Abstract
Apelin-13 and APJ are implicated in different key physiological processes. This work aims at exploring the radioprotective effect of fucoxanthin (FX) on γ-radiation (RAD)-induced changes in the apelin-13/APJ pathway, which causes damage in the liver, kidney, lung and spleen of mice. Mice were administered FX (10 mg kg–1 day–1, i.p) and exposed to γ-radiation (2.5 Gy week–1) for four consecutive weeks. The treatment of irradiated mice by FX resulted in a significant amendment in protein expression of the apelin-13/APJ/NF-κB signalling pathway concurrently with reduced hypoxia (hypoxia-inducible factor-1α), suppressed oxidative stress marker (malondialdehyde), enhanced antioxidant defence mechanisms (reduced glutathione and glutathione peroxidase), a modulated inflammatory response [interleukin-6 (IL-6), monocyte chemoattractant protein-1, IL-10 and α-7-nicotinic acetylcholine receptor) and ameliorated angiogenic regulators [matrix metalloproteinase (MMP-2), MMP-9 and tissue inhibitor of metalloproteinase-1), as well as the tissue damage indicator (lactate dehydrogenase) in organ tissues. In addition, there were significant improvement in serum inflammatory markers tumour necrosis factor-α, IL-10, IL-1β and C-reactive protein compared with irradiated mice. The histopathological investigation of the FX + RAD organ tissues support the biochemical findings where the improvements in the tissues’ architecture were obvious when compared with those of RAD. FX was thus shown to have a noticeable radioprotective action mediated through its regulatory effect on the apelin-13/APJ/NF-κB signalling pathway attributed to its antioxidant and anti-inflammatory activity that was reflected in different physiological processes. It could be recommended to use FX in cases of radiation exposure to protect normal tissues.
Keywords: Fucoxanthin, γ-radiation, MMP-9, Apelin-13/APJ, α-7nAchR
INTRODUCTION
Exposure to ionizing radiation (IR) has occurred during radiology (diagnostic or interventional), radiotherapy and occupational exposure in the radiation field. High radiation doses cause death whereas sublethal doses may induce diverse diseases, such as cancer, cardiovascular diseases and cataracts [1]. The detrimental effects of IR exposure involve the induction of tissue damage mediated through the activation of pro- and antiproliferative endogenous signalling pathways, inflammation and oxidative stress in a synchronized sequence of actions that alter the homeostatic equilibrium between survival and cell death [2]. The mechanism of IR that produces these effects depends mainly on the generation of different free radicals and molecular species within cells, such as superoxide (O2·–), nitric oxide (NO), the hydroxyl radical (OH·), hydrogen peroxide (H2O2) and peroxynitrite (ONOO–), that are produced via direct interactions or subsequent metabolites of IR and cause damage to cellular DNA content, proteins and lipids. In addition, Reisz et al. [3] and Yahyapour et al. [1] stated that, in different organs, IR can robustly affect the immune system, by altering the number and function of immune cells, leading to changes in the normal immune responses. Thus, continuous free radical production and chronic inflammation that occur after radiation exposure can disrupt organ function and subsequently cause several diseases. As exposure to IR is inevitably accompanied by production of high levels of reactive oxygen species (ROS), so the management of this action could provide a method to avoid the deleterious effects on normal tissues upon radiation exposure. Therefore, radiation countermeasure agents should be used to reduce the hazardous effects of IR. Radiation countermeasure agents are classified according to their time of administration into: (i) radioprotectors used before IR exposure to protect cells and tissues from being damaged; (ii) radiomitigators applied soon after the exposure to IR to repair tissues before the appearance of symptoms; and (iii) therapeutic agents administered after IR exposure to enhance healing of injuries via regeneration of tissues. Radioprotectors are various agents that act via different mechanisms involving: scavenging of free radicals and ROS; improvement of the DNA repair process; synchronizing of cells; enhancing antioxidant and redox-sensitive genes; modulating cytokines and growth factors; inhibiting apoptosis; repurposing of drugs; and tissue regeneration. Scavenging of free radicals is the most common mechanism of radioprotection, whereas the alteration of growth factors, cytokines and redox genes appears to be an effective strategy [4].
Apelin and its endogenous ligand APJ (apelin receptor; a member of the G-protein-coupled receptors, similar to angiotensin II receptor-like-1) can exert significant biological effects in different tissues and organs. They can strengthen cardiac contractility, regulate immune defence, gastrointestinal function and insulin sensitivity, and promote cell proliferation, migration and angiogenesis. In addition, apelin produces important effects on the physiology and pathophysiology of liver and kidney function, and plays a crucial role in body fluid homeostasis [5, 6]. A lot of evidence pointed to a strong relationship between oxidative stress and apelin/APJ interaction. Apelin can inhibit the formation and release of ROS [7]. In contrast, Li et al. [8] reported that apelin-13 enhances the generation of ROS linked to the existence of oxidative stress that directly leads to vascular damage and a series of inflammatory reactions. Thus, the function of the apelin/APJ signalling pathway is a double-edged sword in insults involving oxidative stress and inflammatory-related diseases, as reported by Zhou et al. [7]. Therefore, there are still certain controversies and doubts about the exact effect of the apelin/APJ signalling pathway in different conditions. Drugs that target the apelin/APJ pathway might be recommended as a novel therapy for the related oxidative stress and inflammatory diseases.
A great deal of evidence revealed a positive relationship between oxidative stress and inflammation, and each leads to the other in a feedforward mechanism. The overproduction of ROS induces an oxidative modification of biomolecules leading to the enhancement of signalling cascades and activation of transcription factors which are linked to the genes of pro-inflammatory mediators and initiate the inflammatory reactions. Inflammation causes immune cells to secrete various cytokines, which evoke additional immune cells near oxidative stress and generate ROS at the inflammatory site, causing augmented oxidative stress and tissue damage [9]. The study of Ren et al. [10] mentioned that splenic α-7-nicotinic acetylcholine receptor (α-7nAchR) is a primary receptor of the cholinergic anti-inflammatory pathway (CAP) that displays widespread anti-inflammatory reactions and the immune-modulatory response to maintain immune homeostasis. Moreover, Viedt et al. [11] stated that monocyte chemoattractant protein-1 (MCP-1) functions as a pro-inflammatory mediator inducing the production of pro-inflammatory molecules other than just chemokine. It promotes pro-inflammatory reactions in human tubular epithelial cells via up-regulation of the pro-inflammatory interleukin-6 (IL-6) and the adhesion molecule, intercellular adhesion molecule-1 (ICAM-1), through the classical inflammatory pathways, involving sequence-specific DNA binding of nuclear factor-κB (NF-κB) and activating protein-1. Under the normal physiological environment, the regular actions of matrix metalloproteinases (MMPs) are controlled at the level of transcription (activation of the precursor zymogens) and interaction with specific extracellular matrix (ECM) constituents. MMPs are zinc-containing enzymes that destroy the ECM and proteins of connective tissue. This proteolytic effect of MMPs takes part in vascular remodelling, cellular migration and the processing of the ECM. Tissue inhibitor of metalloproteinases (TIMP) elicits a complementary mechanism with MMPs to avoid excessive degradation of the ECM. An imbalance between them could induce exaggerated MMP activity that leads to pathological changes in the structure of the vessel wall inter-related to vascular disease [12]. In addition, Jain et al. [13] showed that lactate dehydrogenase (LDH), an oxidoreductase enzyme, which is found in all living cells and monitors membrane integrity, is released into the cytoplasm upon cell lysis of damaged cells more than of normal cells.
Management of chronic inflammation or inflammation in general is a critical point in the struggle to tame dangerous diseases associated with these undesired disorders. Nowadays, an alternative approach in radiation protection research is oriented towards using natural compounds having many biologically positive influences to overcome several health problems and biological alterations due to their wide safety margin and many beneficial properties (such as antioxidant, immune stimulation, anti-inflammatory and antitumour).
Fucoxanthin (FX), a xanthophyll derivative, is the leading carotenoid formed in brown algae. FX exhibits a variety of pharmacological properties and biological functions including antioxidant, antiviral, anticancer, antidiabetic, UV-preventative, neuroprotective and repressing inflammation without side effects [14–16] because of its unique functional groups, including an infrequent allenic bond and a 5,6-monoepoxide within its molecular construction [15].
Yet, the potential role of the apelin/APJ pathway and how its interference with other mediators might be involved in mediating the deleterious impact of γ-radiation exposure is yet to be elucidated. Therefore, the current study was designed to investigate (i) the effect of γ-radiation on the apelin-13/APJ pathway and its relevance to certain physiological processes in the liver, kidney, lung and spleen of irradiated mice and (ii) whether the effect of FX on the alterations could happen in the apelin-13/APJ pathway in tissues of these organs. To reach these goals, this study monitored the protein expression of the apelin-13/APJ pathway and determined oxidative stress status {hypoxia-inducible factor-1α (HIF-1α), lipid peroxidation [measured as malondialdehyde (MDA)], reduced glutathione (GSH) and glutathione peroxidase (GSH-PX)}. The pro- and anti-inflammatory molecules [NF-κB, α-7nAchR, MCP-1, IL-6, IL-10 and tumour necrosis factor-α (TNF-α)] were determined in different mice groups. Histopathological investigations of the matrix metalloproteinase balance (MMP-2, MMP-9 and TIMP-1) were carried out on the tissue obtained from these four organs.
