Abstract
The aim of this study is to analyze the incidence and risk factors for pneumonitis when immune checkpoint inhibitors (ICIs) are combined with palliative thoracic radiotherapy (RT) for lung cancer. We retrospectively evaluated 29 patients with lung cancer who received ICIs after palliative thoracic RT (30 Gy in 10 fractions). Their ICIs were pembrolizumab (n = 17), nivolumab (n = 8) and atezolizumab (n = 4). Median follow-up period was 10 months. The median interval between starting RT and starting ICI was 25 days. Pneumonitis events were grade 1 (n = 10; 34%), grade 2 (n = 4; 14%) and grade 3 (n = 3; 10%). Obstructive pneumonia was significantly associated with grade ≥ 2 pneumonitis (P = 0.036). Age, sex, ICI agent, interval between RT and ICI and history of ICI before RT were not associated with grade ≥ 2 pneumonitis. Tumor volume; Brinkman index; dosimetric factors, such as lung V5, V10, V20, V30 and mean lung dose (MLD); lactate dehydrogenase; and C-reactive protein did not significantly differ between the grade ≤ 1 and grade ≥ 2 pneumonitis groups. Levels of sialylated carbohydrate antigen KL-6 were evaluated in 27 patients before RT; they significantly differed between patients with grade ≤ 2 pneumonitis (mean: 431 U/ml) and those with grade ≥ 3 pneumonitis (mean: 958 U/ml; P < 0.001). Patients who receive ICI after palliative thoracic RT should be carefully followed-up, especially those who have had obstructive pneumonia or high KL-6 levels.
Keywords: pneumonitis, radiotherapy (RT), immune checkpoint inhibitor (ICI)
INTRODUCTION
Lung cancer is the most common cause of cancer-related mortality in the world [1]. Despite efforts to detect lung cancer in its early stages, some patients were diagnosed as having advanced lung cancer on their first visit to hospital. In the registry study of lung cancer in Japan, approximately 20% of patients were stage IV at diagnosis [2]. Generally, patients with advanced lung cancer are treated with chemotherapy, immune checkpoint inhibitors (ICIs) or their combinations [3–6]. Furthermore, patients with advanced lung cancer frequently present with cancer-related symptoms, such as pain, bleeding and airway obstruction [7–9], which are often treated with palliative radiotherapy (RT) [10]. Recently, RT also has been shown to strengthen the effect of ICIs and stimulate their anti-tumor immune response [11–14]. For these reasons, patients are increasingly treated with ICIs and RT at the same time. One of the concerns after administration of ICIs or RT is pneumonitis. Generally, patients with advanced lung cancer have a poor general condition and development of pneumonitis may directly affect their prognosis. In addition, developing pneumonitis means the discontinuation of chemotherapy or ICIs, which may worsen the prognosis of patients. It is reported that the incidence of pneumonitis is 1.6% to 6% with palliative thoracic RT [15] and 5.9% with nivolumab [16] but the interaction of ICIs and RT on the incidence of pneumonitis remains unclear. Barron et al. reported that 40% of patients developed ICI-related pneumonitis after RT for advanced lung cancer [17]. They concluded that previous RT was a significant risk factor for pneumonitis. However, in their study, RT for the extra-thoracic region was included and doses of RT varied widely. Shaverdian et al. reported that thoracic RT was significantly associated with pulmonary toxicity in a phase I study of pembrolizmab for advanced non-small cell lung cancer (NSCLC) [18]. In their report, doses of RT varied widely, which is similar to Barron et al. In this study, we focused on the patients who received 30 Gy in 10 fractions of palliative RT for the thoracic region followed by ICI. The aim of this study is to analyze the incidence and risk factors for pneumonitis among patients with lung cancer who received ICI after 30 Gy in 10 fractions of palliative thoracic RT.
MATERIALS AND METHODS
Patients
We retrospectively analyzed patients with lung cancer who received ICI after palliative thoracic RT. We included patients for whom the interval between palliative RT and ICI was less than one year. In this study, thoracic RT was defined as radiation directed at tumors in the lung or mediastinum. We excluded patients who received re-irradiation towards the thoracic region while also receiving ICIs. This study was approved by our institutional ethics committee (reference number: 20–091).
