Abstract
Objective: The current study examined instrumental learning in ADHD. Method: A total of 58 children with ADHD and 58 typically developing (TD) children performed a probabilistic learning task using three reward probability conditions (100%, 85%, 70% reward). After a learning phase, application of what was learned was assessed in a test phase. Results: Results showed that children with ADHD performed less accurate compared with TD children during the learning phase, particularly in the 100% and 85% reward probability conditions. These findings were accompanied by a blunted learning rate in the first few task trials. Furthermore, children with ADHD showed poorer application of what was learned. Conclusion: To conclude, children with ADHD show initial learning problems, but increased performance in a similar manner as TD children independent of the probability of reward, although they fail to apply their knowledge. Findings are of clinical relevance as the application of knowledge is important to successfully adapt to daily challenges in life.
Keywords: attention-deficit/hyperactivity disorder, feedback, learning, probabilistic learning task
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity (American Psychiatric Association [APA], 2013). Instrumental learning, also referred to as reinforcement learning, is the ability to change behavior in response to positive and negative feedback, which is essential for adaptive functioning (Rushworth & Behrens, 2008). Difficulties with instrumental learning may result in a number of problems in daily life functioning that are associated with ADHD such as the inability to learn to exhibit “appropriate” behavioral responses (e.g., withhold impulses, await turns) and inability to act according to social rules (Hoza, 2007).
Neurobiological models of ADHD suggest a deficiency in instrumental learning due to diminished dopamine signal in anticipation of, or following, a reinforcer (Frank et al., 2007; Sagvolden et al., 2005; Tripp & Wickens, 2008). Although these models differ in the level of explanation (Luman et al., 2010), they agree on the prediction that children with ADHD show poor instrumental learning compared with controls, particularly when reinforcement is not delivered consistently and frequently. This may be caused by diminished dopamine signal following a reinforcer (Frank et al., 2007; Sagvolden et al., 2005) or because of diminished dopamine signal in anticipation of a reinforcer (Tripp & Wickens, 2008). However, experimental studies that manipulated the consistency of reinforcement delivery to investigate this prediction for individuals with ADHD showed inconsistent results (De Meyer et al., 2019; Frank et al., 2007; Hauser et al., 2014; Luman et al., 2009, 2015).
Two studies focused on instrumental learning using consistent performance feedback (100% reward probability) in ADHD compared with typically developing (TD) children. In a study of Luman et al. (2009), children were required to match four stimuli with two responses using consistent performance feedback under four reward conditions that differed in reward frequency and magnitude (coins on screen that could be exchanged for a present). In a study of Groen et al. (2008), children were presented with two stimulus pairs. One stimulus pair was coupled with consistent performance feedback (points) while the other stimulus pair was coupled with feedback that was independent of performance. In both studies, children were instructed to win as many points or coins as possible. The two studies found comparable results demonstrating that children with ADHD displayed similar learning rates compared with TD children when learning was required from consistent feedback that was dependent on performance. However, despite similar learning rates, in both studies, children with ADHD stayed behind typical controls in terms of overall performance levels.
In many daily life situations, a child is assumed to adapt behavior by learning from inconsistent or probabilistic feedback (Frank et al., 2004; Van Duijvenvoorde et al., 2013). Learning from probabilistic feedback is considered more difficult compared with learning from consistent performance feedback (Eppinger et al., 2009). Studies that compared individuals with ADHD and controls on their performance on probabilistic learning tasks found mixed results (De Meyer et al., 2019; Frank et al., 2007; Hauser et al., 2014; Luman et al., 2015). Frank and colleagues (2007) compared a group of adults with ADHD and controls on a probabilistic reinforcement learning task using three stimulus pairs (Chinese characters) with the following probability rates for the stimuli within a pair: 80%/20% reward, 70%/30% reward, and 60%/40% reward (word “correct!” being presented on screen). Results showed that participants with ADHD showed impaired reinforcement learning, as reflected in reduced performance on the task (Frank et al., 2007). However, that study did not take learning rate into account. In a study by Luman et al. (2015), four stimuli (simple objects) had to be mapped onto two responses using either consistent performance feedback (reward probability 100%) or probabilistic feedback (reward probability of 88%) (thumbs up with monetary reward). They found that children with ADHD were as accurate as TD children and showed similar learning rates. Hauser et al. (2014) examined learning behavior in a probabilistic reversal learning task in adolescents with ADHD and controls. In this task, adolescents were presented with one stimulus pair using a probability rate of 80%/20% reward. Reinforcement probabilities were reversed after six to 10 rewards obtained (money that was paid at the end of the study). Adolescents with ADHD earned less money during the task compared with controls, indicating less efficient learning, although this difference just escaped conventional level significance. In addition, Hauser et al. (2014) found learning rates to be intact. De Meyer and colleagues (2019) compared performance of children with ADHD to that of controls using a probabilistic learning task in which children received a reward (a small candy) when choosing one (out of 10) circles using a probability rate of either 100% or 20% reward. Children with ADHD showed intact acquisition of behavior, but learning rates were not investigated.