MATERIALS AND METHODS
Materials
Fucoxanthin (FX) was obtained from Serene Dew supplements. For western blot analysis, the antibodies against apelin-13 (cat no. CAS 217082-58-1) and β-actin (mouse monoclonal antibody cat no. sc-47778) were obtained from Santa Cruz Biotechnology, and the other antibodies against APJ (rabbit polyclonal antibody cat no. ab214369), NF-κBp65-Ser536 (cat no. ab76302) and α-7nAchR (rabbit polyclonal antibody, cat no. ab10096) were from Abcam. The other chemicals and reagents used in this study are from Sigma-Aldrich Chemical Co. USA.
Radiation facility
Mice were exposed to whole-body γ-irradiation (RAD) using Canadian γ-cell-40 (137 Cs). Irradiation procedures were performed at the NCRRT (Cairo, Egypt) at a dose rate of 0.4 Gy min–1 of γ-rays. The experimental animals were placed in a plastic sample tray with a lid and supports provided for the sample cavity. The unit has ventilation holes, which align with ventilation parts through the main shield to provide a means for uniform irradiation for small animals at a dose rate of 0.403 Gy min–1 at the time of the experiment according to the guidelines of the Protection and Dosimetery Department using a Fricke reference standard dosimeter [17], and confirmed by alanine dosimeters (traceable to the National Institute of Standards and Technology, USA) that were placed along with the irradiated mice inside the irradiation facility. IR weekly doses were given at fixed time intervals each week (in the middle of the week; fourth day during the experimental course to maintain optimum experimental conditions). The irradiation procedures were performed at the NCRRT facility (Cairo, Egypt).
Animals
The Swiss female albino mice adult mice (weighing 22-25 g) used in this study were obtained from the Egyptian Organization for Biological Products and Vaccines (Cairo) breeding unit. Mice were acclimatized and maintained on water ad libitum and a standard commercial pellet diet for 1 week. Experimental animals were used and handled according to the recommendations of the National Institute of Health (NIH no. 85:23, revised 1996) for the care and use of laboratory animals and in accordance with guidelines adopted by the NCRRT ethics committee which approved all experimental procedures (ref no. 10A/20).
Experimental plan
Mice were divided into four equal groups (10 mice/group). (i) Control group: normal mice received only physiological saline i.p. (ii) RAD group: mice were exposed to γ-radiation (2.5 Gy week–1). (iii) FX group: mice were injected i.p with FX at a dose of 10 mg kg–1 day–1 dissolved in physiological saline for 4 weeks according to Ma et al. [8]; and (iv) FX + RAD group: mice were treated with FX and were exposed to γ-radiation. FX was administrated (i.p.) for 3 days before γ-ray exposure to stimulate and impose a pre-conditioning status in normal cells to overcome and sustain the subsequent detrimental effects induced by irradiation in order to achieve radioprotection and adaptive responses of the tissues exposed. The γ-radiation dose was chosen according to the study of Zakaria [18] that aimed to determine the hazardous effects of low successive doses during exposure to γ-irradiation of many workers in the medical, industrial and petroleum fields who may be exposed during a small radiation accident to low or moderate γ-radiation doses (1.5, 2, 2.5, 3 and 3.5 Gy). These doses lead to acute effects on health efficiency and performance of organisms. Thus, in the current study we have chosen 2.5 Gy as a moderate dose to examine its action on the apelin-13/APJ pathway. Twenty-four hours after the last dose of FX, mice were fasted overnight, and then euthanized under light diethyl ether anaesthesia. Cardiac perforation drew blood samples, which were centrifuged for separation of serum and biochemical assessments. The target tissues (liver, kidney, lung and spleen) were excised, then washed in ice-cold saline solution and prepared for the biochemical and histopathological investigations. Upon radiation exposure, these four vital organs were chosen to investigate the interconnection between them in terms of concerted regulation of oxidative stress and inflammatory mediators affected by the dysregulation of the apelin-13/APJ pathway.
Biochemical assays
MDA, the end-product of lipid peroxidation, was assayed according to Yoshioka et al. [19], the GSH content was assayed according to Ellman [20], protein concentration was detected according to the method of Lowry et al. [21] using Folin–Ciocalteu reagent, and the activity of GSH-PX was measured according to the method of Gross et al. [22]. Activities of AST (aspartate aminotransferase) and ALT (alanine aminotransferase) were assayed as described by Reitman and Frankel [23]. Urea and creatinine were measured according to the techniques of Fawcett and Soctt [24] and Bartles et al. [25], respectively. The levels of HIF-1, MCP-1, LDH, the inflammatory mediators IL-10, IL-6, IL-1β, TNF-α and C-reactive protein (CRP), MMP-2, MMP-9 and TIMP-1 were assessed by ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Western blot analysis
For western immunoblotting, the tissue homogenates of liver, kidney, lung and spleen were prepared as defined by Omar et al. [26] using a homogenization lysis buffer (Sigma–Aldrich, St. Louis, MO, USA). Then, the lysate of tissues was centrifuged at 8678 g for 20 min 4°C. The supernatants were taken to measure protein concentration by using the BCA-protein assay kit (Thermo Fisher Scientific). Gel electrophoresis was used to separate aliquots containing 7.5 μg of protein from each sample (SDS–PAGE gel; 8%) and after the run was completed the bands were transferred onto a nitrocellulose acetate membrane by using a semi-dry transfer device (Bio-Rad, Hercules, CA, USA). Thereafter, membranes were incubated with 5% non-fat milk blocking buffer containing Tris–HCl (10 mmol l–1 at pH 7.4), NaCl (150 mmol l–1) and TBST (Tris-buffered saline with Tween-20 at 0.05%) overnight at 4°C. After washing with TBST, the membranes were incubated (overnight, at 4°C with continuous shaking) with 1:1000 diluted antibodies of apelin, APJ, NF-κB and α-7nAchR. After immunoblotting with the primary antibody, horseradish peroxidase- (HRP) conjugated goat immunoglobulin (Amersham Biosciences) was added. Chemiluminescence detection was performed by using the Amersham detection kit according to the manufacturer’s protocols. By using a scanning laser densitometer (Biomed Instruments), the levels of protein were quantified on the autoradiogram. The data were normalized to expression of β-actin, a housekeeping protein [27].
Histopathological examination
Tissue samples from liver, kidney, lung and spleen were fixed in 10% formalin saline for 24 h. The samples were then washed with tap water and subjected to dehydration using a sequential dilution of alcohol (ethyl, absolute and ethyl methyl). Subsequenttly, samples were cleared in xylene and in hot air at 56°C in an oven for 24 h and embedded in paraffin. The paraffin beeswax of tissue blocks was set and sectioned (at 4 mm thickness) using a slide microtome. Finally, on glass slides, the tissue sections were collected and de-paraffinized then stained by H&E stain (haematoxylin and eosin) for routine examination using light microscopy according to the method of Banchroft et al. [28].
Statistical analysis
Statistical analysis by ANOVA (one-way analysis of variance) was carried out, followed by Duncan’s multiple range test using SPSS version 17.0 for Windows. Differences between values were considered significant at P ˂0.05.
RESULTS
Impact of FX on oxidative and antioxidant status of certain organs in γ-irradiated mice
The data illustrated in Fig. 1 showed that the levels of MDA, GSH, GSH-PX and HIF-1α were not significantly changed in the liver, kidney, lung and spleen of the FX group when compared with control mice. However, Fig. 1 showed that radiation exposure according to the current protocol induced significant changes in oxidative stress and the antioxidant status of certain mouse organs. The HIF-1α and MDA levels increased significantly (P < 0.05) in the liver (MDA 3.41-fold and HIF-1α 3.03-fold), kidney (MDA 3.32-fold and HIF-1α 3.13-fold), lung (MDA 3.64-fold and HIF-1α 6.5-fold) and spleen (MDA 2.57-fold and HIF-1α 3.64-fold) when compared with the respective control. In contrast, the GSH content and GSH-PX activities decreased significantly in all organs subjected to investigation in this study as follows: liver (GSH 68.45% and GSH-PX 49.39%), kidney (GSH 67.58% and GSH-PX 54.79%), lung (GSH 51.68% and GSH-PX 43.99%) and spleen (GSH 54.49% and GSH-PX 56.41%). However, in the group of mice treated with FX before exposure to γ-radiation, a considerable amelioration in oxidative and antioxidant status manifested by a significant decrease (P < 0.05) in HIF-1α (liver 32.71%, kidney 48.18%, lung 44.37% and spleen 48.87%) and MDA (liver 42.79%, kidney 43.15%, lung 46.18% and spleen 47.52%) levels, and a substantial increase (P < 0.05) in GSH (liver 2.77-fold, kidney 2.52-fold, lung 1.57-fold and spleen 1.96-fold) content and GSH-PX (liver 1.8-fold, kidney 1.89-fold, lung 1.54-fold and spleen 2.02-fold) activities was observed in all organs subjected to investigation when compared with mice of the RAD group.
Fig. 1.
