Abstract
Diarrhoeal disease attributable to enterotoxigenic Escherichia coli (ETEC) causes substantial morbidity and mortality predominantly in paediatric populations in low- and middle-income countries. In addition to acute illness, there is an increasing appreciation of the long-term consequences of enteric infections, including ETEC, on childhood growth and development. Provision of potable water and sanitation and appropriate clinical care for acute illness are critical to reduce the ETEC burden. However, these interventions are not always practical and may not achieve equitable and sustainable coverage. Vaccination may be the most cost-effective and equitable means of primary prevention; however, additional data are needed to accelerate the investment and guide the decision-making process for ETEC vaccines.
First, to understand and quantify the ETEC disease burden, additional data are needed on the association between ETEC infection and physical and cognitive stunting as well as delayed educational attainment. Furthermore, the role of inappropriate or inadequate antibiotic treatment of ETEC-attributable diarrhoea may contribute to the development of antimicrobial resistance (AMR) and needs further elucidation. An ETEC vaccine that mitigates acute diarrhoeal illness and minimizes the longer-term disease manifestations could have significant public health impact and be a cost-effective countermeasure.
Herein we review the ETEC vaccine pipeline, led by candidates compatible with the general parameters of the Preferred Product Characteristics (PPC) recently developed by the World Health Organization. Additionally, we have developed an ETEC Vaccine Development Strategy to provide a framework to underpin priority activities for researchers, funders and vaccine manufacturers, with the goal of addressing globally unmet data needs in the areas of research, product development, and policy, as well as commercialization and delivery. The strategy also aims to guide prioritization and co-ordination of the priority activities needed to minimize the timeline to licensure and use of ETEC vaccines, especially in in low- and middle-income countries, where they are most urgently needed.
Keywords: Diarrhoeal diseases, Enterotoxigenic Escherichia coli (ETEC), Vaccine research, Childhood growth and development, Disease burden
1. Introduction
Vaccines are needed to reduce the burden of enterotoxigenic Escherichia coli (ETEC) among infants and young children in low-resource settings and among travellers to these regions. Although there are currently no licensed vaccines against this pathogen, rapid progress is being made with promising whole cell and subunit candidates having entered clinical testing. Improved water, sanitation, and hygiene (WASH) and clinical care are critical to reducing the ETEC-attributable disease burden, but these interventions are not always equitable, practical, or sustainable in areas where they are needed the most. Consequently, WASH interventions have a variable impact on reducing the ETEC burden among infants and young children in low-resource settings.
Even when clinical care is accessible, increasing antibiotic resistance among ETEC strains has hampered the effectiveness of antibiotic treatment in both travellers and infants at high-risk for ETEC diarrhoea in endemic areas. In conjunction with inappropriate use, this led the Wellcome Trust to recommend the acceleration of vaccine development for enteric E. coli, like ETEC [1]. In addition, the World Health Organization’s (WHO’s) Product Development for Vaccines Advisory Committee (PDVAC) recently reaffirmed ETEC as a priority vaccine target [2], and WHO’s Immunization, Vaccines, and Biologicals (IVB) Advisory Committee recently developed and posted Preferred Product Characteristics (PPC) to promote the development of ETEC vaccines for use with the primary strategic goal, ‘To develop a safe, effective and affordable ETEC vaccine that reduces mortality and morbidity due to moderate-to-severe diarrhoeal disease in infants and children under 5 years of age in low-middle income countries (LMICs) [3].
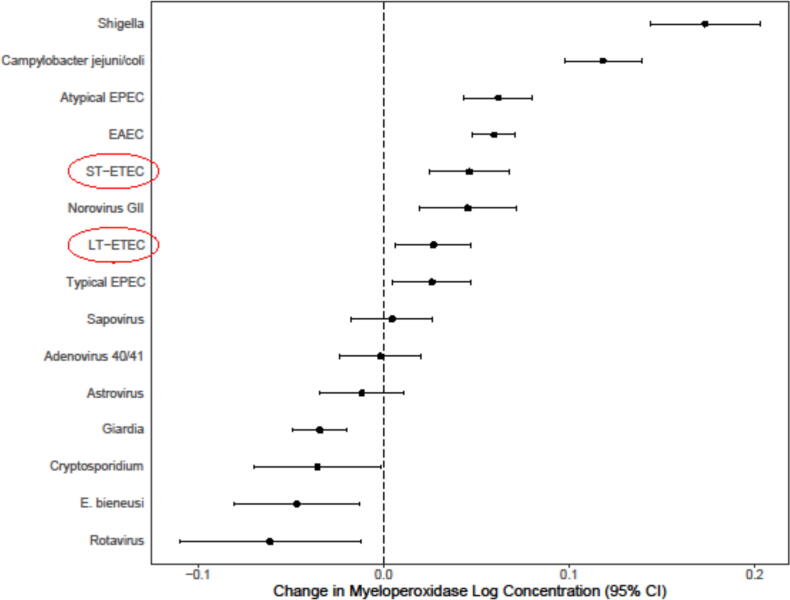
An effective ETEC vaccine would play a critical role in primary disease prevention and in minimizing the acute and chronic disease manifestations of ETEC infection in endemic areas. Improved diagnostic methods for ETEC infections in highly endemic areas have led to the recent recognition that ETEC strains, including those only producing the heat-labile toxin, may contribute to growth stunting (see Fig. 1) and cognitive impairments [4]. This recognition may inform the perceived value of an effective ETEC vaccine among public health stakeholders, policy makers and donors. The Full Value of Vaccines Assessment (FVVA) for ETEC vaccines is considered to be broader than the reduction of acute morbidity and mortality, and includes longer-term morbidity, antimicrobial resistance (AMR), and socio-economic effects at the family, community, and national levels. Future vaccine effectiveness trials incorporating these adverse health outcomes as exploratory or secondary outcomes, could better illustrate the full public health impact of ETEC vaccines on both acute disease outcomes, as well as lowering the risk of asymptomatic infections which also contribute to growth and cognitive deficits in LMICs. This information would add to the FVVA for the vaccine but may be too complex to allow in to drive the clinical development for an unlicensed product until the methodology for assessing these outcomes is more established. Their potential importance is widely recognized and highlighted in the soon to be published ETEC vaccine PPC, as well as in the research gaps outlined in this paper. These same effects could equally apply to combination ETEC (multi-pathogen) vaccines being explored with Shigella and/or Campylobacter [5], [6], [7].
Fig. 1.
Association between enteric pathogens detected by quantitative PCR in monthly non-diarrheal stool samples and stool myeloperoxidase in the multisite MAL-ED birth cohort study. Estimates are per tenfold increase in pathogen quantity from a single linear mixed-effects model including the quantity of each pathogen, sex, and age as fixed effects and site and subject as random effects. A total of 18,365 monthly non-diarrheal stools from 1715 children followed until 2 years of age were included, all of which had valid qPCR results for all included pathogens and were tested for myeloperoxidase (MPO; measured in nanograms per milliliter), a marker of neutrophil activity in the intestinal mucosa (Alpco). (Unpublished data provided courtesy of James Platts-Mills, University of Virginia).
The primary aims of this manuscript are:
-
•
To provide a strategic framework of priority activities for vaccine researchers, funders, and policymakers with the goal of successfully addressing global unmet medical needs, in the categories of research, product development, key capacities and policy, and commercialization and delivery.
-
•
To provide a comprehensive overview of the status of ETEC vaccine development and a gap analysis of R&D needs.
-
•
To create alignment around vaccine development priorities needed to accelerate timelines to licensure and use of ETEC vaccines, especially in LMICs.
2. The public health need for an ETEC vaccine
A driving force for the development of ETEC vaccines is the acute and long-term impact these pathogens have on human health, particularly infants and young children in low-resources settings. With the growing challenge of antimicrobial resistance, efforts are needed to exploit recent advances in our understanding of ETEC pathogenesis and host immunity and disease susceptibility to achieve vaccines against ETEC.