The finding of intact learning rates in ADHD in studies using consistent performance feedback (100% reward probability) (Groen et al., 2008; Luman et al., 2009) and studies using a relatively simple task with a low number of stimuli (De Meyer et al., 2019; Hauser et al., 2014; Luman et al., 2015) suggest that individuals with ADHD may suffer specifically from instrumental learning problems when feedback is probabilistic (i.e., less predictable) and the task requires considerable effort, in line with the predictions by some of the theoretical models (Frank et al., 2007; Sagvolden et al., 2005; Tripp & Wickens, 2008).
Finally, after response acquisition, it is essential to maintain performance when feedback is omitted and to be able to transfer information from the learning context to a novel situation. Both the models of Sagvolden et al. (2005) and Tripp and Wickens (2008) predict that behavioral extinction is altered in ADHD. While Sagvolden et al. (2005) predicted slower extinction of behavior, Tripp and Wickens (2008) (and also Frank et al., 2007) predicted faster extinction of behavior. However, studies evaluating the performance of children with ADHD when feedback is omitted and the application of knowledge is required are scarce. One study by De Meyer et al. (2019) looked at extinction effects in children with ADHD and controls using a probabilistic reinforcement learning task and showed no group differences in exploratory behavior in a 2-min period where reward delivery was omitted.
The aim of the current study was to test whether children with ADHD are impaired in instrumental learning, particularly when feedback is probabilistic (Frank et al., 2007; Sagvolden et al., 2005; Tripp & Wickens, 2008). To this end, we used an adapted, child-friendly version of the probabilistic learning task developed by Frank et al. (2004), in a large and well-defined group of children with ADHD. We improved upon previous studies (De Meyer et al., 2019; Frank et al., 2007; Luman et al., 2015) on the following aspects. First, we examined response acquisition in children in a task where they had to select one stimulus from a pair, using three probability conditions consisting of 85%/15%, 70%/30%, and 100%/0% reward rates (i.e., reflecting consistent feedback). Instead of evaluating learning rate by analyzing bins of trials (Frank et al., 2007; Luman et al., 2015), we performed trial-by-trial analysis to improve the sensitivity in detecting differences between groups in learning from divergent feedback contingencies over the course of the task. Furthermore, in contrast to earlier studies, we investigated the ability to reproduce and apply what had been learned to a new context. We predicted that children with ADHD, compared with their age-matched peers, would show more difficulty in instrumental learning (Frank et al., 2007; Nigg & Casey, 2005; Sagvolden et al., 2005; Tripp & Wickens, 2008; Williams & Dayan, 2005), especially when the reward probability rate was relatively low and thus reinforcement delivery was less continuous (Frank et al., 2007; Sagvolden et al., 2005; Tripp & Wickens, 2008). In addition, after learning, we examined the application of what was learned when feedback is omitted. We predicted that children with ADHD would show poorer application of what was learned compared with their peers (Frank et al., 2007; Tripp & Wickens, 2008).
Method
Participants and Selection Procedure
Participants were 58 children with ADHD and 58 TD children, aged 7 to 13 years. Because previous studies showed developmental improvements in feedback-based learning between the ages of 8 and 13 years (Van den Bos et al., 2009; Van Duijvenvoorde et al., 2013), children from the two groups were closely matched on age (<6 months difference). All children were required to have IQ scores >80 as estimated by four subtests of the Wechsler Intelligence Scale of Children–III (WISC-III): Vocabulary, Arithmetic, Block Design, and Picture Arrangement (Kaufman et al., 1996). In addition, all children were free of neurological impairments. Children with ADHD were recruited from 15 psychiatric outpatient clinics in the west part of the Netherlands. Inclusion criteria for the ADHD group were as follows: (a) a primary diagnosis of ADHD as established by a child psychiatrist using the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; APA, 2000) criteria; (b) elevated parent and teacher ratings on the Disruptive Behavior Disorders Rating Scale (DBDRS; Pelham et al., 1992) with at least one of the scores on the Inattention or Hyperactivity/Impulsivity scale above the 90th percentile for one informant and above the 70th percentile for the other informant; (c) being free of stimulant medication for at least 1 month prior to inclusion in the study. No restrictions were set on other comorbidities. Comorbid disorders were diagnosed according to DSM-IV-TR and retrieved from the medical records. Comorbid disorders in the ADHD group included learning disorders (n = 5), autism spectrum disorders (n = 6), anxiety disorders (n = 2), and mood disorder (n = 1).
TD children were recruited from primary schools, after school programs and sport clubs. TD children were required to obtain parent and teacher ratings <70th percentile on both scales of the DBD to rule out the presence of significant ADHD symptoms. Table 1 gives an overview of the group characteristics.
Table 1.
Group Characteristics.
| Characteristics | ADHD (n = 58) |
TD children (n = 58) |
Group |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | |
| Sex, % male | 67 | 67 | ||||
| Age (years) | 9.7 | 1.3 | 9.9 | 1.2 | 0.44 | .508 |
| Estimated Full-Scale IQ | 99.3 | 12.4 | 108.0 | 11.1 | 15.79 | <.001 |
| DBDRS parent | ||||||
| Inattention | 16.0 | 5.3 | 3.3 | 3.0 | 249.69 | <.001 |
| Hyperactivity/impulsivity | 13.8 | 5.6 | 2.8 | 2.6 | 181.12 | <.001 |
| DBDRS teacher | ||||||
| Inattention | 16.1 | 5.3 | 2.1 | 3.6 | 276.24 | <.001 |
| Hyperactivity/impulsivity | 13.1 | 8.3 | 1.6 | 2.9 | 99.48 | <.001 |
Note. TD = typically developing group; DBDRS = Disruptive Behavior Disorder Rating Scale.
