Abstract
During radiotherapy sessions to treat brain tumors or head-and-neck cancers, some patients experience unusual visual and/or olfactory perceptions. This prospective study sought to answer two questions: (i) what proportion of patients experience these unpleasant sensations?, and (ii) which organs are responsible? Eligible patients had brain or near-orbital tumors treated by helical tomotherapy. All were aged 10 years or older, able to communicate, and interviewed by a radiation oncologist at least once weekly during radiation therapy. If they had experienced such sensations, they were encouraged to join the second phase of the study. The patients were asked to indicate, using a button, when a sensation commenced and ended. The recorded data were collated with the treatment log. Thirty-eight consecutive patients were eligible. Twenty-six experienced visual and 13 olfactory sensations. The radiation doses to the organs related to the visual or olfactory sensations did not differ between patients who reported sensations and those who did not. Seventeen patients were enrolled in the second phase of the study. All 14 with visual sensations reported that the sensations occurred when the X-rays passed at eye level. Olfactory sensations were reported by eight out of nine patients when the X-rays passed through the olfactory epithelium and/or ethmoid sinus level. In conclusion, 68% of patients experienced visual sensations caused by X-rays passing through the level of the eyes, and 34% complained of olfactory sensations. With the exception of one patient, olfactory sensations occurred when the X-rays passed through the levels of the olfactory epithelium and/or ethmoid sinus.
Keywords: olfactory perception, phantosmia, visual sensation, phosphene, brain tumor, radiation
INTRODUCTION
During radiotherapy sessions to treat brain tumors or head-and-neck cancers, some patients experience abnormal visual and/or olfactory perceptions. Of 191 patients in a previous study, 10 and seven with brain tumors experienced visual and olfactory perceptions, respectively, during radiation sessions [1]. However, those numbers may be underestimates because of the retrospective nature of the study. We posed two questions: (i) what proportion of patients experience unpleasant visual and/or olfactory perceptions?, and (ii) which organs are responsible?
Two interesting recent studies on visual sensations have appeared. Tendler et al. in an experimental study, concluded that visual sensations were caused by Cherenkov radiation [2]. However, Wilhelm-Buchstab et al. used a method similar to the one we employed and suggested that interactions between photons and neuronal structures distant from the eyes can trigger phosphenes [3]. Thus, the origin of visual sensations remains controversial. Although two cases of olfactory perceptions were reported by the Memorial Sloan Kettering Cancer Center in 2012 [4], the cause remains unclear. Patients may smell substances such as ozone generated by the radiation [5] or may experience a phantosmia (a sensation of an unpleasant odor that does not in fact exist) [4]. Therefore, we performed this prospective observational study.
MATERIALS AND METHODS
The study protocol was approved by the ethics committee of our institute (approval no. 18-282) and was conducted in accordance with the Declaration of Helsinki and other ethical guidelines for epidemiological research. This was a two-phase study. As the first phase was observational, the need for informed consent was waived. However, informed consent was required from those entering the second phase. Eligible patients had brain or near-orbital tumors treated by helical tomotherapy. All were aged 10 years or older and were able to communicate. During the first phase, all patients were routinely interviewed by a radiation oncologist at least once weekly during radiation therapy and, following a previously prepared list, questioned about their general condition, neurological status, and any unusual visual or olfactory perceptions they had experienced during the radiation sessions. If they had in fact experienced such sensations, they were encouraged to join the second phase of the study. In the second phase, after informed consent was obtained, patients were asked to indicate using a button when a sensation commenced and ended. The data were recorded electronically by a device designed especially for the study (Engineering System, Matsumoto, Japan) and collated with the treatment logs. This process was repeated weekly.
Tomotherapy (The TomoTherapy HD, Accuray, Sunnyvale, CA, USA) was applied using a beam size of 25.1 mm and a pitch of 0.43. The table speed ranged from 0.15 to 1.23 (median 0.50) cm/s during intensity-modulated radiation therapy (IMRT). For one patient who received stereotactic radiation therapy, the beam size, pitch, and table speed were 10.5 mm, 0.29, and 0.06 cm/s, respectively. Patients were treated while supine and allowed to keep their eyes open or closed at their own discretion. All were immobilized using a thermoplastic head fixation shell (Esform; Engineering System). Radiation therapy planning was performed using the TomoHDA System Planning Station, ver. 5.1.1.6 (Accuray).