2.1. Vaccine-relevant pathogen virulence factors associated with disease transmission and enteric illness
ETEC is transmitted by fecal-contaminated food or water. A high dose (approximately 106–108 colony forming units, CFUs) of the bacteria may be required to establish infection and cause disease in healthy adults [8], though this dose may be lower in people who are very young, elderly, or malnourished. The incubation time of ETEC diarrhoea is one to five days (mean two days) [9]. After attachment and colonization, primarily at the epithelial surface of the ileum, ETEC proliferate and produce the larger heat labile-toxin (LT) or the smaller heat stable-toxin (ST) or both enterotoxins. The enterotoxins induce the secretion of fluids and electrolytes, which result in acute watery diarrhoea. Intestinal colonization is mediated by colonization factor (CF) antigens and additional secondary adhesins. The CFs are diverse with at least 25 identified with one or two factors usually expressed per strain. Approximately one third of ETEC strains lack defined colonization factors and may rely on secondary adhesins to facilitate colonization. Among CF expressing strains, some geographic variability has been noted; however due to many shared cross-reactive epitopes, a vaccine formulated to include four to five of these factors could cover most common CFs associated with illness in infants and international travelers [10], [11], [12], [13]. ETEC pathogenesis is also facilitated by a number of conserved proteins that serve as accessory virulence factors [14], [15], [16]; that help modulate adhesion, intestinal colonization and efficient toxin delivery [17]. Animal models and in vitro data suggest that LT may contribute to intestinal barrier dysfunction and environmental enteric dysfunction (EED), as well as help facilitate colonization [18], [19].
The severity of ETEC diarrhoea is highly variable. Recent field and CHIMs data indicated that secretory status and ABO blood group can contribute to disease susceptibility and severity [20], [21]. ETEC infection is usually self-limiting, lasting three to four days; however, some episodes, particularly due to LT-ETEC go on to become persistent diarrhoea lasting longer than 14 days. In addition, evidence from the MAL-ED community-based burden of enteric disease study has also shown that even asymptomatic infection with LT-ETEC can contribute to potentially detrimental gut inflammation (see Fig. 1). Oral rehydration solution (ORS) and other treatments can reduce the severity of ETEC disease. With adequate treatment, including the use of oral rehydration solution (ORS) and dietary improvements, disease severity can be decreased [22]. Furthermore, breastfeeding reduces the risk of diarrhoea and other infectious diseases [23], [24]. However, ETEC morbidity is substantial and given increasing resistance of ETEC isolates to common antibiotics, like trimethoprim-sulfamethoxazole and ampicillin, other primary prevention efforts, like vaccines, are needed [1], [25].
2.2. Disease burden
Quantifying the total disease burden for any pathogen must include assessment of both morbidity and mortality. It is estimated that ETEC causes about 220 million diarrhoea episodes globally, with about 75 million episodes in children under 5 years of age. According to the Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) study estimates, diarrhoea accounts for more than 1 million deaths and about 4% of the total global disability-adjusted life-years (DALYs) per year across all age groups. ETEC was the eighth leading cause of diarrhoea mortality in 2016 among all age groups, accounting for 51,186 (26,757–83,064) deaths – about 3.2% (1.8–4.7) of total diarrhoea deaths [26]. Among children younger than 5 years of age, ETEC was responsible for an estimated 18,700 deaths (9,900–30,659) – about 4.2% (2.2–6.8) of total diarrhoea deaths [27]. More recent estimates suggest that ETEC associated mortality may be slightly higher (an increase of 8–34%) if deaths due to other infectious disease cases among stunted children attributed to ETEC are included [28]. While enteric disease vaccines have traditionally focused solely on acute diarrhoea and resulting deaths, there is an increasing appreciation of long-term consequences on growth and cognitive deficits. These chronic effects are critically relevant in assessing the potential benefits of an effective ETEC vaccine.
The increased sensitivity of molecular diagnostics highlights the high frequency of enteric infections without overt diarrhoea. This is true for ETEC (including LT-ETEC), as well as for other enteropathogens [29], [30], [31]. These “asymptomatic” infections are associated with underappreciated negative health outcomes. In addition to initial growth effects of acute diarrhoea, ETEC may also impair the “catch-up” growth after infection [32], [33]. Additionally, repeated symptomatic and asymptomatic episodes in the first two years of life can lead to a loss of up to 10 IQ points and 12 months of schooling by nine years of age [34], [35]. The heavy burden of enteric infections, including ETEC, early in life can increase the risk of metabolic disorders, including hypertension, obesity and diabetes later in life [36]. The cost of the hypothesized vicious cycle of enteric infections and malnutrition and their potential lasting impact will likely require multiple, synergistic approaches to ameliorate this burden with vaccination being the most equitable and sustainable [37]. A healthy intestinal tract is especially critical in the first two years of life, when most brain and synapse development in humans occurs after birth. Hence, the absorption of key nutrients during this time is critical for optimal growth and development [37], [38]. Enteric infections may also modify the host microbiome and trigger complex immunologic responses resulting in autoimmune dysfunction [39]. Other factors have been suggested as potential mediators of these sequelae for enteric infections, including nutrient deficiencies, other infectious diseases, environmental exposures, lack of adequate stimulation and/or learning opportunities, maternal depressive symptoms, and host genetics [40], [41]. Consequently, this important area of additional research has been highlighted as a “research gap” in the ETEC Vaccine Development Strategy section to follow.
In the community-based Malnutrition and Enteric Disease Study (MAL-ED) study, LT-only expressing ETEC strains were associated with persistent diarrhoea in infants and with fever in 1–2-year-old children, supporting the concept that at least some LT-only strains can be pathogenic in this population [42]. Recent CHIMs data also supports that some LT-only strains can be pathogens and cause acute disease [43]. Furthermore, as shown in Fig. 1 in the MAL-ED cohort study, asymptomatic LT-ETEC were among the top infections independently associated with increased fecal myeloperoxidase (MPO), indicative of an inflammatory enteropathy. These data are supported by new animal model and in vitro studies demonstrating the inflammatory pathogenicity of LT-only strains [44] and their potential contribution to long-term intestinal sequelae including EED and loss of gut barrier function [19], [18].
In addition, IHME conducted a study to quantify the long-term sequelae due to growth faltering, using DALYs, which are the sum of the number of years of life lost to pre-mature mortality (YLL) and the number of years lived with disability (YLD) [45]. The study showed that the global diarrhoea burden is substantially underestimated when only incidence and mortality are considered. When growth impairment is accounted for, the number of diarrhoea DALYs lost increased by approximately 40% among children younger than five years. When accounting for the total burden of disease (both YLLs and YLDs), diarrhoea is the third-leading cause of DALYs among children younger than five years, surpassing malaria and neonatal encephalopathy [46]. ETEC-specific analyses on DALYs due to long-term sequelae are lacking, but such estimates will help to refine the pathogen-specific burden and inform the full value of an ETEC vaccine.
2.3. Potential health and socioeconomic impact of ETEC vaccines
Recent studies have elucidated the health and socio-economic impact that a potential ETEC vaccine could have, as well as estimated cost-effectiveness. These studies include the effects of an ETEC vaccine on acute morbidity and mortality as well as stunting and other infectious disease mortality in stunted children. They first estimated the number of stunting episodes potentially resulting from ETEC infections using data from the Global Enteric Multicentre Study (GEMS) [29] and stunting data from 79 low- and lower-middle-income countries [28]. Based on these data, they predict approximately 13.7 [8.3; 19.1] million additional episodes of stunting, due to ETEC infections over a 10-year period. Childhood stunting is the failure of a child to reach full growth potential as a result of long-term poor diet, health, and/or care, including emotional support. It is identified and measured based on a child’s height and age. Stunting is a marker of a child’s overall lack of health and well-being; it is associated with limits to a child’s physical and cognitive potential [47].
Anderson et al. refined their models to evaluate ETEC vaccine cost-effectiveness sub-nationally in four sub-Saharan countries in Africa, which highlighted an improved cost-effectiveness ratio [48]. These ratios improved even more when the sub-national analysis examined cost-effectiveness by wealth quintile. In almost all provinces, when ETEC vaccine was introduced in the lower wealth quintiles the cost-effectiveness ratio improved substantially. Heterogeneity of ETEC is likely driven by multiple factors that are both geographic and socio-economic in nature, including access to clean water, sanitation, and health care, and increased levels of poverty and stunting.