ICIs
Doctors’ decisions determined the choice of chemotherapy regimen. The ICIs were intravenously administered (nivolumab: 3 mg/kg every two weeks; pembrolizumab: 300 mg/kg every three weeks; atezolizumab: 1200 mg/kg every three weeks).
Radiotherapy
All the patients received 30 Gy in 10 fractions of RT, for palliative intent. RT was performed with a 10 MV X-ray generated by linear accelerator. The RT technique was three-dimensional conformal RT with computed tomography (CT) image simulation. Gross tumor volume (GTV) was defined by a radiation oncologist, this seemed to be the cause of symptoms or was expected to start causing symptoms soon. As a planning target volume margin, a 5–10 mm margin was added to GTV. Usually, antero-posterior, parallel opposite beams were used to create the radiation field with multi-leaf collimator margins of 5 mm. Superposition convolution was the dose calculation algorithm. Dose-volume parameter of the lung, such as mean lung dose (MLD) and volume of the lung receiving more than 5 Gy (V5), 10 Gy (V10), 20 Gy (V20) and 30 Gy (V30), were evaluated [19].
Evaluation
Each patient received a complete blood cell count, differential count, biochemistry measurements and chest X-ray at every administration of ICI. After administration of ICIs, a CT scan was performed when pneumonitis was suspected. For this study, pneumonitis is defined as pulmonary toxicity without evidence of bacterial or viral pneumonia. Bacterial or viral pneumonia was carefully ruled out on the basis of CT imaging; blood cell counts; hemograms; sputum culture; and antigen tests for pneumococcus, legionella; and influenza. Severity of pneumonitis was classified using the Common Terminology Criteria for Adverse Events, version 5. These evaluations were performed by expert physicians in respiratory medicine and a radiation oncologist.
Statistical analysis
Relationships between categorical data and pneumonitis were evaluated by the chi-square test. Mean parameters between two groups were compared using Student’s t-test. Median parameters between two groups were compared using Mann–Whitney U test. P < 0.05 was considered significant. Receiver operating characteristic (ROC) curves analysis and areas under the curve (AUC) were used to find cut-off values to predict pneumonitis. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (SPSS Inc., Armonk, NY, USA).
RESULTS
Patients and treatment characteristics
We analyzed 29 patients with lung cancer who received ICIs after palliative thoracic RT during March 2018 and May 2020. They included 24 men and five women, with a median age of 68 years; the median follow-up period was 10 months, the median interval between starting RT and starting ICI was 25 days and the median number of ICI cycles was five (range: 1–33 cycles). Their target site of irradiation were primary disease in lung (n = 22) and vertebral metastasis (n = 7). Their ICIs were pembrolizumab (n = 17), nivolumab (n = 8) and atezolizumab (n = 4) (Table 1). Twelve patients had received ICIs before starting RT. Two patients received concomitant chemotherapy with ICI. Among them, one patient received carboplatin, pemetrexed plus pembrolizumab and another patient received carboplatin, nab-paclitaxel plus pembrolizumab. The purpose of palliative RT were obstructive pneumonia (n = 11), pain relief (n = 8), superior vena cava syndrome (n = 4), hoarseness (n = 1) and tumor growth suppression (n = 5). The median survival period of patients with grade ≤ 1 pneumonitis was 10 months while that of patients with grade ≥ 2 pneumonitis was eight months (P = 0.838). Obstructive pneumonia was not correlated with the performance status of the patient (P = 0.627).
Table 1.