We treated malignant gliomas using the simultaneous integrated boost technique. The target volumes were contoured as recommended by the ESTRO-ACROP guidelines [6], with some modifications. In brief, the clinical target volume (CTV) for the high-dose area was expanded by an additional 1 cm from residual enhancing lesions and the surgical cavity. The CTV for elective irradiation was expanded by an additional 1 cm from the CTV for the high-dose area. If surrounding edema was evident, we included this in the CTV for elective irradiation. The prescribed CTV doses were 60 and 54 Gy in 30 fractions, respectively. The target volumes and radiation doses for other tumors depended on the pathology and clinical features.
To account for site-specific radiation doses, we analyzed the doses delivered to the following sites: eyes, retina, optic nerves, optic chiasm, visual cortex, olfactory epithelium, olfactory tract and olfactory cortex. The dose distribution was heterogeneous during IMRT, thus we hypothesized that higher doses may cause more unusual sensations. We used the D2% and D50% (Gy)/fraction dose as a representative of that delivered to the organs. The Dx% is the minimum absorbed dose in the hottest x% volume of the region of interest [7].
The olfactory region extends over the upper 10 mm of the septum and over the superior concha and includes the lateral walls above the concha [5, 8]. Therefore, these areas of the nasal cavity were contoured just before the sphenoid sinus and visualized at the lung window/level, as in a previous study [1]. The retina, eyes, optic nerves and optic chiasm were contoured according to a consensus-based atlas [9].
Contour guidelines for other organs such as the olfactory tract, olfactory or visual cortex were not available. The olfactory tract runs along the olfactory sulcus on the orbital surface of the frontal lobe, from the olfactory bulb to the anterior perforated substance, which is located near the optic chiasm [10–13]. Because the structure is hard to identify on MRI, we contoured it as a virtual structure at the base of the olfactory sulcus using a 5 mm-circle brush in each coronal plane. If we could identify the olfactory bulb (located above the nasoethmoidal region) and the olfactory tract, we included these structures in the contour. The olfactory cortex is composed of several areas [10–13]. We contoured the prepiriform areas, located in the anterior part of the uncus and amygdala, to represent the olfactory cortex.
The primary visual cortex is located mostly on the medial aspect of the occipital lobe. Posteriorly, it extends to the occipital pole [14]. Therefore, we contoured the medial surface of the occipital lobe and around the occipital pole to represent the visual cortex.
Student’s t-test was used to compare radiation doses and age distributions, and the chi-squared test was used to compare sex or perception distributions. A p-value <0.05 was considered significant.
RESULTS
Between March 2019 and August 2020, 44 consecutive patients were treated with the TomoTherapy platform in our institution. Of these, one who experienced unusual olfactory perceptions during the radiation sessions was nine years of age and thus ineligible, and the mental status of five patients was too poor to allow communication with the physicians. Therefore, 38 patients were eligible (Table 1), ranging in age from 15 to 86 years; 26 of these patients had malignant gliomas. The fraction dose range was 1.5–5 Gy, the median was 2 Gy, and the mode was 2 Gy (21 patients). Almost all patients with malignant glioma received concurrent chemotherapy (oral temozolomide with or without intravenous bevacizumab).
Table 1.
Patient characteristics
| Total Patients | 38 | ||
|---|---|---|---|
| Sex | Radiation history | ||
| Male | 20 | Yes | 3 |
| Female | 18 | No | 35 |
| Age (years) | 58.5 (15–86)* | Radiation method | |
| Tumor pathology | IMRT | 37 | |
| Glioblastoma | 20 | SRT | 1 |
| AA | 5 | Tumor site | |
| Meningioma | 3 | Intracranial | 36 |
| Germinoma | 2 | Extracranial | 2 |
| Olfactory NB | 2 | Total dose (Gy) | 57 (23.4–60)* |
| Others | 6 | Fraction dose (Gy) | 2 (1.5–5)* |
Note: *median (range)
Abbreviations: AA, anaplastic astrocytoma; NB, neuroblastoma; IMRT, intensity modulated radiotherapy; SRT, stereotactic radiotherapy.