Measures
Probabilistic learning task
We used a child-friendly version of the extensively validated Probabilistic Learning Test (PLT; Frank et al., 2004) to measure instrumental learning, which has successfully been used in typical developing children (Van den Bos et al., 2012). The PLT consisted of a learning phase and a test phase. In the learning phase, children were presented two stimuli in each trial and were instructed to select the stimulus with the greatest probability of reward (Figure 1A). Correctness was defined as selecting the overall most rewarding alternative (Frank et al., 2004). Three fixed pairs (AB, CD, and EF) were presented in a random order, and children had to learn to choose the stimulus that was associated most strongly with reward. Stimuli were represented by randomly chosen characters from the Greek alphabet. Reward delivery was consistent (100% reward probability) in the AB pair (A: 100% reward, B: 100% penalty), and reward delivery was slightly inconsistent (85% reward probability) in the CD pair (C: 85% reward and 15% penalty, D: 15% reward and 85% penalty) and also inconsistent (70% reward probability) in the EF pair (E: 70% reward and 30% penalty, F: 30% reward and 70% penalty). Consequently, A, C, and E are net positive stimuli and B, D, and F are net negative stimuli, and it is more difficult for E than for C and for A to learn that these stimuli are associated with reward more frequently than B, D, and F. Reward consisted of a “thumbs up” symbol and a €0.20 gain, and penalty consisted of a “thumbs down” symbol with a €0.20 loss. All children were aware that they would not receive any of the gained money after they completed the task; however, they were provided with a small present after finishing the task. Each stimulus pair was presented for 1000 ms, followed by a response window of 4,000 ms maximum. The feedback screen appeared for 1500 ms. When no response was given within 4,000 ms, a “too late” message appeared on screen for 1500 ms. The learning phase consisted of learning blocks of 60 trials each (20 trials per feedback condition) with a maximum of five blocks. Children who reached above chance-level performance in any given learning block (AB, CD, and EF pair ≥ 70%, 65%, and 60%, respectively; see Frank et al., 2004) entered the test phase.
Figure 1.
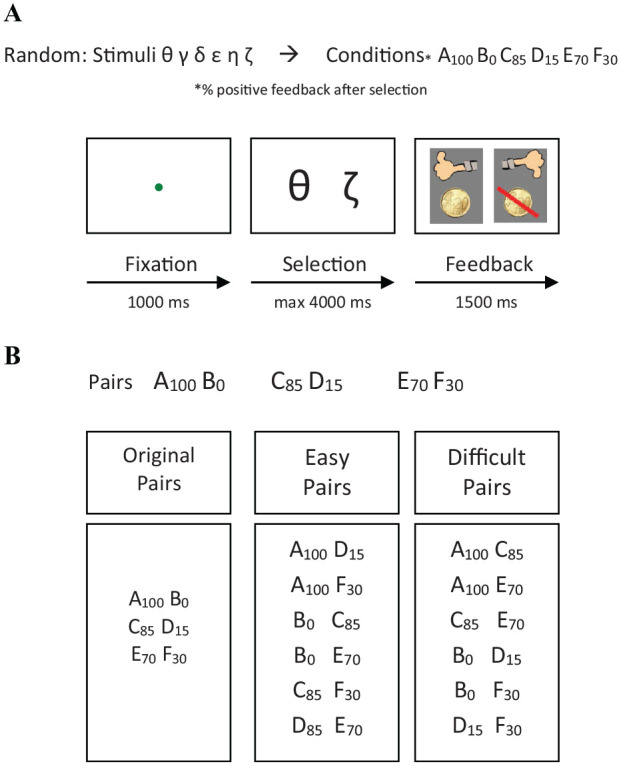
Probabilistic learning task. (A) In the learning phase, children were presented with two stimuli and instructed to select the stimuli with the highest reward probability. During the learning phase stimulus pairs were presented one by one and children had to choose one of both stimuli by pressing ‘1’ or ‘0’ on the keyboard. Reward and penalty were provided as shown in parentheses for each stimulus pair. In stimulus pair AB, A always led to reward whereas B always resulted in penalty. In stimulus pair CD, selecting C led to reward in 85% of trials, whereas selecting D led to reward in only 15% of trials. In stimulus pair EF, selecting E led to reward in 70% of trials, whereas selecting F led to reward in only 30% of trials. (B) In the test phase, novel stimulus pairs were presented to evaluate what was learned in the learning phase. Stimulus pairs were grouped into three categories (original-, easy-, and difficult pairs) according discriminability of the reinforcement values for the two stimuli in a pair during the learning phase (see main text). The difference between the reward probability rates in the learning phase for the easy pairs ranged between 55%-85%, while for the difficult pairs, the difference were smaller ranging between 15%-30%.
