Abstract
Pleural dissemination is a common pattern of failure after initial treatment of thymoma and thymic carcinoma, but there is no standardized treatment. As these tumors are relatively radiosensitive, we investigated the effectiveness of radiotherapy. Twenty patients underwent 33 series of local radiotherapy for 96 pleural dissemination lesions after initial treatment. Conventional radiotherapy (CRT), tomotherapy, and combination of the two were employed in 19, 13, and 1 series, respectively. The median follow-up period after the first irradiation for pleural dissemination was 46 months (range, 14–161). For all 20 patients, overall survival (OS) rates from initial radiotherapy for pleural dissemination were 100% at three years and 86% at five years. Progression-free survival (PFS) rates after 33 series of radiotherapy were 30% at three years and 16% at five years. Local control (LC) rates for 96 lesions were 98% at three years and 96% at five years. In-field recurrence was observed in only two among the 96 lesions. One patient (5%) developed grade 3 radiation pneumonitis and another (5%) developed grade 3 pericardial effusion. No other serious adverse events were observed. When disseminated nodules can be covered within localized fields, local radiotherapy may be a treatment option. Using tomotherapy, multiple lesions can be treated safely.
Keywords: thymic tumors, pleural dissemination, local radiation therapy, intensity modulated radiation therapy
INTRODUCTION
Thymic epithelial tumors are relatively rare, with an incidence of approximately 0.13 per 100 000 person–years [1], but they are the most common primary tumor of the anterior mediastinum in adults. The treatment strategy is determined based on the disease stage; when the tumor is considered resectable, surgery is generally attempted. Radiation therapy and chemotherapy may be considered for unresectable cases. Postoperative radiation therapy is also often employed. Although the recurrence rates depend on the histological subtypes, invasive thymoma and thymic carcinoma relatively frequently develop recurrence after initial treatment; the recurrence rate after initial complete resection of the primary tumor ranges between 10% and 30% [2–4]. Long-term observation is necessary because relapse develops relatively late after initial treatment [5,6]. Completeness of resection is the most significant prognostic factor [2], and incompletely resected cases have a risk of relapse. The recurrent lesions of thymoma are often located in the pleura, anterior mediastinum, and pericardium [2].
There have been few reports analyzing treatment for recurrence of thymic tumors. Several reports demonstrated good survival rates following as much surgical removal of recurrent lesions as possible [7–9], whereas chemotherapy and radiation therapy were also recommended in several reports [10–12]. However, there are only a few reports regarding treatment for pleural dissemination. Many treatments have been tried [13–15], but no standard treatment has been established. Treatment of recurrent thymoma is selected according to the individual situation due to the technical problems of repeated surgery and the relationship with surrounding organs.
As thymic tumors are known to be relatively radiosensitive neoplasms and often exhibit insidious progression, we have employed local radiation therapy for pleural disseminations of thymic tumors since more than 14 years ago. When pleural disseminations are untreated, pleural effusion, local pain, vascular invasion and spinal canal invasion may develop, which may negatively affect the prognosis [16,17]. Also, the presence of pleural dissemination may be a cause of further dissemination. Therefore, we hypothesized that radiation therapy should play a role in controlling the lesion and preventing future occurrence of pleural effusion and other conditions and have the potential to improve the prognosis of the patients, provided that the treatment can be delivered without major complications. There have been no large studies, to our knowledge, that investigated the efficacy of local irradiation for pleural disseminations of thymic tumors; therefore, we investigated the usefulness of local radiotherapy for this condition.