Of the 38 participants, 26 (68%) experienced unusual visual sensations and 13 (34%) unusual olfactory perceptions during the radiation sessions (Table 2). Olfactory sensation was reported by 12 of the 26 patients who experienced light sensation but by only one of the 12 who did not perceive light. Thus, patients who experienced visual sensation were more likely to report concurrent olfactory sensation (p = 0.022).
Table 2.
Frequency of visual and olfactory sensations
| Visual sensation | Olfactory sensation | |||||
|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | |
| Total | 26 | 12 | 13 | 25 | ||
| Sex | ||||||
| Male | 14 | 6 | 0.69 | 6 | 14 | 0.50 |
| Female | 12 | 6 | 7 | 11 | ||
| Age | 52.5 (23–79)* | 60 (15–86)* | 0.46 | 44 (25–78)* | 64 (15–86)* | 0.0073 |
Note: *median (range)
Sixteen reported white or blue lights, and almost all stated that the lights flashed on and off. One reported a visual sensation as follows. First, a small dark purple disk appeared in the center of the visual field, became alternately larger and smaller for some time, and then gradually increased in size. At the same time, white or yellow light (reminiscent of lightning) appeared from the caudal direction, spreading to the entire visual field, which thus became bright. At almost the same time, an unpleasant odor was noted. Then, the lights disappeared in the reverse order of their appearance. Another patient reported that the light was akin to a disk, the center of which was white and the periphery blue. The light usually moved from right to left and flashed on and off. Sometimes, the light came from the opposite direction. Another patient also reported movement of a light from right to left, and another reported such movement via the upper side. Two patients reported that the light moved from side to side.
All patients but one who experienced olfactory perceptions reported them as unpleasant/foul. Six described an odor of burning plastics (three patients), rubber (one patients) or ‘something’ (two patients). One patient reported the smell of chloride. Two reported that the smell was difficult to describe, and two described the smell as ‘stimulating.’ One patient simply reported an unpleasant smell and another an ‘aura of forest’ (moss?).
There was no significant difference in sex or age between patients who reported visual sensations and those who did not. There was no significant difference in sex between patients who experienced olfactory perceptions and those who did not. The patients who experienced unusual olfactory perceptions were younger than those who did not (p = 0.0073; Table 2).
Tables 3 and 4 report the results of the site-specific dosimetric analyses. No significant differences were detected in the D2% or D50% fraction dose in each organ between the patients who reported visual or olfactory sensations during radiation and those who did not.
Table 3.
Dosimetric analyses of visual sensations
| All patients | Perceived | Not perceived | p | ||
|---|---|---|---|---|---|
| Eye | D2% (Gy) | 0.70 (0.42) *1 | 0.77 (0.42) | 0.55 (0.39) | 0.14 |
| D50%(Gy) | 0.41 (0.35) | 0.44 (0.38) | 0.32 (0.27) | 0.34 | |
| Retina | D2% (Gy) | 0.75 (0.45) | 0.84 (0.45) | 0.56 (0.41) | 0.080 |
| D50%(Gy) | 0.38 (0.25) | 0.40 (0.25) | 0.33 (0.26) | 0.46 | |
| Lens | D2% (Gy) | 0.33 (0.33) | 0.37 (0.37) | 0.25 (0.18) | 0.30 |
| D50%(Gy) | 0.27 (0.33) | 0.30 (0.38) | 0.20 (0.16) | 0.39 | |
| Optic | D2% (Gy) | 1.24 (0.68) | 1.32 (0.61) | 1.06 (0.80) | 0.29 |
| nerve | D50%(Gy) | 0.84 (0.50) | 0.88 (0.46) | 0.75 (0.55) | 0.47 |
| Optic*2 | D2% (Gy) | 1.54 (0.72) | 1.57 (0.64) | 1.46 (0.86) | 0.69 |
| chiasm | D50%(Gy) | 1.24 (0.71) | 1.26 (0.69) | 1.18 (0.77) | 0.77 |
| Visual | D2% (Gy) | 1.20 (0.72) | 1.27 (0.66) | 1.33 (0.82) | 0.81 |
| cortex | D50%(Gy) | 0.96 (0.63) | 0.91 (0.54) | 1.06 (0.77) | 0.51 |
| Olfactory*3 | D2% (Gy) | 1.00 (0.54) | 1.03 (0.48) | 0.93 (0.65) | 0.59 |
| epithelium | D50%(Gy) | 0.72 (0.47) | 0.77 (0.44) | 0.64 (0.51) | 0.45 |
| Olfactory*4 | D2% (Gy) | 1.43 (0.73) | 1.47 (0.60) | 1.34 (0.93) | 0.63 |
| tract | D50%(Gy) | 1.02 (0.57) | 1.02 (0.48) | 1.02 (0.74) | 0.99 |
| Olfactory*5 | D2% (Gy) | 1.77 (0.79) | 1.86 (0.66) | 1.57 (1.00) | 0.32 |
| cortex | D50%(Gy) | 1.14 (0.65) | 1.27 (0.62) | 0.86 (0.62) | 0.078 |
Notes: D2% or D50%: the minimum absorbed dose in the hottest 2% or 50% volume of the region of interest, respectively.