Patient characteristics (n = 29)
| Characteristic | ||
|---|---|---|
| Age, years, median (range) | 68 (52–85) | |
| Sex, n (%) | Male | 24 (83) |
| Female | 5 (17) | |
| Performance status, n (%) | 0 | 9 (31) |
| 1 | 16 (55) | |
| 2 | 3 (10) | |
| 3 | 1 (4) | |
| T classification, n (%) | 1a | 1 (3) |
| 1c | 2 (7) | |
| 2a | 2 (7) | |
| 2b | 1 (3) | |
| 3 | 12 (41) | |
| 4 | 11 (38) | |
| N classification, n (%) | 0 | 7 (24) |
| 1 | 2 (7) | |
| 2 | 8 (27) | |
| 3 | 12 (41) | |
| M classification, n (%) | 0 | 4 (14) |
| 1a | 8 (27) | |
| 1b | 8 (27) | |
| 1c | 9 (31) | |
| Immune checkpoint inhibitor, n (%) | Pembrolizumab | 17 (59) |
| Nivolumab | 8 (27) | |
| Atezolizumab | 4 (14) | |
| Interval between RT and ICI, days, median (range) | 25 (0–300) | |
| Gross tumor volume, cc, median (range) | 110 (5–872) | |
| V5 of the lung, %, median (range) | 15 (0–26) | |
| V10 of the lung, %, median (range) | 12 (0–21) | |
| V20 of the lung, %, median (range) | 9 (0–16) | |
| V30 of the lung, %, median (range) | 1 (0–5) | |
| Mean lung dose, Gy, median (range) | 3.6 (0.7–5.5) |
RT: radiotherapy; ICI: immune checkpoint inhibitors; lung V5: lung volume receiving >5 Gy; lung V10: lung volume receiving >10 Gy; lung V20: lung volume receiving >20 Gy; lung V30: lung volume receiving >30 Gy.
Incidence of pneumonitis
The observed pneumonitis incidence among these patients was grade 1 (n = 10; 34%), grade 2 (n = 4; 14%) and grade 3 (n = 3 patients; 10%). Grade 4 or grade 5 pneumonitis was not observed. Representative patients who developed grade 2 pneumonitis are shown in Figs 1 and 2. The median interval between administration of the first cycle of ICI after RT and development of pneumonitis was three months for patients with grade 3 pneumonitis and five months for patients with grade 2 pneumonitis. Among three patients with grade 3 pneumonitis, two patients received 1–1.5 mg/kg of prednisolone and one patient received steroid pulse therapy. Pneumonitis of these three patients was improved after a gradual decrease of steroids. Four patients with grade 2 pneumonitis received 0.5 mg/kg of predonisolone. Grade 2 pneumonitis was also improved after gradual decrease of steroid. Among three patients with grade 3 pneumonitis, two patients developed out-of-irradiated field pneumonitis and one patient developed intra-irradiated field pneumonitis. Among four patients with grade 2 pneumonitis, three patients developed intra-irradiated field pneumonitis and one patient developed out of irradiated field pneumonitis.
Fig. 1.
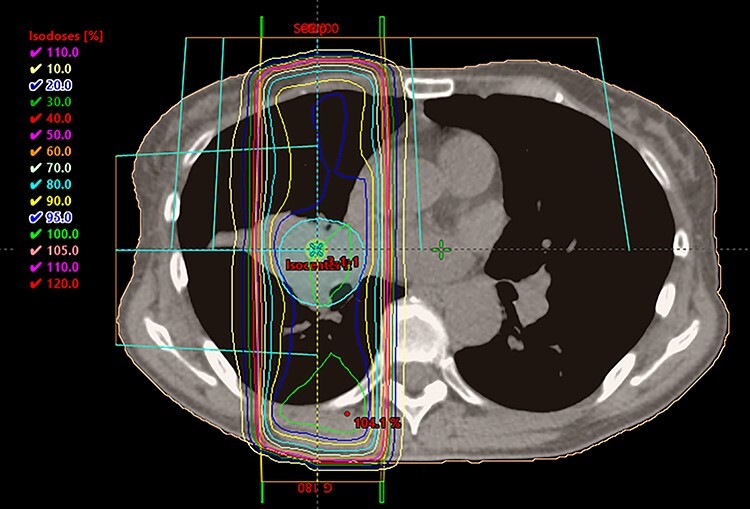
Dose distribution of representative patients who developed grade 2 pneumonitis. There is a tumor on hilum of right lung. A blue line showed 95% of prescribed dose (30Gy).
Fig. 2.
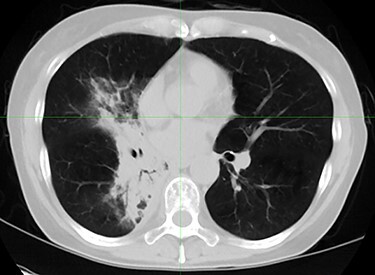
Chest computed tomography image of same patient. Consolidation shadow appeared in irradiated field.