In the test phase, no feedback was provided, and children had to select the stimulus most frequently associated with reward in the learning phase from all possible pair configurations of stimuli. The test phase consisted of both easy and difficult pairs. Easy pairs (AD, AF, BC, BE, CF, and DE in 60 trials) consisted of one stimulus that was most frequently associated with reward (and thus selected) in the training phase and one stimulus that was most frequently associated with penalty (and thus rejected) in the training phase. The difficult pairs (AC, AE, CE, BD, BF, and DF in 60 trials) in the test phase consisted of stimuli that were either both most frequently associated with reward (and should thus be selected) or both most frequently associated with penalty (and thus rejected) in the training phase. During the test phase, eight blocks were presented, with each block containing all 15 possible stimulus pairs presented in random order (each pair was presented eight times). Task duration ranged between 16 and 42 min.
Dependent variables in the learning phase were as follows: (a) the number of learning blocks required to reach the entry criterion for the test phase and (b) learning in the first learning block across trials (2–20) for the three reward probability conditions (reward probability 100%, 85%, and 70%). We removed the first trial of each stimulus pair (guess trials, resulting in 19 trials per condition) and excluded trials with reaction times <200 ms (anticipatory responses; 0.68%) and trials without a response (omissions; 0.36%). We only examined the first learning block as we were interested in response acquisition. Dependent variables in the test phase were the overall percentage correct and percentage correct of original, easy, and difficult pairs (Figure 1B). In the test phase, trials with reaction times <200 ms (0.47%) were excluded from the analysis and omissions (0.79%) were interpreted as incorrect responses.
Procedure
The PLT was part of a larger assessment battery that was used as part of a treatment trial for ADHD (Ref. No. NCT01363544, https://clinicaltrials.gov/show/NCT01363544). The study was approved by the national medical ethics committee (NL 31641.029.10 CCMO). All children verbally agreed to participate, and written informed consent was obtained before participation from all parents and children aged 11 years and older. Children received a small gift to thank them for participating in the study.
Statistical Analysis
Analyses were performed with Statistical Package for the Social Sciences version 18 (SPSS 18; IBM Corporation, Armonk, NY, USA). Group comparability in terms of background characteristics was analyzed using a chi-square (χ2) test or analyses of variance (ANOVAs).
Learning phase
To investigate group differences (ADHD vs. TD children) in learning performance, we compared (a) the number of learning blocks required to reach the entry criterion for the test phase using ANOVA and (b) accuracy and learning rate in the first block of trials (2–20) for the three reward probability conditions using generalized estimating equations (GEE) with an identity link function and an autoregressive, AR(1), working correlation matrix. Main effects were group, trial, and condition. Possible significant interactions of group × trial were followed-up by post hoc analysis to check in which part of the task the interaction took place; significant interactions of group × condition were followed-up by post hoc analyses including pairwise comparisons to examine possible group differences within each condition and to examine possible differences between conditions within groups (ADHD and TD).
Test phase
If children did not reach the performance criterion to proceed to the test phase for all three stimulus pairs after a maximum of five learning blocks (300 trials), their test phase results were omitted from the statistical analysis. We evaluated group differences in the ability to reproduce and apply what had been learned during the learning phase, using data derived from the test phase. In the test phase, we compared groups on the overall percentage correct for the original pairs (AB, CD, and EF) using analysis of covariance (ANCOVA). Results on new (i.e., easy and difficult) pairs (Figure 1B) were examined using a repeated measures (RM) ANCOVA with pairs (easy and difficult pairs) as within-subject factor and group (ADHD and TD) as between-subject factor. Because we assumed that the overall percentage correct in the test phase would be influenced by the learning phase, percentage correct for the last block of the learning phase was used as a covariate in the analysis to control for possible group differences in accuracy during the learning phase.
Results
Group characteristics are summarized in Table 1. As a result of the selection procedure, children with ADHD obtained higher scores on all the ADHD symptom measures (Table 1). Children with ADHD had lower IQ scores than TD children, but did not differ in age or sex. To investigate whether IQ influenced the results, sensitivity analyses were performed (see below).