Given its predominance as the leading etiology of travellers’ diarrhoea (TD), a standalone vaccine for ETEC is more cost-effective than for other TD pathogens, and a viable commercial market also likely exists. For military populations, an ETEC-focused vaccine is more cost-effective than stand-alone vaccines for Campylobacter or Shigella, and it was estimated that, from a business case perspective, a global market of over US$600 million is achievable for ETEC alone predominately driven by travellers and middle-income markets [49]. However, a multi-pathogen vaccine covering for ETEC along with Campylobacter and Shigella would be highly cost-effective for military as well as international travelers. In addition, the growing appreciation that combination ETEC vaccines, particularly with Shigella may be possible. Recent evidence indicating that candidate oral and parenteral ETEC vaccine may be more immunogenic in target age-groups than previously developed cholera, typhoid and shigella vaccines because of improved formulations and the inclusion of the new mucosal adjuvant, dmLT, will likely improve their prospects for commercialization and use [5], [7] Additional issues and factors impacting on commercialization, uptake and delivery of ETEC vaccines are included in the recently developed ETEC vaccine PPC from WHO [3] and in the research gaps section below [49].
2.4. AMR impact
AMR among ETEC isolates is increasing globally. As a result, Enterobacteriaceae are listed on the critical WHO pathogen priority list for the development of new antimicrobials [50] and classified as an urgent AMR threat by the US Centers for Disease Control and Prevention (CDC) [51]. Examples of AMR to the major antibiotics have now been reported widely [52].
Infection with AMR strains can result in treatment failure, increased patient mortality or morbidity, prolonged hospitalizations, reduced productivity, and increased treatment costs and can prolong an individual’s inability to fully participate in social activities, school, or work. An efficacious ETEC vaccine may directly prevent an AMR infection and may reduce the number of antibiotic-requiring infections, further reducing the risk of AMR strains developing [53]. Several recent studies also indicate that many infants and children presenting with diarrhoea are treated inappropriately with antibiotics [54], [55], which also contributes to the further development of resistance. Consequently, an ETEC vaccine capable of preventing medically attended diarrhoea may have an AMR impact beyond just ETEC.
In a recent report from the Wellcome Trust and the Boston Consulting Group on vaccine impact on AMR development and control, it was recommended that vaccine development for enteric E. coli, including ETEC, should be accelerated [1]. WHO is developing a comprehensive framework to consider the value of a future ETEC vaccine (and other vaccines) on AMR and is expecting to publish its findings in 2021 [56]. There are currently limited data on ETEC-associated antibiotic use and the health and economic impact of AMR ETEC. The paucity of data is a major roadblock to estimating the effects of an ETEC vaccine on AMR; however, datasets are available that may inform important parameters such as the proportion of strains that are resistant to common antibiotics as well as the use of antibiotics for treating ETEC infections. To this point, a recent Phase 2b trial of the ETEC candidate vaccine ETVAX in Finnish travellers to Benin demonstrated a reduction in antibiotic use among vaccinated subjects compared to placebo recipients (p = 0.033) [2]. Additional studies may be needed to more fully inform parameter estimates for the ETEC-attributable contribution to AMR.
3. Current status of ETEC vaccine development
Field studies and CHIMs data indicate that ETEC vaccine development is feasible [57], [15], [16] and public health stakeholders, such as the WHO, have encouraged and supported ETEC vaccine development over the last 20 years. Today, the probability of developing a safe and effective ETEC vaccine that will meet the WHO’s current strategic goal is considered very high [3]. With continued funding and stakeholder support, licensure and WHO prequalification may be obtainable for ETVAX, the most advanced candidate (see Table 1), within the next five years.
Table 1.
Overview of the ETEC vaccine development landscape.
| Type of Vaccine | Candidate | Description of ETEC Candidate | Developmental stage | Developer |
|---|---|---|---|---|
| Inactivated whole cell vaccine | ETEC cells with LT components ETVAX | Recombinant E. coli (one E. coli K12 and three O78 positive E. coli) strains that over-express CFA/I, CS3, CS5 and CS6 antigens combined with hybrid LT/CTB, adjuvanted with dmLT | Phase 2b | Scandinavian Biopharma |
| Live attenuated Shigella-vectored | ShigETEC toxin hybrid | ShigETEC LPS-free cell expressing conserved ETEC and Shigella antigens | Phase 1 | EveliQure |
| Shigella hybrid (1208S-122) | Attenuated Shigella vaccine strains engineered to express ETEC CF and LT | Phase 1 | University of Maryland’s Center for Vaccine Development (CVD) | |
| Subunit | Fimbrial tip adhesin (FTA) | Class 5 fimbriae combined with other CF/CS, designed to block adhesion of ETEC to the intestinal epithelium by inducing antibodies to the tips of fimbriae | Phase 1/2b | US Naval Medical Research Center (NMRC) |
| Multi-Epitope Fusion Antigen (MEFA) (MecVax) | On CFA/I backbone express CS1-CS6, consensus peptide fused to 3xST-dmLTA-1LTB dmLT-ST toxoid | Preclinical | University of Illinois; Johns Hopkins University | |
| Potential Subunits | Alternative or complementary to known virulence factors | YghJ, a protein secreted by the same pathway as ETEC LT; EatA, a serine protease that degrades mucin and promotes ETEC access to mucosal surfaces, EtpA, a secondary adhesin factor | Preclinical | Washington University, St Louis; CVD; University of Bergen; GlyProVac |
| ST | Multiple constructs designed to improve immunogenicity with no toxicity or autoreactivity | Preclinical | University of Bergen; Tulane University |
Definitions: CF/CS, colonization factor antigens; ETEC, enterotoxigenic Escherichia coli; LT, heat-labile toxin; ST, heat-stable toxin; dmLT, double-mutant heat-labile toxin (LTR192G/L211A).
Current ETEC vaccine development efforts focus on inducing immune responses against CFs and one or both ETEC toxins which are documented to contribute to protective immunity [58], [7], [59]. CF/CS antigens are highly diverse, so vaccines have been designed to express some of the most epidemiologically prevalent CF/CS antigens and toxin components [60], [10], [7]. A vaccine formulated to include four to five of these factors would cover for 70–80% of the most common strains associated with illness in infants and international travelers [11], [12], [13]. In addition, CF/CS antigens in many of the current candidates may induce antibodies that cross-react with related CF/CS antigens which may also help expand coverage [61]. Table 1 provides a landscape summary of the ETEC vaccine candidates currently in development. A more detailed listing of novel ETEC antigens that may warrant further evaluation as potential candidates for inclusion in ETEC vaccines is shown in Table 2.
Table 2.
Expanded list of novel antigens of ETEC.
| Antigen/Epitope | Description/Role in Pathogenesis |
|---|---|
| EtpA | Secreted adhesin, intestinal colonization, toxin delivery |
| EatA and Ag43 | Mucin degrading serine protease, toxin delivery |
| EaeH | Outer membrane adhesin, toxin delivery |
| ECP | E. coli common pilus, role in pathogenesis not known |
| Flagellin | Flagellar H subunit involved in motility, toxin delivery |
| Type 1 Fimbriae | Involved in toxin delivery |
| YghJ Metalloprotease1 | T2SS, mucin degrading, toxin release |
| MipA2 | Remodelling peptidoglycan, role in pathogenesis not known |
| Skp2 | Molecular chaperon, OMP, role in pathogenesis not known |
| ETEC_24792 | OMP, LCFA transport |
| EspB | Effector protein; part of the Type 3 secretory system in EPEC |
| LPS (O) | Sero-determinant, role in pathogenesis is not clear |
EatA and EtpA are plasmid encoded, all other putative novel antigens are chromosomally encoded.
YjhJ also may be designated as SslE and can be expressed as a cell surface associated and secreted lipoprotein with mucinase activity in some ETEC, typical EPEC and extraintestinal E. coli, like uropathogenic (UPEC) and neonatal sepsis associated (NSEC) E. coli strains.
Conserved epitope also present in commensal E. coli. Impact of using subunit vaccines consisting of proteins encoded by commensal bacteria are not known. However, vaccination with these proteins did not significantly alter host intestinal microbiome in animals.
3.1. Cellular ETEC candidate vaccines
The most advanced cellular ETEC vaccine candidate is ETVAX [62], [63] which had an excellent safety and immunogenicity profile in adult Swedish subjects when given with or without dmLT [64]. ETVAX was also generally safe in an age-descending trial in Bangladesh with vomiting being the most common event in 6–11 month old infants [63]. Vomiting was reduced by using fractional adult doses without loss of immunogenicity. The inclusion of the dmLT adjuvant in the vaccine improved the frequency, magnitude and kinetics of mucosal antibody responses in both Swedish adults and Bangladeshi infants [63], [64].