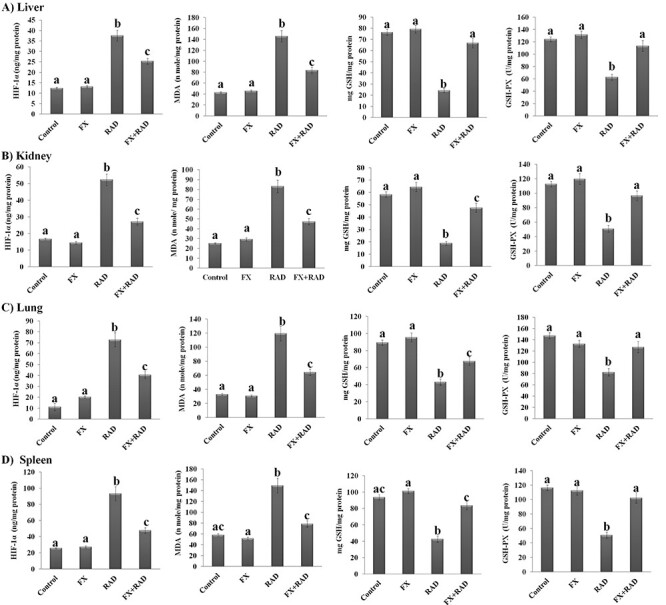
Impact of FX on oxidative (HIF-1α and MDA) and antioxidant status (GSH and GSH-PX) of (A) liver, (B) kidney, (C) lung and (D) spleen in γ-irradiated mice. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns having the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Impact of FX on inflammatory responses of certain organs in γ-irradiated mice
The data obtained from the present study showed that the inflammatory response (IL-6, MCP-1 and IL-10) in the liver, kidney, lung and spleen of the FX group was not altered significantly compared with the control mice. Also, the protein expression of splenic α-7nAchR was not changed significantly in the FX group in comparison with control mice. In contrast, the data of the inflammatory response mediators in certain organs significantly changed in mice exposed to γ-irradiation compared with the normal mice (Fig. 2). Among them, MCP-1 and IL-6 increased significantly (P < 0.05) in the liver (MCP-1 3.75-fold and IL-6 3.38-fold), kidney (MCP-1 3.12-fold and IL-6 4.80-fold), lung (MCP-1 2.39-fold and IL-6 4.75-fold) and spleen (MCP-1 2.45-fold and IL-6 3.37-fold) in irradiated mice compared with the control mice. The IL-10 level was significantly decreased in both lung (54.77%) and spleen (44.35%) of irradiated mice, associated with a considerable decrease (P < 0.05) in the protein expression of α-7nAchR in the spleen (55%) when compared with its equivalent value in control mice (P < 0.05). We observed significant changes in all inflammatory response parameters in all organs subjected to investigation in the current study when mice were injected with FX before exposure to γ-radiation. As shown, MCP-1 and IL-6 in all organs (liver, MCP-1 53.68% and IL-6 41.21%; kidney, MCP-1 52.78% and IL-6 58.69%; lung, MCP-1 54.17% and IL-6 51.62%; and spleen, MCP-1 42.92% and IL-6 70.33%) of the FX + RAD group were significantly (P < 0.05) decreased when compared with the RAD group. The IL-10 level in the lung (1.86-fold) and spleen (1.60-fold) significantly increased, associated with a significant increase in the splenic α-7nAchR protein expression (1.93-fold) compared with the RAD group.
Fig. 2.
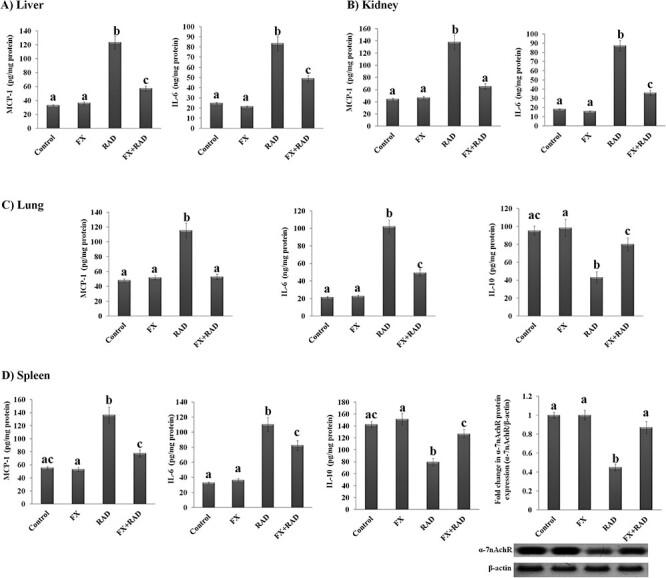
Impact of FX on inflammatory responses in (A) liver (MCP-1 and IL-6), (B) kidney (MCP-1 and IL-6), (C) lung (MCP-1, IL-6 and IL-10) and (D) spleen (MCP-1, IL-6 and IL-10) with representative western blot analysis of α-7nAchR (54 kDa) with its SDS–PAGE normalized to β-actin (43 kDa) protein expression in γ-irradiated mice. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns havimg the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Impact of FX on γ-irradiation-induced alteration in apelin-13/APJ/NF-κB signalling
The data exemplified in Fig. 3 (histograms and western blot output) showed that the protein expression of apelin-13 and its receptor APJ and a complex protein NF-κB (an inducible transcriptional factor) was not altered in the liver, kidney, lung and spleen of mice administered FX when compared with normal mice. However, the protein expression of apelin-13, PJ and NF-κB increased significantly (P < 0.05) in the four organs of the RAD group as compared with the control mice as follows: liver (5.62-, 6.4- and 5.15-fold), kidney (3.7-, 4.2- and 6.8-fold), lung (2.77-, 3.1- and 5.3-fold) and spleen (5.3-, 6.8- and 6.01-fold), respectively. Nevertheless, with FX administration, the protein expression of apelin-13, APJ and NF-κB was significantly decreased (P < 0.05) in the liver (68.91, 50.16 and 44.23%), kidney (48.65, 50.24 and 54.70%), lung (53.57, 34.19 and 52.83%) and spleen (58.49, 50.15 and 74.15%), respectively, in the FX + RAD group compared with the RAD group (Fig. 3).
Fig. 3.
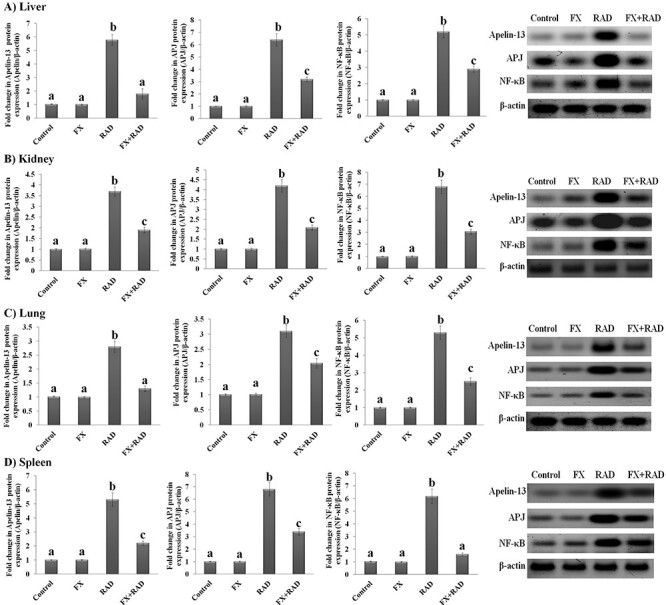
Impact of FX on the protein expression of apelin-13/APJ/NF-κB signalling in (A) liver, (B) kidney, (C) lung and (D) spleen with representative western blot analysis of apelin-13 (17 kDa), APJ (60 KDa) and NF-κB (65 kDa) with its SDS–PAGE normalized to β-actin (43 kDa) in mice exposed to γ-irradiation. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns havimg the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Impact of FX on the changes induced in MMP-2, MMP-9, TIMP-1 and LDH of certain organs in γ-irradiated mice
Data shown in Fig. 4 revealed that the activities of MMP-2 and MMP-9, the TIMP-1 level and LDH activity were not significantly changed (P < 0.05) in liver, kidney, lung and spleen of the FX mice group when compared with the control mice. In the RAD mice group, the MMP-2, MMP-9 and LDH activities increased significantly (P < 0.05) in the liver (2.47-, 2.32- and 1.65-fold), kidney (2.35-, 3.76- and 1.31-fold), lung (3.18-, 1.91- and 1.85-fold) and spleen (2.73-, 2.03- and 2.23-fold), respectively, when compared with the control mice, while a substantial decrease in TIMP-1 concentration (liver 53.26%, kidney 43.51%, lung 46.77% and spleen 54.09%) was observed when compared with controls. Mice treated with FX before exposure to γ-radiation showed a significant (P < 0.05) reduction in the changes induced by γ-radiation on MMP-2, MMP-9 and LDH as compared with the RAD group, as follows: liver (35.95, 50.85 and 24.56%), kidney (35.71, 38.65 and 13.69%), lung (54.86, 30.34 and 29.18%) and spleen (33.33, 42.67 and 30.49%), respectively. On the other hand, a significant elevation (P < 0.05) in TIMP-1 concentration of all organs (liver 1.70-fold, kidney 1.38-fold, lung 1.57-fold and spleen 1.59-fold) was observed when compared with the RAD group.
Fig. 4.
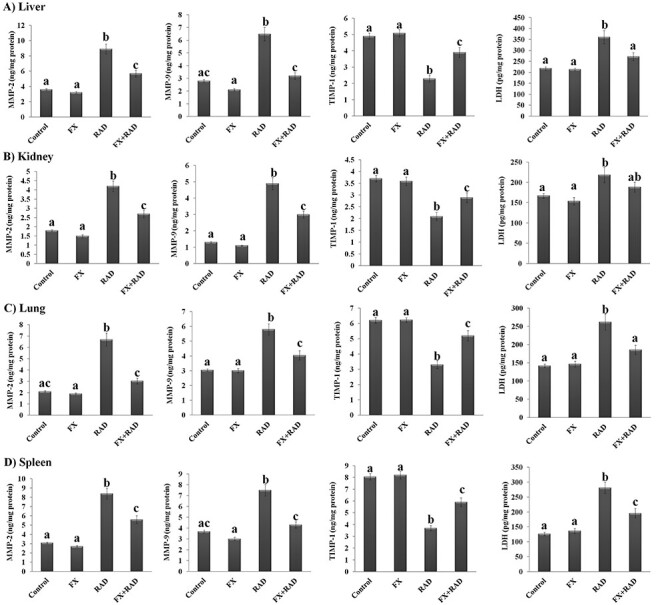
Impact of FX on the changes induced in MMP-2, MMP-9, TIMP-1 and LDH of (A) liver, (B) kidney, (C) lung and (D) spleen in irradiated mice. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns having the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Impact of FX on the changes induced in the physiological function of liver and kidney in γ-irradiated mice
Liver function, as shown by the results of ALT and AST enzymes in serum of mice who received FX, was not significant changed (P < 0.05) when compared with the normal mice (Fig. 5). However, in the mice group exposed to γ-radiation, the activities of these two enzymes (ALT 1.32-fold and AST 1.84-fold) increased significantly (P < 0.05) as compared with the control mice. However, the activities of ALT (15.78%) and AST (34.56%) were significantly (P < 0.05) decreased in mice who received FX and were exposed to γ-radiation when compared with the RAD group (Fig. 5).
