Summary
Objective:
Viral encephalitis increases the risk for developing seizures and epilepsy. Indoleamine 2,3-dioxygenase 1 (Ido1) is induced by inflammatory cytokines and functions to metabolize tryptophan (Trp) to kynurenine (Kyn). Kynurenine can be further metabolized to produce kynurenic acid (KynA) and the N-methyl-D-aspartate (NMDA) receptor agonist quinolinic acid (QuinA). In the present study we sought to determine the role of Ido1 in promoting seizures in an animal model of viral encephalitis.
Methods:
C57BL/6J and Ido1 knockout mice (Ido1-KO) were infected with Theiler’s murine encephalomyelitis virus (TMEV). Quantitative RT-PCR was used to evaluate hippocampal expression of proinflammatory cytokines, Ido1, and viral RNA. Body weights and seizure scores were recorded daily. Elevated zero maze was used to assess differences in behavior and hippocampal pathology was determined by immunohistochemistry.
Results:
Infected C57BL/6J mice upregulated proinflammatory cytokines, Ido1, and genes encoding the enzymatic cascade responsible for QuinA production in the kynurenine pathway prior to the onset of seizures. Seizure incidence was elevated in Ido1-KO compared to C57BL/6J mice. Infection increased locomotor activity in Ido1-KO compared to C57BL/6J mice. Furthermore, the occurrence of seizures was associated with hyperexcitability. Neither expression of proinflammatory cytokines nor viral RNA was altered as a result of genotype. Immunohistochemical analysis revealed increased hippocampal pathology in Ido1-KO mice.
Significance:
Our findings suggest that Ido1 deletion promotes seizures and neuropathogenesis during acute TMEV encephalitis.
Keywords: acute seizures; Viral encephalitis; Theiler’s murine encephalomyelitis virus; Indoleamine 2,3-dioxygenase 1; Infection-induced seizures; Epilepsy and neuropathogenesis
1. Introduction
Viral encephalitis is a serious medical condition that commonly results from infection of the brain by members of Herpesviridae (herpes simplex virus 1, varicella zoster virus), Flaviviridae (i.e. West Nile virus)), and also Picornaviridae (enterovirus)1. Regardless of the etiological agent, survivors are at risk for neurological sequelae including, but not limited to, postencephalitic epilepsy2. In fact, the risk of developing an unprovoked seizure two years after a diagnosis of encephalitis was reportedly 22% if the patient experienced an acute seizure during encephalitis and 10% in those who had not exhibited seizures during acute encephalitis3. While viral-induced epilepsy represents a substantial burden, the mechanisms whereby viral infection promotes the development of seizures (ictogenesis) and persistence of seizures (epileptogenesis) are incompletely understood.
Theiler’s murine encephalomyelitis virus (TMEV) is a member of the family Picornaviridae. Intracerebral inoculation of C57BL/6J mice with TMEV causes loss of neurons in the hippocampal CA1 region. Neuronal death is dependent on neuroinflammation (monocyte influx and pro-inflammatory cytokine production within the brain), but does not involve direct viral lysis of neurons4–6. Unlike other models of viral encephalitis, almost all mice survive infection, but depending on the strain and titer of TMEV, between 40–70% of infected mice will develop seizures between 2–7 days p.i. Despite a lack of viral persistence, some of the mice will develop epilepsy with unprovoked seizures evident for at least seven months post-infection7. In this model, ictogenesis is associated with the upregulation of cytokines, including IFNs, TNF and IL-68–10. Together, these traits make the use of TMEV an excellent model with which to study the pathogenesis of viral-induced seizures and concomitant neuropathology.
Indoleamine 2,3-dioxygenase 1 (Ido1) metabolizes tryptophan (Trp) to kynurenine (Kyn). While basal levels of Ido1 vary between tissue location and cell type, the expression of this enzyme is highly inducible by inflammatory mediators, in particular type II (IFN-γ) interferons11. Ido1 induction may lead to focal depletion of Trp at the site of inflammation or infection, which can directly limit viral replication12. The intracellular Kyn generated by Ido1 is either released or metabolized into downstream ‘kynurenines’ (kynurenic acid or quinolinic acid) which have both neuro- and immune-modulatory consequences13; 14. Extracellular kynurenic acid (KynA) serves as an N-methyl-D-aspartate (NMDA) receptor antagonist and is considered neuroprotective. On the other hand quinolinic acid (QuinA) is a glutamatergic NMDA receptor agonist and can evoke neurotoxic and ictogenic responses15. Thus during viral encephalitis, Ido1 may exerpt a positive effect (via Trp depletion and KynA production) or negative effect (via QuinA production).
With this dichotomy in mind, the objective of the current study was to determine the role of Ido1 during the pathogenesis of TMEV-induced seizures and neuropathology. The current literature would suggest that Ido1 induction would drive seizure onset, incidence, and severity by a mechanism involving the production of QuinA. Surprisingly, global inactivation of Ido1 was associated with an increased incidence of seizures during infection with TMEV.
