Abstract
Background:
Diamond Blackfan anemia (DBA) is an inherited bone marrow failure syndrome characterized by anemia, short stature, congenital anomalies and cancer predisposition. Most cases are due to mutations in genes encoding ribosomal proteins (RP) leading to RP haploinsufficiency. Effective treatments for the anemia of DBA include chronic red cell transfusions, long-term corticosteroid therapy or hematopoietic stem cell transplantation. In a small patient series and in animal models there have been hematologic responses to L-leucine with amelioration of anemia. The study objectives of this clinical trial were to determine feasibility, safety and efficacy of L-leucine in transfusion-dependent patients with DBA.
Procedure:
Patients ≥2 years of age received L-leucine 700 mg/m2 orally three times daily for 9 months to determine a hematologic response and any improvement in growth. (NCT01362595).
Results:
This multicenter, phase I/II study enrolled 55 subjects; 43 were evaluable. There were 21 males; median age at enrollment was 10.4 years (range, 2.5–46.1 years). No significant adverse events were attributable to L-leucine. Two subjects had a complete erythroid response and five had a partial response. Nine of 25, and 11 of 25, subjects experienced a positive weight and height percentile change, respectively, at the end of therapy.
Conclusions:
L-leucine is safe, resulted in an erythroid response in 16% of subjects with DBA, and led to an increase in weight and linear growth velocity in 36% and 44% of evaluable subjects, respectively. Further studies will be critical to understand the role of L-leucine in the management of patients with DBA.
Keywords: Diamond Blackfan anemia, L-leucine, erythropoiesis, clinical trial
Introduction
Diamond Blackfan anemia (DBA), a rare inherited bone marrow failure syndrome, is characterized by hypoproliferative, pro-apoptotic erythropoiesis and red cell failure as well as poor growth, birth defects and a predisposition to cancer (1). The vast majority of cases of DBA are due to inactivating mutations in one of 26 genes encoding either a large or small subunit-associated ribosomal protein (RP) resulting in RP haploinsufficiency (2). Most RP-associated DBA is inherited as autosomal dominant with variable penetrance; de novo cases represent sporadic new dominant mutations. Apart from RP mutations, there are few cases of DBA caused by mutations in X-linked genes encoding TSR2, a direct binding partner of RPS26 (3) and the erythroid transcription factor GATA1 (4). Since the 1950s corticosteroids have been the mainstay of treatment for the hematopoietic manifestations of DBA (5). While effective in approximately 80% of patients, only about half of the initial responders can be weaned to a tolerable, yet effective, dose. Some patients may remit, often remaining transfusion or corticosteroid independent for years. However, the majority of steroid non-responders require chronic red blood cell (RBC) transfusions with iron chelation or hematopoietic stem cell transplantation; each therapy associated with significant morbidity and the risk of death (6). In addition, none of these treatments address the issues of poor growth, birth defects or cancer predisposition. A number of agents have been tested in clinical trials, often without a strong scientific rationale, and have been proven to be largely ineffective in patients with DBA (7). Thus, despite considerable effort over the past two decades, these traditional approaches have remained the only therapeutic options effective in mitigating the red cell failure of DBA.
Prompted by the initial discovery in 1999 that DBA is a disorder of ribosome biosynthesis and function (1, 8), there is mounting experimental evidence suggesting that therapy directed at improving translational efficiency could possibly ameliorate both the erythroid failure and the growth retardation in DBA. The branched chain amino acid L-leucine had been shown to improve translational efficiency through activation of the eukaryotic translation initiation factor 4E-binding protein 1 as well as by upregulating ribosome biosynthesis through the mTOR signaling pathway (9). In 2007, Pospisilova and colleagues administered L-leucine to six patients from the Czech DBA registry resulting in one complete and two partial erythroid responses (10). Results from that cohort, and further in vivo responses in both murine (11) and zebrafish models (12) and in vitro evidence in CD34+ models (12), have provided substantial evidence supporting the performance of a multi-institutional phase I/II clinical trial of L-leucine in patients with DBA. The single-arm, open-label study reported here describes the feasibility, safety and efficacy of administering L-leucine to achieve a hematologic response and improve growth in red cell transfusion-dependent subjects with DBA.