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board of Nagoya City University Graduate School of Medical Sciences (approval number: 60-19-0128) and was conducted in compliance with the guidelines of the Helsinki Declaration. As this study is a retrospective study, informed consent was obtained from all patients in the form of opt-out on the website. We reviewed medical records of patients who had been radiologically or histologically diagnosed with pleural dissemination of thymoma or thymic carcinoma between March 2005 and May 2018, and received radiation therapy for pleural dissemination at our institution. Indications for the treatment were considered as follows: (i) pleural disseminations enlarging over time, (ii) patient refusal of other treatment options including surgery and chemotherapy, and (iii) no pleural effusion. Because of the criterion (1), the largest diameter of tumors in the axial plane was usually ≥10 mm, but smaller tumors were treated simultaneously when the tumors were multiple and the largest one was ≥10 mm. Hereinafter, the tumor size is defined as the maximum thickness from the pleura (mm) × diameter orthogonal to its line in the axial plane (mm). Twenty patients were identified for inclusion in this study. Our criteria for radiological diagnosis of pleural dissemination were previously published [18]. Local pleural recurrence was defined as a new pleural (parietal, visceral, or interlobar) nodule detected on computed tomography (CT) after initial surgical or chemoradiotherapy treatment. For this analysis, two patients palliatively treated for pain relief were excluded. No patient had active double cancer. No patient received concurrent chemoradiotherapy for pleural dissemination, but three received chemotherapy after radiation. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0–2 in all patients. Seven of the 20 patients received irradiation twice and two received irradiation four times in total for pleural disseminations at different sites. Multiple pleural dissemination lesions were treated simultaneously in 11 patients. As a result, 33 treatment series were carried out and 96 tumors were treated in total. The patient characteristics and treatment history are shown in Table 1.
Table 1.
Patient characteristics and treatment history
| Patient and treatment characteristics | Number of patients |
|---|---|
| Age at diagnosis (years) Median (range) | 60 (32–81) |
| Sex (male/female) | 9 / 11 |
| Masaoka stage at diagnosis (III/IVa/IVb/unknown) | 10 / 8 / 1 / 1 |
| WHO histology (A/AB/B1/B2/B3/TC/unknown) | 1 / 2 / 1 / 8 / 4 / 3 / 1 |
| Myasthenia gravis (yes/no) | 6 / 14 |
| Initial surgical resection (yes/no) | 19 / 1 |
| Previous radiotherapy (yes/no) | 16 / 4 |
| Mediastinum irradiation (yes/no) | 12 / 8 |
| Hemithorax irradiation (yes/no) | 4 / 16 |
| Previous chemotherapy (yes/no) | 14 / 6 |
| Steroid pulse before radiotherapy (yes/no) | 6 / 14 |
Planning and treatment
Our treatment planning method was described in detail previously [19]. Briefly, all patients underwent planning CT with a slice thickness of 2.5 or 3.2 mm under shallow free breathing. When respiratory movements of the lesions were large, CT during inspiratory and expiratory phases was added. The clinical target volume (CTV) was the visible tumor volume and that on CT during the three phases was superimposed on 3D radiation treatment planning systems. The mean planning target volume (PTV) margin was 7 mm (standard deviation, SD, 2) for the lung and chest wall sides, and 8 mm (SD, 2) for the craniocaudal direction. It was intended to cover 95% of the PTV with ≥95% of the prescribed dose. The maximum dose allowed within the PTV was 107%. Regarding the dose constraints of organs at risk, the volume of the lung receiving ≥20 Gy (V20Gy), mean lung dose, heart V30Gy and mean heart dose were set to be <35%, <20 Gy, <45% and < 26 Gy, respectively. In tomotherapy planning, lung V5Gy was kept below 65%.
All radiation therapy was carried out using 6- or 10-MV X rays with fractionation every weekday. In the treatment series using conventional radiotherapy (CRT), tangential or parallel opposing fields or multiple fields whenever necessary were used to treat the lesion. More recently, tomotherapy (TomoTherapy® or Radixact® System, Accuray, Sunnyvale, CA, USA) was used in 14 series to simultaneously treat multiple lesions; the TomoHelical mode was used in 12 series and the TomoDirect mode was used in 1. In the remaining 1 series, CRT was used first and then TomoDirect radiation was used after 40 Gy. In general, CRT was used to treat one or two lesions, and tomotherapy was used to simultaneously treat three or more lesions. However, depending upon the location and shape of the tumors, tomotherapy was also used to treat one or two lesions; especially tomotherapy was considered useful when the tumors existed near the previously irradiated regions. Our method of tomotherapy for pleural disseminations was similar to those used for breast cancer as described in detail previously [20]. The details of radiation therapy and tumor characteristics are shown in Table 2. Using CRT, the median tumor number treated per series was 1 (range 1–5), whereas it was 3 (range 1–20) for tomotherapy. In cases treated by the combination of CRT and tomotherapy, the target number was 4. The median dose per fraction was 2.0 Gy (range, 1.5–3.0) and the median total dose was 50 Gy (range, 39–60).