Learning Phase
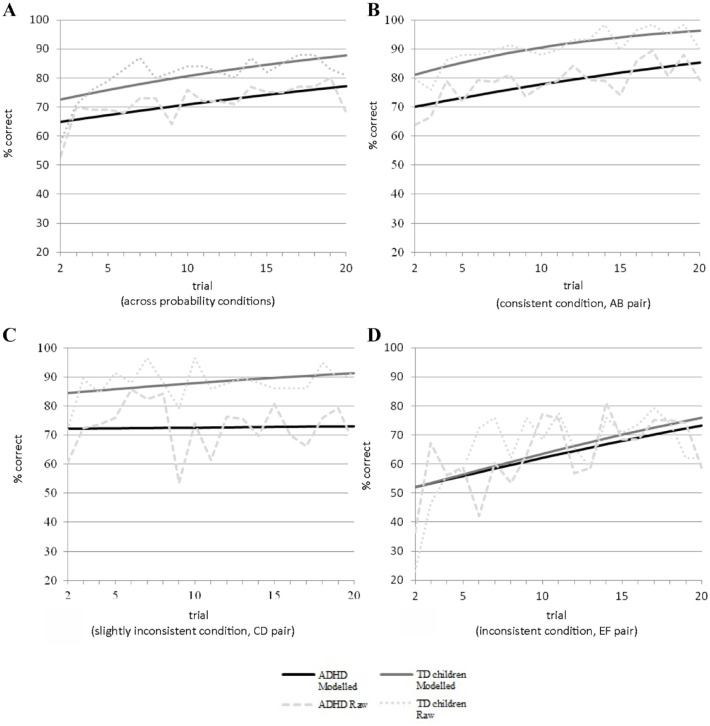
The prediction that children with ADHD would show more difficulty in reinforcement learning compared with their age-matched peers was confirmed by the finding that children with ADHD needed on average a half learning block extra compared with TD children to reach the entry criterion for the test phase, F(1, 114) = 4.71, p = .032 (see Table 2). Next, we examined performance rates in the first learning block (see Figure 2 and Table 2). Across trials and conditions, children with ADHD achieved a lower learning performance than TD children, Wald χ2(1) = 19.54, p < .001. We performed a trial-by-trial analysis to analyze possible group differences in learning rate. The interaction between group and trial was significant, Wald χ2(1) = 3.97, p = .046, indicating that across conditions the learning rate differed between groups (Figure 2A, upper left panel). Performance increased over the course of the first block as indicated by a significant main effect of trial, Wald χ2(1) = 41.93, p < .001. Post hoc tests, in which we consecutively removed trials, showed that the group × trial interaction was no longer significant after removal of the first 7 trials, Wald χ2(1) = 0.001, p = .98, indicating that the interaction effect was attributable to the first few trials. Whereas performance of the TD and ADHD group did not differ overall during trials 2–7, Wald χ2(1) = 0.227, p = .634, the TD group showed superior performance compared with the ADHD group during the subsequent trials, Wald χ2(1) = 6.52, p = .011.
Table 2.
Accuracy in the Fist Block of the Learning Phase for Children With ADHD and TD children.
| Measure | ADHD (n = 58) |
TD children (n = 58) |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Learning blocks to enter test phase | 2.2 | 1.5 | 1.7 | 1.0 |
| Percentage correct | ||||
| Across conditions | 71.4 | 21.0 | 81.2 | 19.9 |
| 100%/0% (consistent) reward condition (AB) | 78.2 | 20.3 | 90.4 | 12.9 |
| 85%/15% (slightly inconsistent) reward condition (CD) | 72.6 | 22.0 | 87.9 | 12.6 |
| 70%/30% (inconsistent) reward condition (EF) | 63.3 | 18.1 | 65.3 | 21.9 |
Note. TD = typically developing group.
Figure 2.
Learning curves based on performance in the first learning block administered to children with ADHD and TD with performance data collapsed over probability conditions (A) and for the 100% reward probability condition (B), the 85% reward probability condition (C), and the 70% reward probability condition (D). Raw data represents the actual data while modelled data shows data as modelled by the generalized estimating equations (GEE) analyses that were used to compare the learning curves between groups over trials and conditions. The upper left graph represents the learning curves of children with ADHD and TD children across conditions, showing a significant interaction between group and trial which seems to driven by the shallower learning curve of children with ADHD at the first few trials of the learning phase. Please note that because we omitted the first (guess) trial from our analyses, the learning curve starts at trial 2.
To test whether group differences in performance and learning rate were more pronounced when reward probability rates were lower, we examined interactions between group, trial, and condition. We found a significant interaction between group and reward probability condition, Wald χ2(2) = 20.40, p < .001. Overall, performance differed between reward probability conditions, Wald χ2(2) = 103.68, p < .001: children achieved a higher performance rate in the 100% reward probability condition compared with the 85% reward probability condition, Wald χ2(1) = 7.69, p = .006, and the 70% reward probability condition, Wald χ2(1) = 91.69, p < .001. Learning performance in the 85% reward probability condition was higher compared with the 70% reward probability condition, Wald χ2(1) = 56.10, p < .001. However, pairwise comparisons between groups showed that children with ADHD achieved a lower learning performance in the 100% reward probability condition, F(1, 114) = 15.04, p < .001, and 85% reward probability condition, F(1, 114) = 21.25, p < .001, than TD children. No group differences were observed in the 70% reward probability condition, F(1, 114) = 0.27, p = .604. The three-way interaction of group × trial × condition was not significant, Wald χ2(2) = 1.89, p = .388.
We tested whether the results found in the learning phase may be explained by lower IQ scores observed in the ADHD group (see Table 1). Therefore, we conducted a sensitivity analysis adding IQ as a covariate. Sensitivity analyses including IQ as covariate did not change our main findings.
Test Phase
In the test phase, we examined whether children with ADHD are impaired in applying what was learned when feedback is omitted. Data of seven children (ADHD, n = 5; TD, n = 2) were not included in these analyses, because these children did not reach the performance criterion, leaving a total of 53 children with ADHD and 56 TD children for analysis. Attrition analyses showed no differences in group characteristics between the initial sample (ADHD, n = 58; TD, n = 58) and the sample that reached the performance criterion of the learning phase (ADHD, n = 53; TD, n = 56) (p values > .762). Compared with TD children, children with ADHD were less able to apply what was learned in the learning phase (see Table 3), by showing a lower accuracy rate in the test phase when adjusting for their performance in the learning phase, F(1, 106) = 5.72, p = .018, . Further we tested whether the type of stimulus pair influenced group differences in task performance. Children with ADHD obtained lower percentage correct on the original pairs (AB, CD, and EF) compared with TD children, F(1, 106) = 4.94, p = .028, . Groups also differed on the new pairs, F(1, 106) = 5.00, p = .027, , indicating that children with ADHD performed less accurately compared with TD children, but there was no significant interaction between easy and difficult pairs and group, F(1, 106) = 0.33, p = .565, .