2. Materials and Methods
Animals
All animal care protocols were in accordance with NIH Guidelines for Care and Use of Laboratory Animals and were approved by the University of Illinois Laboratory Animal Care and Use Committee. Male 5–6 week-old mice were used for all experiments. C57BL/6J (Jackson Laboratories No. 000664) and indoleamine 2,3 dioxygenase 1 knockout (Ido1-KO; Jackson Laboratories No. 005867) mice on a C57BL/6J background were group-housed in solid-bottom caging with standard bedding (Teklad 1/8” corncob) under temperature-controlled conditions (23 ± 1°C) with 12-hour reversed light/dark cycles (10am-10pm). Rodent diet (Teklad No. 8640) and water were provided ad libitum. At the end of each experiment mice were anesthetized via intraperitoneal injection of ketamine (100mg/kg) and xylazine (10mg/kg). After reaching a surgical plane of anesthesia they were perfused through the heart with sterile phosphate buffer saline (PBS, pH 7.4).
Theiler’s murine encephalomyelitis virus growth, titration and infection
The BeAn 8386 strain of TMEV (Dr. Jane Welsh, Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX) was used for all experiments. The virus was propagated in baby hamster kidney (BHK)-21 cells. Briefly, monolayers were incubated with TMEV (1 multiplicity of infection, MOI) diluted in Delbecco’s modified eagle medium supplemented with antibiotics and 0.5% bovine serum albumin (Sigma-Aldrich St. Louis, MO) for one hour at room temperature with constant rocking. The virus was aspirated then fresh medium added. After 48 h, medium was frozen then thawed. Cell debris was removed by centrifugation at 12,000 x g for 15 min at 4°C. Virus was aliquoted and stored at −80°C. Viral titer was determined by standard plaque assay on BHK-21 cells16. Mice were infected as described previously17. In brief, mice were anesthetized with 4% isoflurane (MWI, Meridian, ID), then inoculated with either sterile viral growth medium or with medium containing 1.0×106 plaque forming units (PFU) into the right mid-parietal cortex at a depth of approximately 1.5 mm. The infection volume was 20µl.
Body weights and seizure scoring
Daily observations of mice were performed under red light (between 10am and 1pm). Following body weight measurement, mice were single-housed in solid-bottom caging without bedding. Mice were monitored for a duration of 15 min in an undisturbed fashion by two persons blinded to genotype. The total observation time per day was approximately three hours. Observed seizure scores were recorded. Seizures were graded using the Racine Scale as follows: 1, mouth and facial movements; 2, head nodding; 3, forelimb clonus; 4, rearing with forelimb clonus; 5, rearing and falling with forelimb clonus18. Seizure incidence was calculated by dividing the number of mice with seizures, ≥ 3 at any time during the study, by the number of mice infected in that genotype18. Cumulative seizure burden for a particular day was calculated by adding the Racine score of that day to the sum of all preceding scores; any day without an observed seizure (Racine 0) maintained the previous day’s cumulative burden score19.
Elevated Zero Maze (EZM)
The elevated zero maze test was performed under white-light illumination during the dark phase in order to assess differences in anxiety-like behavior between control and infected C57BL/6J and Ido1-KO mice. The EZM is composed of two walled arms and two open illuminated areas. The experiment commenced with mouse placement in the walled arm of the maze. Mice were video-recorded for a duration of 5 min and analyzed using EthoVision XT 7 automated behavior recognition software (Noldus Information Technology, Lessburg, VA). Data were derived from 3-point analysis and are reported as a measure of total distance traveled (cm), latency time to the first exploration of the open area (s), percent (%) time spent in the open area, and number of entries into the open area.
Tissue preparation, RNA isolation and Real-time PCR (RT-qPCR)
For experiments shown in Fig. 1, hippocampal RNA was isolated using the EZNA Total RNA Kit II (Omega Bio-Tek, Norcross, GA, USA) and complementary DNA (cDNA) was prepared with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Grand Island, NY). RT-qPCR with probe-based assays was performed using TaqMan Universal PCR Master Mix (ThermoFischer Scientific, Waltham, MA) on a Prism 7900 thermocycler (Applied Biosystems, Foster City, CA) to determine expression levels of proinflammatory cytokines (Tnf, Il6, Il1b, and Ifng), Ido1 (Ido1-FL), and genes encoding enzymes of the kynurenine pathway essential for either quinolinic acid (Kmo, Kynu, Haao) or kynurenic acid (Kat2) production. Gene expression was normalized using Gadph.
Figure 1. Effect of TMEV infection on hippocampal cytokines and Ido1 mediated pathway activation.

A–C, C57BL/6J mice were either sham-innoculated (blue; n = 7) or TMEV-infected (orange; n = 6). Hippocampal expression was determined by RT-qPCR at day 2 post infection. Levels of Tnf, Il6, Il1b, and Ifng (A), Ido1-FL (B), and Kmo, Kynu, Haao, and Kat2 (C) are shown. **p < 0.01, ***p < 0.001 sham versus TMEV.
