Key words: Besnoitia besnoiti, Ezetimibe, Neospora caninum, NPC1L1, Toxoplasma gondii
Coccidia are obligate apicomplexan parasites that affect humans and animals. In fast replicating species, in vitro merogony takes only 24–48 h. In this context, successful parasite proliferation requires nutrients and other building blocks. Coccidian parasites are auxotrophic for cholesterol, so they need to obtain this molecule from host cells. In humans, ezetimibe has been applied successfully as hypolipidaemic compound, since it reduces intestinal cholesterol absorption via blockage of Niemann−Pick C-1 like-1 protein (NPC1L1), a transmembrane protein expressed in enterocytes. To date, few data are available on its potential anti-parasitic effects in primary host cells infected with apicomplexan parasites of human and veterinary importance, such as Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti. Current inhibition experiments show that ezetimibe effectively blocks T. gondii, B. besnoiti and N. caninum tachyzoite infectivity and replication in primary bovine endothelial host cells. Thus, 20 μm ezetimibe blocked parasite proliferation by 73.1−99.2%, via marked reduction of the number of tachyzoites per meront, confirmed by 3D-holotomographic analyses. The effects were parasitostatic since withdrawal of the compound led to parasite recovery with resumed proliferation. Ezetimibe-glucuronide, the in vivo most effective metabolite, failed to affect parasite proliferation in vitro, thereby suggesting that ezetimibe effects might be NPC1L1-independent.
Introduction
Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti are cyst-forming species belonging to the Apicomplexa phylum, which consists of a large group of obligatory intracellular protozoan parasites that affect both humans and animals. Despite morphological similarities between coccidian species, host specificity and clinical consequences greatly differ among them. In this context, T. gondii is considered a major public health problem and an abortive agent especially in ovines (Benavides et al., 2017) and humans (Nayeri et al., 2020). The closely related coccidian parasite N. caninum is currently considered as a major cause of abortions in cattle (Reichel et al., 2013). In contrast, B. besnoiti causes bovine besnoitiosis, an emerging disease within Europe, which is characterized by massive alterations of skin and mucosas and also bull infertility (Alvarez-Garcia et al., 2013).
During the acute stage of infection, coccidian parasites undergo asexual replication within host cells. In this context, host endothelial cells have shown high permissiveness for tachyzoite infection and proliferation in vivo (Alvarez-Garcia et al., 2013; Konradt et al., 2016). Likewise, primary bovine endothelial cells have consistently been reported as suitable for in vitro replication of T. gondii, N. caninum and B. besnoiti (Taubert et al., 2006, 2016; Silva et al., 2019; Velásquez et al., 2019), allowing high tachyzoite proliferation rates in an experimental set up close to the in vivo scenario. During the fast proliferation phase, tachyzoites need significant amounts of nutrients for offspring development, which may be obtained from the host cell or newly synthesized. Specifically during coccidian replication high amounts of cholesterol are needed for new membrane biosynthesis (Coppens, 2013). Given that apicomplexan parasites are considered auxotrophic for cholesterol (Coppens, 2013), their replication within the parasitophorous vacuole (PV) highly depends on cholesterol supply by the host cell. In general, cellular cholesterol supply may be achieved either by enhancement of cellular endogenous de novo biosynthesis or by an increased cholesterol uptake from extracellular sources (Luo et al., 2020). In line, apicomplexan parasites can differentially exploit cholesterol sources depending on host cell type and parasite species. LDL internalization appears the main pathway for cholesterol uptake, and cholesterol esterification allows for storage in lipid-rich organelles (Luo et al., 2020). Recently, LDL-mediated cholesterol incorporation was described as pivotal, but not exclusive mechanism to fulfil cholesterol requirement during fast replicating coccidia proliferation (Nolan et al., 2015; Silva et al., 2019).