In a double-blind, randomized placebo-controlled, efficacy trial in Finnish travellers to Benin, West Africa, ETVAX demonstrated good immunogenicity with strong serum IgA and IgG responses to LTB and excellent safety. Preliminary results indicate significant efficacy against moderate-to-severe ETEC-attributable disease (p = 0.006) as well as significant protection against severe diarrhoea due to any infectious cause (p = 0.03). Antibiotic or antisecretory drug treatment was given to significantly fewer vaccine recipients than to placebo recipients (p = 0.03), suggesting that ETVAX reduced disease severity [2], [57]. In follow-on studies a Phase 1 trial demonstrating the safety and immunogenicity of the vaccine in Zambian infants and young children has recently been completed and a Phase 2B efficacy trial is scheduled to begin in the Gambia in 2021.
Other promising cellular approaches to ETEC vaccine development have been the construction of attenuated, hybrid Shigella vaccine vectors expressing ETEC antigens. The two lead candidates based on this approach are ShigETEC and CVD 1208S-122 [7], [65], [66], Phase 1 testing of ShigETEC and CVD 1208S-122 will likely begin in 2021.
3.2. Subunit ETEC candidate vaccines
Subunit candidates have also focused primarily on the induction of anti-CF/CS and anti-toxin immunity by the parenteral route of administration. The most advanced program employs a fusion of representative adhesin-pilin subunits (Table 1): class 5a (CfaEB), class 5b (CsbDA-CooA), class 5c (CotDA), and CS6 (CssBA). It is anticipated that these components, along with an LT-based adjuvant, like dmLT, could provide over 75% coverage against circulating ETEC strains [13]. Parenteral immunization with pilot lot preparations of the individual vaccine adhesins have all induced protection against illness in non-human primates (Aotus nancymaae) [66], [7]. Two of the components (CfaE and CssBA) have undergone clinical evaluation and demonstrated safety, robust systemic and mucosal immunogenicity, including heterologous within sub-class responses and mucosally-homing antibody secreting cell responses, as well as significant antigen-specific fecal IgA responses [67]. The CfaE component also demonstrated qualified efficacy in a CHIM, reducing both the incidence and severity of disease following challenge with ETEC strain H10407 [2], [60]. Given the parenteral nature of these vaccine constructs and their ability to be combined with other enteric vaccines under development including Shigella and typhoid conjugates adds to the FVVA for this vaccine approach [5], [6], [68].
Another subunit approach uses a novel toxoid fusion strategy and epitope- and a structure-based multiepitope-fusion-antigen (MEFA) platform to construct a toxoid fusion protein, MecVax. Immunization with this candidate induces antibodies against both ETEC enterotoxins (STa and LT) and the seven most common ETEC adhesins (CFA/I, CS1 to CS6) [69], [2], [7]. MecVax induces antibodies significantly inhibiting adherence of E. coli bacteria producing any of the seven adhesins and neutralized STa and LT enterotoxicity. Moreover, MecVax demonstrated efficacy against STa + ETEC and LT + ETEC in a pig challenge model [70], [71].
Not all epidemiologically relevant ETEC express CF/CS and there is no clear evidence that anti-toxin immunity alone can be broadly protective. Consequently, it is important to identify novel antigens that may be included in current vaccine formulations to develop a more broadly protective vaccine. The application of several “Omics” technologies has facilitated the identification of several novel protein antigens including flagellin, EtpA, EatA, EaeH, EspB, and YghJ, that warrant consideration for inclusion in future vaccine approaches (see Table 2) [16], [72], [73], [74]. For example, immunization of mice with both EtpA and EatA significantly reduced intestinal ETEC and antibodies against both antigens appear to work synergistically to help neutralize LT enterotoxin activity by interfering with its delivery to the epithelial cell surface [16]. Additional studies are needed to evaluate these antigens as additional components in other vaccine candidates.
4. The future of ETEC vaccine development
All of the leading ETEC vaccine candidates appear compatible with the general parameters of the recent WHO PPC for an acceptable ETEC vaccine in that they are suitable for the prevention of MSD in infants and young children 6 to 24 months of age and are compatible for use in travellers visiting ETEC-endemic areas. It remains unclear whether current vaccine approaches will be efficacious in infants and young children since this age group has proven difficult to effectively immunize against enteric diseases by either the oral or parenteral routes. Several reasons for poor vaccine effectiveness in the target age-group have been suggested [39], [75], [76], [77]. However, both ETVAX and the adhesin-based candidate include dmLT which may help to overcome this barrier. For example, recent data from 6- to 11-month-old Bangladeshi infants show that dmLT improves the mucosal immune response to ETEC antigens following immunization with ETVAX [63] and the kinetics of the immune response was accelerated by inclusion of dmLT in the vaccine. A mucosal adjuvant, like dmLT, may also facilitate dose-sparing [78], [63], as well as improve cost of goods (COGs) and the FVVA for both candidates. ETVAX has been evaluated using the Vaccine Technology Impact Assessment Tool, developed by PATH and WHO, which identified an all-in-one presentation of this five-component vaccine as optimal for use in LMICs and, as indicated at the 2020 ETEC PDVAC meeting, efforts are underway to develop this formulation [2]. ETEC vaccines intended for use in paediatric populations in LMICs must be formulated in such a way that their costs are reasonable, their delivery is programmatically suitable and their tolerability and immunogenicity are optimized [79], [80], [81].
4.1. New tools and enabling technologies supporting ETEC vaccine development
As outlined above, the development and application of new or improved research tools and models facilitates development, evaluation and enhancement of promising ETEC vaccines. Improvements to the CHIM and development of the mouse oral challenge model provide early opportunities for assessments of vaccine efficacy and may enhance understanding of ETEC’s role in EED and stunting and help identify vaccines most likely to reduce ETEC-associated gut inflammation [4]. Clinical evaluations will certainly benefit from work identifying immune correlates of protection [82]. The availability of ETEC proteomic arrays may enable a more in-depth characterization of the antigen specific responses during infection. Novel adjuvants may also play an important role in vaccine development. Early data indicate that dmLT may adjuvant mucosal immune responses to co-administered antigens in young children in LMICs. Additionally, anti-LT immunity induced by dmLT may contribute to protection against other diarrheal diseases as previous field trials have demonstrated with prior ETEC vaccines (i.e. Dukoral and the LT-patch) [83], [84]; however, the basis for this expanded efficacy is unclear. Recent data demonstrating that dmLT can be administered parenterally to improve intestinal immune responses opens new opportunities for subunit-based ETEC vaccines. Enhanced studies of the fecal microbiome may also point to an important role of intestinal microbiota in ETEC susceptibility [85], [86]. These data may enable an evaluation of the interplay between vaccination and potential modifications of microbiome inducing a less susceptible profile. Finally, the recent rapid production and high protective efficacy of mRNA-based vaccines against the COVID-19 virus will likely encourage investigators to consider this approach for ETEC and other enteric pathogens.
5. The ETEC vaccine development strategy
We have identified a number of strategic research gaps that are hampering ETEC vaccine development, that if not addressed could hinder policy recommendations, and eventual broad uptake. We recommend prioritizing the following activities to bridge these gaps in the near-term to enable product development, licensure, and global access for ETEC vaccines.
5.1. Conduct a formal FVVA to improve awareness of full ETEC burden and assess potential vaccine demand, as well as identify potential benefits and challenges of vaccine development to help optimize vaccine profile, clinical efficacy endpoints, licensure, and uptake
It cannot be taken for granted that the licensure of an effective, low-cost ETEC vaccine that requires multiple doses would be greeted with strong demand and immediate uptake. LMICs face increasingly difficult decisions about how to prioritize new vaccine introductions, and they would benefit from a well-articulated and objective assessment of the potential public health and economic case for ETEC vaccines.