For experiments shown in Fig. 5, hippocampal RNA was isolated using TriReagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions. Total RNA was treated with DNase I (ThermoFischer Scientific, Waltham, MA) and cDNA was obtained using the Reverse Transcription System (Promega, Madison, WI) according to the manufacturer’s instructions. Samples were then screened for contaminating DNA by PCR using β-actin-specific primers and Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) using a C1000 Touch Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). RT-qPCR was performed to determine expression levels of proinflammatory cytokines (Ifng, Tnf, and Il6) and virus (Tmev). Products were amplified using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) on a StepOnePlus Real-Time PCR System thermocycler (Applied Biosystems, Foster City, CA). Gene expression was normalized using β-actin. For all experiments fold change was calculated using the formula 2−∆∆Ct. Materials are shown in Supplemental Table 1.
Figure 5. Effects of Ido1 deficiency on hippocampal proinflammatory cytokine and viral RNA expression.

A–D, Hippocampal RNA was isolated from TMEV-infected C57BL/6J (blue; n = 8–9) and Ido1-KO (orange; n = 7–8) mice at day 8 (left) and 16 (right) p.i. Fold change of Tnf (A), Il6 (B), Ifng (C), and Tmev (D). Mice that displayed at least one clinical seizure are depicted with red symbols.
Immunohistochemistry
Following perfusion, whole brains were immersed in 4% paraformaldehyde in PBS. After 24 h, brains were transferred into PBS containing 30% sucrose (w/v) and stored at 4°C until cryoprotected. Brain tissue was then bisected coronally and frozen in Tissue-Tek O.C.T. Compound (VWR International, Randor, PA). Next, 10µm-thick sections were prepared using a CM1950 cyrostat (Leica Biosystems, Wetzlar, Germany), collected on Superfrost Plus Microscope Slides (ThermoFischer Scientific, Waltham, MA) and stored at −80° C. At the time of staining, sections were rehydrated with PBS for 15 min then blocked and permeabilized with PBS containing 0.3% Triton-X 100 (PBST, Sigma-Aldrich, St. Louis, MO) supplemented with 5% goat serum (Abcam, Cambridge, United Kingdom) for 1 h at room temperature. Sections were then incubated with primary antibody (NeuN; 3µg/ml; Abcam, Cambridge, United Kingdom) overnight at 4°C. The following day, the slides were washed three times with PBST then incubated with an AlexaFluor-488 labeled antibody in PBST (goat anti-rabbit IgG; 2µg/ml; ThermoFischer Scientific, Waltham, MA) for 1 h at room temperature. After washing, nuclei were then counterstained for 1 min (Hoechst33342; 5µg/ml; ThermoFischer Scientific, Waltham, MA). Slides were washed then glass coverslips were added using Fluoromount-G solution (SouthernBiotech, Birmingham, AL). Fluorescent images were captured on a Zeiss Observer.Z1 fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany) using AxioVision (Release 4.6) software. Images were opened in Image J (National Institutes of Health, Bethesda, MD) and the number of NeuN+Hoechst+ double-positive cells per field of vision (20X magnification) were obtained from C57BL/6J (n = 3) and Ido1-KO (n = 3) mice using the counter application. Analysis was performed across anatomically similar (Bregma −1.34 to −2.06) hippocampal images (n = 5–17). For each mouse, the average number of double-positive hippocampal CA1 neurons obtained from the left and right cerebral hemisphere was determined.
Statistical Analysis
Data are expressed as mean ± standard error. Statistical analysis was performed using GraphPad Prism (7.0) software. Differences in seizure incidence were determined by the chi-square test. Unpaired t-test was used to test the effect of treatment on Ido1-FL expression. Three-way analysis of variance (ANOVA) was used to test the effects of genotype (C57BL/6J and IDO1-KO), treatment (sham and TMEV), and time (day post infection) on body weight. Two-way ANOVA followed by Bonferroni post-hoc test was used to determine differences in behavior and neuropathology. One-way ANOVA followed by Kruskal–Wallis post-hoc was used to assess differences in cytokine data. In all cases p < 0.05 was considered statistically significant.
3. Results
TMEV induces cytokines, Ido1, and genes that encode enzymes in the kynurenine pathway.
IDO1 is upregulated following stimulation with proinflammatory cytokines. Therefore, we first measured cytokine expression in the hippocampus prior to the development of seizures. In C57BL/6J mice, compared to sham-inoculation, TMEV-infection increased expression of Tnf, Il6, Il1b, and Ifng at day 2 p.i. (Fig. 1A).
Next, we questioned if TMEV induces Ido1 expression in the brains of mice. Ido1 expression is essentially absent in hippocampi of naïve mice. However Ido1 expression was dramatically upregulated during infection (Fig. 1B). Ido1 is rate-limiting for Trp metabolism to Kyn, which is subsequently catabolized to QuinA by the enzymes kynurenine 3-monooxygenase (KMO), kynureninase (KYNU), and 3-hydroxyanthranilate 3,4-dioxygnase (HAAO). Alternatively, Kyn can be converted to KynA by kynurenine aminotransferase II (KAT2). Interestingly, Kynu, Kmo, and Haao expression was increased in the hippocampus of mice infected with TMEV (Fig. 1C). However, expression of Kat2 did not differ from sham-inoculated C57BL/6J mice. These data indicate that TMEV may activate the enzymatic pathway responsible for QuinA production.
Ido1 deletion promotes TMEV-induced seizures.