Based on pathophysiological consequences of human hyperlipidaemia, several pharmacological lipid-lowering compounds have been developed (Barter and Rye, 2016). Amongst these, ezetimibe is one of the most common hypolipidaemic drugs, which is capable of reducing intestinal cholesterol absorption by its interaction with Niemann−Pick C-1 like-1 protein (NPC1L1) in enterocytes (Davis et al., 2004; Garcia-Calvo et al., 2005). In detail, ezetimibe binds to NPC1L1, resulting in the blockage of NPC1L1 endocytosis into clathrin-coated vesicles and thereby diminishing cholesterol internalization into enterocytes (Ge et al., 2008; Wang et al., 2009). Despite that, the participation of other potential ezetimibe targets as the class B type 1 scavenger receptor (SR-BI) and the aminopeptidase N (CD13) have been linked to its hypolipidaemic effect (Kramer et al., 2005; Labonté et al., 2007). In general, the efficacy and safety of ezetimibe has been reported in mice and human studies (Bays et al., 2001; van Heek et al., 2001). As such, ezetimibe might represent a promising anti-parasitic drug candidate (Andrade-Neto et al., 2016). In line, ezetimibe treatments significantly reduced Cryptosporidium parvum growth in Caco-2 cells (Ehrenman et al., 2013). However, other evidences are incongruent: whilst ezetimibe reduced the parasite burden of Leishmania amazonensis in vivo, and diminished the L. infantum replication in vitro and in vivo (alone or in binary and ternary combination with miltefosine and itraconazole) (Andrade-Neto et al., 2016, 2021), this treatment did not affect Plasmodium yoelii parasitaemia in mice, while reduced the intraerythrocytic proliferation of P. falciparum in vitro (Kume et al., 2016; Hayakawa et al., 2021), thereby suggesting parasite-specific effects for this compound.
So far, no data are available on the impact of this drug on typical fast replicating coccidian parasites. Therefore, the aim of this study was to evaluate anti-parasitic efficacy of ezetimibe in T. gondii, N. caninum and B. besnoiti-infected primary bovine host endothelial cells.
Materials and methods
Host cell culture
Primary bovine umbilical vein endothelial cells (BUVEC) were isolated as described elsewhere (Taubert et al., 2006). BUVEC were cultured at 37°C and 5% CO2 atmosphere in modified endothelial cell growth medium (modECGM), by diluting ECGM medium (Promocell®) with M199 (Sigma-Aldrich) at a ratio of 1:3, supplemented with 500 U/mL penicillin (Sigma-Aldrich) and 50 μg/mL streptomycin (Sigma-Aldrich) and 5% FCS (foetal calf serum; Biochrom). BUVEC of less than three passages were used in this study.
Parasites
Toxoplasma gondii (strain RH) and Neospora caninum (strain NC-1) tachyzoites were cultivated in vitro as described elsewhere (Taubert et al., 2006; Velásquez et al., 2019), by maintaining them at several passages in permanent African green monkey kidney epithelial cells (MARC 145) in Dubelcco's modified eagle medium (DMEM) (Sigma-Aldrich). Besnoitia besnoiti (strain Bb Evora04) tachyzoite stages were propagated in Madin−Darby bovine kidney cells (MDBK) (Velásquez et al., 2020) in Roswell Park Memorial Institute (RPMI) medium (Sigma-Aldrich). All culture media were supplemented with 500 U/mL penicillin and 50 μg/mL streptomycin and 5% foetal calf serum (FCS; Sigma-Aldrich). Infected and non-infected cells were cultured at 37°C and 5% CO2 atmosphere. Vital tachyzoites were collected from supernatants of infected host cells (800 × g; 5 min) and re-suspended in modECGM for further experiments.
For infection rate-related experiments, tachyzoites of each species were pre-incubated in 20 μm ezetimibe for 1 h. After washing in modECGM (800 × g; 5 min), tachyzoites were used for infection experiments.
Treatments of host cells and infections
BUVEC (n = 5) were seeded in 12-well plates (Sarstedt) pre-coated with fibronectin (1:400; Sigma-Aldrich). Ezetimibe (Cayman Chemical) and ezetimibe-glucuronide (Santa Cruz Biotechnology) stock solutions were prepared in dimethyl sulphoxide (DMSO; Sigma-Aldrich, 33 mm), diluted in modECGM at 2.5, 5, 10 and 20 μm and administered to fully confluent cell monolayers 48 h before infection. ModECGM with DMSO (0.06%) served as vehicle control. Following pre-treatments, the medium was entirely removed and cells were infected with tachyzoites of T. gondii, B. besnoiti or N. caninum at a multiplicity of infection of 1:5 for 4 h under inhibitor-free conditions. Then, extracellular tachyzoites were removed and fresh medium with inhibitors was re-administered. At 4 h post infection (p. i.), phase-contrast images for infection rate estimation [(infected cells/total cells) × 100] were acquired by an inverted microscope (IX81, Olympus®) equipped with a digital camera (XM10, Olympus®). Tachyzoites present in cell culture supernatants were collected (800 × g; 5 min) at 48 h p. i. and counted in a Neubauer chamber.