Fig. 5.
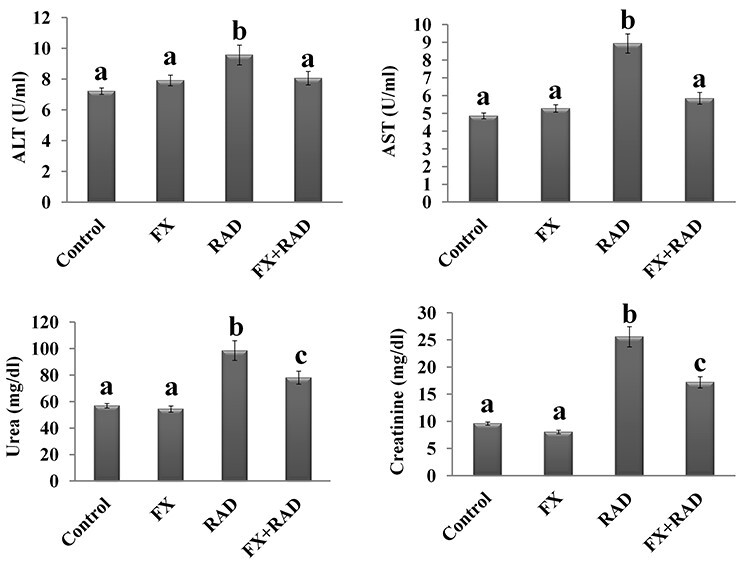
Impact of FX on the changes induced in physiological function of (A) liver (ALT and AST) and (B) kidney (urea and creatinine) in serum of irradiated mice. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns having the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Regarding kidney function, the data illustrated by Fig. 5 show that the concentration of urea and creatinine in the serum of mice who received FX treatment was not significantly changed (P < 0.05) as compared with the normal mice. The exposure of mice to γ-radiation stimulates a significant increase (P < 0.05) in the serum content of urea (1.73-fold) and creatinine (2.66-fold) compared with control mice. However, in mice who received FX treatment before γ-radiation exposure, the content of urea (20.73%) and creatinine (32.79%) showed a significant (P < 0.05) reduction as compared with the RAD group (Fig. 5).
Impact of FX on the changes induced in systemic inflammation of γ-irradiated mice
Figure 6 reveals that there were no significant changes (P < 0.05) in the serum inflammatory markers (TNF-α, IL-1β, CRP and IL-10) of mice who received FX when compared with the control mice. As expected, there were significant increases in the levels of TNF-α (2.56-fold), IL-1β (2.08-fold) and CRP (4.12-fold), and a significant decline in the level of IL-10 (38.65%), observed in mice exposed to γ-irradiation when compared with controls. Treatment with FX before exposure to γ-radiation brought about an incredible improvement in serum levels of the four measured inflammatory markers when compared with the RAD group, with a significant decrease in TNF-α (30.34%), IL-1β (30.89%) and CRP (42.73%), and a significant increase in the level of IL-10 (1.35-fold).
Fig. 6.
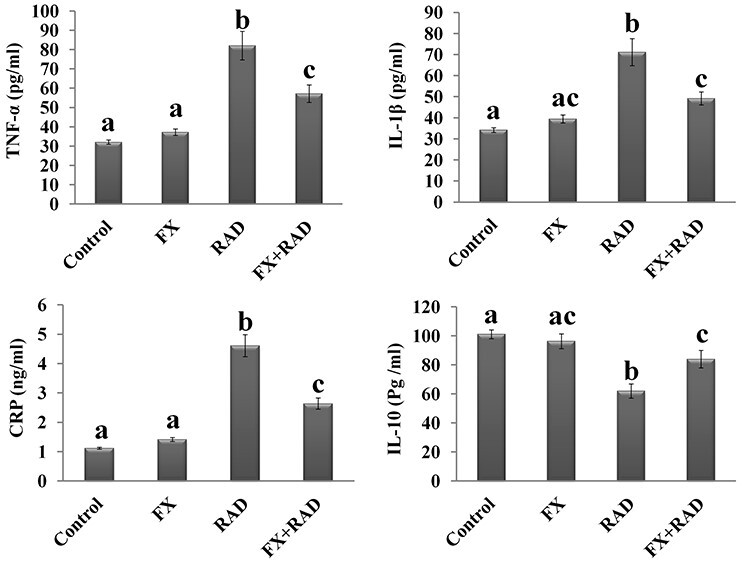
Impact of FX on the changes induced in TNF-α, IL-1β, CRP and IL-10 in serum of γ-irradiated mice. Data are expressed as mean values ± SEM (n = 6 independent values). Columns with different letters (a, b, c…) within the same histogram are significantly different and columns having the same letters are not significantly different at P < 0.05. Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Histopathological study
The histopathological inspection of the liver, kidney, lung and spleen tissues of different animal groups is presented in Figs 7, 8, 9 and 10, respectively.
Fig. 7.
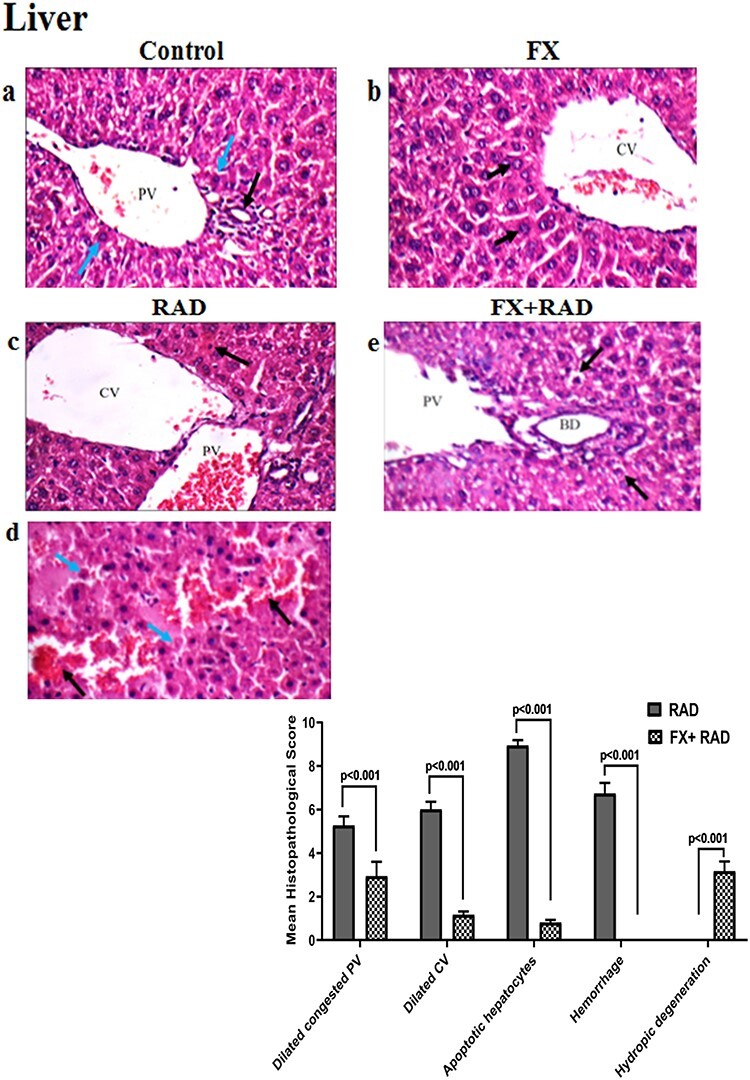
Histopathological examination of the liver in different animal groups. All tissues sections are stained with haematoxylin and eosin, magnification ×400 (H&E ×400). Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Fig. 8.