*1Mean and standard deviation;
*2Two patients were excluded because the optic chiasm could not be identified;
*3One patient who underwent two olfactory resections was excluded;
*4One patient who did not receive MRI was excluded because olfactory sulcus identification was not possible;
*5Only one of the paired olfactory cortexes was included for three patients who underwent resection of the other.
Table 4.
Dosimetric analyses of olfactory sensations
| All patients | Perceived | Not perceived | p | ||
|---|---|---|---|---|---|
| Eye | D2% (Gy) | 0.70 (0.42) *1 | 0.72 (0.32) | 0.69 (0.47) | 0.85 |
| D50%(Gy) | 0.41 (0.35) | 0.41 (0.19) | 0.41 (0.41) | 0.99 | |
| Retina | D2% (Gy) | 0.75 (0.45) | 0.79 (0.37) | 0.73 (0.49) | 0.73 |
| D50%(Gy) | 0.38 (0.25) | 0.45 (0.24) | 0.34 (0.25) | 0.21 | |
| Lens | D2% (Gy) | 0.33 (0.33) | 0.32 (0.14) | 0.33 (0.39) | 0.90 |
| D50%(Gy) | 0.27 (0.33) | 0.25 (0.14) | 0.28 (0.39) | 0.75 | |
| Optic | D2% (Gy) | 1.24 (0.68) | 1.37 (0.47) | 1.17 (0.76) | 0.42 |
| nerve | D50%(Gy) | 0.84 (0.50) | 0.97 (0.35) | 0.78 (0.55) | 0.26 |
| Optic*2 | D2% (Gy) | 1.54 (0.72) | 1.57 (0.39) | 1.52 (0.83) | 0.85 |
| chiasm | D50%(Gy) | 1.24 (0.71) | 1.25 (0.47) | 1.23 (0.81) | 0.94 |
| Visual | D2% (Gy) | 1.20 (0.72) | 1.21 (0.74) | 1.33 (0.70) | 0.62 |
| cortex | D50%(Gy) | 0.96 (0.63) | 0.81 (0.49) | 1.03 (0.68) | 0.32 |
| Olfactory*3 | D2% (Gy) | 1.00 (0.54) | 1.09 (0.44) | 0.96 (0.58) | 0.50 |
| epithelium | D50%(Gy) | 0.72 (0.47) | 0.84 (0.44) | 0.67 (0.47) | 0.32 |
| Olfactory*4 | D2% (Gy) | 1.43 (0.73) | 1.52 (0.43) | 1.38 (0.84) | 0.60 |
| tract | D50%(Gy) | 1.02 (0.57) | 1.18 (0.44) | 0.94 (0.62) | 0.24 |
| Olfactory*5 | D2% (Gy) | 1.77 (0.79) | 1.88 (0.59) | 1.71 (0.88) | 0.56 |
| cortex | D50%(Gy) | 1.14 (0.65) | 1.37 (0.50) | 1.02 (0.69) | 0.12 |
Notes: D2% or D50%: the minimum absorbed dose in the hottest 2% or 50% volume of the region of interest, respectively
*1Mean and standard deviation;
*2Two patients were excluded because the optic chiasm could not be identified;
*3One patient who underwent two olfactory resections was excluded
*4One patient who did not receive an MRI was excluded because olfactory sulcus identification was not possible;
*5Only one of the paired olfactory cortexes were included for three patients who underwent resection of the other.