Additionally, withdrawal experiments were carried out. Therefore, ezetimibe-containing medium was replaced by control medium at 24 h p. i., and parasite replication was estimated 24 h later (n = 5). Finally, further assays were performed to estimate the effect of ezetimibe over time as cells were treated as described above with a daily replacement of medium containing ezetimibe (20 μm) at 24, 48 and 72 h p. i., and tachyzoite proliferation was observed at 48, 72 and 96 h p. i., respectively (n = 5).
Live cell 3D holotomographic microscopy to illustrate parasite development
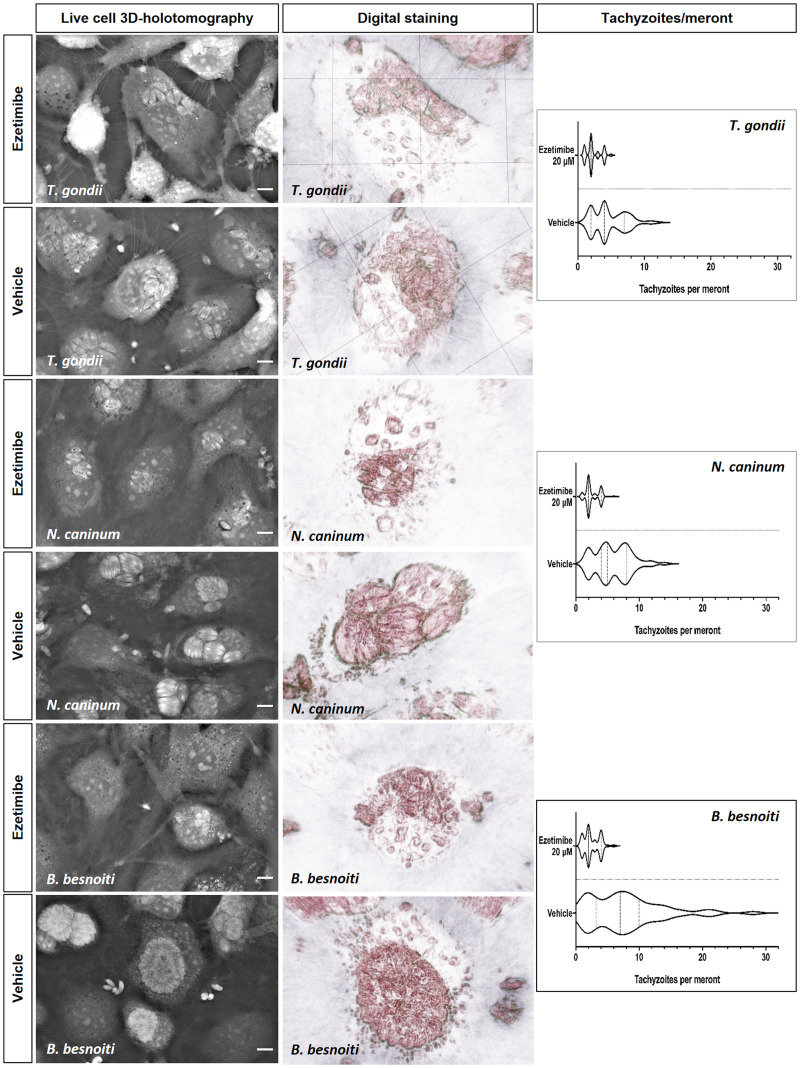
BUVEC were seeded into 35 mm tissue culture μ-dishes (Ibidi®) and cultured (37°C, 5% CO2) until confluence. Ezetimibe treatment (20 μm) was performed as described above. Thereafter, T. gondii, N. caninum and B. besnoiti tachyzoites were used to infect cell layers (MOI = 3:1). At 24 h p. i., holotomographic images were obtained by using 3D Cell-Explorer-fluo microscope (Nanolive) equipped with a 60 × magnification (λ = 520 nm, sample exposure 0.2 mW/mm2) and a depth of field of 30 μm. Images were analysed using STEVE software (Nanolive) to obtain refractive index (RI)-based z-stacks (Silva et al., 2019). Additionally, digital staining was applied according to the RI of intracellular tachyzoites. Finally, intracellular meront development was evaluated by counting intra-meront tachyzoites in at least six 3D holotomographic z-stacks of infected host cells ( = 50 cells per condition) in presence or absence of ezetimibe (20 μm).