Methods
Study Design
This open-label study, “A pilot phase I/II study of amino acid leucine in treatment of patients with transfusion-dependent Diamond Blackfan anemia”, was conducted at 12 study sites in the United States. The study objectives were to determine the feasibility and safety of administering the amino acid L-leucine and to determine the efficacy of L-leucine to produce a hematologic and growth response in transfusion-dependent subjects with DBA.
Subjects received L-leucine (Ajinomoto Aminoscience LLC, Raleigh, NC) 700 mg/m2/dose orally three times daily for 9 months. The dose was restricted by the Food and Drug Administration (FDA) to that used by Pospisilova et al.(10) Compliance was monitored at each monthly visit by a subject/parent report. L-leucine was dispensed at initiation of study and at the 3-month and 6-month time points. This was an intent-to-treat study therefore, noncompliant subject participation was truncated and they were considered non-responders. Furthermore, subjects were queried about concomitant medications and dietary supplements. Transfusion guidelines were set (Table 1) to preserve erythroid drive and maintain a physiologically acceptable hemoglobin (Hb). Subjects unable to comply with these parameters were withdrawn from the study. Other aspects of patient care were determined by local institutions based upon current standards of care for patients with Diamond Blackfan anemia (7). Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events v4.0 (http://ctep.cancer.gov/reporting/ctc.html). Hematologic response criteria including complete response (CR), partial response (PR), and no response (NR) were precisely defined (Table 2) and were evaluated monthly throughout nine months of therapy. Weight and height were obtained at baseline and at end of therapy for all patients. Percentiles were calculated using the CDC growth calculator for 2– 20 years (https://peditools.org/growthpedi/). For changes in height velocity, females less than 16 years of age and males less than 18 years of age were evaluated.
TABLE 1.
Transfusion therapy guidelines during L-leucine administration
| Hemoglobin (gm/dL) | Reticulocyte count (%) | Transfusion outcome |
|---|---|---|
| ≤8 | ≤0.5 (or baseline) | Transfuse as usual |
| >8 | >0.5 (or >baseline) | Hold transfusion for one week, if clinically stable, and recheck in one week |
| 7–8 | >0.5 or (>baseline) | Hold transfusion for one week, if clinically stable, and recheck in one week OR Transfuse 10ml/kg, or 1 unit, if >60 kg |
TABLE 2.
On-study response criteria
| Complete Response | Hb1 ≥9 gm/dL without need for transfusion |
| Partial Response | Hb <9 gm/dL with an increase in reticulocyte count |
| No Response | No change in Hb or reticulocyte count |
Hb, hemoglobin
Statistical considerations
In this standard Phase I/II pilot study with the goal of determining the feasibility, safety and efficacy of L-leucine in transfusion-dependent patients with DBA, the statistical approach is primarily descriptive in nature. The sample size was too small to correlate genotype with response to L-leucine.
Subjects
Eligible subjects were ≥2 years of age with a diagnosis of RBC transfusion-dependent Diamond Blackfan anemia, as previously published (7). Transfusion dependence was defined as ≥10 ml/kg or, if over 60 kg, 2 units of packed RBC per 28 days averaged over 84 days prior to study entry. The study was restricted to subjects who were receiving no DBA specific therapy other than packed RBC transfusions and had never undergone a hematopoietic stem cell transplant. Subjects were required to be off therapeutic corticosteroids or any other DBA-directed therapy for 30 days prior to study enrollment. Hydrocortisone for treatment of adrenal insufficiency was permitted. Iron chelation medications were allowed as standard of care for transfused patients. Renal and hepatic function were required to be adequate (creatinine clearance or glomerular filtration rate ≥ 70ml/min/1.73 M2 or a serum creatinine based on age and gender; total bilirubin ≤ 1.5 × upper limit of normal (ULN) for age, alanine aminotransferase < 5 × ULN for age, and serum albumin ≥ 2 g/dL.