The recently published WHO ETEC vaccine PPC3 stressed the importance of assessing the FVVA to incentivize ETEC vaccine development. An ETEC FVVA would provide a critical portrait of the potential public health and socioeconomic value of vaccines, from both an individual and population based perspective, for intended beneficiaries. The FVVA will also help guide funding agencies and vaccine manufacturers in investment prioritization and prepare the ground for policy considerations, thereby accelerating introduction of ETEC vaccines. Consequently, it is important, as several promising candidate vaccines move forward into more advanced clinical development, that the evidence-based research and analyses associated with a formal FVVA be prioritized and pursued to address this strategic information gap and impact analysis in ETEC vaccine development. This analysis is important to increasing stakeholder awareness of the ETEC burden and its acute and more long-term health impact and also can influence vaccine design and manufacturing priorities by helping to estimate likely demand for a vaccine, and ultimately facilitate policy recommendations and financing decisions by WHO, PAHO, and Gavi, the Vaccine Alliance. This was recently initiated for Shigella vaccines (WHO PDVAC meeting on Shigella vaccines, May 2020) and it is anticipated that the ETEC vaccine FVVA analysis should be focused on similar research questions. In addition, the targeted use of an ETEC vaccine [48] has the potential to improve vaccine cost-effectiveness ratios so that they are closer to those of other childhood vaccinations and may improve equity by targeting the most vulnerable. Another consideration is the development of a multi-pathogen vaccine, with consideration of epidemiology and population. Recent publications have indicated that combination with Shigella and/or Campylobacter vaccines is feasible [87], [6].
5.2. Develop fieldable, rapid, point-of -care diagnostics to improve ETEC disease burden estimates, support efficacy trials, and increase disease awareness
The development of rapid and fieldable diagnostics for rotavirus was instrumental in documenting the widespread burden of disease, facilitated efficacy testing and helped drive policy decisions recommending uptake [88]. However, similar rapid diagnostics for ETEC have lagged behind. The recent application of new TaqMan-based, real-time quantitative PCR technology in support of the GEMS and MAL-ED studies, as well as in several recent TD studies [89], [29], [31] has been shown it to be extremely helpful in detecting ETEC and other pathogens directly from stool specimens [90]. However, detection of ETEC infection has not been feasible outside well-equipped laboratories in major urban centres, hence the real burden of ETEC infections is likely under-estimated. ETEC diagnostics, together with other enteric pathogen detection, are important for treatment of outbreaks, for public health surveillance and for use in vaccine field efficacy trials [90]. It remains unclear whether molecular-based diagnostics can be used successfully in Phase 3 trials or whether regulatory agencies will accept these data for vaccine licensure.
More rapid, simpler, and lower-cost methods, like rapid loop-mediated isothermal amplification assays (RLDT), have shown promise for a number of infectious diseases, including TB and COVID-19. and should be considered for ETEC and other enteric pathogens [91], [92]. They warrant field evaluation in this context and comparison to other quantitative PCR methods. The speed, simplicity and point-of-care potential of RLDT are intriguing in that results could be used to rapidly screen samples for ETEC and presumptive positives (based on quantitative cycle thresholds) could be targeted in a hybrid approach for more in-depth culture-based assessment.
5.3. Improve coverage of ETEC strains in candidate vaccines and explore combination vaccine options
Alignment of vaccine developers on ETEC antigen(s) composition is important. Candidate vaccines including anti-LT toxin and anti-CF/CS antigens have been effective in preventing MSD, and potentially colonization, in travelers and CHIMs [60]. Consequently, antigens like EatA and YghJ/SslE, which may also contribute to reductions in ETEC colonization and interfere with effective toxin delivery and/or triggering of gut inflammation may need further consideration. An added benefit of including these novel antigens in future ETEC vaccines is that may help to broaden coverage and improve the breadth of protection. For example, YghJ, also designated SslE is also expressed in typical EPEC strain and in extraintestinal E. coli strains associated with URI and neonatal sepsis and meningitis.
Consequently, ETEC vaccines containing these antigens may have some impact on the incidence and severity of these other E. coli infections. Further exploration of mucosal adjuvants is indicated by the encouraging results observed in Bangladeshi infants with dmLT and the encouraging safety and immunogenicity results when dmLT is administered parenterally with subunit ETEC vaccines. The latter findings offer a viable alternative delivery route that may have advantages over oral vaccination. Finally, preclinical studies are needed to explore combination vaccine strategies that might help drive multi-pathogen vaccine efforts considering the additions of Shigella and Campylobacter [6], [7], [93].
5.4. Accelerate efforts to identify correlates of protective ETEC immunity
WHO’s recent decision for ETEC to remain a priority vaccine target highlights the importance of better defining correlates of protection to aid and accelerate vaccine development. Field studies and CHIMs demonstrated that antibodies against LT and CF/CS antigens can contribute to protection [2]. T cells may also contribute to immunity [82], [94]; however, mucosal-based threshold levels associated with protection in infants and young children are unknown. Standardized antigens, like those made available by NIH, and harmonized assays, like those being developed by the National Institute for Biological Standards and Control (NIBSC) for anti-Vi serology and anti-Shigella LPS serology, are needed for ETEC. One new research tool that may help with identifying immune correlates or immune profiles associated with ETEC immunity is the ETEC proteomic array. The promising recent data generated with the Shigella proteomic array [95] suggesting that conserved invasion proteins may also contribute to protection highlights the need to more fully explore this platform for ETEC.
5.5. Identify new endpoints and trial designs for assessing ETEC candidate vaccines’ impact on a broader array of morbidity outcomes
While pivotal field trials of an ETEC candidate vaccine will primarily focus on prevention of acute diarrhoea, these studies should also consider incorporating anthropologic measurements to assess the vaccine’s effect on age-specific height and weight for age Z (HAZ and WAZ, and WHZ) scores. Additionally, studies should consider including an assessment of vaccine direct effects on stunting and malnutrition, as well as, indirect effects, including decreased AMR, herd protection, and financial risk protection. Such secondary endpoints would highlight the full public health value of ETEC vaccines and provide key data to justify vaccine uptake. These secondary endpoints may support a broader public health benefit of an ETEC vaccine by preventing not only the symptomatic disease but other negative consequences of symptomatic and asymptomatic infection. This becomes even more applicable for multi-pathogen vaccines. The combinatorial array of infections that occur during early childhood is complex and the possibility of interactions between pathogens is probable. This complexity limits the capacity of reliable estimates of etiology-specific burdens. A simple measure, such as an initial and even a single follow-up height and weight measurement at the end of the study period, could help start capturing at least some of the potential importance of an effective ETEC vaccine in preventing not only overt diarrhoeal illnesses but also, and perhaps even more likely, effects on the far more common silent (‘asymptomatic’) ETEC infections. ETEC vaccine-related characteristics and potential endpoints for future trials that need to be addressed in future clinical and operational research are outlined in Table 3.
Table 3.
ETEC vaccine-related characteristics and potential endpoints for future trials.
| Research area | Current assumptions | Research gaps | Recommendations to address gaps |
|---|---|---|---|
| Target population | Timing is constrained by EPI schedule and need to provide protection in the first year of life when the case fatality rate (CFR) for acute diarrheal illness is greatest | Immunogenicity as a function of age for principal candidates in infants in LMICs is a question; target age-group has proven difficult to immunize effectively | Trial size sufficient to permit age stratification of results based on age of initial dose; novel approaches to improve mucosal immunogenicity in infants need to be exploded; also explore combination approaches |
| Efficacy | Innate immunity is equal across populations | Histo-blood group antigens (HBGA) appear to influence rates of infection and severity of disease in children to strains expressing canonical vaccine antigens CFA/I, CS3, CS5 and CS6 (PMID: 30768135) | Efficacy studies in populations should test subject HBGA to account for heterogeneity between populations in multi-site studies and within study populations |
| Epidemiologically important CFA and non-canonical antigenic determinants, like EtpA and EatA are stable over time and place and are compelling candidates for inclusion in vaccines | Combination needs to be optimized to broaden coverage and efficacy | Multi-site Phase 2/3 trials should confirm this supposition and robust nature of protection | |
| ETEC vaccine need to have more public health benefit than preventing mortality and MSD diarrhoea. Reduction in other negative acute or more long-term negative health outcomes need to be considered | WHO ETEC vaccine PPC has called for new pre-clinical, CHIMs and field research to evaluate vaccine impacts on disease severity beyond diarrhoea and their ability to interfere with the pathogenic pathway to stunting and poor neurodevelopment | New secondary/exploratory endpoints targeting vaccine impact on reducing intestinal inflammation; growth deficits, markers of cognitive development and disease severity scores need to be evaluated in future field trials and if validated be used future pivotal Phase 3 supporting vaccine licensure | |
| Formulation and Presentation | Improved temperature stability and simple vaccine presentation facilitating delivery are important parameters that contribute to a favourable FVVA and increase uptake | Lead cellular and subunit ETEC vaccine candidates need additional formulation and presentation research to ensure they meet WHO PPC specifications and improve their prospect for widespread uptake following licensure | Strategies need to be explored to improve the temperature stability and cold-chain foot print of the most advanced ETEC candidates, ensuring that vaccine and antigen co-formulations are more standardized, stable and also develop presentations that facilitate use in Phase 3 trials. Use of the VTIA tool recently developed by PATH and WHO can help in optimizing vaccine presentation |
Overall, more public health research and macroeconomic research is needed to better understand and quantify the full value of ETEC vaccines. Specifically, additional data are needed on: the sub-national heterogeneity of disease burden including in different socioeconomic populations; the association of ETEC infection and stunting including the stunting that may result from mild diarrhoea episodes; the long-term impact on cognition and educational attainment, and; finally, the macroeconomic consequences associated with decreased educational attainment. If these measures were included, it could have a significant impact on the cost-effectiveness and public health impact of ETEC vaccines.