Factors related to pneumonitis
Patient and treatment factors, such as age, sex, ICI agent, interval between RT and ICI and history of ICI before RT, were not significantly associated with grade ≥ 2 pneumonitis. However, existence of obstructive pneumonia was significantly associated with grade ≥ 2 pneumonitis (P = 0.036; Table 2). Tumor volume, Brinkman index and dosimetric factors (such as lung V5, V10, V20, V30 and MLD), lactate dehydrogenase and C-reactive protein did not significantly differ between patients with grade ≤ 1 pneumonitis and those with grade ≥ 2 pneumonitis (Table 3); these factors also did not significantly differ between patients with grade ≤ 2 pneumonitis and those with grade ≥ 3 pneumonitis. Levels of sialylated carbohydrate antigen KL-6 (KL-6) were evaluated in 27 patients before RT, with a median interval between evaluation of KL-6 and RT of 25 days. Mean KL-6 did not significantly differ between patients with grade ≤ 1 pneumonitis and those with grade ≥ 2 pneumonitis (P = 0.095). However, it was significantly different between patients with grade ≤ 2 pneumonitis (431 U/ml) and those with grade ≥ 3 pneumonitis (958 U/ml; P < 0.001). Median KL-6 was 389 U/ml (range: 153–853 U/ml) among the patients with grade ≤ 2 pneumonitis and 539 U/ml (range: 360–1971 U/ml) among patients with grade ≥ 3 pneumonitis. In the ROC analysis, 535 U/ml was the optimal KL-6 cut-off value to predict grade ≥ 3 pneumonitis (AUC: 0.764). Results of ROC analysis according to KL-6 and grade ≥ 2 pneumonitis is shown in Fig. 3.
Table 2.
Patient factors related to grade ≥ 2 radiation pneumonitis (chi-square test)
| Patient characteristics | No. of patients with grade ≤ 1 pneumonitis | No. of patients with grade ≥ 2 pneumonitis | p value | |
|---|---|---|---|---|
| Age | ≥ 68 years old | 11 | 4 | 0.742 |
| < 68 years old | 11 | 3 | ||
| Sex | Female | 19 | 5 | 0.362 |
| Male | 3 | 2 | ||
| Performance status | 0 | 8 | 1 | 0.271 |
| 1–3 | 14 | 6 | ||
| ICI | Pembrolizmab | 13 | 4 | 0.996 |
| Nivolumab | 6 | 2 | ||
| Atezolizmab | 3 | 1 | ||
| Interval between RT and ICI | ≥ 25 days | 12 | 3 | 0.590 |
| < 25 days | 10 | 4 | ||
| History of ICI before RT | Yes | 8 | 4 | 0.331 |
| No | 14 | 3 | ||
| Obstructive pneumonia | Yes | 6 | 5 | 0.036 |
| No | 16 | 2 |
ICI: immune checkpoint inhibitors; RT: radiotherapy.
Table 3.
Comparison of clinical factors and dosimetric parameters between patients with grade ≤ 1 pneumonitis and those with grade ≥ 2 pneumonitis
| Parameter | Grade ≤ 1 pneumonitis group (n = 22) | Grade ≥ 2 pneumonitis group (n = 7) | p-value |
|---|---|---|---|
| Tumor volume (ml) | 194 (±200) | 79 (±35) | 0.144 |
| Lung V5 (%) | 14 (±7) | 13 (±9) | 0.611 |
| Lung V10 (%) | 12 (±6) | 10 (±7) | 0.588 |
| Lung V20 (%) | 8 (±4) | 7 (±5) | 0.489 |
| Lung V30 (%) | 2 (±1) | 1 (±1) | 0.15 |
| Mean lung dose (cGy) | 334 (±150) | 290 (±164) | 0.512 |
| LDH (U/L) | 287 (±189) | 243 (±75) | 0.560 |
| CRP (mg/L) | 3.4 (±4.3) | 3.1 (±4.5) | 0.869 |
| * KL-6 (U/ml) | 388 (±246) | 659 (±590) | 0.095 |
lung V5: lung volume receiving >5 Gy; lung V10: lung volume receiving >10 Gy; lung V20: lung volume receiving >20 Gy; lung V30: lung volume receiving >30 Gy; LDH: lactate dehydrogenase; CRP: C-reactive protein; KL-6: Sialylated carbohydrate antigen KL-6.
* KL-6 was evaluated in 27 patients, including 20 who developed grade ≤ 1 pneumonitis and seven who developed grade ≥ 2 pneumonitis.
Fig. 3.