The study was approved by the FDA and the Department of Defense Telemedicine and Advanced Technology Research Center and conducted in accordance with the protocol submitted to and approved by the United States Army Medical Research & Materiel Command Office of Research Protections Human Research Protection Office and the Institutional Review Board at each participating center. Written informed consent as per the Declaration of Helsinki was obtained from all study subjects or their representatives, if minors. The study was registered with ClinicalTrials.gov (NCT02386267).
Results
Fifty-five subjects consented to the trial between June 2013 and April 2016. Ten subjects were screen failures: two subjects had elevated liver enzymes; one was already on over-the-counter L-leucine; one was on corticosteroids; one was not transfusion-dependent for the prescribed time period; one due to history of severe non-compliance; one for inability to obtain prior medical records; one local hematologist was unable to comply with study requirements; and two subjects were misdiagnosed. Two additional subjects decided to not participate after signing consent.
Forty-three subjects were evaluable. The characteristics of the 43 subjects enrolled and treated in the study are described in Table 3. There were 21 males and 22 females; the median age of all subjects was 10.4 years (range 2.5–46.1 years). Four subjects withdrew from the study early. Two subjects were withdrawn early by parental decision due to the transfusion requirements. Two subjects experienced severe adverse events (SAEs). Both subjects who experienced a SAE required hospitalization: one subject presented with vomiting, dehydration and transaminitis deemed secondary to deferasirox toxicity; the other subject presented with emesis and bloody diarrhea and was found to have a gastrointestinal bleed, also deemed secondary to deferasirox. Both subjects recovered without any further events; however, their parents decided not to restart L-leucine.
TABLE 3.
Subject characteristics
| Evaluable patients (n=43) | |
|---|---|
| Gender, n1 | |
| Male | 21 |
| Female | 22 |
| Age at enrollment, n | |
| 2 to <10 years | 21 |
| 10 to <20 years | 13 |
| 20 to <30 years | 4 |
| 30 to < 40 years | 2 |
| >40 years | 3 |
| Prior steroid response, n | |
| Had a prior response | 19 |
| Never responded | 17 |
| Unknown | 7 |
| Genotypes (n) | |
| Complete response to L-leucine | RPL35A (1), Unknown (1) |
| Partial response to L-leucine | RPS17 (1), RPS24 (1), Unknown (3) |
| No response to L-leucine | RPS19 (11), RPS17 (4), RPS24 (2), RPS26 (1), RPS29 (1), RPL5 (1), RPL11 (2), RPL35A (2), Unknown (12) |
n, number of subjects
Two subjects experienced a hematologic CR and 5 subjects had a PR. The two subjects who had a CR did so at 1 month and 3 months after the start of L-leucine, respectively. The first subject was a 9-year-old male who had been transfusion-dependent since 5 weeks of age (Hb 2.3 gm/dL) and was never steroid responsive. His last transfusion was one month into the trial. At 9 months his Hb was 13.2 gm/dL with a reticulocyte count of 1.1%. Three years later he remains on L-leucine with Hb 11–12 gm/dL. The second subject was a 16-year-old male who presented at one week of age (Hb 7 gm/dL), was steroid responsive until age 12 years, and then became transfusion-dependent. His last transfusion was 3 months into the trial. At the end of the trial his Hb ranged from 8.7–9.5 gm/dL with a reticulocyte count of 0.9–1.9%. Five years later he remains transfusion independent and on daily L-leucine.