Table 3.
Accuracy in Test Phase for children With ADHD and TD children.
| Measure | ADHD (n = 53) |
TD children (n = 56) |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Overall % correct | 72.3 | 10.6 | 77.3 | 8.5 |
| Original % correct | 80.8 | 15.4 | 88.2 | 12.1 |
| Easy % correct | 80.8 | 14.7 | 87.1 | 12.2 |
| Difficult % correct | 59.3 | 13.3 | 62.1 | 12.3 |
Note. Stimulus pairs were grouped into three categories (original, easy, and difficult pairs) according to the discriminability of the reinforcement values for the two stimuli in a pair during the learning phase. TD = typically developing group.
Discussion
In the present study, we investigated whether children with ADHD showed difficulties in instrumental learning compared with age-matched TD children using a probabilistic learning task with relatively difficult stimuli. We expected the largest group differences in the probabilistic reward conditions. Our findings indicated that, compared with TD controls, children with ADHD needed more learning blocks to achieve the performance criterion to enter the test phase (above chance-level performance). Analyses of learning performance in the first learning block, revealed a slower learning rate in the first few trials in children with ADHD compared with TD children, resulting in overall poorer performance of the ADHD group in this block. In addition, children with ADHD achieved significantly lower learning performance in the 100% and 85% reward probability conditions, but not in the 70% reward probability condition. Finally, in the test phase, children with ADHD performed less accurate than TD children in applying what they had learned to a novel context. Findings were unrelated to estimated IQ scores.
The increase in performance over the course of the first learning block (main effect of trial) validated the assumption that learning took place. As expected, children reached highest levels of accuracy in the 100% reward probability condition, followed by the 85% and 70% reward probability conditions, respectively. These results confirmed that learning is more difficult when the reward probability rate decreased (see also Eppinger et al., 2009; Luman et al., 2015). Overall, like adults with ADHD (Frank et al., 2007), compared with TD children, children with ADHD needed more learning blocks to achieve the performance criterion to enter the test phase, supporting the hypothesis of impairments in response acquisition in ADHD. To provide more insight in group differences in terms of learning curves within and between reward probability conditions, we analyzed trial-by-trial learning performance in the first learning block.
In the first learning block and regardless of condition, learning rate was lower in children with ADHD than their TD peers. Post hoc analyses showed that this difference was driven by the first few trials within the block. Within an instrumental learning task, the first few trials are essential to map the stimulus–response relation. Visual inspection of Figure 2A, displaying the learning curves across the reward probability conditions, indicates a similar learning rate in the first few trials after the guess trial. However, after trial 3, differences in performance appeared as TD children showed a faster learning rate compared with children with ADHD. In the subsequent trials, performance of children with ADHD stayed behind that of TD children, as learning curves in both groups increased at a similar rate. Note that previous studies only detected lower accuracy levels in children with ADHD compared with TD children and found no group differences in learning rates (Groen et al., 2008; Hauser et al., 2014; Luman et al., 2009, 2015). We speculate that impaired learning rates in ADHD may only be present when memory load is high, for example, when relatively difficult stimuli are used (Greek letters in our study as compared with simple concrete visual stimuli in other studies: De Meyer et al., 2019; Groen et al., 2008; Luman et al., 2009) or more than two stimulus pairs are involved in the task (three stimulus pairs in our study as compared with either one or two stimulus pairs in previous work: Groen et al., 2008; Hauser et al., 2014; Luman et al., 2015). Under these conditions, one might speculate that impaired fundamental processes, such as attention and working memory, known to be disturbed in ADHD, possibly contribute to the explanation of impaired performance on the initial learning trials in children with ADHD (van Duijvenvoorde et al., 2013).
In the current study, children with ADHD achieved a lower learning performance in the 100% and 85% reward probability conditions compared with TD children. Visual inspection of Figure 2B and 2C, displaying the learning curves for these more consistent reward conditions, shows that children with ADHD started at a lower learning performance after the guess trial. In subsequent trials, both groups showed parallel learning curves and children with ADHD did not catch up with TD children. This finding supports our hypothesis of children with ADHD being impaired in instrumental learning. However, learning performance in the 70% reward probability condition did not differ between groups. The lack of a group difference in the 70% reward probability condition is in line with Frank et al. (2007), who found no group differences in learning from 60% reward probability in sample of adults with ADHD. Possibly, this result is caused by a floor effect in the initial learning trials of the probabilistic reward condition (which cannot be lower than chance level). We speculate that because of the inconsistent feedback (70% reward probability) in this condition, it took children in both groups long to choose the stimulus with the greatest probability of positive feedback.