QuinA is an N-methyl-D-aspartate receptor agonist and has been known for a long time to promote ictogenesis15. We hypothesized that inactivation of Ido1 would confer protection against virus-induced seizures. To determine if Ido1 modulated seizure induction during viral encephalitis, we inoculated C57BL/6J and Ido1-KO mice with vehicle or TMEV and monitored daily for seizures. Importantly, sham-inoculation did not cause seizures (data not shown). However, 41% of TMEV-infected C57BL/6J mice exhibited seizures within 6 days versus 74% infected Ido1-KO mice (Fig. 2A). Examination of cumulative seizure burden has been efficacious in evaluating seizure severity and number over time19. We found that cumulative seizure burden was greater in Ido1-KO mice compared to C57BL/6J mice at day 6 p.i. (Fig. 2B). There were no significant differences in cumulative seizure burden amongst mice that experienced seizures (Fig. 2C). These data indicate that although the lack of Ido1 increased the incidence of ictogenesis, it did not affect either the frequency or severity of seizures.
Figure 2. Ido1 deficient mice exhibit increased seizure incidence compared to C57BL/6J mice.

A–C, C57BL/6J (blue; n = 34) and Ido1-KO (orange; n = 31) mice were intracerebrally infected with TMEV. Seizure incidence, percent mice having experienced at least one seizure (Racine score ≥3) (A). ***p < 0.001 C57BL/6J versus Ido1-KO. Cumulative seizure burden compounded daily for all mice (B) and only for mice that developed seizures (C). ###p < 0.001 C57BL/6J versus Ido1-KO.
Effect of Ido1 on body weight and behavior during TMEV infection.
Infection caused a transient reduction in body weight when compared to sham-inoculation. However, the absence of Ido1 neither affected weight loss nor recovery (Fig. 3).
Figure 3. Intracerebral infection decreased body weight.

C57BL/6J (blue) and Ido1-KO (orange) mice were either sham-inoculated (n = 10) or TMEV-infected (n = 32–38). Body weight is expressed as percent change relative to baseline from 3 independent experiments.
To determine if genetic deletion of Ido1 influenced behavior, C57BL/6J and Ido1-KO mice were subjected to two separate trials of EZM spaced 8 days apart in order to mimic analyses performed on infected animals. There were no significant differences in distance traveled, although there was a strong trend towards an effect of genotype (Suppl. Fig. 1A–B). Ido1-KO mice entered the open areas fewer times and spent less time in the open areas than C57BL/6J mice (Suppl. Fig. 1C–D). These data indicate that Ido1-KO mice exhibit slightly more anxiety-like behavior than C57BL/6 mice. Anxiety-like behavior between C57BL/6J and Ido1-KO mice was evaluated using an EZM at day 6 and 14 p.i. At day 6 p.i. TMEV-inoculated mice spent more time in open areas of the maze and entered the open areas more often than sham inoculated controls (Fig 4B–C). At day 14 p.i. TMEV-inoculated mice were less hesitant in their first exploration of the open area, spent more time in the open areas, and entered the open areas more often than sham inoculated controls (Fig. 4E–G). Moreover, infected Ido1-KO mice traveled further than infected C57BL/6 mice (Fig 4H). Collectively, these data suggest that acute TMEV infection may decrease anxiety-like behavior. However, an alternative explanation is that mice with seizures are more hyperactive. Indeed, it appeared that mice that developed seizures were generally more mobile than mice in which seizures were not observed (Fig. 4A–H).