Five subjects, ranging in age from 2–43 years, had a PR, with an increase in reticulocyte counts from baseline (range, 0.1–0.7%) to the end of the study (range, 1.7–4.0%), however a Hb >9 gm/dL was unable to be maintained. Two of the 5 subjects with PR had a prior response to steroids, two had never had a response to steroids, and one subject’s response to steroids is unknown. Of note, one of the subjects who experienced a PR had a baseline reticulocyte count of 0.3% which increased to 4.0% by month 4 of L-leucine. One year after study completion he restarted L-leucine off study, responded again and has been transfusion independent for over 2 years.
Genotypes were known for 27 (63%) of the evaluable 43 subjects (Table 3). The most common DBA associated gene RPS19 was reported in 11 subjects. One of the subjects that experienced a CR was found to have a RPL35A mutation and 2 of the subjects with a PR had a RPS17 mutation and a RPS24 mutation, respectively. Of the subjects without a response to L-leucine, 24 had genetic mutations identified while 12 did not.
Weight and height were compared pre- and post-treatment with L-leucine for 25 subjects under 18 years of age for boys and under 16 years for girls, deemed on the basis of age to have theoretical growth potential (22 subjects at 9 months and 3 subjects at 8 months of therapy, respectively). Nine subjects (36%) experienced an increase in weight velocity (median 3.9 percentiles, range 1.1–8.4); 6 of these were 2 years to less than 9 years of age, with no difference by gender. The remainder of the subjects had either no change (n=4) or a decrease (n=12) in weight velocity. With regard to the hematologic response to L-leucine in these subjects, one had a hematologic CR, 2 had a hematologic PR and 6 had no hematologic response. Eleven subjects (44%) had an increase in height velocity ranging from 0.7–24.7 percentiles (median 3.5) (Figure 1), while 56% had no change (n=3) or a decrease (n=11) in height velocity. As with the increase in weight velocity, seven of the 11 subjects with an increase in height velocity were aged 2 to less than 9 years. The two patients who experienced a hematologic CR increased their height 2.6 and 8.6 percentiles, respectively. Of the other 9 patients with a growth response, 1 had a hematologic PR and 8 had no hematologic response. Five patients had both an increase in weight and height velocity, including one subject with a CR and one with a PR.