6. Conduct advocacy efforts to improve country awareness and ‘pull’ (demand) for an ETEC vaccine
Endemic-country awareness of the total disease burden is fundamental to informing health-policy decisions. Policymakers in some endemic nations are unaware of the significance of ETEC and the burden of diarrhoeal illness [27]. Considering that the first vaccines maybe five to ten years from licensure, the level of awareness must improve in the near term, otherwise, vaccine uptake and utilization may be severely limited.
6.1. Assess potential impact of ETEC vaccines on antibiotic use and the development of AMR
The spread of AMR bacteria constitutes a major threat to global health. Among children in LMICs, diarrhoea is the second-leading cause of antibiotic consumption and ETEC strains producing ST or both ST and LT toxins have been shown to be a prominent cause of clinically attended, antibiotic-treated diarrhoea across all age groups over the first five years of life [55]. In general, vaccines can limit the rise of AMR by reducing the carriage and transmission of drug-resistant pathogens, and by reducing the presentation of clinical symptoms, that often lead to antibiotic use. However, the role and value of vaccines in reducing AMR, including averting the antibiotic use, has not been well studied.
Recent observations from ETEC candidate vaccine efficacy studies and CHIMs and in the field indicate that an effective vaccine can reduce the need for antibiotic intervention [2], [96]. However, critical data gaps remain to understand the impact of vaccines on the reduction of antibiotic use. The required data is rarely collected, and if so, the data collection is not standardized, not tailored to measure the impact of vaccines against AMR, and as a result, often limited in the information that it provides. There is a need to develop consensus guidelines and methodologies to measure the disease, syndrome, or pathogen associated antibiotic use in future clinical trials and research studies to inform the impact of vaccines and other interventions on antibiotic use. International convenings of experts and stakeholders are recommended to help WHO develop these guidelines.
7. Conclusions
Increases in donor and industry investment in ETEC vaccine development over the last 10 years have led to the development of a number of promising vaccine candidates in early and late-stage clinical testing. In addition, the growing appreciation of ETEC’s contribution to the pathogenic pathway contributing to more long-term negative health outcomes in infant and children in LMICs, including poor cognitive development, has increased urgency around ETEC vaccine development and led to WHO’s recent decision to retain ETEC as a priority vaccine target. Further, growing AMR concerns led to the Wellcome Trust recommending acceleration of vaccine development against enteric E. coli including ETEC.
ETEC vaccine development is likely to be successful if overall funding remains stable; however, there is a need for better diagnostics to quantify burden and increased stakeholder awareness of ETEC’s impact on infant and child health, including growth, cognitive development, and antibiotic use. Any vaccine should optimize strain coverage, and dmLT or other promising adjuvants may help ensure mucosal immunogenicity and improved efficacy in infants and young children. The anticipated uptake of an effective ETEC vaccine is unclear. Gavi, the Vaccine Alliance has indicated an interest in enteric vaccines including ETEC; however, debates are ongoing whether a standalone or multi-pathogen vaccine would be optimal. From a market assessment standpoint, ETEC is one enteric pathogen that has a recognized travel medicine market. However, it is also clear that the travel medicine industry would look more favourably on the use of a combined ETEC-Shigella-Campylobacter vaccine than a standalone product. Recent vaccine impact and cost-effectiveness estimates have also started to build an even stronger public health case for the development of an ETEC vaccine that could eventually be used together with vaccines against other enteric pathogens like Shigella, Campylobacter, cholera or Typhoid.
Finally, human-made and natural disasters, such as the COVID-19 pandemic, could have substantial negative effects on the nutritional status of infants and children, highlighting the need to protect these populations from other infections, like ETEC that negatively affect growth and development. A vaccine preventing ETEC-attributable diarrhoea could minimize negative growth and development effects in children affected by other infectious diseases and public health crises. Consequently, it is important that ETEC vaccine development be accelerated [97].
Our manuscript is not meant to discuss both recommendations for research gaps and vaccine implementation policy, but rather to point out important priorities that the authors think are urgent. This report should potentiate the focused pursuit of current options for ETEC vaccine development. In addition, it has outlined the findings that can lead to success for this pursuit. It highlights new disease burden information and provides insights to ETEC disease pathogenesis and immunity that strengthens the case for ETEC vaccine development and improves the probability of success. This report also provides developers and stakeholders with a strategy for prioritized research activities that will not only seek sustained funding but also ensure more rapid product development, licensure and global access to an effective and affordable ETEC vaccine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors want to thank of Fred Cassels, Allison Clifford, Emily Hsu and Tori Rivera for their help in editing this manuscript.
Disclaimer
The opinions expressed in this article are those of the authors and do not reflect the view of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the Department of Health and Human Services, the Department of the Navy, Department of Defense or the United States Government.
Copyright Statement
Two of the authors are U.S. Government employees (SB and CP). This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1.Vaccines_for_AMR.pdf. https://vaccinesforamr.org/wp-content/uploads/2018/09/Vaccines_for_AMR.pdf (accessed June 6, 2020).
- 2.PDVAC_ETEC_18-June_Executive-Summary.pdf. https://www.who.int/immunization/research/meetings_workshops/PDVAC_ETEC_18-June_Executive-Summary.pdf (accessed Oct 9, 2020).
- 3.PPC_ETEC_April_2020_Public_Consultation.pdf. https://www.who.int/immunization/research/ppc-tpp/PPC_ETEC_April_2020_Public_Consultation.pdf?ua=1 (accessed Dec 12, 2020).