The second phase of the study included 17 patients (six for both types of sensations, eight for visual sensations only, and three for olfactory perceptions only) (Table 5). All 14 experiencing visual sensations reported that they occurred when the X-rays passed through the level of the eyes (Fig. 1, Supplementary Fig. 1). Olfactory perceptions were reported when the X-rays passed through the olfactory epithelium and/or ethmoid sinus level(s) in eight out of nine patients (Fig. 2, Supplementary Fig. 2). However, one patient experienced sensations when the rays passed through the frontal lobe level, thus above the olfactory epithelium (Supplementary Fig. 2, patient 4). This patient underwent surgery twice to treat an olfactory neuroblastoma and thus had no olfactory perception at all prior to commencing radiation therapy. However, she reported unpleasant phantosmia during and after treatment sessions. The details will be presented elsewhere.
Table 5.
Details of patients who participated to the second phase study
| # | Sex | Age | Tumor pathology | Main site of the lesion | Total Dose(Gy) | Fraction Dose(Gy) | Eye D2 (Gy) | OE D2 (Gy) | 2nd phase study | |
|---|---|---|---|---|---|---|---|---|---|---|
| Visual | Olfactory | |||||||||
| 1 | M | 25 | GB | Lt F | 60 | 2 | 0.72 | 0.41 | Yes | Yes |
| 2 | M | 71 | GB | Rt T | 60 | 2 | 0.76 | 0.69 | Yes | No |
| 3 | F | 72 | Meningioma | Lt T | 54 | 1.8 | 0.78 | 0.94 | Yes | No |
| 4 | F | 35 | olfactory NB | ethmoid | 60 | 2 | 1.2 | -* | No | Yes |
| 5 | M | 70 | AA | Rt BG-brainstem | 60 | 2 | 0.51 | 1.1 | Yes | No |
| 6 | M | 63 | GB | brainstem | 60 | 2 | 0.41 | 1.1 | Yes | No |
| 7 | M | 23 | GB | Rt F | 60 | 2 | 0.93 | 0.11 | Yes | No |
| 8 | F | 49 | GB | Rt T | 60 | 2 | 0.61 | 0.82 | Yes | Yes |
| 9 | F | 70 | Meningioma | suprasellar | 54 | 1.8 | 0.32 | 1.2 | Yes | No |
| 10 | F | 47 | GB | Rt T-F | 60 | 2 | 0.81 | 1.4 | Yes | Yes |
| 11 | F | 73 | Merkel cell ca | Rt cheek | 60 | 2 | 0.20 | 0.99 | Yes | No |
| 12 | F | 44 | metastasis | brainstem | 30 | 3 | 0.26 | 0.74 | No | Yes |
| 13 | F | 67 | GB | Lt P | 60 | 2 | 0.26 | 0.053 | Yes | No |
| 14 | F | 48 | Pituitary ad | Pituitary gland | 50 | 2 | 0.74 | 1.4 | Yes | Yes |
| 15 | M | 28 | recurrence of germinoma | MO & CC | 24 | 1.5 | 1.5 | 1.5 | Yes | Yes |
| 16 | M | 54 | olfactory NB | ethmoid | 54 | 1.8 | 0.63 | 1.3 | No | Yes |
| 17 | M | 40 | AA | Lt P | 60 | 2 | 0.45 | 0.34 | Yes | Yes |
Abbreviations: M, male; F, female; OE, olfactory epithelium; GB, glioblastoma; NB, neuroblastoma; ca, carcinoma; ad, adenoma; AA, anaplastic astrocytoma; Lt, left; Rt, right; F, frontal lobe; T, temporal lobe; P, parietal lobe; BG, basal ganglia; MO, Medulla oblongata; CC, Corpus callosum.
Note: *Not determined due to surgical resection of the olfactory epithelium.
Fig. 1.