FIGURE 1.
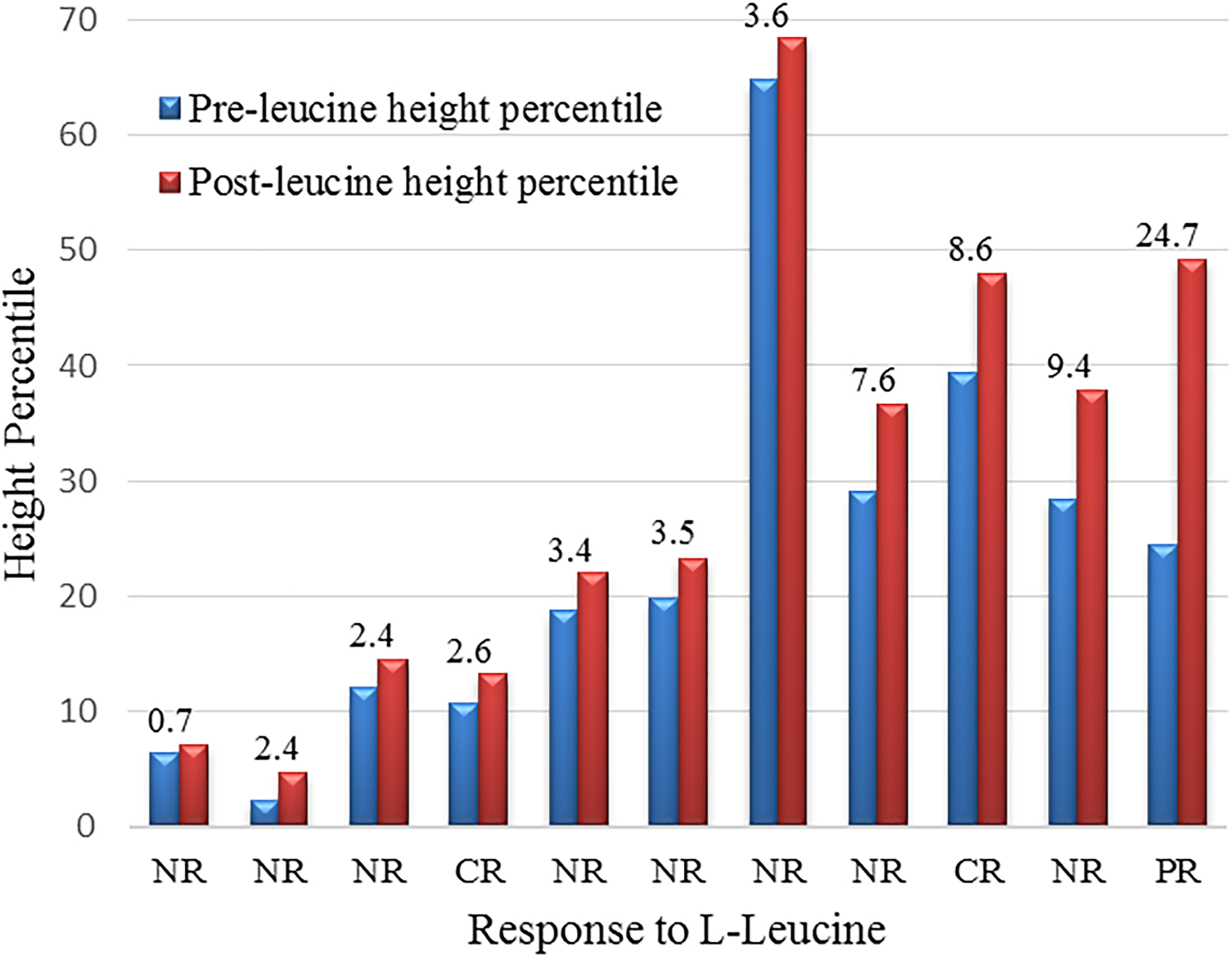
Change in height percentiles in subjects receiving L-leucine.
Comparison of pre- to post-L-leucine height percentiles in 11 patients with growth potential (males and females up to age 18 years and 16 years, respectively). Numbers above pre- and post- bars are the number of percentiles of accelerated growth during the study duration of 8–9 months. Each subject’s response to L-leucine is categorized below each set of bars. NR, no response; CR, complete response; PR, partial response.
Discussion
Historically the treatment of Diamond Blackfan anemia has been empiric and directed exclusively to ameliorating anemia. Since the 1950s (5), for those patients who responded, corticosteroids have supplanted red blood cell transfusions as the treatment of choice for DBA. After over 60 years of corticosteroid use in DBA, their significant untoward effects on bone mineralization as well as development and growth were recognized as dose limiting. In particular, it was noted that patients with DBA were inordinately sensitive to these corticosteroid side effects (6, 7). As red blood cell transfusions became safer, with regard to the elimination of blood-borne viruses combined with the availability of effective iron chelators and a reduced incidence of allosensitization due to extended red cell typing, RBC transfusions have been used more liberally to mitigate the consequences of long-term chronic corticosteroid exposure in DBA. The adverse consequences of prolonged corticosteroid administration, even at lower doses, and iron overload in transfused patients pointed to the urgent need for alternative therapies. Trials of immunosuppressive agents, cytokines and others drugs, based upon anecdotal observations, have failed (7). Other than treating the anemia of DBA, there was no evidence to suggest that corticosteroids, non-specific in their action, could serve to ameliorate growth failure found in DBA.