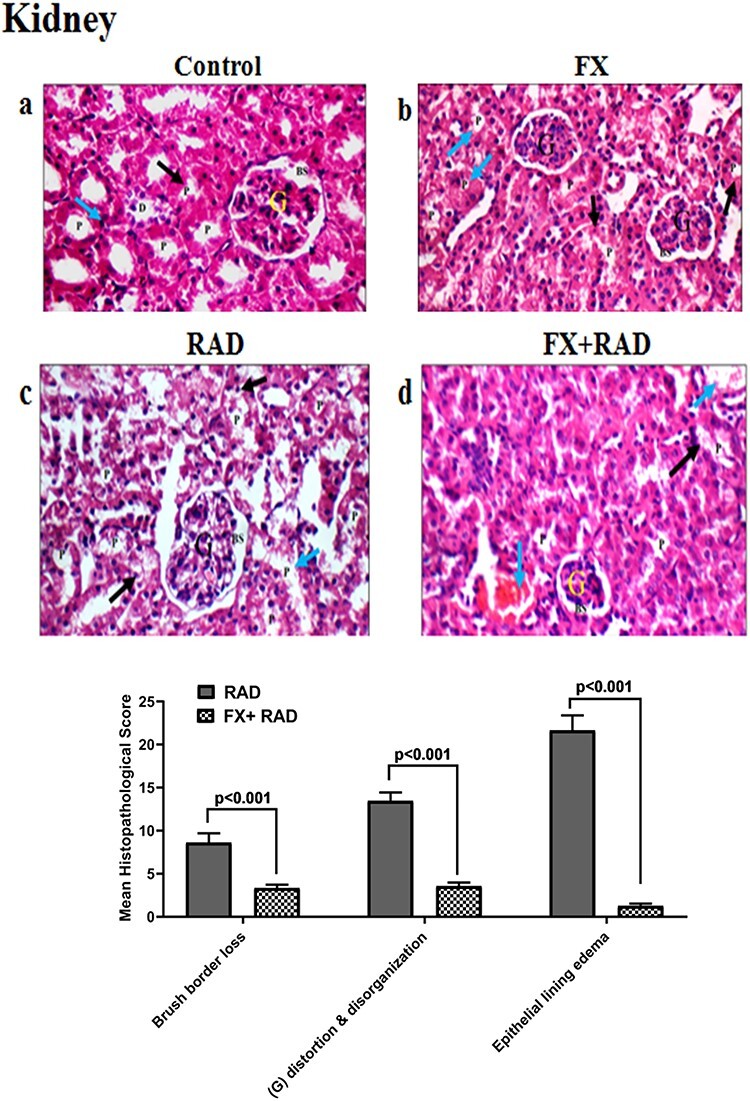
Histopathological examination of the kidney in different animal groups. All tissues sections are stained with haematoxylin and eosin, magnification ×400 (H&E ×400). Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Fig. 9.
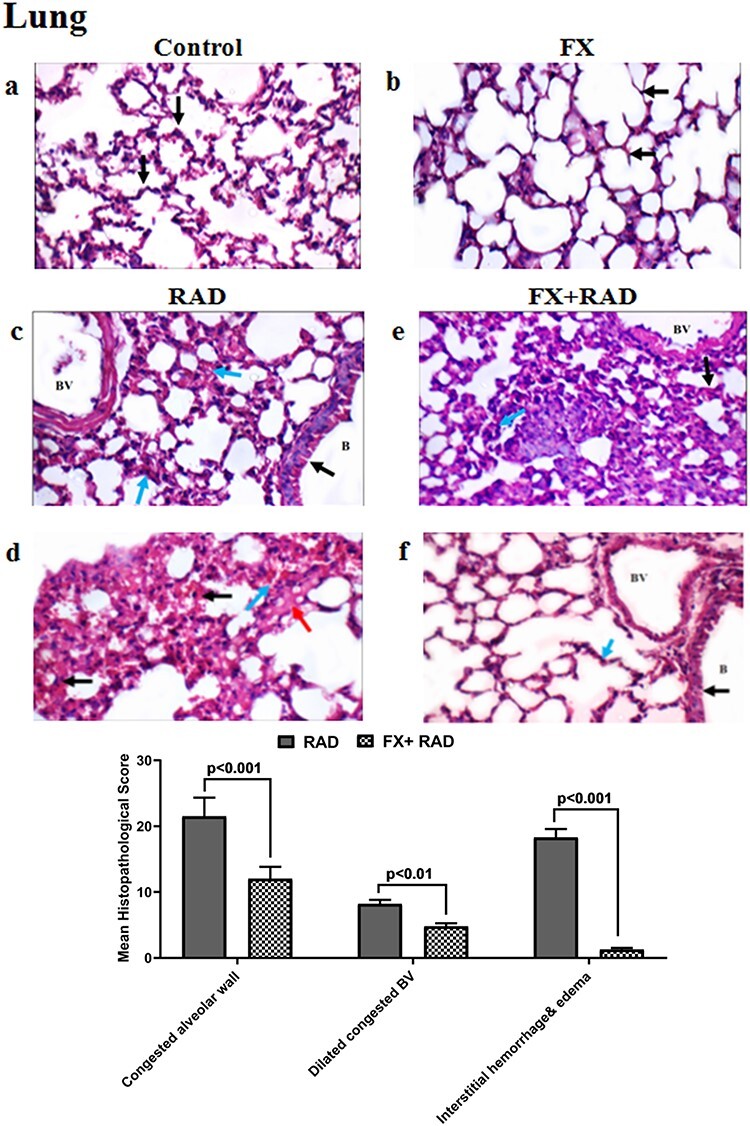
Histopathological examination of the lung in different animal groups. All tissues sections are stained with haematoxylin and eosin, magnification ×400 (H&E ×400). Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Fig. 10.
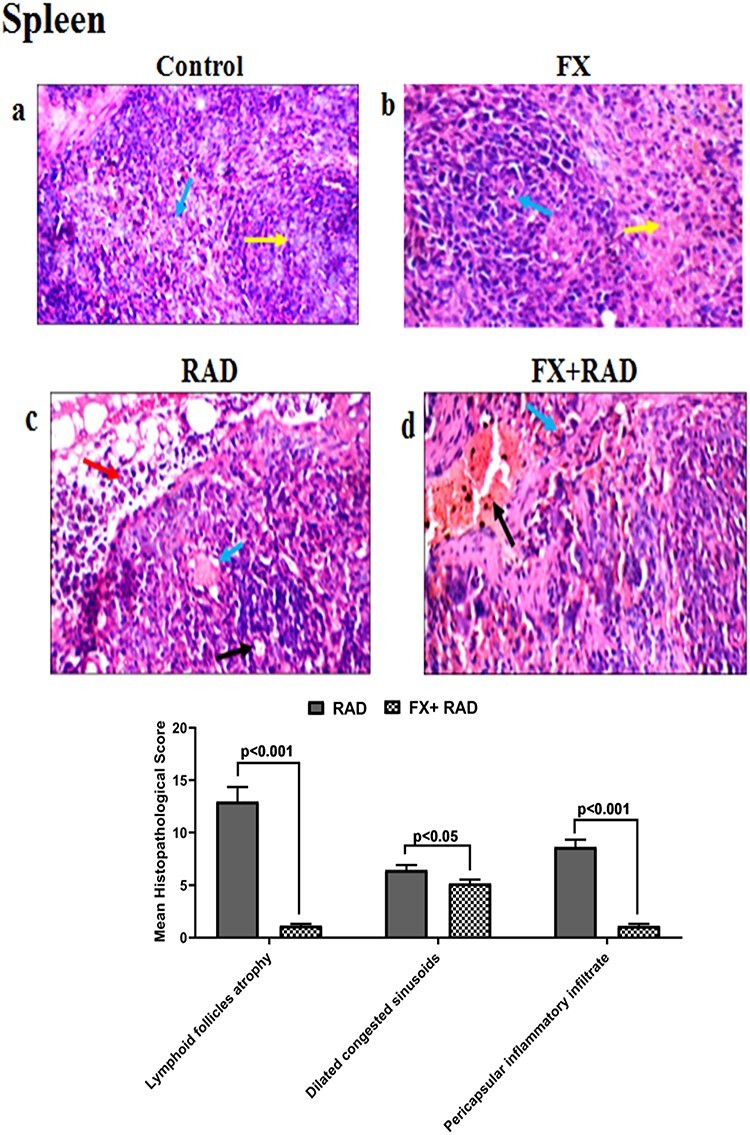
Histopathological examination of the spleen in different animal groups. All tissues sections are stained with haematoxylin and eosin, magnification ×400 (H&E ×400). Control group, normal mice; RAD group, mice exposed to γ-radiation; FX group, mice treated with fucoxanthin; and FX + RAD group, mice treated with FX and exposed to γ-radiation.
Liver tissues
The control group showed a normal portal tract with a normal portal vein (PV), bile ducts (BDs) (black arrow) and hepatocytes in the periportal area (blue arrows) (Fig. 7a). The FX group showed a normal central vein (CV) and regular hepatocytes in the perivenular area (black arrow) (Fig. 7b). The RAD group showed portal tracts with a mildly widened congested PV, mildly widened CV and scattered apoptotic hepatocytes in the perivenular zone (black arrows) (Fig. 7c), and areas of haemorrhage (black arrows) with scattered apoptotic hepatocytes (blue arrow) (Fig. 7d). The FX + RAD group showed portal tracts with a mildly widened PV, normal BDs and mild hydropic change of hepatocytes in the periportal zone (black arrows) (H&E ×400) as shown in Fig. 7e.
Kidney tissues
The control group showed normal glomeruli (G) with normal Bowman’s spaces (BS), normal proximal tubules (P) with preserved brush borders (black arrow), normal distal tubules (D) and normal interstitium (blue arrow) as represented in (Fig. 8a). The FX group showed normal G with normal BS, and P with scattered apoptotic epithelial lining (black arrow) and preserved brush borders (blue arrow) (Fig. 8b). The RAD group showed distorted G with average BS, and P with markedly oedematous epithelial lining (black arrow) and partial loss of brush borders (blue arrow) (Fig. 8c). The FX + RAD group showed small-sized G with normal BS, and P with partial loss of brush borders (black arrow) and mildly widened congested interstitial blood vessels (blue arrow) (H&E ×400) as shown in Fig. 8d.
Lung tissues
The control group showed normal alveolar walls (black arrows) and normal interstitium (blue arrow) (Fig. 9a). The FX group showed normal alveolar walls (black arrow) (Fig. 9b). The RAD group showed bronchioles (B) with regular epithelial lining (black arrow), mildly dilated blood vessels (BV) and markedly congested alveolar walls (blue arrow) (Fig. 9c). and congested alveolar walls (black arrows) with interstitial haemorrhage (blue arrow) and oedema (red arrow) (Fig. 9d). The FX + RAD group showed mildly dilated congested BV, thickened alveolar walls (black arrow) with mild interstitial inflammatory infiltrate (blue arrow) (Fig. 9e), and another bronchiole (B) showed normal epithelial lining (black arrow), normal BV and normal alveolar walls (blue arrow) (H&E ×400) as revealed in (Fig. 9f).