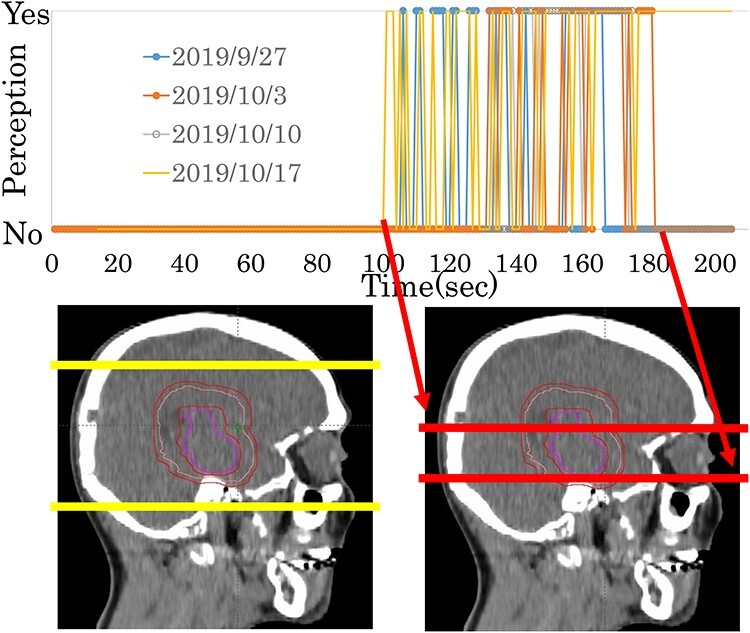
A representative case (in Patient #5 of Table 5) of a visual sensation experienced during radiation therapy. The upper column indicates when the patient experienced the sensation. The lower column shows the beam positions that overlapped the sagittal views of treatment-planning CT. Red lines indicate the start and the end of the sensation. Yellow lines show the start and end of the full treatment session.
Fig. 2.
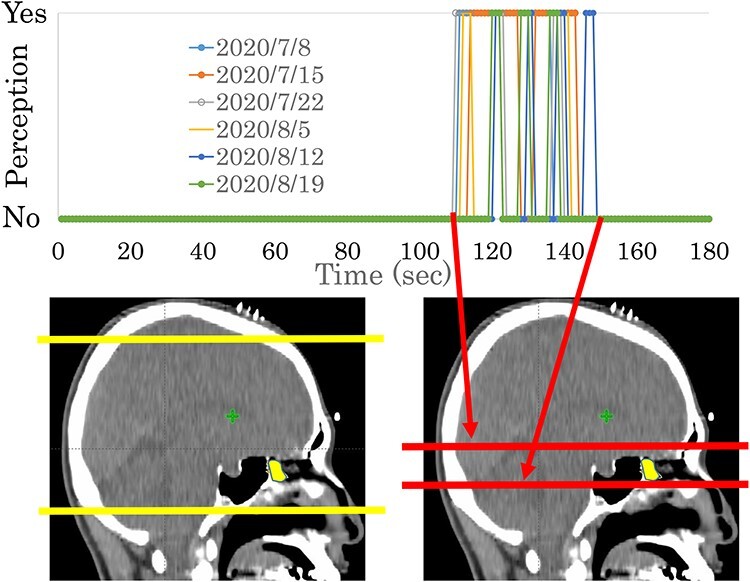
A representative case (in Patient #17 of Table 5) of an olfactory perception experienced during radiation therapy. The upper column indicates when the patient experienced the sensation. The lower column shows the beam positions that overlapped the sagittal views of treatment-planning CT. Red lines indicate the start and end of the sensation. Yellow lines indicate the start and end of the full treatment session. The yellow area indicates the olfactory epithelium.
We also analyzed the beam positions in patients who did not report sensations (Table 6). In 10 of the 12 patients without visual sensation and in 20 of the 25 without olfactory sensation, the beam passed through the eyes and olfactory epithelium/ethmoid sinuses, respectively.
Table 6.