In 1999 DBA was identified as a ribosomopathy (8), with haploinsufficiency of at least 26 DBA-linked RPs, small and large subunit-associated (RPS and RPL), now confirmed (2). As a disorder of ribosome biogenesis and function, a number of theories have been proposed linking faulty translation to the manifestations of DBA. Many of the postulated negative effects of RP haploinsufficiency on the erythron are operational in other tissues in DBA (13). L-leucine is the first therapy with a rationale based upon an understanding of the consequence of ribosomal protein haploinsufficiency resulting in markedly inefficient and defective translation and resultant nucleolar stress.
Like erythropoiesis, bone development and growth, both intramembranous and endochondral, require high translational capacity to support cell proliferation, matrix formation and ossification (14). These features likely underlie common abnormalities in craniofacial development, skeletal defects and/or poor linear growth observed in DBA (6, 7). With an understanding of the molecular pathophysiology of DBA, it is not surprising to see a salutary effect of L-leucine on growth in patients with DBA (14). In this study, a substantial number of patients with growth potential demonstrated accelerated growth during their treatment window. All patients were on chronic transfusion schedules and had not been on steroid therapy for more than one year so the height velocity increase cannot be attributed to discontinuing steroid therapy. Notably, growth acceleration was much more frequent at the study dose of L-leucine than were improvements in erythropoiesis.
This study demonstrates that the administration of L-leucine is safe and effective. L-leucine administration resulted in an erythroid response (CR and PR) in 16% of subjects, two subjects becoming transfusion independent and 5 subjects increasing their reticulocyte count over baseline. Patients with DBA have reticulocytopenia regardless of the degree of anemia due to the erythroid hypoplasia therefore the increase in reticulocyte count observed is not secondary to the prescribed change to a lower hemoglobin threshold in some patients (Table 1) driving erythropoiesis. L-leucine therapy also led to an increase growth velocity, as weight percentile in 36% and height percentile in 44% of evaluable subjects. The results indicate that L-leucine can ameliorate both anemia and, to a greater extent, growth failure characteristic of DBA. Experimental murine and zebrafish models of DBA were found to respond to L-leucine however the mouse model, based upon weight, was given about four times the dose prescribed to the subjects in this study and the fish were swimming in leucine added to the water (11, 12). Thus, it is likely that the dose of L-leucine in this trial was suboptimal. We therefore hypothesize that higher doses of L-leucine will lead to hematologic responses and improve growth velocity in additional RBC transfusion-dependent patients in anticipation of a dose escalation study.
The number of patients were too small to draw any inferences regarding a genotype-phenotype correlation to the L-leucine response. Also there was no indication of a prior response to corticosteroids correlating with L-leucine response.
L-leucine is found in many nutritional supplements and the initial hypothesis included a potential increase in growth for these patients. Patients with DBA are often shorter and smaller than others their age. Both weight and height percentiles were increased in over one-third of the patients, more often in the younger group of children taking L-leucine. Given these preliminary findings, further studies should continue to include young children as the greatest effect may be in that age group. The increase in weight percentiles did not often correlate with an increase in height percentile. However the increase in weight percentile was associated with a positive effect, with many caregivers expressing that their children seemed to be less fatigued and were more energetic when taking L-leucine (10). This effect was not measured in our study but should be evaluated in future dose escalation studies of L-leucine in patients with DBA. The follow-up study will also include steroid-dependent patients with DBA to evaluate the effect of L-leucine on erythropoiesis and growth in those patients as well.
Acknowledgements
We are grateful to the patients with Diamond Blackfan anemia (DBA), their parents and families who participated in this trial and the Diamond Blackfan Anemia Foundation for their continued cooperation and recruitment efforts for the Diamond Blackfan Anemia Registry. This work was supported by the Department of Defense Telemedicine and Advanced Technology Research Center (W81XWH-10-2-0 177; AV, JML), the National Institutes of Health National Heart, Lung, Blood Institute ([R01 HL079571; JML, AV, EA], [U01 HL16021; AV, JML], [R01 HL150194, JEF]; [R01 HL144436, AN]); National Institutes of Health National Institute of General Medical Sciences (P20 GM121293; JEF); Arkansas Biosciences Institute (JEF); Feinstein Institutes for Medical Research (JML, AV, EA), Gambino Medical Science Foundation (JML), Pediatric Cancer Foundation (JML).