RT-qPCR for relative quantification of NPC1L1 mRNA
BUVEC (n = 5) grown in 25 cm2 culture tissue flasks (Greiner Bio-One) were infected with T. gondii, N. caninum or B. besnoiti tachyzoites (MOI = 5:1). Infected- and non-infected host cells were processed for total RNA isolation at four different time points after infection (3, 6, 12, 24 h p. i.). Tissue samples from bovine small intestine obtained at a local slaughterhouse were used as positive controls for NPC1L1. For total RNA isolation, the RNeasy kit (Qiagen) was used according to the manufacturer's instructions. Total RNAs were stored at −80°C until further use. In order to remove any genomic DNA leftover, DNA digestion step was performed. Therefore, 1 μg of total RNA was treated with 10 U DNase I (Thermo Scientific) in 1× DNase reaction buffer (37°C, 30 min). DNase was inactivated by heating the samples (65°C, 10 min). The efficiency of genomic DNA digestion was confirmed by no-RT-controls in each RT-qPCR experiment. cDNA synthesis was performed using the SuperScript IV (InvitrogenTM) according to the manufacturer's instructions. Briefly, for first-strand cDNA synthesis, 1 μg of DNase treated total RNA was added to 0.5 μL of 50 μm oligo(dt), 1 μL of 50 ng/μL random hexamer primer, 1 μL of 10 mm dNTP mix in a total volume of 10 μL. Thereafter, the samples were incubated at 65°C for 5 min and then immediately cooled on ice. Additionally, 4 μL of 5× SSIV buffer, 1 μL 0.1 m DTT, 1 μL RNAse free H2O and 0.5 μL SuperScript IV enzyme were added obtaining a total volume of 20 μL. The samples were incubated at 23°C for 10 min followed by 50°C for 10 min and an 80°C inactivation step for 10 min.
Probes were labelled at the 5′-end with a reporter dye FAM (6-carboxyfluorescein) and at the 3′-end with the quencher dye TAMRA (6-carboxytetramethyl-rhodamine). bNPC1L1 primer sequences were designed as follows: Bos taurus NPC1L1 forward 5′- CTTCCCTGATATGTCTTAC −3′; reverse 5′- GACCAGAGATATAAAGGC-3′ probe AGCCAGTCAATGAAGTCGTCCA. qPCR amplification was performed on a Rotor-Gene Q Thermocycler (Qiagen) in duplicates in a 10 μL total volume containing 400 nm forward and reverse primers, 200 nm probe, 10 ng cDNA and 5 μL 2× PerfeCTa qPCR FastMix (Quanta Biosciences). The reaction conditions were as follows: 95°C for 10 min, 40 cycles at 95°C for 10 s, 60°C for 15 s and 72°C for 30 s. No-template controls and no-RT reactions were included in each experiment. As reference gene GAPDH was used as previously reported (Taubert et al., 2006; Hamid et al., 2014; Hamid et al., 2015).
Viability assessment
For experiments on parasite viability, 5 × 105 tachyzoites of each parasite species were treated for 1 h with vehicle (DMSO 0.06%) or ezetimibe (20 μm) (37°C, 5% CO2). Thereafter, viability of tachyzoites was determined by the trypan blue (Sigma-Aldrich®) exclusion staining assay as described elsewhere (Cervantes-Valencia et al., 2019). Non-stained parasites were considered as viable. Additionally, cell viability after compound treatments was assessed by the colorimetric XTT test (Promega®) according to the manufacturer instructions. Briefly, BUVEC seeded in 96-well plate (Greiner) were incubated with DMSO, ezetimibe or ezetimibe-glucuronide (both 20 μm) in a total volume of 50 μL for 72 h. Thereafter, 50 μL of XTT working solution was added, and samples were incubated for 4 h (37°C, 5% CO2 atmosphere). The resulting formazan product was estimated via optical density (OD) measurements at 590 nm and reference filter 620-nm wavelength using VarioskanTM Flash Multimode Reader (Thermo Scientific).
Statistical analysis
For statistical analyses, the statistical software GraphPad® Prism 8 (version 8.4.3.) was used. Data description was performed by presenting arithmetic mean ± standard deviation. In addition, the non-parametric statistical test Mann−Whitney for comparison of two experimental conditions was applied. In cases of three or more conditions, Kruskal−Wallis test was used. Whenever global comparison by Kruskal−Wallis test indicated significance, post hoc multiple comparison tests were carried out by Dunn tests to compare test with control conditions. Outcomes of statistical tests were considered to indicate significant differences when P ⩽ 0.05 (significance level).