Figure 4. Ido1 deficient mice display decreased anxiety-like behavior and increased locomotor activity.
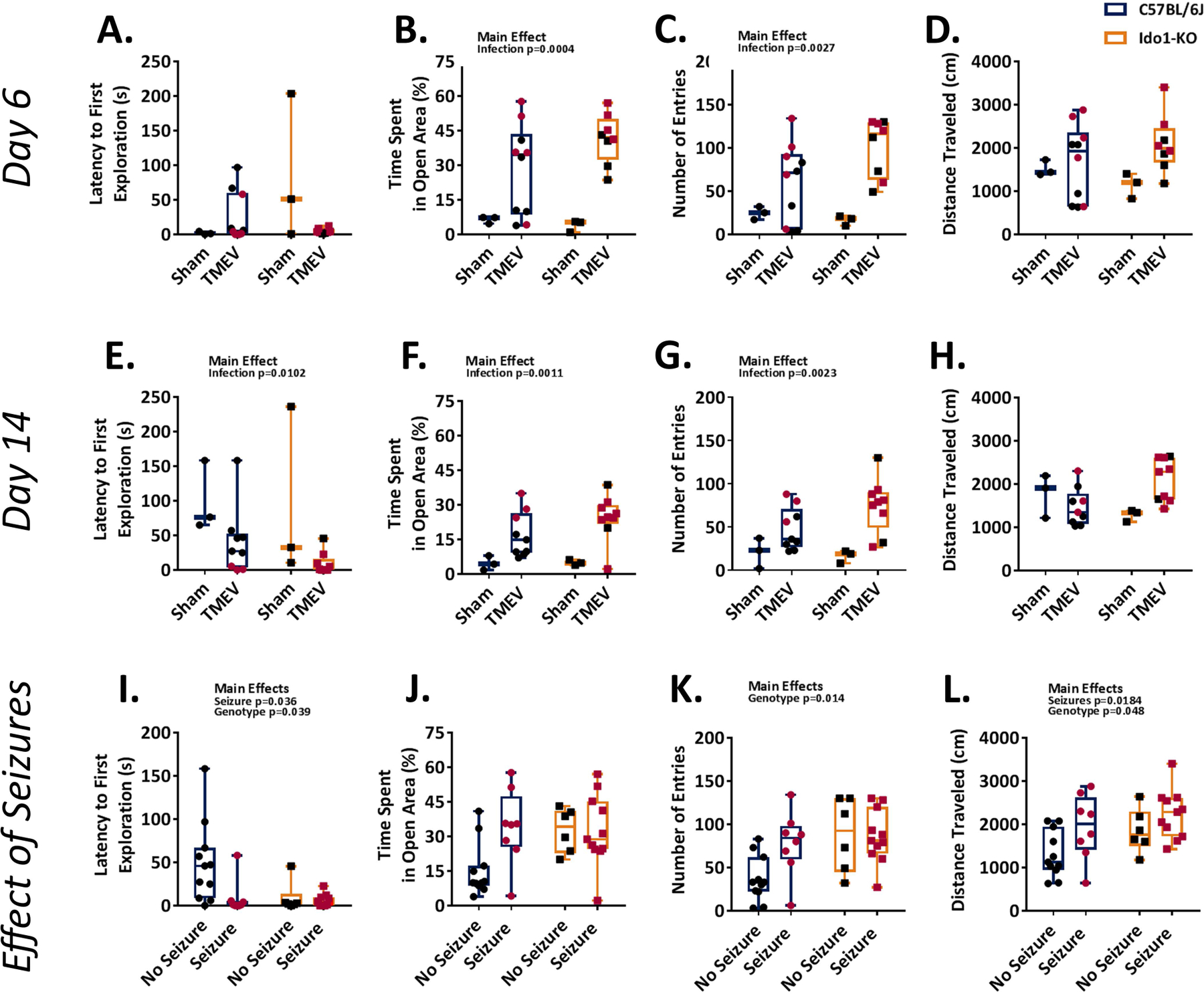
A–H, Behavior was assessed for TMEV-infected C57BL/6J (blue; n = 9–10) and Ido1-KO (orange; n = 8–9) mice using an elevated zero maze at day 6 and 14 p.i. A–D, Differences in anxiety-like behavior between infected C57BL/6J (left, circles) and Ido1-KO (right, squares) mice at day 6 p.i. Latency time to the first entry of the open area (A), percent time spent in the open area (B), number of entries into the open area (C), and total distance traveled (D) are shown. E–H, Differences in anxiety-like behavior between infected C57BL/6J (left, circles) and Ido1-KO (right, squares) mice at day 14 p.i. Latency time to the first entry of the open area (E), percent time spent in the open area (F), number of entries into the open area (G), and total distance traveled (H). Results of individual mice are shown. Mice that displayed at least one clinical seizure are depicted with red symbols. I–L, Behavioral differences between infected C57BL/6J and Ido1-KO mice with at least one observed seizure and those without. Latency time to the first entry of the open area (I), percent time spent in the open area (J), number of entries into the open area (K), and total distance traveled (L) are shown.
To dissect the relationship between seizure incidence and hyperactivity we evaluated locomotion of mice with or without seizures. To perform this analysis, we collapsed the data across time since only two Ido1-KO mice in this cohort remained seizure-free at day 14 p.i.. Using this approach we found that mice that displayed clinical seizures were less hesitant in their first exploration of the open area than those without seizures (Fig. 4I). Furthermore, C57BL/6J mice that seized spent more time in the open area than those that had not seized. This effect was not apparent in Ido1-KO mice (Fig. 4J). Likewise, it appeared that C57BL/6J mice that experienced seizures had an increased number of entries into the open area than those that had not seized, but this effect did not reach statistical significance (Fig. 4K). Finally, mice with documented seizures moved a greater distance than those for which we did not observe seizures (Fig. 4L). When collapsing the data in this fashion infected Ido1-KO mice were less hesitant to explore open areas of the maze, entered the open areas more often and traveled more distance than infected C57BL/6J mice (Fig. 4I, K&L). Collectively, these data indicate that during acute TMEV infection hyperexcitability is associated with the occurrence of seizures20 and generally lend support for our observation that seizure incidence is increased in Ido1-KO mice.
The effect of IDO1 on proinflammatory cytokine and viral RNA levels in the hippocampus.
We next questioned if the lack of Ido1 altered expression of proinflammatory cytokines in the hippocampus at day 8 and 16 p.i. Expression of proinflammatory cytokines were not different between genotypes (Fig. 5A–C). Moreover, viral RNA levels were not different between C57BL/6J and Ido1-KO mice at day 8 p.i.. At day 16 p.i. viral RNA appeared to be reduced in Ido1-KO mice compared with C57BL/6J controls but the effect did not reach statistical significance (Fig. 5D).