An interesting alternative explanation of the lower accuracy of the ADHD group in the learning phase is that individuals with ADHD may show a greater “exploration rate” (or greater choice stochasticity) rather than a slower learning rate (Hauser et al., 2014, 2016). Using computational modeling, Hauser et al. (2014, 2016), and also alternative studies by Williams and Dayan (2005), showed that instrumental learning performance in ADHD is characterized by more exploratory behavior meaning that individuals with ADHD would examine the alternative options more frequently than controls. Although exploratory behavior is necessary to detect changes in reinforcement delivery, according to the computational model of Hauser et al., individuals with ADHD show too much exploratory behavior causing them to underachieve compared with healthy controls. To disentangle reduced learning from increased choice stochasticity, future studies should incorporate computational modeling to examine mechanisms behind the increased exploratory behavior in ADHD, which was beyond the scope of the current study.
The application of learned behavior is an important skill to successfully adapt behavior to daily challenges in life (Gershman & Niv, 2015). In the test phase, we examined the application of what was learned when feedback is omitted. We found that children with ADHD had more difficulty in applying what they had learned to novel stimulus–pair combinations, independent of whether stimulus pairs were easy or difficult, controlling for impairments in response acquisition. Our findings are in accordance with predictions of neurobiological models of ADHD that suggest impaired dopamine signaling in ADHD involved in instrumental learning (Frank et al., 2007; Tripp & Wickens, 2008) may lead to faster extinction of behavior (Tripp & Wickens, 2008).
The present study carries some limitations that should be addressed. First, children with ADHD had lower IQ scores compared with TD children that may have influenced learning performance. However, we found performance in both groups independent of IQ, making it unlikely that the observed group differences might be related to the differences in IQ. Second, in the learning phase, we used a performance criterion to enter the test phase. However, performance of children with ADHD (M = 85.7% SD = 7.6%) was worse compared with TD children (M = 89.3% SD = 5.4%) in the last block of the learning phase: F(1, 107) = 8.29, p = .005. Therefore, one may argue that not every child had the chance to get equally familiar with the stimulus–response couplings before entering the test phase. To control for this group difference, the analyses on the data of the test phase were adjusted for performance on the last block of the learning phase. Third, children in the current study were aware that they would not receive any of the gained money. Instead, they would only receive a small gift after they completed the task. In children with ADHD, this might have affected their motivation to learn (Sagvolden et al., 2005; Sonuga-Barke, 2003) and may have drawn their attention away from making an effort. However, the modest feedback contributes to the ecological validity of the paradigm.
Summary and Clinical Implications
Results in the current study indicate that children with ADHD are capable of learning using probabilistic feedback, although they need more trials to reach the performance criterion compared with TD children. Analyses of the first learning block showed slower learning rates in children with ADHD compared with TD children, and subsequent post hoc analyses revealed that this result is driven by impaired performance on initial trials. Children with ADHD stayed behind controls after these “start-up problems,” followed by a parallel learning curve. This confirms that children with ADHD are able to adjust their behavior to meet task demands (Fosco et al., 2016). The prediction of specific impairments in learning when feedback is probabilistic was not supported. More specifically, our results did not demonstrate a difference in learning between groups when feedback was highly inconsistent (70% reward probability). We did find that children with ADHD, compared with TD children, show a poorer ability to apply what was learned to a new situation when rewards are omitted, in line with the prediction of two important neurobiological models of instrumental learning in ADHD (Frank et al., 2007; Tripp & Wickens, 2008). Although effects were small, the latter finding might add to an explanation of, for example, impaired school performance in children with ADHD. However, more research on the application of what is learned when feedback is omitted is necessary to offer practical recommendations to enhance learning performance in children with ADHD.
Acknowledgments
We like to thank all participating children and their families for their contribution, as well as all research interns for their valuable support. Furthermore, we would like to thank the participating centers of child and adolescent psychiatry: Yulius Academie, Groene Hart ziekenhuis, Lucertis, Alles Kits, GGZ Delfland, Maasstad ziekenhuis, RIAGG Schiedam, Kinderpraktijk Zoetermeer, Albert Schweitzer ziekenhuis, Groos Mentaal Beter Jong, ADHD behandelcentrum, GGZ inGeest, and PuntP.
Author Biographies
Marjolein Luman is an associate professor in clinical neuropsychology. She studies the etiology, assessment and treatment of childhood externalizing behavior.
Tieme W. P. Janssen is an assistant professor clinical neurodevelopmental psychology. His research covers healthy and clinical populations and neuroscience-based interventions.
Marleen Bink is senior program manager and specialized in comparative effectiveness research and clinical neuropsychology
Rosa van Mourik works as a researcher in mental health care supporting professionals to integrate patient-reported measures of recovery into treatment.
Athanasios Maras is a child psychiatrist and director of the Yulius Academy for research into child and adolescent psychiatry.
Jaap Oosterlaan is a full professor in Paediatric Neuropsychology and director of the Follow Me program at the University Medical Centre.
Footnotes
Ethical Approval: All parents (and children >11 years old) completed a written informed consent prior to the study, which was approved by the local medical ethics committee (VU Medical Center). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendment.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was funded by the Netherlands Organisation for Health Research and Development (ZonMw; grant no. 157 003 012). ZonMw funded the trial, but had no role in the data analysis, manuscript preparation, or decision to publish.