Results
Ezetimibe treatments effectively block T. gondii, N. caninum and B. besnoiti tachyzoite proliferation
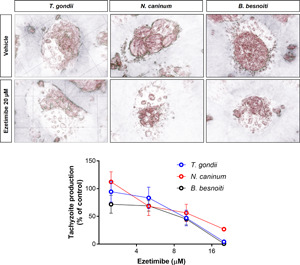
To analyse the effects of ezetimibe on intracellular tachyzoite replication, functional inhibition experiments were performed, thereby evaluating the number of freshly released tachyzoites at 48 h p. i. from cells pre-treated and exposed to ezetimibe during the intracellular parasite proliferation stage. Overall, ezetimibe treatments significantly inhibited tachyzoite replication of T. gondii (10 μm, P = 0.0397; 20 μm, P = 0.0010; Figure 1A), N. caninum (20 μm, P = 0.0078; Figure 1B) and B. besnoiti (10 μm, P = 0.0059; 20 μm, P < 0.0001; Figure 1C) in BUVEC in a dose-dependent manner. Overall, the strongest effect of ezetimibe treatments at 20 μm was observed in case of B. besnoiti (99.2 ± 0.5% replication reduction), followed by T. gondii (95.7 ± 2.3% reduction) and N. caninum (73.1 ± 2.8% reduction). In line, phase-contrast microscopy showed an impairment in meront development for T. gondii- (Fig. 1A1 and 1A2), N. caninum- (Fig. 1B1 and 1B2) and B. besnoiti- (Fig. 1C1 and 1C2) infected BUVEC at 24 h p. i. To better visualize ezetimibe-based effects on parasite development, additionally live cell 3D holotomographic microscopy were performed. As illustrated in Fig. 2, treatments with ezetimibe led to reduced meront sizes in T. gondii, N. caninum and B. besnoiti infections (Fig. 2), without apparently affecting the morphology of non-infected host cells (data not shown). Additionally, the number of tachyzoites per PV was determined to better understand ezetimibe-derived impact on parasite development (Fig. 2). Ezetimibe treatments markedly reduced the number of tachyzoites per meront in all three parasite species (all: P < 0.0001), however, the strongest effect was observed for B. besnoiti, with a reduction of 68.2% on the mean number of tachyzoites per meront, followed by T. gondii and N. caninum showing more than 50% reduction (56.5% and 50.2%, respectively).
Fig. 1.
Ezetimibe treatments inhibit T. gondii, N. caninum and B. besnoiti tachyzoite proliferation in primary endothelial cells. BUVEC were treated with ezetimibe (2.5, 5, 10 and 20 μm) 48 h before (A) T. gondii, (B) N. caninum or (C) B. besnoiti infection (MOI 1:5). 48 h after infection, the number of tachyzoites present in cell culture supernatants were counted (A–C). Exemplary illustration of T. gondii (A1−A2) N. caninum (B1−B2) or B. besnoiti (C1−C2) meront development at 24 h post infection. Scale bar represents 5 μm. Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
Fig. 2.
Ezetimibe treatment affects intracellular meront formation and reduces the number of T. gondii, N. caninum and B. besnoiti intra-meront tachyzoites. Ezetimibe-pretreated BUVEC were infected with T. gondii, N. caninum and B. besnoiti tachyzoites and live cell 3D holotomographic microscopy was performed at 24 h p. i. Digital staining was achieved via STEVE software analysis. Violin plots depict the distribution of absolute T. gondii, N. caninum and B. besnoiti tachyzoite number per meront.
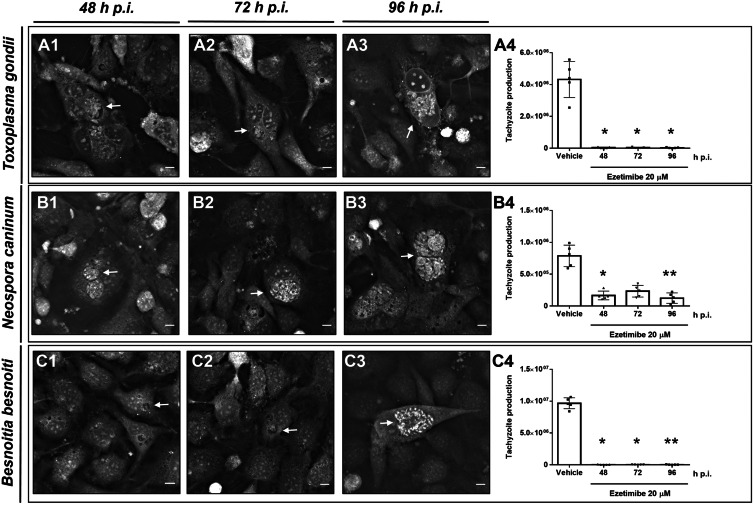
Fast replicating coccidian fulfil their replication cycle within 36–48 h p. i. in BUVEC layers in vitro (Taubert et al., 2006; Silva et al., 2019; Velásquez et al., 2019). In this context, the sustained inhibitory effect of ezetimibe over time was evaluated by counting tachyzoite production daily at 48, 72 and 92 h p. i. As depicted in Fig. 3, ezetimibe (20 μm) effectively blocked T. gondii (99.1 ± 0.0% reduction; A1−A4), N. caninum (75.9 ± 7.6% reduction; B1−B4) and B. besnoiti (99.6 ± 0.1% reduction; C1–C4) replication over time (48, 72 and 96 h p. i.).