Deletion of Ido1 exacerbates hippocampal damage
Infection of C57BL/6J mice with TMEV causes cell death of hippocampal CA1 neurons21. Having demonstrated seizure incidence is affected by the lack of Ido1, we next questioned whether the absence of this enzyme altered hippocampal neuropathology. To test if this was the case, we counted the number of NeuN+Hoechst+ cells in hippocampal CA1 and CA2 of infected C57BL/6J and Ido1-KO mice. In control mice, the number of NeuN+ cells did not differ between mouse strains (Suppl. Fig. 2A–B). At day 14 p.i. Ido1-KO mice had reduced numbers of CA1 hippocampal neurons compared to C57BL/6J mice (Fig. 6A&B). In contrast, there was no effect of genotype on the numbers of neurons in CA2 (Fig. 6C&D). These findings suggest that during acute viral encephalitis the lack of Ido1 decreases survival of hippocampal neurons.
Figure 6. Hippocampal pathology following TMEV infection.

C57BL/6J (blue; n = 3) and Ido1-KO (orange; n = 3) mice were infected with TMEV. At day 14 p.i. coronal brain sections were stained for NeuN (green) and nuclei (blue). Representative pathology in CA1 for each genotype (A) and number of NeuN+ neurons in CA1 per 20X field (B). Representative pathology in CA2 for each genotype (C) and number of NeuN+ neurons in CA2 per 20X field (D). Individual mice are shown. Mice that displayed at least one clinical seizure are red. **p < 0.01 C57BL/6J versus Ido1-KO.
4. Discussion
In this study we investigated the role of Ido1 in an animal model of virus-induced ictogenesis. We found that TMEV infection of C57BL/6J mice increased hippocampal expression of the proinflammatory cytokines Tnf, Il6, Il1b, and Ifng. Moreover, we increased hippocampal expression of full-length Ido1 as well as Kynu, Kmo, and Haao as a result of infection whereas Kat2 levels remained unchanged. These results suggest that TMEV infection induces the enzymatic pathway responsible for QuinA production. Injection of the NMDA receptor agonist QuinA can cause seizures15. Therefore, we disrupted the kynurenine pathway using an Ido1 global knockout mouse model to test if inhibition of an enzyme upstream of QuinA would decrease seizure susceptibility or neuropathogenesis during TMEV infection. We demonstrated that mice lacking Ido1 had an increased seizure incidence compared to C57BL/6J mice. Finally, we found that infected Ido1-KO mice displayed increased neuronal pathology within hippocampal CA1 compared with C57BL/6J mice. Collectively, our data suggest Ido1 plays a protective role during virus-induced ictogenesis.
The current data is the first to provide evidence that indicates genetic deletion of Ido1 increases the incidence of seizures during acute TMEV infection of C57BL/6J mice. Genetic background is known to play a role in the pathogenesis of TMEV-induced neurological diseases, which has been recently exemplified through the use of the Collaborative Cross mouse population22. Importantly, all mice used in this study were on the C57BL/6J background. While it is beyond the scope of the current study, given the seemingly protective role of Ido1 in mitigating the occurrence of TMEV-induced seizures during acute infection, it would be interesting to compare the expression of this gene in mouse strains that are resistant to seizures (i.e. SJL). It has been reported that Ido1 promotes depression-like behavior in a rat model of temporal lobe epilepsy23. However, its ability to modulate seizure incidence and severity in animal models of epilepsy other than TMEV has not been fully elucidated. An important next step will be to determine the effects of Ido1 deletion on TMEV-induced epileptogenesis and the development of depression-like behaviors in mice that exhibit unprovoked seizures following viral clearance.
As reported previously, infection with the BeAn 8386 strain of TMEV caused a transient loss in body weight17. Despite that the absence of Ido1 increased the incidence of seizures, we did not observe changes in body weights between Ido1-KO and C57BL6/J mice. Nonetheless, our results indicate that locomotor behavior was increased in mice with documented seizures. These results appear to be consistent with Theiler and Gard’s characterization of disease following infection with the FA strain insofar as mice with seizures exhibited hyperexcitability20. Our behavioral analysis indicated that mice that exhibited at least one observed seizure moved greater distances than mice that remained ‘seizure free’. In a previous study, TMEV-inoculated mice exhibited increased anxiety-like behavior when compared to sham inoculated mice24. Anxiety-like behavior in this study was assessed using the light/dark box test and open field analysis, during the light phase. In contrast, we found that TMEV inoculated mice displayed increased activity in the elevated zero maze compared to sham inoculated controls. A potential important difference between these studies is that our testing occurred during the dark phase, when mice are typically active. Furthermore, our findings suggest that increased activity amongst infected animals was associated with the occurrence of seizures. This observation is interesting and may be indicative of a manic state rather than decreased anxiety. Mania is associated with both increased activity (here measured as distance traveled) and risk taking behaviors (here measured as time spent in open, lighted areas of an elevated maze). Moreover, mania occurs as a result of temporal lobe seizures as well as during viral encephalitis25.