Table 2.
Treatment details and tumor characteristics
| Treatment / tumor details | Number |
|---|---|
| Total treatment patients / series / lesions | 20 / 33 / 96 |
| CRTa / tomotherapy / CRT + tomotherapy (series) | 19 / 13 / 1 |
| Total prescribed dose (Gy) Median (range) | 50 (39–60) |
| Dose per fraction (Gy) Median (range) | 2.0 (1.5–3.0) |
| BED10b (Gy) Median (range) | 65 (48–75) |
| Maximum tumor thickness from pleura (mm) Median (range) | 9 (3–40) |
| Maximum tumor diameter (mm) Median (range) | 20 (5–76) |
| Tumor sizec (mm2) Median (range) | 181 (15–2240) |
aCRT, conventional radiation therapy.
bBED10, biologically effective dose with an α/β ratio of 10 Gy.
cTumor size is defined by the maximum thickness from the pleura (mm) × diameter (mm) orthogonal to its line in the axial plane.
Evaluation
All patients were followed up by regular CT after radiation therapy. The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used to evaluate tumor response. Toxicity was rated according to the Common Terminology Criteria for Adverse Events version 5.0. Overall survival (OS) rates for 20 patients, progression-free survival (PFS) rates after 33 series of treatment, and local control (LC) rates for 96 tumors were calculated from the date of starting radiation therapy using the Kaplan–Meier method. Differences between subgroups in these curves were analyzed by the log-rank test. The data were evaluated by adopting the WHO histological classification before revision [21] that was actually used at the time of definitive diagnosis. Results were regarded as significant if the probability value was less than 5% (p < 0.05). All statistical analyses were carried out using the open source software EZR version 1.40. The multivariate Cox proportional hazard model was used to evaluate the variables showing associations with OS in the univariate analyses.
RESULTS
Survival, progression-free survival and local control
The median follow-up period after the first radiotherapy session for pleural dissemination was 46 months (range, 14–161). The mean ages (± SD) at the time of initial treatment and the time of irradiation for pleural dissemination were 53 ± 12 and 59 ± 12 years, respectively. The median time from initial treatment to recurrence was 36 months (range, 5–171), and that to local radiation therapy for pleural dissemination was 52 months (range, 15–221).
The OS, PFS, and LC curves for the 20 patients, 33 series of treatment, and 96 tumors are shown in Fig. 1. For all 20 patients, the OS was 100% at three years and 86% at five years. Two patients died of the disease and one died of an unknown cause. The PFS after 33 series of treatment was 30% at three years and 16% at five years. Only one patient developed distant metastases in the peritoneum, and pleural recurrence at different sites was observed after 23 series of treatment. The median time to recurrence was 18 months (range, 2–113). LC rates for the 96 lesions were 98% at three years and 96% at five years.
Fig. 1.
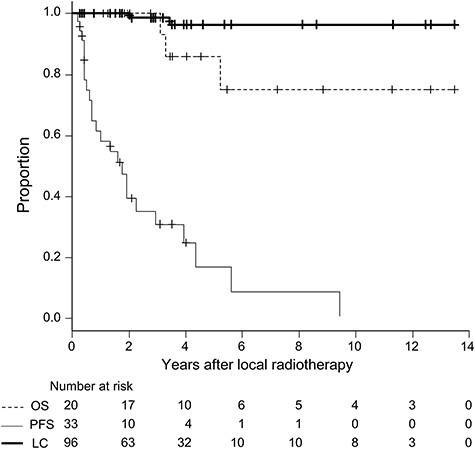
Curves of overall survival (dotted line) for all 20 patients, progression-free survival after 33 treatment series (thin line) and local control for 96 lesions of pleural dissemination (bold line).