Fig. 3.
Ezetimibe blocks T. gondii, N. caninum and B. besnoiti tachyzoite proliferation over time. Effect of daily ezetimibe treatments on tachyzoite proliferation over time: BUVEC were treated with ezetimibe (20 μm) 48 h before infection and then infected with T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. Exemplary live cell 3D holotomographic illustration of T. gondii (A1−A3) N. caninum (B1−B3) or B. besnoiti (C1−C3) meront development (arrows) at 48, 72 and 96 h p. i., respectively. At 48, 72 and 96 h p. i., the number of tachyzoites present in cell culture supernatants were counted (A4, B4, C4). Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
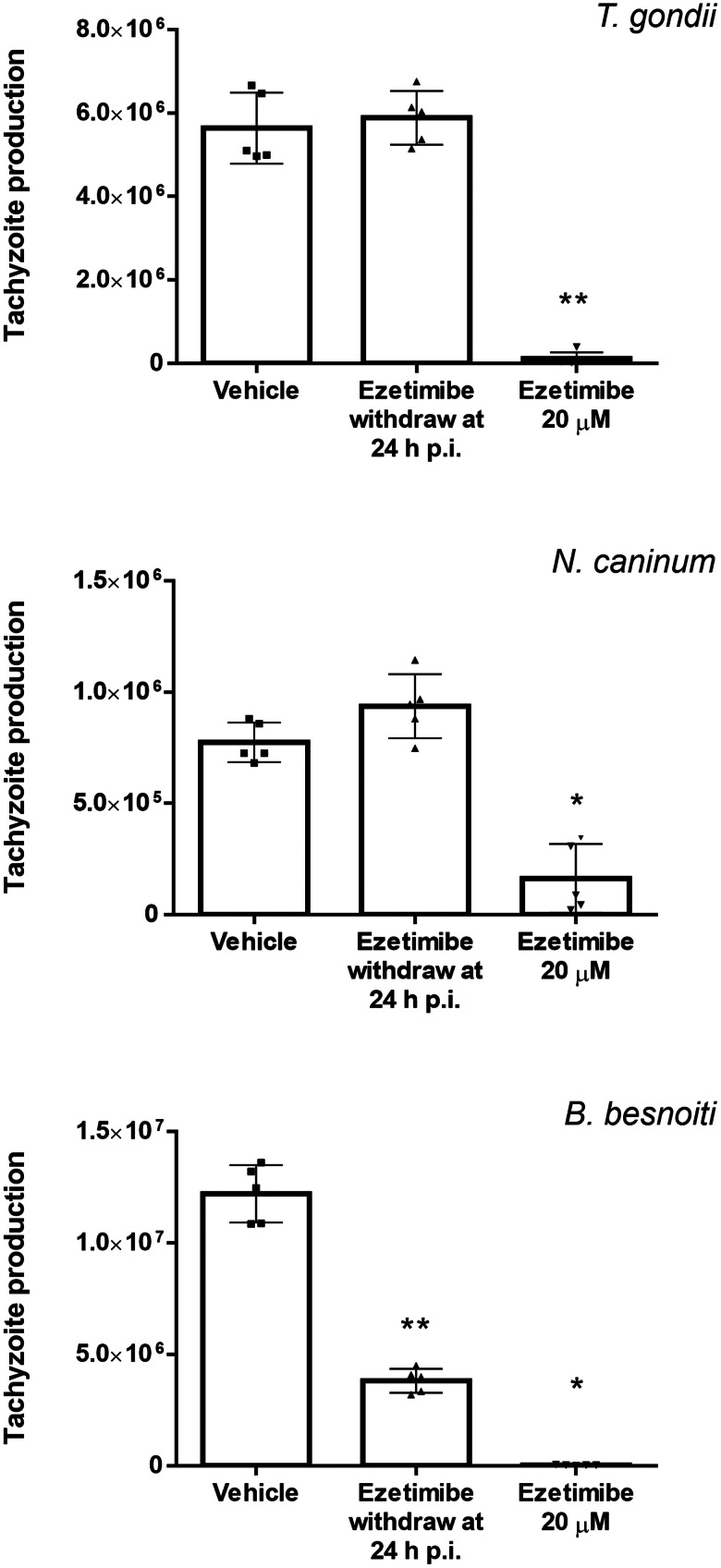
To estimate whether ezetimibe induces either parasitostatic or parasitocidal effects, compound withdrawal experiments were performed at 24 h p. i. As illustrated in Fig. 4, remnant T. gondii and N. caninum tachyzoites quickly recovered and regained proliferative capacities 24 h after ezetimibe withdrawal. In contrast, B. besnoiti proved more sensitive for ezetimibe treatments showing an ongoing reduction (31.6 ± 5.3%) of tachyzoite production when compared to non-treated cells (P = 0.15).