The relationships between visual and olfactory sensations and treatment range
| Eyes | Olfactory epithelium / ethmoid sinus | ||||
|---|---|---|---|---|---|
| Within the treatment range | Outside the treatment range | Within the treatment range | Outside the treatment range | ||
| Visual sensation | Yes | 26 | 0 | 24 | 2 |
| No | 10 | 2 | 9 | 3 | |
| Olfactory sensation | Yes | 13 | 0 | 13 | 0 |
| No | 23 | 2 | 20 | 5 | |
DISCUSSION
Of all patients, 68% reported visual sensations during the radiation sessions, as expected. Steidley reported that all patients who received radiation to the eyes experienced phosphenes [15]. No significant differences were detected by dosimetric analysis between patients who reported the sensations and those who did not. Although the radiation dose to the eye was not predictive of sensations, all second-phase patients reported sensations when the X-ray beam passed through the eyes level, suggesting that the eyes caused the sensations. Since no patients reported sensations during megavolt CT, it is likely that the production of sensations depends more on the radiation dose-rate than the cumulated-dose.
Wilhelm-Buchstab et al. reported that some of the phosphenes were caused by radiation-induced stimulation of the visual nerve [3]. However, we did not encounter this phenomenon. Conversely, Tendler et al. found that the X-rays caused ocular Cherenkov emissions, explaining the visual sensations [2]. Our data are consistent with those results. Some participants reported that the light disk moved from right to left. The linac of the TomoTherapy turns clockwise in the gantry during treatment; the light movement probably reflects the trajectory of the X-rays, thus explaining the eye phenomena. Two patients (not included in the present study) reported that the light moved along the path of the sound produced by the treatment machine (data not shown). The sound may result from movement of the binary collimator at the aperture of the machine, which follows the direction of the beam.
Eight out of nine second-phase participants reported olfactory perceptions when the X-rays passed through the olfactory epithelium and/or ethmoid sinus level. Some stated that the smell was like that of burning plastics or chemicals. Such sensations were reported in studies from the early 1990s [5, 16]. According to Sagar et al. ozone is detectable even at low concentrations if it is produced adjacent to the olfactory epithelium [5]. Other investigators found that the measured concentrations of ozone synthesized during X-irradiation reached the human sensory threshold [16]. The ethmoid sinuses lie adjacent, and are connected, to the olfactory epithelial region; we suspect that a substance generated by X-irradiation of these areas was detected.
However, it is possible that the X-rays stimulate the olfactory nerve system. The olfactory nerves arise from the olfactory mucosa, passing through foramina in the cribriform plate of the ethmoid bone to the olfactory bulb situated on the orbital surface of the frontal lobe. The olfactory tracts, which lie between the inferior surfaces of the frontal lobes and the skull base, travel from the bulb to the ipsilateral piriform cortex, amygdala, and rostral entorhinal cortex [14]. Temporal lobe seizures have been associated with a reported foul odor such as that of burning tires [11]. Some parts of the neural course are included in the area through which X-rays passed when the patients reported the sensations. One patient whose olfactory epithelial region was resected reported sensations as the X-rays passed through the frontal lobe. It is thus probable that the X-rays stimulated the neural course.
In a previous report, almost all patients who reported olfactory perceptions during radiation sessions were young [1], and our study confirmed this. The reason is unclear, but in general, the sense of smell declines progressively over the human lifespan [17].
Patients who experienced visual sensation were significantly more likely to report olfactory sensations. The eyes and olfactory organs are in close proximity, and higher dose-rate beams pass through these organs simultaneously. Therefore, it is reasonable that patients might experience both sensations simultaneously.
A limitation of this study is that we analyzed the X-ray beam levels only when visual and/or olfactory perceptions were reported. As mentioned above, Wilhelm-Buchstab et al. used a similar method [3], but their work was more precise. They reported the relationships of visual sensations with both the beam level and beam direction. We have the treatment logs, and we can analyze the relationship between beam direction and olfactory perception to identify the organ(s) involved more precisely. However, small differences in beam position may greatly affect such analysis. Patients reported the sensation at different time points over the course of the study. The subjective nature of sensation reporting contributed to variable data on the timing of sensation onset and end. Therefore, we described only the relationship between the treatment beam level and the sensations.
CONCLUSION
We found that 68% of patients experienced phosphenes when the X-rays passed through the eye level, and 34% complained of olfactory perceptions. Eight out of nine patients reported such sensations when the X-rays passed through the levels of the olfactory epithelium and/or ethmoid sinus. One patient reported such a sensation when the X-rays passed through the frontal lobe.