In-field local recurrence developed for two lesions; one was type B3 thymoma with a size of 442 mm2 treated by 50 Gy in 25 fractions and another was type A with a size of 551 mm2 treated by 60 Gy in 30 fractions. A scatter diagram of LC status according to the size and biologically effective dose with an α/β ratio of 10 Gy (BED10), excluding 62 lesions without local recurrence within four years, are shown in Fig. 2. The two recurrent lesions were located at the upper middle to right areas. Tumors with a size <400 mm2 did not develop in-field local recurrence with a BED10 of 60 Gy or less.
Fig. 2.
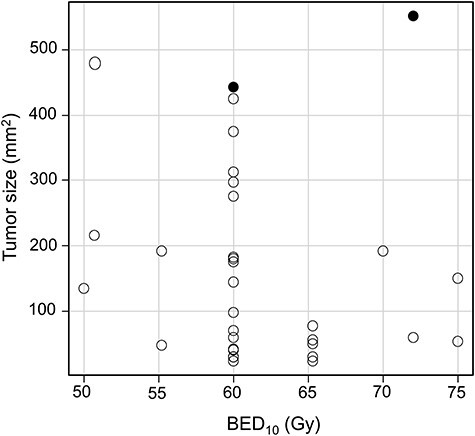
Tumor size (maximum thickness from the pleura × diameter orthogonal to its line in the axial plane) and BED10 in 32 cases with no local recurrence within four years (○) and in two cases with local recurrence (●).
Adverse events
Complications of treatment are summarized in Table 3. One patient developed grade 3 radiation pneumonitis (5%) and another developed grade 2 (5%) pneumonitis. All other pneumonitis was grade 1. The patient developing grade 3 pneumonitis received treatment for two relatively large tumors (tumor size: 418 and 312 mm2, respectively) simultaneously. One patient had grade 3 pericardial effusion (5%); the patient received local radiotherapy twice for relatively large tumors (size: 2240 and 480 mm2, respectively). In addition, she had received steroid-pulse therapy and systemic chemotherapy before the local radiation treatments. Among the nine patients who underwent two or more series of treatment, only one developed grade ≥ 2 toxicity. As local pleural irradiation usually does not produce myelosuppression, hematological toxicities were not evaluated.
Table 3.
Adverse events
| Grade 1 | Grade 2 | Grade 3 | Total | |
|---|---|---|---|---|
| Pneumonitis | 18 (90%) | 1 (5%) | 1 (5%) | 20 (100%) |
| Nausea | 3 (15%) | 1 (5%) | 0 | 4 (20%) |
| Dysphagia | 1 (5%) | 1 (5%) | 0 | 2 (10%) |
| Pericardial effusion | 0 | 0 | 1 (5%) | 1 (5%) |
| Pleural effusion | 3 (15%) | 1 (5%) | 0 | 4 (20%) |
| Dermatitis radiation | 5 (25%) | 1 (5%) | 0 | 6 (30%) |
Statistical analysis
We analyzed age (< vs ≥ 65 years), gender, WHO PS (0 vs ≥1), Masaoka stage [22] (III vs IVA/B), WHO histological classification (A/AB/B1/B2 vs B3/C), presence of myasthenia gravis (MG), chemotherapy history, history of postoperative radiation, radiation method (CRT vs tomotherapy), tumor size (< vs ≥ 400 mm2), number of irradiated lesions (1 vs ≥ 2), number of radiation series (1 vs ≥ 2), and total radiation doses in BED10 (< vs ≥ 65 Gy) as potential prognostic factors. Regarding OS, PS 0 was associated with better OS (P < 0.001), and patients who underwent two or more series of radiation for pleural dissemination had a better OS than those undergoing only one series (five-year OS, 100% vs 67%; seven-year OS, 100% vs 33%; P = 0.018). The former patients had a five-year OS of 100% after the second treatment for pleural dissemination. Regarding PFS, younger age was associated with better rates (P = 0.024). Regarding LC, smaller tumor size was associated with better rates (P = 0.00021). In multivariate analyses, no factor was associated with OS.