Fig. 4.
Ezetimibe withdrawal restores T. gondii and N. caninum tachyzoite replication but hardly affects B. besnoiti recovery. Ezetimibe-treated BUVEC were infected with T. gondii, N. caninum and B. besnoiti tachyzoites. At 24 h p. i., ezetimibe was removed from cultures and tachyzoites present in supernatants 24 h after withdrawal were counted. Bars represent means of five biological replicates, standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
Ezetimibe treatments reduce tachyzoite infectivity but fail to affect host cell permissiveness
To fulfil intracellular replication tachyzoites must first actively invade the host cells. To determine if anti-parasitic effects of ezetimibe also relied on reduced infection rates, both compartments, i. e. host cells and parasites, were separately treated with ezetimibe and then tested for infection rates 4 h after infection. Therefore, BUVEC were pre-treated with ezetimibe for 48 h before infection. At 4 h p. i. non-treated control cells presented an infection rate of 50.2% (Fig. 5A), 51.8% (Fig. 5B) and 47.0% (Fig. 5C), for T. gondii, N. caninum and B. besnoiti, respectively. In pre-treated cells, similar infection rates were observed for each parasite species (Fig. 5A–C), thereby denying any effect of ezetimibe pre-treatments. In contrast, ezetimibe pre-treatments of fresh tachyzoites significantly reduced invasive capacities of T. gondii (P = 0.0079; Figure 5A), N. caninum (P = 0.0159; Figure 5B) and B. besnoiti (P = 0.0079; Figure 5C) tachyzoites, when compared to non-treated control stages. Here, species-dependent effects were observed since the impact of ezetimibe pre-treatments were more prominent in case of B. besnoiti (28.1% reduction) than in T. gondii (22.2% reduction) or N. caninum (17.3% reduction).
Fig. 5.
Ezetimibe treatment affects the infection capacity of T. gondii, N. caninum and B. besnoiti tachyzoites. Non-treated or ezetimibe-treated BUVEC were infected with ezetimibe-treated or non-treated T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. After 4 h, infection rates were estimated. Bars represent infection rate mean of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01.
Ezetimibe glucuronidation causes loss of anti-parasitic efficacy
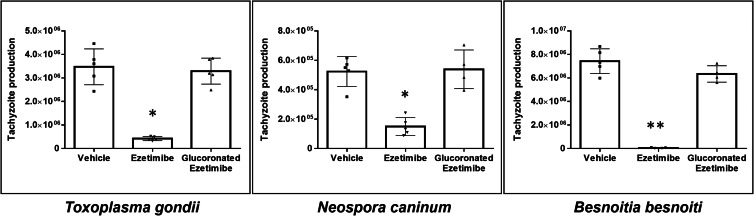
Ezetimibe-glucuronide is the major and pharmacologically active metabolite of ezetimibe following in vivo liver biotransformation. Thus, a functional assay to evaluate the effect of this chemically modified molecule on tachyzoite proliferation in vitro was performed (Fig. 6). Here, only ezetimibe but not its glucuronated derivative led to a reduction of T. gondii, N. caninum nor B. besnoiti tachyzoite proliferation.
Fig. 6.
Ezetimibe-mediated anti-parasitic effects are abolished by glucoronation. Ezetimibe- or ezetimibe-glucoronide-pre-treated BUVEC were infected with T. gondii (A), N. caninum (B) and B. besnoiti (C) tachyzoites. 48 h after infection, the number of tachyzoites present in cell culture supernatants were counted. Bars represent means of five biological replicates ± standard deviation. * P ⩽ 0.05; ** P ⩽ 0.01; *** P ⩽ 0.001; **** P ⩽ 0.0001.
