:173–191. [DOI] [PubMed] [Google Scholar]
- 5.Kant A, Gupta PK, Zohar S, Chevret S, Hopkins PM. Application of the continual reassessment method to dose-finding studies in regional anesthesia: An estimate of the ed95 dose for 0.5% bupivacaine for ultrasound-guided supraclavicular block. Anesthesiology 2013;119:29–35. [DOI] [PubMed] [Google Scholar]
- 6.Song JG, Jeon DG, Kang BJ, Park KK. Minimum effective volume of mepivacaine for ultrasound-guided supraclavicular block. Korean J Anesthesiol 2013;65:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal JM, Moore JM, Kopacz DJ, Liu SS, Kramer DJ, Plorde JJ. Quantitative analysis of respiratory, motor, and sensory function after supraclavicular block. Anesth Analg 1998;86:1239–44. [DOI] [PubMed] [Google Scholar]
- 8.Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979;7:346–9. [DOI] [PubMed] [Google Scholar]
- 9.Renes SH, Spoormans HH, Gielen MJ, Rettig HC, van Geffen GJ. Hemidiaphragmatic paresis can be avoided in ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2009;34:595–9. [DOI] [PubMed] [Google Scholar]
- 10.Sivashanmugam T, Maurya I, Kumar N, Karmakar MK. Ipsilateral hemidiaphragmatic paresis after a supraclavicular and costoclavicular brachial plexus block: A randomised observer blinded study. Eur J Anaesthesiol 2019;36:787–795. [DOI] [PubMed] [Google Scholar]
- 11.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009;135:391–400. [DOI] [PubMed] [Google Scholar]
- 12.Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013;47:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med 2001;20:597–604. [DOI] [PubMed] [Google Scholar]
- 14.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase i cancer clinical trials. J Natl Cancer Inst 2009;101:708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares LG, Brull R, Lai J, Chan VW. Eight ball, corner pocket: The optimal needle position for ultrasound-guided supraclavicular block. Reg Anesth Pain Med 2007;32:94–5. [DOI] [PubMed] [Google Scholar]
- 16.Petrar SD, Seltenrich ME, Head SJ, Schwarz SK. Hemidiaphragmatic paralysis following ultrasound-guided supraclavicular versus infraclavicular brachial plexus blockade: A randomized clinical trial. Reg Anesth Pain Med 2015;40:133–8. [DOI] [PubMed] [Google Scholar]
- 17.Tedore TR, YaDeau JT, Maalouf DB et al. Comparison of the transarterial axillary block and the ultrasound-guided infraclavicular block for upper extremity surgery: A prospective randomized trial. Reg Anesth Pain Med 2009;34:361–5. [DOI] [PubMed] [Google Scholar]
- 18.May S, Bigelow C. Modeling nonlinear dose-response relationships in epidemiologic studies: Statistical approaches and practical challenges. Dose Response 2006;3:474–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stundner O, Meissnitzer M, Brummett CM et al. Comparison of tissue distribution, phrenic nerve involvement, and epidural spread in standard- vs low-volume ultrasound-guided interscalene plexus block using contrast magnetic resonance imaging: A randomized, controlled trial. Br J Anaesth 2016;116:405–12. [DOI] [PubMed] [Google Scholar]
- 20.Kang RA, Chung YH, Ko JS, Yang MK, Choi DH. Reduced hemidiaphragmatic paresis with a “corner pocket” technique for supraclavicular brachial plexus block: Single-center, observer-blinded, randomized controlled trial. Reg Anesth Pain Med 2018;43:720–724. [DOI] [PubMed] [Google Scholar]
- 21.Aliste J, Bravo D, Fernandez D, Layera S, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and small-volume supraclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med 2018;43:590–595. [DOI] [PubMed] [Google Scholar]
- 22.Boezaart AP. The sweet spot of the nerve: Is the “paraneural sheath” named correctly, and does it matter? Reg Anesth Pain Med 2014;39:557–8. [DOI] [PubMed] [Google Scholar]
- 23.Karmakar MK, Shariat AN, Pangthipampai P, Chen J. High-definition ultrasound imaging defines the paraneural sheath and the fascial compartments surrounding the sciatic nerve at the popliteal fossa. Reg Anesth Pain Med 2013;38:447–51. [DOI] [PubMed] [Google Scholar]
- 24.Dube BP, Dres M. Diaphragm dysfunction: Diagnostic approaches and management strategies. J Clin Med 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoser B, Fong E, Geberhiwot T et al. Maximum inspiratory pressure as a clinically meaningful trial endpoint for neuromuscular diseases: A comprehensive review of the literature. Orphanet J Rare Dis 2017;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.al-Kaisy AA, Chan VW, Perlas A. Respiratory effects of low-dose bupivacaine interscalene block. Br J Anaesth 1999;82:217–20. [DOI] [PubMed] [Google Scholar]
- 27.Borgeat A, Perschak H, Bird P, Hodler J, Gerber C. Patient-controlled interscalene analgesia with ropivacaine 0.2% versus patient-controlled intravenous analgesia after major shoulder surgery: Effects on diaphragmatic and respiratory function. Anesthesiology 2000;92:102–8. [DOI] [PubMed] [Google Scholar]
- 28.Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2008;101:549–56. [DOI] [PubMed] [Google Scholar]
- 29.Wong AK, Keeney LG, Chen L, Williams R, Liu J, Elkassabany NM. Effect of local anesthetic concentration (0.2% vs 0.1% ropivacaine) on pulmonary function, and analgesia after ultrasound-guided interscalene brachial plexus block: A randomized controlled study. Pain Med 2016;17:2397–2403. [DOI] [PubMed] [Google Scholar]
- 30.Braun TM. The current design of oncology phase i clinical trials: Progressing from algorithms to statistical models. Chin Clin Oncol 2014;3:2. [DOI] [PubMed] [Google Scholar]