Spleen tissues
The control group showed normal lymphoid follicles (yellow arrow) and blood sinusoids (blue arrow) (Fig. 10a). The FX group showed normal lymphoid follicles with central arterioles (blue arrow), and normal blood sinusoids (red bulb) (yellow arrow) (Fig. 10b). The RAD group showed small-sized lymphoid follicles with central arterioles (black arrow) and dilated congested blood sinusoids (blue arrow) with pericapsular inflammatory infiltrate (red arrow) (Fig. 10c). The FX + RAD group showed dilated congested blood vessels (black arrow) with congested blood sinusoids (red bulb) (blue arrow) (H&E ×400) as revealed in Fig. (10d).
DISCUSSION
The focus of the current study was FX, due to its bioactive properties; we examined its action against RAD exposure-induced changes in the apelin-13/APJ pathway which could affect or be affected by the inflammatory reactions and changes in redox status.
The data of the current study showed that exposure of mice to IR led to a remarkable induction in the oxidative burden in liver, kidney, lung and spleen tissues that are manifested by a significant up-regulation of the hypoxia biomarker, HIF-1α, elevation in MDA (the end-product of lipid peroxidation) and reduction in antioxidant markers (GSH-PX and GSH). These data were associated with a considerable elevation of pro-inflammatory molecules (MCP-1 and IL-6) in the examined organ tissues, down-regulation in the splenic α-7nAchR and disturbance in systemic inflammatory mediators (TNF-α, IL-1β, CRP and IL-10). These findings were supported by the histopathological investigation, whereas the changes in the architecture of tissues that responded to the oxidative damages and inflammation are obvious.
These results could be attributed to the development of oxidative stress, a status that arises from the abundant generation of ROS or malfunction of the antioxidant defence system. Yahyapour et al. [1] reported that inflammation and oxidative damage are strongly related to IR exposure. Chatterjee [9] revealed that progressive chronic inflammation and oxidative stress are implicated in a variety of pathological processes and can lead to dangerous diseases. Radiation-induced lung injury via post-radiation hypoxia is a causative factor mediating continuous generation of ROS, a surge in leukocyte migration, vascular permeability, stimulation of collagen formation and up-regulation of release of inflammatory cytokines by various cells such as endothelial cells, alveolar macrophages, pneumocytes and fibroblasts [29]. The data of the current study might indicate a case of hypoxia. The increase of HIF-1α in organ tissues of irradiated mice could signify the occurrence of hypoxia after radiation exposure, which could take part in the development of oxidative stress and inflammation. The study of Azab et al. [30] specified that exposure to γ-radiation causes exaggerated ROS formation and directs the irradiated cells into a condition of oxidative stress that has been implicated in diverse processes of natural and pathological origin. This overproduction of ROS is accompanied by the reduction of cellular antioxidant activities and lipid peroxidation, protein oxidation indices and inflammatory markers in the liver of irradiated rats. In the study of Moustafa and Thabet [31], the irradiation of rats by γ-rays (6 Gy) elevated the MDA level and reduced the antioxidant enzymes [superoxide dismutase (SOD) and catalase (CAT)] and eventually caused liver tissue damage. Also, the exposure of brain tissue to 5 Gy of γ-radiation increased MDA, IL-1β and IL-6, coupled with abrogated antioxidant enzyme activity (glutathione S-transferase) that led to brain injury [32].
It is noteworthy that the IL-6 produced in response to liver and kidney injury is a signal in the event of tissue damages [33–35]. The IL-6 produced in response to kidney damage directly causes inflammation and injury to lung tissue [35], which is consistent with the results shown in the present study. However, IL-6 activates splenocytes to produce IL-10 via α-7nAchR that is required to temper tissue injury, as reported in the study of Kinsey [35]. The γ-irradiation microenvironment could direct the persistent activation of the IL-6 in tissues and systemic pro-inflammatory mediators (TNF-α and IL-1β) to be detrimental rather than beneficial, as manifested by the down-regulation of splenic α-7nAchR and IL-10 levels as observed in the present study. Supporting this view, Linard et al. [36] stated that after whole-body γ-irradiation of rats at 10 Gy, cascades of inflammatory responses were induced through the increased concentrations of IL-6 and IL-8 associated with a decrease in the IL-10 level. Moreover, Galal et al. [37] stated that 7 Gy of γ-radiation increased the biomarkers of hepatotoxicity and diminished ROS-detoxifying enzymes in the liver and spleen associated with the down-regulation of splenic α-7nAchR. The down-regulation of splenic α-7nAchR in the RAD group might be related to cumulative pro-inflammatory and diminished anti-inflammatory cytokine release through the NF-κB pathway [38]. Further, Wang et al. [39] revealed that exposure of human umbilical vein endothelial cells to γ-radiation disrupts the cellular junctions through the activation of the inflammatory NF-κB signalling pathway manifested by enhancement of oxidative and nitrosative stresses and increases in the cytokines IL-6 and TNF-α.
In contrast, the administration of several doses of FX before γ-radiation exposure in the FX + RAD group diminished the disturbances in the oxidant/antioxidant defence system and pro-/anti-inflammatory balance as observed in the present study. This result might be due to the antioxidant capacity of FX. Rodrigues et al. [40] reported that FX, a marine carotenoid, has a potent free radical-scavenging capacity which explains its antioxidant abilities. These abilities might be credited to its distinctive allenic bond and 5,6-monoepoxide which are critical for free radical scavenging and protection of cells from damage induced by H2O2 and UV-B [41]. FX showed improvements in the traumatic brain injury model by reducing the MDA content and restoring the activity of GSH-PX [15]. It could exert its cytoprotective effects counter to H2O2 oxidative injury in L02 cells (normal human hepatic cell line) through the phosphatidylinositol 3-kinase-dependent induction of Nrf-2 (nuclear-factor erythroid related factor-2) signalling that is manifested by reduced leakage of LDH and intracellular ROS with enhanced intracellular GSH [42]. FX is responsible for the amendments of cellular redox tone and inflammation that were observed in the liver of irradiated rats at 8 Gy of fractionated (2 Gy ×4; 2 Gy every 3 days) γ-rays via regulation of TNF-α levels, MDA production and preservation of GSH concentration [43]. Also, FX restores the reduced antioxidant system (GSH, GSH-PX, SOD and CAT) and inhibits the high level of generation of the inflammatory molecules IL-1β and TNF-α which are linked to obesity [44]. Furthermore, Grasa-López et al. [45] showed that FX has a progressive effect via increasing the anti-inflammatory cytokine adiponectin and diminishing the pro-inflammatory cytokines leptin and CRP. The anti-inflammatory effect of FX might be attributed to the inhibition of NF-κB induction and the suppression of mitogen-activated protein kinase (MAPK) phosphorylation which leads to decreasing the level of pro-inflammatory mediators involving NO, prostaglandin E2, IL-1β, TNF-α and IL-6 in lipopolysaccharide-stimulated murine macrophage cells [14].
The protein expression of the apelin-13/APJ/NF-κB pathway increased in the liver, kidney, lung and spleen tissues of the irradiated mice group concurrently with the development of oxidative stress and inflammation, as observed in the current study. The increase observed in apelin-13, APJ and NF-κB protein expression could be credited to the action of highly inflammatory mediators such as IL-6 or the development of oxidative stress because of the large amounts of MDA formed in response to the excessively generated ROS in organ tissues due to radiation exposure. Han et al. [46] showed that the inflammation-induced rise in apelin mRNA expression was mediated via IL-6 and interferon-γ by stimulation of apelin promoter activity, which in turn was mediated through the JAK/STAT pathway. Helmi et al. [47] described that the damage to the heart observed during chronic systemic hypoxia is because of a rise in the relative apelin mRNA expression, high MDA levels and substantial increases in the formation of ROS during hypoxia. The up-regulation of inflammatory mediators and the activation of NF-κB are key events behind the inflammatory process. Xu et al. [48] established that the transcription factor NF-κB controlled serious cellular responses to stress and injury through activation of cytokines (such as TNF-α and IL-1) and oxygen free radicals. Binding of apelin-13 to its receptor APJ can prompt the expression of the transcription factor NF-κB, which intensifies the manifestation of the chemotactic pro-inflammatory molecule MCP-1 [49, 50] and is associated with down-regulation of α-7nAchR protein expression [38].
The anti-inflammatory effect of FX against γ-radiation-induced tissue inflammation could be interpreted in the light of its ability to control the apelin/APJ/NF-κB axis. The present data pointed out the significant amelioration in the apelin/APJ/NF-κB signalling pathway in mice who received FX and then were exposed to γ-radiation. It could be assumed that the management of this signalling pathway might be attributed to the role of NF-κB as a regulator of the apelin pathway linked mechanistically to apelin’s inhibition of inflammatory mediator up-regulation and suppression of NF-κB activation in tissues suffering from inflammation. Kim et al. [14] and Choi et al. [51] postulated that FX is beneficial as anti-inflammatory therapy because of its inhibitory influence on NF-κB activation and MAPK phosphorylation.