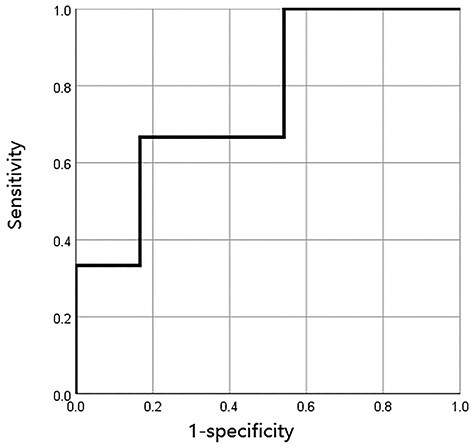
Results of receiver operating characteristics analysis was shown. Optimal cut-off value of KL-6 to predict grade 3 or greater pneumonitis was 535 U/ml.
DISCUSSION
We analyzed the incidence and risk factors of pneumonitis after palliative thoracic RT followed by ICI. We found that the incidence of grade 2 pneumonitis was 24% and obstructive pneumonia at RT was a significant factor for grade ≥ pneumonitis. In addition, KL-6 was significantly higher in patients with grade ≥ 3 pneumonitis compared with patients with grade ≤ 2 pneumonitis. The optimal KL-6 cut-off value for predicting grade ≥ 3 pneumonitis was 535 U/ml. Our findings indicate that all patients who received ICI after palliative thoracic RT should be followed-up carefully, especially patients who had obstructive pneumonia or high KL-6 levels.
In this study, the incidence of pneumonitis at any grade was 58%. For the phase I study of pembrolizumab for advanced NSCLC (KEYNOTE-001), Shaverdian et al. reported that 63% of patients who received thoracic RT and 40% of patients with no previous thoracic RT, developed pulmonary toxicity of any grade [18]. They also reported that 17% of patients developed grade ≥ 3 pulmonary toxicity. The current study had comparable results (any grade of pneumonitis: 58%; grade ≥ 3 pneumonitis: 10%). In radical treatment settings, durvalumab after concurrent chemoradiotherapy (CCRT) for locally advanced NSCLC (LA-NSCLC) is effective and widely used [20]. In our previous report on real-world outcomes from durvalumab after CCRT for LA-NSCLC [21], the incidence of grade ≥ 2 pneumonitis was 36%, which was higher than in the current study (24%); this was quite reasonable, because the total RT dose is lower in palliative treatment than in radical intent-to-cure treatment. However, we have to pay enough attention because 24% of patients developed ≥2 pneumonitis even after palliative RT.
In this study, patient factors, including age, sex, ICI agent, interval between RT and ICI and history of ICI before RT, were not significantly associated with grade ≥ 2 pneumonitis. However, obstructive pneumonia at RT was significantly associated with grade ≥ 2 pneumonitis (P = 0.036). We believe that obstructive pneumonia itself does not directly cause pneumonitis, but reduces the pulmonary reserve, leading to more severe pneumonitis than in patients with normal lung reserve. Other factors, such as tumor volume, Brinkman index, dosimetric factors (lung V5, V10, V20, V30 and MLD), lactate dehydrogenase and C-reactive protein were not significantly different between patients with grade ≤ 1 pneumonitis and those with grade ≥ 2 pneumonitis. Our median V5, V10, V20, V30 MLD values were much lower than those reported for the radical treatment cohort. We believe this is why lung dose-volume parameters were not significantly correlated with pneumonitis in the current study, although many studies have indicated relationships between dose-volume parameters and pneumonitis in radical treatment settings. However, KL-6 levels significantly differed between patients with grade ≤ 2 pneumonitis and those with grade ≥ 3 pneumonitis (P < 0.001), with an optimal KL-6 cut-off value of 535 U/ml for predicting grade ≥ 3 pneumonitis. It is reported that pre-treatment KL-6 value is a significant predictive factors for pneumonitis after thoracic stereotactic RT [22]. This study suggested that KL-6 value is also a predictive factor after palliative thoracic RT. We believe particular care should be exerted in following-up patients with obstructive pneumonia or high KL-6.
This study had some limitations. First, this was a retrospective analysis, with a small study cohort. Second, as all the patients were treated with the same RT fractionation schedule (30 Gy in 10 fractions), incidence and risk factors of ICIs combined with different palliative RT doses (e.g. 8 Gy in 1 fraction or 20 Gy in 5 fractions) were not explored. Further studies with larger cohorts and longer follow-up are necessary to evaluate optimal combinations of palliative RT and ICIs.