DISCUSSION
Only a few groups have evaluated radiotherapy for pleural dissemination of thymic epithelial tumors [23–25]. Ichinose et al. [23] reported good LC of pleural dissemination in 8 patients treated by radiation therapy. Other groups focused on hemithoracic irradiation rather than local treatment of pleural dissemination [24,25]. Therefore, our report may be the largest series evaluating the efficacy of local radiotherapy for pleural dissemination of thymoma and thymic carcinoma. LC rates were good. Although PFS was poor because patients developed other pleural dissemination lesions with a median interval of 18 months, OS was favorable (86% at five years). We consider that the favorable OS was due to debulking of tumors and prevention of the conditions associated with enlargement of pleural disseminations, also reflecting the insidious course of thymic tumors. Patients undergoing retreatment for pleural dissemination had a better OS than those undergoing only one series in univariate analysis, although it was not significant in multivariate analysis. Therefore, we consider this treatment, including repeat treatment, to be a useful option for this disease.
Several groups reported outcomes of surgery for recurrence [5,6,8,9,26,27]. The five- and 10-year OS rates were 37–91% and 16–82%, respectively, with some differences among the studies. Our OS results are consistent with these results. PFS rates after surgery for pleural dissemination were 13–29% at five years and 19% at 10 years [28,29]. Therefore, the PFS rates are also similar between our study and others evaluating surgical results. In a small series, the OS did not differ between patients receiving surgery plus radiation and those receiving radiation alone for intrathoracic recurrence of thymoma [30]. These tumors often cause pleural recurrence at different sites, but repeat treatment of the recurrences was generally tolerable and retreated patients had favorable OS. Only one patient undergoing repeat radiation therapy had grade 2 pulmonary toxicity in the present study.
This study demonstrated that stable LC can be achieved for most tumors. Only two developed local recurrence. Both were relatively large, and despite the relatively high doses delivered (BED10 > 60 Gy, corresponding to 50 Gy in 25 fractions), they developed recurrence within four years. On the other hand, lesions of 400 mm2 (tumor size) or less have not yet developed recurrence with a BED10 of 60 Gy or less within four years, as shown in Fig. 2. Therefore, doses corresponding to 50 Gy in 25 fractions may be recommended for tumors of 400 mm2 or less. As there are no data regarding the α/β ratio of thymic tumors, employing a ratio lower than 10 Gy may also be considered when using a more hypofractionated schedule [31].
Some reports stated the WHO histological classification as a prognostic factor [16,26], but no significant effects on OS or LCR were noted in our study with a small sample size. MG is the autoimmune disease most frequently (> 30%) associated with thymoma [32,33]; these reports suggested a good prognosis of MG patients. Although 30% of our patients also had MG, we found no difference in OS and LC rates between the MG and non-MG groups. The small sample size may be related to this, and it is the largest limitation of this study.
Before installation of tomotherapy at our institution, we used CRT to treat this disease. Using localized tangential radiation, the normal lung can be effectively spared and lung damage may be minimized. However, only a few (up to five in our study) lesions were able to be treated simultaneously, and treating multiple sites was time-consuming. The advent of tomotherapy solved this problem; multiple lesions can be effectively and safely treated. We successfully treated a patient with 20 disseminated lesions with mild toxicity (grade 2 pneumonitis). It was previously reported that the rate of grade 2 or higher radiation pneumonitis was less than 20% after whole pleural intensity-modulated radiotherapy for mesothelioma [34,35]. Therefore, the indication of local radiotherapy for pleural disseminations of thymic tumors may be expanded to include more than five lesions per treatment series.
In conclusion, our study revealed that local radiotherapy with a BED10 of 60 Gy may result in LC for tumors with a size smaller than 400 mm2. Although these patients may develop pleural recurrence at different sites, they can again be treated using the same technique. As a result, a relatively long OS can be expected with this approach.