Neuroinflammation has emerged as a contributor to both ictogenesis and epileptogenesis26. Specifically, toll-like receptor agonists (i.e. high mobility group box 1), danger associated molecular pattern receptor agonists (i.e. P2X7), inflammatory cytokines (i.e. IL-1β, TNF, IL-6 and TGFβ1), chemokines (i.e. CCL2, CCL5, CCL3), COX-2 and prostaglandins have recently received attention for their ability to modulate seizure onset and progression in humans and in preclinical models27; 28. Given their association with neuroinflammation, it is not surprising that many viruses that infect the CNS can contribute to seizure onset and increase the risk for developing epilepsy29. However, how viruses convey this effect has not been fully elucidated. Cytokines increase astrocyte chemokine production30. Subsequent upregulation of chemokines attract inflammatory monocytes to the CNS which contribute to neuronal death and seizure onset31. Alternatively, cytokines and chemokines may interact with neurons and/or astrocytes to directly modulate synaptic activity27; 32. It is likely that there is no single molecular event that entirely accounts for the effects of neuroinflammation on seizure risk. Rather, it appears that a cascade of events underlie this effect. In the current study we build on the understanding of how inflammation contributes to seizure onset during viral infection by demonstrating that deficiency of cytokine-dependent Ido1 is associated with increased seizure incidence and greater neuron loss but had no effect on hippocampal cytokine expression. A caveat of this study is that hippocampal cytokines were measured at day 8 p.i., after the generation of seizures. It is not yet known if deletion of Ido1 affects immune cell trafficking to the brain or cytokine production in the brain prior to seizure onset. Furthermore, it is not known if global Ido1 depletion affects the generation of TMEV-specific adaptive immunity. This is the subject of current investigation.
Given that Ido1 is a known interferon stimulated gene, it is not surprising that it is upregulated during viral encephalitis. In fact, Ido1 expression is increased by other viruses that cause encephalitis and post-encephalitic epilepsy33, but the outcome of this effect is obscure. Interestingly, concomitant stimulation of both human astrocytoma (86HG39) and cervical cancer epithelial (HeLa) cell lines with TNF and IFN-γ induced Ido1 expression and inhibited herpes simplex 2 virus replication34. Moreover, during experimental West Nile Virus infection, Ido1 was upregulated within cells adjacent to virally infected cells in a manner dependent on type I interferon production. The increase in Ido1 expression corresponded to decreased intracellular Trp, which inhibited viral replication33. Data derived from in vivo studies also indicate that Ido1 is upregulated during viral encephalitis. For instance, intraperitoneal injection with encephalomyocarditis virus (ECMV) increased Ido1 expression in the cerebellum where it co-localized to areas of reactive gliosis35. Moreover, peripheral infection with Japanese encephalitis virus (JEV) increased Ido1 expression in the periphery and brain, and Ido1-KO mice were more resistant to encephalitis12. While the authors from the latter study mentioned the occurrence of seizures caused by JEV, the effect of Ido1 on the incidence and/or severity of these seizures was not reported. Therefore, it remains to be determined whether Ido1 protection against viral encephalitic pathogenesis is specific for members of Picornaviridae.
In conclusion, Ido1 is increased in the hippocampus of mice infected with TMEV. Preventing this induction by global deletion of Ido1 was associated with increased incidence of seizures, altered behavior, and exacerbated neuronal pathology following TMEV infection. While we have not yet determined the mechanism governing these observations, our data strongly suggest that a lack of Ido1 is detrimental during TMEV infection as it promoted ictogenesis and neuropathology.
Supplementary Material
A–C, Behavior was assessed for control C57BL/6J (blue; n = 4) and Ido1-KO (orange; n = 4) mice using an elevated zero maze at 6 and 7 weeks of age which corresponded to day 6 and day 14 post infection of our infection studies. A–C, The effects of genotype on distance traveled (A), number of entries into the open area (B) and time spent in the open area (C). Results from individual mice are shown. Statistical main effects are shown.
A–C, Adult C57BL/6J (blue) and Ido1-KO (orange) mice were aged 7 weeks were euthanized (n=3 per genotype), and coronal sections of the brains stained for neurons using antibodies specific to NeuN. The number of NeuN+ cells were counted in CA1 and CA2 regions of the hippocampus (A) using ImageJ. The average number of NeuN+ cells in CA1 (B) and CA2 (C) regions of the right and left hippocampi per genotype are shown. Scale bar in A is 200µm. DG is dentate gyrus. Arrows demark boundaries wherein neurons were counted.
Key Points:
Seizure incidence was greater in TMEV infected Ido1-KO compared to C57BL/6J mice.
Infection increased hippocampal pathology in Ido1-KO compared to C57BL/6J mice.
These data indicate that genetic deletion of Ido1 promotes ictogenesis and hippocampal damage during TMEV encephalitis.
Acknowledgements
The authors thank Dr. Jane Welsh for kindly providing the BeAn 8386 strain of TMEV and BHK-21 cells. This research was funded in part by the USDA National Institute of Food and Agriculture, HATCH Project ILLU-538–932 (A.J.S.), University of Illinois start-up funds (A.J.S) and by NIH via R01 MH101145 (R.H.M.).