In conclusion, the incidence of grade ≥ 2 pneumonitis was 24%; obstructive pneumonia at RT was a significant predictor of grade ≥ 2 pneumonitis; and mean KL-6 was significantly higher in patients with grade ≥ 3 pneumonitis than in patients with grade ≤ 2 pneumonitis. Patients who receive ICIs after palliative thoracic RT, especially those with obstructive pneumonia or high KL-6, should be carefully followed up.
Contributor Information
Satoshi Saito, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Takanori Abe, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Misaki Iino, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Tomomi Aoshika, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Yasuhiro Ryuno, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Tomohiro Ohta, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Mitsunobu Igari, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Ryuta Hirai, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Yu Kumazaki, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Ou Yamaguchi, Respiratory Medicine, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Kyoichi Kaira, Respiratory Medicine, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Hiroshi Kagamu, Respiratory Medicine, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Shin-ei Noda, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Shingo Kato, Departments of Radiation Oncology, International Medical Center, Saitama Medical University, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
ACKNOWLEDGEMENTS
The institutional review board of the Saitama Medical University International Medical Center approved this study (20-091). We thank Marla Brunker, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
FUNDING
There is no funding source to be declared.
CONFLICT OF INTEREST
K. Kaira, O. Yamaguchi and H. Kagamu have received research grants and a speaker honorarium from Ono Pharmaceutical Company, Bristol-Myers Company and Chugai Pharmaceutical company.
References
- [1]. Cheng TY, Cramb SM, Baade PD et al. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol 2016;11:1653–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Sawabata N. Prognosis of lung cancer patients in Japan according to data from the Japanese Joint Committee of Lung Cancer Registry. Respir Investig 2014;52:317–21. [DOI] [PubMed] [Google Scholar]
- [3]. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Non-Small Cell Lung Cancer, Version 7. 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (4 April 2021, date last accessed).
- [4]. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Reck M, Rodríguez-Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- [6]. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- [7]. Tsuya A, Kurata T, Tamura K et al. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007;57:229–32. [DOI] [PubMed] [Google Scholar]
- [8]. Gaito S, Hughes C, Woolf D et al. Radiotherapy in the control of bleeding from primary and secondary lung tumours. Br J Hosp Med (Lond) 2019;80:211–5. [DOI] [PubMed] [Google Scholar]
- [9]. Higginson DS, Chen RC, Morris DE et al. Predicting the need for palliative thoracic radiation after first-line chemotherapy for advanced nonsmall cell lung carcinoma. Cancer 2012;118:2744–51. [DOI] [PubMed] [Google Scholar]
- [10]. Moeller B, Balagamwala EH, Chen A et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol 2018;8:245–50. [DOI] [PubMed] [Google Scholar]
- [11]. Dovedi S, Adlard AL, Lipowska-Bhalla G et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–68. [DOI] [PubMed] [Google Scholar]
- [12]. Deng L, Liang H, Burnette B et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rückert M, Deloch L, Fietkau R et al. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Onkol 2018;194:509–19. [DOI] [PubMed] [Google Scholar]
- [14]. Yamaguchi O, Kaira K, Hashimoto K et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer 2019; 10:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Stevens R, Macbeth F, Toy E et al. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev 2015;1:CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Barrón F, Sánchez R, Arroyo-Hernández M et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol 2020;10:570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Shaverdian N, Lisberg AE, Bornazyan K et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Tsujino K, Hirota S, Endo M et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2003;55:110–5. [DOI] [PubMed] [Google Scholar]
- [20]. Antonia S, Villegas A, Daniel D et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- [21]. Saito S, Abe T, Kobayashi N et al. Incidence and dose-volume relationship of radiation pneumonitis after concurrent chemoradiotherapy followed by durvalumab for locally advanced non-small cell lung cancer. Clin Transl Radiat Oncol 2020;23:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Hara R, Itami J, Komiyama T et al. Serum levels of KL-6 for predicting the occurrence of radiation pneumonitis after stereotactic radiotherapy for lung tumors. Chest 2004;125:340–4. [DOI] [PubMed] [Google Scholar]