ACKNOWLEDGEMENTS
The authors wish to thank Drs. Kotoha Tatekawa, Chikao Sugie, Natsuo Tomita, Yoshihiko Manabe, Shinya Otsuka, Taiki Takaoka, Shiho Ayakawa, Chisa Hashizume, Kaoru Uchiyama, Akifumi Miyakawa, Shinya Takemoto, Maho Yamada, Akihiro Hayashi, Maiko Nagasawa, Mikiko Imai, Akira Torii, Nozomi Kita, Tomoki Mizuno, Kimihito Iida and Taro Murai for their valuable help in this study.
Contributor Information
Dai Okazaki, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan.
Yuta Shibamoto, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan.
Takeshi Yanagi, Narita Memorial Proton Center, 78 Shirakawa-cho, Toyohashi, Aichi 441-8021, Japan.
Satoshi Ishikura, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan.
Takuhito Kondo, Department of Radiology, Nagoya Ekisaikai Hospital, 4-66 Syonen-cyo, Nakagawa-ku, Nagoya, Aichi 454-8502, Japan.
Yuki Yamada, Department of Radiation Oncology, Kasugai Municipal Hospital, 1-1-1 Takaki-cho, Kasugai, Aichi 486-8510, Japan.
Masanari Niwa, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
PRESENTATION AT A CONFERENCE
Part of this work was presented at 2016 annual meeting of ASTRO, Boston, USA
References
- 1. Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regnard JF, Magdeleinat P, Dromer C et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376–84. [DOI] [PubMed] [Google Scholar]
- 3. Maggi G, Casadio C, Cavallo A et al. Thymoma: Results of 241 operated cases. Ann Thorac Surg 1991;51:152–6. [DOI] [PubMed] [Google Scholar]
- 4. Margaritora S, Cesario A, Cusumano G et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg 2010;89:245–52. [DOI] [PubMed] [Google Scholar]
- 5. A1 S, Cusumano G, Lococo F et al. Long-term results after treatment for recurrent thymoma: A multicenter analysis. J Thorac Oncol 2014;9:1796–804. [DOI] [PubMed] [Google Scholar]
- 6. Lucchi M, Davini F, Ricciardi R et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185–9. [DOI] [PubMed] [Google Scholar]
- 7. Hosaka Y, Tsuchida M, Toyabe S. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann Thorac Surg 2010;89:912–7. [DOI] [PubMed] [Google Scholar]
- 8. Regnard JF, Zinzindohoue F, Magdeleinat P et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593–8. [DOI] [PubMed] [Google Scholar]
- 9. Ruffini E, Mancuso M, Oliaro A et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55–63. [DOI] [PubMed] [Google Scholar]
- 10. Giaccone G, Wilmink H, Paul MA et al. Systemic treatment of malignant thymoma: a decade experience at a single institution. Am J Clin Oncol 2006;29:336–44. [DOI] [PubMed] [Google Scholar]
- 11. Loehrer PJ Sr, Kim K, Aisner SC et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma. Final results of an intergroup trial. J Clin Oncol 1994;12:1164–8. [DOI] [PubMed] [Google Scholar]
- 12. Bott M, Wang H, Travis W et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg 2011;92:1984–91. [DOI] [PubMed] [Google Scholar]
- 13. Taguchi T, Suehiro T, Toru K et al. Pleural dissemination of thymoma showing tumor regression after combined corticosteroid and tacrolimus therapy. Eur J Intern Med 2006;17:575–7. [DOI] [PubMed] [Google Scholar]
- 14. Terada Y, Kambayashi T, Okahashi S et al. Trans-arterial infusion chemotherapy for recurrence of pleural dissemination after thymectomy. Ann Thorac Surg 2005;79:e32–3. [DOI] [PubMed] [Google Scholar]
- 15. De Bree E, van Ruth S, Baas P et al. Cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy in patients with malignant pleural mesothelioma or pleural metastases of thymoma. Chest 2002;121:480–7. [DOI] [PubMed] [Google Scholar]
- 16. Lewis JE, Wick MR, Scheithauer BW et al. Thymoma: a clinicopathologic review. Cancer 1987;60:2727–43. [DOI] [PubMed] [Google Scholar]