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References:
- 1.George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS ONE 2014;9:e104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol 2016;131:211–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Beghi E, et al. The risk of unprovoked seizures after encephalitis and meningitis. Neurology 1988;38:1407–1410. [DOI] [PubMed] [Google Scholar]
- 4.Howe CL, Lafrance-Corey RG, Sundsbak RS, et al. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J Neuroinflammation 2012;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buenz EJ, Sauer BM, Lafrance-Corey RG, et al. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol 2009;175:668–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusick MF, Libbey JE, Patel DC, et al. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol 2013;87:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart KA, Wilcox KS, Fujinami RS, et al. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol 2010;69:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkman NJ, Libbey JE, Wilcox KS, et al. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 2010;51:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libbey JE, Kirkman NJ, Wilcox KS, et al. Role for complement in the development of seizures following acute viral infection. J Virol 2010;84:6452–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel DC, Wallis G, Dahle EJ, et al. Hippocampal TNFalpha Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SB, Choi JY, Uyangaa E, et al. Blockage of indoleamine 2,3-dioxygenase regulates Japanese encephalitis via enhancement of type I/II IFN innate and adaptive T-cell responses. J Neuroin flammation. 2016;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell BM, Charych E, Lee AW, et al. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 2014;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner R, Forteza MJ, Ketelhuth DFJ. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2017. [DOI] [PubMed]
- 14.Schwarcz R, Brush GS, Foster AC, et al. Seizure activity and lesions after intrahippocampal quinolinic acid injection. Exp Neurol 1984;84:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Steelman AJ, Alford E, Young CR, et al. Restraint stress fails to render C57BL/6 mice susceptible to Theiler’s virus-induced demyelination. Neuroimmunomodulation 2010;17:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steelman AJ, Dean DD, Young CR, et al. Restraint stress modulates virus specific adaptive immunity during acute Theiler’s virus infection. Brain Behav Immun 2009;17:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libbey JE, Kirkman NJ, Smith MC, et al. Seizures following picornavirus infection. Epilepsia 2008;49:1066–1074. [DOI] [PubMed] [Google Scholar]
- 18.Barker-Haliski ML, Heck TD, Dahle EJ, et al. Acute treatment with minocycline, but not valproic acid, improves long-term behavioral outcomes in the Theiler’s virus model of temporal lobe epilepsy. Epilepsia 2016;57:1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theiler M, Gard S. ENCEPHALOMYELITIS OF MICE : I. CHARACTERISTICS AND PATHOGENESIS OF THE VIRUS. J Exp Med 1940;72:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkmeyer-Langford CL, Rech R, Amstalden K, et al. Host genetic background influences diverse neurological responses to viral infection in mice 2017;7:12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie W, Cai L, Yu Y, et al. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflammation 2014;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umpierre AD, Remigio GJ, Dahle EJ, et al. Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol Dis 2014;64:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc 1988;63:906–912. [DOI] [PubMed] [Google Scholar]
- 24.Vezzani A, Lang B, Aronica E. Immunity and Inflammation in Epilepsy. Cold Spring Harb Perspect Med 2015;6:a022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronica E, Bauer S, Bozzi Y, et al. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 2017;58 Suppl 3:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auvin S, Walker L, Gallentine W, et al. Prospective clinical trials to investigate clinical and molecular biomarkers. Epilepsia 2017;58 Suppl 3:20–26. [DOI] [PubMed] [Google Scholar]
- 27.Getts DR, Balcar VJ, Matsumoto I, et al. Viruses and the immune system: their roles in seizure cascade development. J Neurochem 2008;104:1167–1176. [DOI] [PubMed] [Google Scholar]
- 28.Choi SS, Lee HJ, Lim I, et al. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS ONE 2014;9:e92325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaenisch R, Varvel NH, Neher JJ, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Nat Med 2016;113:E5665–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulter DA, Steinhauser C. Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med 2015;5:a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung AW, Wu W, Freewan M, et al. Flavivirus infection induces indoleamine 2,3-dioxygenase in human monocyte-derived macrophages via tumor necrosis factor and NF-kappaB. J Leukoc Biol 2012;91:657–666. [DOI] [PubMed] [Google Scholar]
- 32.Adams O, Besken K, Oberdorfer C, et al. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect 2004;6:806–812. [DOI] [PubMed] [Google Scholar]
- 33.Taguchi A, Niwa M, Hoshi M, et al. Indoleamine 2,3-dioxygenase 1 is upregulated in activated microglia in mice cerebellum during acute viral encephalitis. Neurosci Lett 2014;564:120–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–C, Behavior was assessed for control C57BL/6J (blue; n = 4) and Ido1-KO (orange; n = 4) mice using an elevated zero maze at 6 and 7 weeks of age which corresponded to day 6 and day 14 post infection of our infection studies. A–C, The effects of genotype on distance traveled (A), number of entries into the open area (B) and time spent in the open area (C). Results from individual mice are shown. Statistical main effects are shown.
A–C, Adult C57BL/6J (blue) and Ido1-KO (orange) mice were aged 7 weeks were euthanized (n=3 per genotype), and coronal sections of the brains stained for neurons using antibodies specific to NeuN. The number of NeuN+ cells were counted in CA1 and CA2 regions of the hippocampus (A) using ImageJ. The average number of NeuN+ cells in CA1 (B) and CA2 (C) regions of the right and left hippocampi per genotype are shown. Scale bar in A is 200µm. DG is dentate gyrus. Arrows demark boundaries wherein neurons were counted.