In the present study, an imbalance between MMP-2, MMP-9 and TIMP-1 was detected in the liver, kidney, lung and spleen of mice exposed to γ-irradiation compared with the control mice. This was accompanied by a significant increase in LDH activity in all organs, disturbance in liver function enzymes (as shown by increased AST and ALT activities) and kidney function (as shown by the raised urea and creatinine levels) and alterations in the architectures of organ tissues that were observed in tissues photomicrographs of the RAD group. The induction of oxidative stress, inflammation and up-regulation of the apelin/APJ/NF-κB signalling pathway after γ-irradiation might contribute to the above consequences and the subsequent breakdown of organ functions in irradiated mice. Yahyapour et al. [1] stated that the situation of oxidative destruction, chronic inflammation and its consequences which resulted after IR may interrupt the functions of irradiated organs. Galis and Khatri [52] related the imbalance between MMPs and TIMPs, and the promotion of vascular remodelling to the induction of oxidative stress and the development of inflammatory responses. Also, Nguyen et al. [53] observed the induction of MMP-2 and MMP-9 and reduction of TIMP-1 in response to the up-regulation of NF- B in PMA (phorbol 12-myristate 13-acetate)-induced human fibrosarcoma. In addition, in colorectal carcinoma patients, the development of oxidative stress enhances the cell membrane lipid peroxidation, leading to impairment in membrane permeability and leakage of LDH and malate dehydrogenase (MDH) into the circulation [54]. The outflow of the cytosolic enzyme LDH is related to cellular viability, and hence it is a convenient gauge of membrane destruction. Several studies confirmed that LDH is a marker of organ injury and correlated its increase in tissues with oxidative stress and inflammatory conditions [55–58]. Thus, the increases in LDH that were found in all organs of the present study emphasize the damage of those organs after exposure to γ-radiation.
B in PMA (phorbol 12-myristate 13-acetate)-induced human fibrosarcoma. In addition, in colorectal carcinoma patients, the development of oxidative stress enhances the cell membrane lipid peroxidation, leading to impairment in membrane permeability and leakage of LDH and malate dehydrogenase (MDH) into the circulation [54]. The outflow of the cytosolic enzyme LDH is related to cellular viability, and hence it is a convenient gauge of membrane destruction. Several studies confirmed that LDH is a marker of organ injury and correlated its increase in tissues with oxidative stress and inflammatory conditions [55–58]. Thus, the increases in LDH that were found in all organs of the present study emphasize the damage of those organs after exposure to γ-radiation.
From the data revealed in the present study, FX administration before γ-radiation exposure reduces oxidative stress and at the same time prevents the MMP-2, MMP-9/TIMP-1 imbalance and maintains cellular integrity as perceived by diminished LDH leakage from the liver, kidney, lung and spleen of irradiated mice. These findings might be credited to the antioxidant and anti-inflammatory actions of FX, which resulted in regulation of the apelin-13/APJ/NF-κB signalling axis as previously interpreted. The FX administration reduced cellular MMP-2 and MMP-9 activities concomitant with increases in the tissue MMP inhibitors, such as as TIMP-1 in PMA-induced human fibrosarcoma cells via inhibition of NF- B, JNK and p38-MAPK [53]. Also, the pre-treatment by FX resulted in a reduction of LDH leakage, decreased intracellular ROS content and enhanced intracellular GSH [42]. The inhibition of LDH reduced hepatic necrosis, apoptosis and the appearance of pro-inflammatory mediators in a mouse model with acute liver failure [58] which could explain the improvement that was observed in the organ of the FX + RAD group. Histopathological examination of the liver, kidney, lung and spleen in the current investigation revealed that FX possesses a remarkable radioprotective potential as implied by its mitigation of the harmful alterations induced by IR on the biochemical profile. These data are in agreement with those of Bharathriraja et al. [59], Zheng et al. [60] and Wang et al. [61] who postulated the cytoprotective efficacy of FX against various deleterious factors in different organs and attributed these effects to its antioxidant and anti-inflammatory properties. Of note, the current study sheds light on a novel mechanism that might contribute to the cytoprotective efficacy of FX via the concerted regulation of the splenic cholinergic anti-inflammatory nicotinic receptor (α-7nAchR) and the apelin-13/APJ pathway in the target organs.
B, JNK and p38-MAPK [53]. Also, the pre-treatment by FX resulted in a reduction of LDH leakage, decreased intracellular ROS content and enhanced intracellular GSH [42]. The inhibition of LDH reduced hepatic necrosis, apoptosis and the appearance of pro-inflammatory mediators in a mouse model with acute liver failure [58] which could explain the improvement that was observed in the organ of the FX + RAD group. Histopathological examination of the liver, kidney, lung and spleen in the current investigation revealed that FX possesses a remarkable radioprotective potential as implied by its mitigation of the harmful alterations induced by IR on the biochemical profile. These data are in agreement with those of Bharathriraja et al. [59], Zheng et al. [60] and Wang et al. [61] who postulated the cytoprotective efficacy of FX against various deleterious factors in different organs and attributed these effects to its antioxidant and anti-inflammatory properties. Of note, the current study sheds light on a novel mechanism that might contribute to the cytoprotective efficacy of FX via the concerted regulation of the splenic cholinergic anti-inflammatory nicotinic receptor (α-7nAchR) and the apelin-13/APJ pathway in the target organs.
As reported in Mun et al. [62], there are several radioprotectors which have been investigated with different mechanisms of protective actions such as: bergenin (Caesalpinia digyna) which activates MAPK and ERK pathways to modulate the radiation damage effect; N-acetyl tryptophan glucopyranoside (Bacillus subtilis) which overcomes radiation-induced damage by recovering cytoprotective cytokines and antioxidant enzymes; zymosan A (Saccharomyces cerevisiae) which protects against radiation-induced DNA damage by up-regulating the levels of cytokines, and psoralidin (Psoralea corylifolia) which inhibits the radiation-induced PI3K–IKK–IκB signalling pathway, COX-2 and expression of pro-inflammatory cytokines. N-acetyl cysteine and resveratrol have been shown to decrease DNA damage via induction of natural antioxidants (GSH, SOD and CAT); vitamin C, inapoyl-Eglucoside and quercetin-3-O-rhamnoside-7-O-glucoside showed protection against radiation through reduction in lipid peroxidation, as reported in Smith et al. [63]. Oh et al. [64] reported that algal natural products such as dieckol, fucoxanthin, astaxanthin and algae extracts have radioprotective effects including radical scavenging and antioxidant properties. However, the new findings of the current study shed light on a novel mechanism of FX as a radioprotector against γ-radiation-induced damage in the organs studied through its concerted regulation of the apelin/APJ/NF-κB signalling pathway and splenic α-7nAchR, a primary receptor of the cholinergic anti-inflammatory pathway, as it has antioxidant and anti-inflammatory effects that led to modulation of the structural damage markers (MMP-2, MMP-9, TIMP-1 and LDH) and the function of the organs.
Collectively, the results of the current study indicate that radiation exposure-induced activation of apelin-13/APJ signalling which could be attributed to the induction of oxidative stress (as manifested in this study by an increase of HIF-1α and MDA, and decreases of GSH and GSH-PX) as well as inflammation (as manifested by increases in MCP-1, IL-6 and NF-κB and decreases in IL-10 and α-7nAchR). Furthermore, the elevation of oxidative stress and inflammatory mediators led to structural damage in the organs examined (as manifested by an increase in MMP-2, MMP-9 and LDH, and a decrease in TIMP-1 confirmed by histopathological examination). In turn, the recorded activation of apelin signalling in the irradiated group appeared to participate in the surge observed in oxidative stress and inflammation. We could suggest that the systemic administration of FX has considerable beneficial effects against oxidative damage and the inflammatory status. The regulation of the apelin-13/APJ/NF-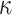 B signalling axis might represent the cornerstone of the protective mechanisms of FX against radiation hazards that lead to the cellular damage and collapse of organ functions. As a recommendation, FX might be of use as a potential radioprotector in cases of radiation exposure.
B signalling axis might represent the cornerstone of the protective mechanisms of FX against radiation hazards that lead to the cellular damage and collapse of organ functions. As a recommendation, FX might be of use as a potential radioprotector in cases of radiation exposure.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr Sayed Abdel Raheem (Associate Professor of Histopathology, Faculty of Medicine, Al-Azhar University) for carrying out the histopathological examination for this study.
Contributor Information
Nermeen M El Bakary, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
Noura Magdy Thabet, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
Neama M El Fatih, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
Mohamed Khairy Abdel-Rafei, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
Ghada El Tawill, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
Khaled Shaaban Azab, Radiation Biology Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest concerning the publication of this article.