- 17. Shintani Y, Kanzaki R, Kusumoto H et al. Pleuropneumonectomy for a large thymoma with multiple pleural dissemination using median sternotomy followed by posterolateral thoracotomy. Surg Case Rep 2015;1:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omata S, Ozawa Y, Nakagawa M et al. Useful computed tomography features for differentiating between focal atelectasis and pleural dissemination on preoperative evaluations of thymic epithelial tumors. Eur J Radiol 2018;103:38–43. [DOI] [PubMed] [Google Scholar]
- 19. Shibamoto Y, Naruse A, Fukuma H et al. Influence of contrast materials on dose calculation in radiotherapy planning using computed tomography for tumors at various anatomical regions: a prospective study. Radiother Oncol 2007;84:52–5. [DOI] [PubMed] [Google Scholar]
- 20. Shibamoto Y, Murai T, Suzuki K et al. Definitive radiotherapy with SBRT or IMRT boost for breast cancer: excellent local control and cosmetic outcome. Technol Cancer Res Treat 2018;17:1533033818799355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller-Hermelink HK, Engel P, Kuo TT et al. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart, (Third edition) in Tumors of the Thymus. Lyon: IARC, 2004. [Google Scholar]
- 22. Koga K, Matsuno Y, Noguchi M et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359–67. [DOI] [PubMed] [Google Scholar]
- 23. Ichinose Y, Ohta M, Yano T et al. Treatment of invasive thymoma with pleural dissemination. J Surg Oncol 1993;54:180–3. [DOI] [PubMed] [Google Scholar]
- 24. Yoshida H, Uematsu M, Itami J et al. The role of low-dose hemithoracic radiotherapy for thoracic dissemination of thymoma. Radiat Med 1997;15:399–403. [PubMed] [Google Scholar]
- 25. Sugie C, Shibamoto Y, Ikeya-Hashizume C et al. Invasive thymoma: postoperative mediastinal irradiation, and low-dose entire hemithorax irradiation in patients with pleural dissemination. J Thorac Oncol 2008;3:75–81. [DOI] [PubMed] [Google Scholar]
- 26. Safieddine N, Liu G, Cuningham K et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018–22. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura S, Kawaguchi K, Fukui T et al. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg 2019;67:524–9. [DOI] [PubMed] [Google Scholar]
- 28. Kimura K, Kanzaki R, Kimura T. Long-term outcomes after surgical resection for pleural dissemination of thymoma. Ann Surg Oncol 2019;26:2073–80. [DOI] [PubMed] [Google Scholar]
- 29. Yano M, Sasaki H, Yukiue H. Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg 2009;33:1425–31. [DOI] [PubMed] [Google Scholar]
- 30. Urgesi A, Monetti U, Rossi G et al. Aggressive treatment of intrathoracic recurrences of thymoma. Radiother Oncol 1992;24:221–5. [DOI] [PubMed] [Google Scholar]
- 31. Shibamoto Y, Miyakawa A, Otsuka S et al. Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Res 2016;57:i76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rimner A, Gomez DR, Wu AJ et al. Failure patterns relative to radiation treatment fields for stage II-IV thymoma. J Thorac Oncol 2014;9:403–9. [DOI] [PubMed] [Google Scholar]
- 33. Maggi G, Casadio C, Cavallo A et al. Thymoma: Results of 241 operated cases. Ann Thorac Surg 1991;51:152–6. [DOI] [PubMed] [Google Scholar]
- 34. Kenneth E, Rosenzweig MG et al. Pleural intensity-modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM). Int J Radiat Oncol Biol Phys 2012;83:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rimner A, Zauderer MG, Gomez DR et al. Phase II study of hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) as part of lung-sparing multimodality therapy in patients with malignant pleural mesothelioma. J Clin Oncol 2016;34:2761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