ORCID iD: Marjolein Luman  https://orcid.org/0000-0002-1539-2831
https://orcid.org/0000-0002-1539-2831
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- De Meyer H., Beckers T., Tripp G., Van der Oord S. (2019). Reinforcement contingency learning in children with ADHD: Back to the basics of behavior therapy. Journal of Abnormal Child Psychology, 47, 1889–1902. 10.1007/s10802-019-00572-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. (2009). Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology, 46(5), 1043–1053. [DOI] [PubMed] [Google Scholar]
- Fosco W. D., White C. N., Hawk L. W. J. (2016). Acute stimulant treatment and reinforcement increase the speed of information accumulation in children with ADHD. Journal of Abnormal Child Psychology, 45, 911–920. 10.1007/s10802-016-0222-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. J., Santamaria A., O’Reilly R. C., Willcutt E. (2007). Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology, 32(7), 1583–1599. 10.1038/sj.npp.1301278 [DOI] [PubMed] [Google Scholar]
- Frank M. J., Seeberger L. C., O’reilly R. C. (2004). By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science, 306(5703), 1940–1943. 10.1126/science.1102941 [DOI] [PubMed] [Google Scholar]
- Gershman S. J., Niv Y. (2015). Novelty and inductive generalization in human reinforcement learning. Topics in Cognitive Science, 7, 391–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen Y., Wijers A. A., Mulder L. J. M., Waggeveld B., Minderaa R. B., Althaus M. (2008). Error and feedback processing in children with ADHD and children with autistic spectrum disorder: An EEG event-related potential study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 119(11), 2476–2493. 10.1016/j.clinph.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Hauser T. U., Fiore V., Moutoussis M., Dolan R. J. (2016). Computational psychiatry of ADHD: Neural gain impairments across Marrian levels of analysis. Trends in Neurosciences, 39, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T. U., Iannaccone R., Ball J., Mathys C., Brandeis D., Walitza S., Brem S. (2014). Role of the medial prefrontal cortex in impaired decision making in juvenile attention-deficit/hyperactivity disorder. JAMA Psychiatry, 71, 1165–1173. 10.1001/jamapsychiatry.2014.1093 [DOI] [PubMed] [Google Scholar]
- Hoza B. (2007). Peer functioning in children with ADHD. Journal of Pediatric Psychology, 32, 655–663. 10.1093/jpepsy/jsm024 [DOI] [PubMed] [Google Scholar]
- Kaufman A. S., Kaufman J. C., Balgopal R., Mclean J. E. (1996). Comparison of three WISC-III short forms: Weighing psychometric, clinical, and practical factors. Journal of Clinical Child Psychology, 25(1), 97–105. 10.1207/s15374424jccp2501 [DOI] [Google Scholar]
- Luman M., Goos V., Oosterlaan J. (2015). Instrumental learning in ADHD in a context of reward: Intact learning curves and performance improvement with methylphenidate. Journal of Abnormal Child Psychology, 43, 681–691. 10.1007/s10802-014-9934-1 [DOI] [PubMed] [Google Scholar]
- Luman M., Tripp G., Scheres A. (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience and Biobehavioral Reviews, 34, 744–754. 10.1016/j.neubiorev.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Luman M., Van Meel C. S., Oosterlaan J., Sergeant J. A., Geurts H. M. (2009). Does reward frequency or magnitude drive reinforcement-learning in attention-deficit/hyperactivity disorder? Psychiatry Research, 168(3), 222–229. 10.1016/j.psychres.2008.08.012 [DOI] [PubMed] [Google Scholar]
- Nigg J. T., Casey B. J. (2005). An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17, 785–806. [DOI] [PubMed] [Google Scholar]
- Pelham W. E., Gnagy E. M., Greensalade K. E., Milich R. (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of American Academy of Child and Adolescent Psychiatry, 31(2), 210–218. [DOI] [PubMed] [Google Scholar]
- Rushworth M. F. S., Behrens T. E. J. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11(4), 389–397. 10.1038/nn2066 [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Johansen E. B., Aase H., Russell V. A. (2005). A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences, 28, 397–419. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J. (2003). The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews, 27, 593–604. [DOI] [PubMed] [Google Scholar]
- Tripp G., Wickens J. R. (2008). Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines, 49(7), 691–704. 10.1111/j.1469-7610.2007.01851.x [DOI] [PubMed] [Google Scholar]
- Van den Bos W., Cohen M. X., Kahnt T., Crone E. A. (2012). Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex, 22(6), 1247–1255. 10.1093/cercor/bhr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W., Güroğlu B., Van den Bulk B. G., Rombouts S. A. R. B., Crone E. A. (2009). Better than expected or as bad as you thought? The neurocognitive development of probabilistic feedback processing. Frontiers in Human Neuroscience, 3, Article 52. 10.3389/neuro.09.052.2009 [DOI] [PMC free article] [PubMed]
- Van Duijvenvoorde A. C. K., Jansen B. R. J., Griffioen E. S., Van der Molen M. W., Huizenga H. M. (2013). Decomposing developmental differences in probabilistic feedback learning: A combined performance and heart-rate analysis. Biological Psychology, 93(1), 175–183. 10.1016/j.biopsycho.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Williams J., Dayan P. J. (2005). Dopamine, learning, and impulsivity: A biological account of attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology, 15, 160–179. [DOI] [PubMed] [Google Scholar]