References
- 1. Yahyapour R, Amini P, Rezapour S et al. Radiation-induced inflammation and autoimmune diseases. Mil Med Res 2018;5:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Maggio FM, Minafra L, Forte GI et al. Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond) 2015;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reisz JA, Bansal N, Qian J et al. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 2014;21:260–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra KN, Moftah BA, Alsbeih GA. Appraisal of mechanisms of radioprotection and therapeutic approaches of radiation countermeasures. Biomed Pharmacother 2018;106:610–7. [DOI] [PubMed] [Google Scholar]
- 5. Huang Z, Wu L, Chen L. Apelin/APJ system: a novel potential therapy target for kidney disease. J Cell Physiol 2018;233:3892–900. [DOI] [PubMed] [Google Scholar]
- 6. Lv SY, Cui B, Chen WD et al. Apelin/APJ system: a key therapeutic target for liver disease. Oncotarget 2017;8:112145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Q, Cao J, Chen L. Apelin/APJ system: a novel therapeutic target for oxidative stress-related inflammatory diseases (review). Int J Mol Med 2016;37:1159–69. [DOI] [PubMed] [Google Scholar]
- 8. Li L, Li F, Li F et al. NOX4-derived reactive oxygen species drive Apelin-13-induced vascular smooth muscle cell proliferation via the ERK pathway. Int J Pept Res Ther 2011;17:307–15. [Google Scholar]
- 9. Chatterjee S. Oxidative stress, inflammation, and disease. In: Dziubla T, Butterfield DA (eds). Oxidative Stress and Biomaterials. New York: Academic Press, 2016, 35–58. [Google Scholar]
- 10. Ren C, Tong YL, Li JC et al. The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int J Biol Sci 2017;13:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viedt C, Dechend R, Fei J et al. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappa B and activating protein-1. J Am Soc Nephrol 2002;13:1534–47. [DOI] [PubMed] [Google Scholar]
- 12. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008;75:346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain AK, Singh D, Dubey K et al. Models and methods for in vitro toxicity. In: Dhawan A, Kwon S (eds). In Vitro Toxicology. Cambridge, MA: Academic Press, 2018, 45–65. [Google Scholar]
- 14. Kim KN, Heo SJ, Yoon WJ. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol 2010;649:369–75. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Wang H, Fan Y et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE and Nrf2–autophagy pathways. Sci Rep 2017;7:46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma SY, Park WS, Lee DS. Fucoxanthin inhibits profibrotic protein expression in vitro and attenuates bleomycin-induced lung fibrosis in vivo. Eur J Pharmacol 2017;811:199–207. [DOI] [PubMed] [Google Scholar]
- 17. ISO/ASTM E 51026 . Practice for using the Frick dosimetry system. West Conshohocken, PA: ASTM International, Switzerland, 2015. [Google Scholar]
- 18. KM Z. Effect of gamma ray on reactive oxygen species at experimental animals. OMICS J Radiol 2017;6:283. [Google Scholar]
- 19. Yoshioka T, Kawada K, Shimada T et al. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 1979;135:372–6. [DOI] [PubMed] [Google Scholar]
- 20. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–7. [DOI] [PubMed] [Google Scholar]
- 21. Lowry OH, Rosenbrough NJ, Farr AL et al. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 22. Gross RT, Bracci R, Rudolph N et al. Hydrogen peroxide toxicity and detoxification in the erythrocytes of newborn infants. Blood 1967;29:481–93. [PubMed] [Google Scholar]
- 23. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic, oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 24. Fawcett JK, Soctt JE. A rapid and precise method for the determination of urea. J Clin Pathol 1960;13:156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartles H, Bohmer M, Heirli C. Serum creatinine determination without protein precipitation. Clin Chem Acta 1972;37:193–7. [DOI] [PubMed] [Google Scholar]
- 26. Omar HA, Sargeant AM, Weng JR et al. Targeting of the Akt-nuclear factor-kappa B signaling network by [1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol (OSU-A9), a novel indole-3-carbinol derivative, in a mouse model of hepatocellular carcinoma. Mol Pharmacol 2009;76:957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mingone CJ, Gupte SA, Quan S et al. Influence of heme and heme oxygense-1 transfection of pulmonary microvascular endothelium on oxidant generation and cGMP. Exp Biol Med (Maywood) 2003;228:535–9. [DOI] [PubMed] [Google Scholar]
- 28. Banchroft JD, Stevens A, Turner DR. Theory and Practice of Histological Techniques, 4th edn. New York: Churchil Livingstone, 1996. [Google Scholar]
- 29. Vujaskovic Z, Anscher MS, Feng QF et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 2001;50:851–5. [DOI] [PubMed] [Google Scholar]
- 30. Azab Kh S, Mostafa AH, Ali EM et al. Cinnamon extract ameliorates ionizing radiation-induced cellular injury in rats. Ecotoxicol Environ Saf 2011;74:2324–9. [DOI] [PubMed] [Google Scholar]
- 31. Moustafa EM, Thabet NM. Beta-sitosterol upregulated paraoxonase-1 via peroxisome proliferator-activated receptor-γ in irradiated rats. Can J Physiol Pharmacol 2017;95:661–6. [DOI] [PubMed] [Google Scholar]
- 32. Thabet NM, Moustafa EM. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch Physiol Biochem 2018;124:185–93. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol 2016;64:1403–15. [DOI] [PubMed] [Google Scholar]
- 35. Kinsey GR. The spleen as a bidirectional signal transducer in acute kidney injury. Kidney Int 2017;91:1001–3. [DOI] [PubMed] [Google Scholar]
- 36. Linard C, Marquette C, Mathieu J et al. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappa B inhibitor. Int J Radiat Oncol Biol Phys 2004;58:427–34. [DOI] [PubMed] [Google Scholar]
- 37. Galal SM, Abdel-Rafei MK, Hasan HF. Cholinergic and cytoprotective signaling cascades mediate the mitigative effect of erythropoietin on acute radiation syndrome. Can J Physiol Pharmacol 2018;96:442–58. [DOI] [PubMed] [Google Scholar]
- 38. Xu H, Shi Q, Mo Y et al. Downregulation of α7 nicotinic acetylcholine receptors in peripheral blood monocytes is associated with enhanced inflammation in preeclampsia. BMC Pregnancy Childbirth 2019;19:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Chandra-Segaran R, Chan LY et al. Gamma radiation-induced disruption of cellular junctions in HUVECs is mediated through affecting MAPK/NF-κB inflammatory pathways. Oxid Med Cell Longev 2019;2019:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodrigues E, Mariutti LR, Mercadante AZ. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar Drugs 2012;10: 1784–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sachindra NM, Sato E, Maeda H et al. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem 2007;55:8516–22. [DOI] [PubMed] [Google Scholar]
- 42. Wang X, Cui YJ, Qi J et al. Fucoxanthin exerts cytoprotective effects against hydrogen peroxide-induced oxidative damage in L02 cells. Bio Med Research International 2018;1085073:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Azab Kh S, Meky NH, El-Deghidy EAM et al. Response of COX2/PGE2 inflammatory pathway to brown seaweed extract in rats exposed to gamma radiation. WJNST 2017;7:189–205. [Google Scholar]
- 44. Tan CP, Hou YH. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 2014;37:443–50. [DOI] [PubMed] [Google Scholar]
- 45. Grasa-López A, Miliar-García Á, Quevedo-Corona L et al. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar Drugs 2016;14:148–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han S, Wang G, Qi X et al. Involvement of a Stat3 binding site in inflammation-induced enteric Apelin expression. Am J Physiol Gastrointest Liver Physiol 2008;295:G1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helmi HR, Ferdinal F, Prijanti AR et al. Expression of Apelin is related to oxidative damage in heart tissue of rats during chronic systemic hypoxia. Acta Bio Ina 2018;1:68–78. [Google Scholar]
- 48. Xu Y, Rojkind M, Czaja MJ. Regulation of monocyte chemoattractant protein 1 by cytokines and oxygen free radicals in rat hepatic fat-storing cells. Gastroenterology 1996;110:1870–7. [DOI] [PubMed] [Google Scholar]
- 49. Mao XH, Tao S, Zhang XH et al. Apelin-13 promotes monocyte adhesion to human umbilical vein endothelial cell mediated by phosphatidylinositol 3-kinase signaling pathway. Prog Biochem Biophys 2011;38:1162–70. [Google Scholar]
- 50. Lu Y, Zhu X, Liang GX et al. Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-κB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids 2012;43:2125–36. [DOI] [PubMed] [Google Scholar]
- 51. Choi JH, Kim NH, Kim SJ et al. Fucoxanthin inhibits the inflammation response in paw edema model through suppressing MAPKs, Akt, and NFκB. J Biochem Mol Toxicol 2016;30:111–9. [DOI] [PubMed] [Google Scholar]
- 52. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002;90:251–62. [PubMed] [Google Scholar]
- 53. Nguyen V-T, Qian Z-J, Lee B et al. Fucoxanthin derivatives from Sargassum siliquastrum inhibit matrix metalloproteinases by suppressing NF-kB and MAPKs in human fibrosarcoma cells. Algae 2014;29:355–66. [Google Scholar]
- 54. Gopcevic K, Rovcanin B, Kekic D et al. Matrix metalloproteinase-2 and -9, lactate, and malate dehydrogenase and lipid peroxides in sera of patients with colorectal carcinoma. Folia Biologica (Praha) 2017;63:190–6. [DOI] [PubMed] [Google Scholar]
- 55. Drent M, Cobben NA, Henderson RF et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 1996;9:1736–42. [DOI] [PubMed] [Google Scholar]
- 56. Zager RA, Johnson ACM, Becker K. Renal cortical lactate dehydrogenase: a useful, accurate, quantitative marker of in vivo tubular injury and acute renal failure. PLoS One 2013;8:e66776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dkhil MA, Al-Quraishy S, Al-Khalifa MS. The effect of Babesia divergens infection on the spleen of Mongolian gerbils. Biomed Res Int 2014;2014:483854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferriero R, Nusco E, De Cegli R et al. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol 2018;69:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bharathiraja K, Hari BL, Vijayabrakash S et al. Fucoxanthin, a marine carotenoid protects cadmium-induced oxidative renal dysfunction in rats. Biomed Prev Nutr 2013;3:201–17. [Google Scholar]
- 60. Zheng J, Tian X, Zhang W et al. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar Drugs 2019;17:552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang PT, Sudirman S, Hsieh MC et al. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed Pharmacother 2020;125:109992. [DOI] [PubMed] [Google Scholar]
- 62. Mun GI, Kim S, Choi E et al. Pharmacology of natural radioprotectors. Arch Pharm Res 2018;41:1033–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith TA, Kirkpatrick DR, Smith S et al. Radioprotective agents to prevent cellular damage due to ionizing radiation. J Transl Med 2017;15:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oh J-Y, Fernando IPS, Jeon Y-J. Potential applications of radioprotective phytochemicals from marine algae. Algae 2016;31:403–14. [Google Scholar]