Abbreviations:
- CR
Complete response
- DBA
Diamond Blackfan anemia
- FDA
Food and Drug Administration
- Hb
Hemoglobin
- NR
No response
- PR
Partial response
- RBC
Red blood cell
- RP
Ribosomal protein
- RPL
Ribosomal protein, large subunit-associated
- RPS
Ribosomal protein, small subunit-associated
- SAE
Severe adverse event
Footnotes
Conflict of Interest
The authors do not report any conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
- Vlachos A, Atsidaftos E, Muir E, Lababidi ML, Alhushki W, Farrar JE, Glader B, Gruner BA, Hartung H, Knoll C, Lowe TW, Nalepa G, Narla A, Panagrahi A, Rogers ZR, Sieff CA, Walkovich K, Lipton JM. Leucine for the Treatment of Transfusion Dependence in Patients with Diamond Blackfan Anemia. American Society of Hematology. December 2018. San Diego, CA. Oral Presentation.
- Vlachos A, Atsidaftos E, Muir E, Rogers Z, Lababidi ML, Alhushki W, Bernstein J, Farrar J, Glader B, Gruner B, Hartung H, Knoll C, Nalepa G, Panigrahi A, Sieff C, Walkovich K, Narla A, Lipton J. Leucine for the Treatment of Transfusion Dependence in Patients with Diamond Blackfan Anemia. American Society of Pediatric Hematology/Oncology, April 2019. New Orleans, LA. Selected to be presented as a special “Highlights of ASH” oral presentation.
References
- 1.Vlachos A, Blanc L, Lipton JM. Diamond Blackfan anemia: a model for the translational approach to understanding human disease. Expert Rev Hematol 2014;7:359–72. [DOI] [PubMed] [Google Scholar]
- 2.Ulirsch JC, Verboon JM, Kazerounian S, et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am J Hum Genet 2018;103:930–947. Erratum in: Am J Hum Genet 2019;104:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gripp KW, Curry C, Olney AH, et al. Diamond-Blackfan anemia with mandibulofacial dystostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. Am J Med Genet A 2014;164A:2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sankaran VG, Ghazvinian R, Do R, et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest 2012;122:2439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasser C Aplastic anemia (chronic erythroblastophthisis) and cortisone. Schweiz Med Wochenschr 1951;81:1241–2. [PubMed] [Google Scholar]
- 6.Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood 2010;116:3715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol 2008;142:859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustavsson P, Andersson B, Pettersson M, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 1999;21:169–75. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Gogol M, Gaudenz K, Gerton JL. Improved transcription and translation with L-leucine stimulation of mTORC1 in Roberts syndrome. BMC Genomics 2016;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pospisilova D, Cmejlova J, Hak J, Adam T, Cmejla R. Successful treatment of a Diamond-Blackfan anemia patient with amino acid leucine. Haematologica 2007;92:e66–7. [DOI] [PubMed] [Google Scholar]
- 11.Jaako P, Debnath S, Olsson K, Bryder D, Flygare J, Karlsson S. Dietary L-leucine improves the anemia in a mouse model for Diamond-Blackfan anemia. Blood 2012;120:2225–8. [DOI] [PubMed] [Google Scholar]
- 12.Payne EM, Virgilio M, Narla A, et al. L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway. Blood 2012;120:2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattangadi S, Lipton JM: Diamond Blackfan anemia. In: Pediatric Oncology, Bone Marrow Failure. New York: Springer; 2018. pp 137–152. [Google Scholar]
- 14.Trainor PA, Merrill AE. Ribosome biogenesis in skeletal development and the pathogenesis of skeletal disorders. Biochim Biophys Acta 2014;1842:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