- 4.Kosek M.N., Ahmed T., Bhutta Z. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine. 2017;18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker R., Dull P. 11. Combination vaccine strategies to prevent enteric infections. Vaccine. 2017;35:6790–6792. doi: 10.1016/j.vaccine.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 6.Laird R.M., Ma Z., Dorabawila N. 12. of a conjugate vaccine platform against enterotoxigenic Escherichia coli (ETEC), Campylobacter jejuni and Shigella. Vaccine. 2018;36:6695–6702. doi: 10.1016/j.vaccine.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry E., Cassels F., Riddle M., Walker R., Wierzba T. Vaccines Against Shigella and Enterotoxigenic Escherichia coli: A summary of the 2018 VASE Conference. Vaccine. 2019;37:4768–4774. doi: 10.1016/j.vaccine.2019.02.070. [DOI] [PubMed] [Google Scholar]
- 8.Qadri F, Khan AI, Faruque ASG, et al. 24. Enterotoxigenic Escherichia coli and Vibrio cholerae Diarrhea, Bangladesh, 2004. Emerg Infect Dis 2005; 11: 1104–7. [DOI] [PMC free article] [PubMed]
- 9.Levine M.M., Nalin D.R., Hoover D.L., Bergquist E.J., Hornick R.B., Young C.R. 38. Immunity to Enterotoxigenic Escherichia coli. Infect Immun. 1979;23:729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svennerholm A.-M., Tobias J. Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vacc. 2008;7:795–804. doi: 10.1586/14760584.7.6.795. [DOI] [PubMed] [Google Scholar]
- 11.Von Mentzer A., Connor T.R., Wieler L.H. Identification of enterotoxigenic Escherichia coli (ETEC) clades with long-term global distribution. Nat Genet. 2014;46:1321–1326. doi: 10.1038/ng.3145. [DOI] [PubMed] [Google Scholar]
- 12.Vidal R.M., Muhsen K., Tennant S.M. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS) PLoS NeglTrop Dis. 2019;13:e0007037. doi: 10.1371/journal.pntd.0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isidean S.D., Riddle M.S., Savarino S.J., Porter C.K. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. 2011;29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 14.Fleckenstein J.M., Roy K., Fischer J.F., Burkitt M. Identification of a Two-Partner Secretion Locus of Enterotoxigenic Escherichia coli. Infect Immun. 2006;74:2245–2258. doi: 10.1128/IAI.74.4.2245-2258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy K., Hamilton D., Allen K.P., Randolph M.P., Fleckenstein J.M. The EtpA Exoprotein of Enterotoxigenic Escherichia coli Promotes Intestinal Colonization and Is a Protective Antigen in an Experimental Model of Murine Infection. Infect Immun. 2008;76:2106–2112. doi: 10.1128/IAI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein J.M., Sheikh A., Qadri F. Novel Antigens for enterotoxigenic Escherichia coli (ETEC) Vaccines. Expert Rev Vacc. 2014;13:631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleckenstein J.M., Hardwidge P.R., Munson G.P., Rasko D.A., Sommerfelt H., Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikh A., Tumala B., Vickers T.J. CEACAMs serve as toxin-stimulated receptors for enterotoxigenic Escherichia coli. PNAS. 2020;117:29055–29062. doi: 10.1073/pnas.2012480117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreisberg R.B., Harper J., Strauman M.C., Marohn M., Clements J.D., Nataro J.P. Induction of increased permeability of polarized enterocyte monolayers by enterotoxigenic escherichia coli heat-labile enterotoxin. Am J Trop Med Hyg. 2011;84:451–455. doi: 10.4269/ajtmh.2011.10-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colston J.M., Francois R., Pisanic N. Effects of child and maternal histo-blood group antigen status on symptomatic and asymptomatic enteric infections in early childhood. J Infect Dis. 2019;220:151–162. doi: 10.1093/infdis/jiz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P., Kuhlmann F.M., Chakraborty S. Enterotoxigenic Escherichia coli–blood group A interactions intensify diarrheal severity. J Clin Invest. 2018;128:3298–3311. doi: 10.1172/JCI97659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mottram L., Wiklund G., Larson G., Qadri F., Svennerholm A.-M. FUT2 non-secretor status is associated with altered susceptibility to symptomatic enterotoxigenic Escherichia coli infection in Bangladeshis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-10854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victora C.G., Bahl R., Barros A.J.D. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 24.Lamberti L.M., Fischer Walker C.L., Noiman A., Victora C., Black R.E. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11:S15. doi: 10.1186/1471-2458-11-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wennerås C., Erling V. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Popul Nutr. 2004;22:370–382. [PubMed] [Google Scholar]
- 26.Troeger C., Blacker B.F., Khalil I.A. 18. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016 - The Lancet Infectious Diseases. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(18)30475-4/fulltext (accessed April 1, 2020). [DOI] [PMC free article] [PubMed]
- 28.Anderson J.D., Bagamian K.H., Muhib F. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff K.L., Nataro J.P., Blackwelder W.C. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 30.Kotloff K.L., Nasrin D., Blackwelder W.C. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS) The Lancet Global Health. 2019;7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platts-Mills J.A., Liu J., Rogawski E.T. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schorling J., Guerrant R., Moy R.J.D., Choto R., Booth I.W., Mcneish A.S. Diarrhoea and catch-up growth. The Lancet. 1990;335:599–600. doi: 10.1016/0140-6736(90)90378-i. [DOI] [PubMed] [Google Scholar]
- 33.Steiner T.S., Lima A.M., Nataro J.P., Guerrant R.L. Enteroaggregative Escherichia coli Produce Intestinal Inflammation and Growth Impairment and Cause Interleukin-8 Release from Intestinal Epithelial Cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 34.Lima A.A., Guerrant D.I., Patrick P.D., Schorling J.B., Moore S.R., Guerrant R.L. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Tropical Med Hygiene. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 35.Lorntz B., Soares A.M., Moore S.R. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 36.Guerrant R.L., DeBoer M.D., Moore S.R., Scharf R.J., Lima A.A.M. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petri W.A., Miller M., Binder H.J., Levine M.M., Dillingham R., Guerrant R.L. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorntz B., Lima A.A., Guerrant R.L. 33. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Tropical Med Hygiene. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 39.Chronic Health Consequences of Acute Enteric Infections in the Developing World - ProQuest. https://search.proquest.com/openview/a98727686cee8f24379fdb26f979e7b7/1?pq-origsite=gscholar&cbl=2041980 (accessed April 1, 2020).
- 40.Riddle M, Walker R. Persisting consequence of intestinal infection: summary of the seminar. 13.
- 41.Walker S.P., Wachs T.D., Gardner J.M. Child development: risk factors for adverse outcomes in developing countries. The Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 42.Platts-Mills J.A., Babji S., Bodhidatta L. Pathogen-specific burdens of community diarrhoea in developing countries (MAL-ED): a multisite birth cohort study. Lancet Glob Health. 2015;3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenzie R., Porter C.K., Cantrell J.A. Volunteer challenge with enterotoxigenic Escherichia coli that express intestinal colonization factor fimbriae CS17 and CS19. J Infect Dis. 2011;204:60–64. doi: 10.1093/infdis/jir220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolick D.T., Medeiros P.H.Q.S., Ledwaba S.E. Critical Role of Zinc in a New Murine Model of Enterotoxigenic Escherichia coli Diarrhea. Infect Immun. 2018;86 doi: 10.1128/IAI.00183-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Troeger C., Colombara D.V., Rao P.C. 28. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health. 2018;6:e255–e269. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 - The Lancet. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)32130-X/fulltext (accessed April 8, 2020). [DOI] [PMC free article] [PubMed]
- 47.Leroy J.L., Frongillo E.A. Perspective: What Does Stunting Really Mean? A Critical Review of the Evidence. Adv Nutrit. 2019;10:196–204. doi: 10.1093/advances/nmy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson J.D., Muhib F., Rheingans R., Wierzba T. Heterogeneity in potential impact and cost-effectiveness of ETEC and Shigella vaccination in four sub-Saharan African countries. Vaccine X. 2019;3 doi: 10.1016/j.jvacx.2019.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Case for Investment in Enterotoxigenic Escherichia coli Vaccines. https://www.path.org/resources/the-case-for-investment-in-enterotoxigenic-escherichia-coli-vaccines/ (accessed Dec 20, 2020).
- 50.Tacconelli E., Carrara E., Savoldi A. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.), 2019. doi:10.15620/cdc:82532.
- 52.ResistanceMap - Antibiotic Resistance. https://resistancemap.cddep.org/ (accessed Dec 14, 2020).
- 53.Atkins K.E., Lafferty E.I., Deeny S.R., Davies N.G., Robotham J.V., Jit M. Use of mathematical modelling to assess the impact of vaccines on antibiotic resistance. Lancet Infect Dis. 2018;18:e204–e213. doi: 10.1016/S1473-3099(17)30478-4. [DOI] [PubMed] [Google Scholar]
- 54.Bielicki J.A., Fink G. Measuring antibiotic use in children: piecing together the puzzle. The Lancet Global Health. 2020;8:e742–e743. doi: 10.1016/S2214-109X(20)30209-6. [DOI] [PubMed] [Google Scholar]
- 55.Lewnard J.A., McQuade E.T.R., Platts-Mills J.A., Kotloff K.L., Laxminarayan R. Incidence and etiology of clinically-attended, antibiotic-treated diarrhea among children under five years of age in low- and middle-income countries: Evidence from the Global Enteric Multicenter Study. PLoS NeglTrop Dis. 2020;14:e0008520. doi: 10.1371/journal.pntd.0008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasso_Prudden_AMR_PDVAC_2019.pdf. https://www.who.int/immunization/research/meetings_workshops/5_Hasso_Prudden_AMR_PDVAC_2019.pdf?ua=1 (accessed Dec 14, 2020).