FUNDING
Funding was provided by Juntendo University.
CONFLICT OF INTEREST
The authors declare they have no conflict of interests.
PRESENTATION
Presented in part at the 2020 ASTRO annual meeting.
Supplementary Material
REFERENCES
- 1. Obinata M, Yamada K, Sasai K. Unusual olfactory perception during radiation sessions for primary brain tumors: a retrospective study. J Radiat Res 2019;60:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tendler II, Hartford A, Jermyn M et al. Experimentally observed Cherenkov light generation in the eye during radiation therapy. Int J Radiat Oncol Biol Phys 2020;106:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilhelm-Buchstab T, Buchstab BM, Leitzen C et al. Extraretinal induced visual sensations during IMRT of the brain. PLoS One 2015;10:e0123440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang JC, Khakoo Y, Lightner DD et al. Phantosmia during radiation therapy: a report of 2 cases. J Child Neurol 2013;28:791–4. [DOI] [PubMed] [Google Scholar]
- 5. Sagar SM, Thomas RJ, Loverock LT et al. Olfactory perceptions produced by high-energy photon irradiation of the olfactory receptor mucosa in humans. Int J Radiat Oncol Biol Phys 1991;20:771–6. [DOI] [PubMed] [Google Scholar]
- 6. Niyazi M, Brada M, Chalmers AJ et al. ESTRO-ACROP guideline ‘target delineation of glioblastomas’. Radiother Oncol 2016;118:35–42. [DOI] [PubMed] [Google Scholar]
- 7. International Commission on Radiation Units and Measurements . Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). ICRU report 83. JICRU 2010;10:27–40. [Google Scholar]
- 8. Standring S. Nose, nasal cavity and paranasal sinuses. In: Standring S (ed). Gray's Anatomy: The Anatomical Basis of Clinical Practice, 41st edn. Amsterdam: Elsevier, 2015, 556–70. [Google Scholar]
- 9. Eekers DB, in't Ven L, Roelofs E et al. The EPTN consensus-based atlas for CT- and MR-based contouring in neuro-oncology. Radiother Oncol 2018;128:37–43. [DOI] [PubMed] [Google Scholar]
- 10. Duprez TP, Rombaux P. Imaging the olfactory tract (cranial nerve #1). Eur J Radiol 2010;74:288–98. [DOI] [PubMed] [Google Scholar]
- 11. Berkowitz AL. Cranial nerves 1, 9, 10, 11, and 12. In: Berkowitz AL (ed) Clinical Neurology and Neuroanatomy: A Localization-based Approach (Kindle ed). New York: McGraw-Hill, 2017. [Google Scholar]
- 12. Mancall EL. Special senses. In: Mancall EL, Brock DG (eds). Gray's clinical neuroanatomy: the anatomic basis for clinical neuroscience. Amsterdam, Elsevier: Elsevier Health Sciences, 2011, 209–25. [Google Scholar]
- 13. Harnsberger HR, Osborn AG, Ross JS. CN1 (olfactory nerve). In: Macdonald AJ (ed) Diagnostic and Surgical Imaging Anatomy. Brain, Head & Neck, Spine. Salt Lake City: Amirsys, 2011, 186–9. [Google Scholar]
- 14. Standring S. Cerebral hemispheres. In: Standring S (ed). Gray's Anatomy: The Anatomical Basis of Clinical Practice, 41st edn. Philadelphia: Elsevier, 2015, 373–98. [Google Scholar]
- 15. Steidley KD, Eastman RM, Stabile RJ. Observations of visual sensations produced by Cerenkov radiation from high-energy electrons. Int J Radiat Oncol Biol Phys 1989;17:685–90. [DOI] [PubMed] [Google Scholar]
- 16. Costello SA, Wynne CJ, Faid A et al. High energy photon irradiation of the olfactory mucosa in humans. Int J Radiat Oncol Biol Phys 1992;23:477. [DOI] [PubMed] [Google Scholar]
- 17. Mullol J, Alobid I, Marino-Sanchez F et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open 2012;2:e001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