- 57.Evans D.G., Silver R.P., Evans D.J., Chase D.G., Gorbach S.L. 35. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975;12:656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourgeois A.L., Wierzba T.F., Walker R.I. 9. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 59.Qadri F., Svennerholm A.-M., Faruque A.S.G., Sack R.B. 34. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourgeois A.L., Wierzba T.F., Walker R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 61.Leach S., Lundgren A., Carlin N., Löfstrand M., Svennerholm A.-M. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent enterotoxigenicEscherichia coli (ETEC) vaccine ETVAX. Vaccine. 2017;35:3966–3973. doi: 10.1016/j.vaccine.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Akhtar M., Chowdhury M.I., Bhuiyan T.R. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine. 2019;37:5645–5656. doi: 10.1016/j.vaccine.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qadri F., Akhtar M., Bhuiyan T.R. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lundgren A., Bourgeois L., Carlin N. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine. 2014;32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 65.Harutyunyan S., Neuhauser I., Mayer A. Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC. Vaccines. 2020;8:689. doi: 10.3390/vaccines8040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rollenhagen J.E., Jones F., Hall E. Establishment, validation, and application of a new world primate model of enterotoxigenic Escherichia coli disease for vaccine development. Infect Immun. 2019;87 doi: 10.1128/IAI.00634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maciel M., Bauer D., Baudier R.L. Intradermal or sublingual delivery and heat-labile enterotoxin proteins shape immunologic responses to a CFA/I fimbria-derived subunit antigen vaccine against enterotoxigenic Escherichia coli. Infect Immun. 2019;87 doi: 10.1128/IAI.00460-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker R.I., Clifford A. 13. Recommendations regarding the development of combined enterotoxigenic Eschericha coli and Shigella vaccines for infants. Vaccine. 2015;33:946–953. doi: 10.1016/j.vaccine.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W., Sack D.A. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vacc. 2012;11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 70.Nandre R.M., Duan Q., Wang Y., Zhang W. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine. 2017;35:552–556. doi: 10.1016/j.vaccine.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nandre R., Ruan X., Lu T., Duan Q., Sack D., Zhang W. Enterotoxigenic Escherichia coli Adhesin-Toxoid Multiepitope Fusion Antigen CFA/I/II/IV-3xSTaN12S-mnLTG192G/L211A-Derived Antibodies Inhibit Adherence of Seven Adhesins, Neutralize Enterotoxicity of LT and STa Toxins, and Protect Piglets against Diarrhea. Infect Immun. 2018;86 doi: 10.1128/IAI.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chakraborty S., Randall A., Vickers T.J. Interrogation of a live-attenuated enterotoxigenic Escherichia coli vaccine highlights features unique to wild-type infection. npj Vaccines. 2019;4 doi: 10.1038/s41541-019-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hazen T.H., Nagaraj S., Sen S. Genome and Functional Characterization of Colonization Factor Antigen I- and CS6-Encoding Heat-Stable Enterotoxin-Only Enterotoxigenic Escherichia coli Reveals Lineage and Geographic Variation. mSystems. 2019;4 doi: 10.1128/mSystems.00329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuhlmann F.M., Martin J., Hazen T.H. Conservation and global distribution of non-canonical antigens in Enterotoxigenic Escherichia coli. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feikin DR, Flannery B, Hamel MJ, Stack M, Hansen PM. Vaccines for Children in Low- and Middle-Income Countries. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development/The World Bank, 2016. http://www.ncbi.nlm.nih.gov/books/NBK361927/ (accessed April 7, 2020).
- 76.Church J.A., Parker E.P., Kosek M.N. Exploring the relationship between environmental enteric dysfunction and oral vaccine responses. Future Microbiol. 2018;13:1055–1070. doi: 10.2217/fmb-2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parker E.P., Ramani S., Lopman B.A. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13:97–118. doi: 10.2217/fmb-2017-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holmgren J., Bourgeois L., Carlin N. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine. 2013;31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Odevall L., Hong D., Digilio L. The Euvichol story – Development and licensure of a safe, effective and affordable oral cholera vaccine through global public private partnerships. Vaccine. 2018;36:6606–6614. doi: 10.1016/j.vaccine.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White J.A., Lal M. Technical product attributes in development of an oral enteric vaccine for infants. Vaccine. 2019;37:4800–4804. doi: 10.1016/j.vaccine.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 81.WHO | Principles and considerations for adding a vaccine to a national immunization programme. WHO. http://www.who.int/entity/immunization/programmes_systems/policies_strategies/vaccine_intro_resources/nvi_guidelines/en/index.html (accessed April 7, 2020).
- 82.Holmgren J., Parashar U.D., Plotkin S. Correlates of protection for enteric vaccines. Vaccine. 2017;35:3355–3363. doi: 10.1016/j.vaccine.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peltola H., Siitonen A., Kataja M.J. Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. The Lancet. 1991;338:1285–1289. doi: 10.1016/0140-6736(91)92590-x. [DOI] [PubMed] [Google Scholar]
- 84.Glenn G.M., Francis D.H., Danielsen E.M. Toxin-mediated effects on the innate mucosal defenses: implications for enteric vaccines. Infect Immun. 2009;77:5206–5215. doi: 10.1128/IAI.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walters W.A., Reyes F., Soto G.M. Epidemiology and associated microbiota changes in deployed military personnel at high risk of traveler’s diarrhea. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leonard M.M., Karathia H., Pujolassos M. Multi-omics analysis reveals the influence of genetic and environmental risk factors on developing gut microbiota in infants at risk of celiac disease. Microbiome. 2020;8:130. doi: 10.1186/s40168-020-00906-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medeiros P.H.Q.S., Bolick D.T., Ledwaba S.E. A bivalent vaccine confers immunogenicity and protection against Shigella flexneri and enterotoxigenic Escherichia coli infections in mice. npj Vaccines. 2020;5:1–5. doi: 10.1038/s41541-020-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malek M.A., Teleb N., Abu-Elyazeed R. The epidemiology of rotavirus Diarrhea in Countries in the Eastern Mediterranean Region. J Infect Dis. 2010;202:S12–S22. doi: 10.1086/653579. [DOI] [PubMed] [Google Scholar]
- 89.Lalani T., Tisdale M.D., Liu J. Comparison of stool collection and storage on Whatman FTA Elute cards versus frozen stool for enteropathogen detection using the TaqMan Array Card PCR assay. PLoS One. 2018;13:e0202178. doi: 10.1371/journal.pone.0202178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taniuchi M, Islam K, Sayeed MA, et al. Etiology of Diarrhea Requiring Hospitalization in Bangladesh by Quantitative Polymerase Chain Reaction, 2014–2018. Clin Infect Dis 2020; published online June 27. doi:10.1093/cid/ciaa840. [DOI] [PMC free article] [PubMed]
- 91.Global Tuberculosis Programme. The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. 2016 http://www.ncbi.nlm.nih.gov/books/NBK384520/ (accessed Dec 17, 2020). [PubMed]
- 92.Ganguli A., Mostafa A., Berger J. Rapid isothermal amplification and portable detection system for SARS-CoV-2. PNAS. 2020;117:22727–22735. doi: 10.1073/pnas.2014739117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker R.I. New vaccines against enteric bacteria for children in less developed countries. Expert Rev Vacc. 2005;4:807–812. doi: 10.1586/14760584.4.6.807. [DOI] [PubMed] [Google Scholar]
- 94.McArthur M.A., Chen W.H., Magder L., Levine M.M., Sztein M.B. Impact of CD4+ T Cell Responses on Clinical Outcome following Oral Administration of Wild-Type Enterotoxigenic Escherichia coli in Humans. PLoS NeglTrop Dis. 2017;11:e0005291. doi: 10.1371/journal.pntd.0005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ndungo E., Randall A., Hazen T.H. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere. 2018;3 doi: 10.1128/mSphere.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harro C., Louis Bourgeois A., Sack D. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine. 2019;37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fore H.H., Dongyu Q., Beasley D.M., Ghebreyesus T.A. Child malnutrition and COVID-19: the time to act is now. The Lancet. 2020;396:517–518. doi: 10.1016/S0140-6736(20)31648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]