NPC1L1 gene is inconsistently transcribed in T. gondii-, N. caninum- and B. besnoiti-infected BUVEC
Given that NPC1L1 is described as the main target of ezetimibe in humans, the profile of gene transcription of NPC1L1 was estimated over infection kinetics (3–24 h p. i.) on T. gondii-, N. caninum- and B. besnoiti-infected BUVEC by qRT-PCR. Using bovine small intestine tissue samples, the functionality of the qPCR system was proved. However, infection-related data showed that neither T. gondii-, N. caninum- and B. besnoiti-infected BUVEC nor non-infected controls have a reliable amplification of NPC1L1 mRNAs. Specifically, as illustrated in supplementary Table 1, NPC1L1 was not detected in a consistent manner, thereby showing amplification only in some of the replicates at a rather high threshold cycle (CT > 30). Consequently, no infection-driven effect on NPC1L1 gene transcription was assumed.
Treatments does not cause cytotoxic damage to host cells or tachyzoites
To evaluate if ezetimibe (20 μ) treatment induced tachyzoite dead trypan blue exclusion test was performed. Our data showed an average viability of 95.2% ± 1.3, 90.7% ± 1.9 and 93.7% ± 2.1 for T. gondii, N. caninum and B. besnoiti tachyzoites treated for 1 h with vehicle control (DMSO 0.06%) without significant effects provoked by ezetimibe (Fig. S1 A−C). Moreover, the cytotoxicity of ezetimibe or ezetimibe-glucuronide on endothelial host cells XTT test was performed. As illustrated in Fig. S1 D, treatments with ezetimibe or ezetimibe-glucuronide did not induce significant colorimetric changes in the formazan product compared to the vehicle control (DMSO 0.06%).
Discussion
Cholesterol is a major component of eukaryotic cell membranes (Luo et al., 2020). Given that apicomplexan parasites are generally considered as defective in cholesterol synthesis, they need to obtain this molecule from the host cell. Thus, LDL-driven cholesterol uptake is considered as a key pathway to fulfil cholesterol requirements during parasite merogony in different parasite species (Labaied et al., 2011; Coppens, 2013; Ehrenman et al., 2013; Hamid et al., 2014; Hamid et al., 2015; Nolan et al., 2015; Taubert et al., 2018; Silva et al., 2019). Additionally, in case of C. parvum-infected Caco-2 cells, cholesterol is incorporated via NPC1L1-mediated micellar uptake (Ehrenman et al., 2013). NPC1L1 is a trans-membrane protein highly expressed in enterocytes that mediates sterol internalization via clathrin-coated vesicles and it has widely been accepted as main target of the lipid-lowering drug ezetimibe (Altmann et al., 2004; Garcia-Calvo et al., 2005; Betters and Yu, 2010).
Current data demonstrate for the first time that ezetimibe has inhibitory effects on T. gondii, N. caninum, and B. besnoiti tachyzoite replication in primary host endothelial cells, i.e. a host cell type that is parasitized in vivo during the acute phase of toxoplasmosis, neosporosis and besnoitiosis (Maley et al., 2003; Alvarez-Garcia et al., 2013; Konradt et al., 2016). Here, 10 μm ezetimibe treatment effectively blocked T. gondii and B. besnoiti proliferation, while N. caninum revealed less sensitive and inhibition needed a higher concentration of 20 μm. However, both concentrations are in range or even lie below the concentration known to block effectively NPC1L1 endocytosis (25–100 μm, Ehrenman et al., 2013). Applying 20 μm ezetimibe as effective concentration to all species studied, the anti-parasitic effects of this compound over time were explored. Its inhibitory effect on tachyzoite proliferation was consistent over time, since the number of newly released tachyzoites at 48, 72 and 96 h p. i. was consistently low. Thus, an overall reduction of tachyzoite production of 95.7, 73.1 and 99.2% was obtained for T. gondii, N. caninum and B. besnoiti, respectively. These findings in principle match data from C. parvum-infected Caco-2 cells (permanent cell line), where a growth reduction of 65% was achieved at 25–100 μm concentrations (Ehrenman et al., 2013). In vitro anti-parasitic efficacy of ezetimibe was also reported for L. amazonensis where 10 μm ezetimibe reduced promastigote replication, however, amastigote production was only affected at double doses (20 μm; Andrade-Neto et al., 2016). This stage-specific discrepancy might be explained by a lower sensitivity of intracellular stages to ezetimibe treatments. Still, the concentrations here used do not necessarily support this assumption, since high effects were found at 10 μm ezetimibe in case of T. gondii and B. besnoiti-infected BUVEC. Thus, stage- and species-related or even host cell type-related sensitivities may play a role. In line, P. falciparum-merozoite replication was effectively blocked only at much higher concentrations of 80 μm ezetimibe in vitro (Hayakawa et al., 2020).
Successful parasite offspring formation relies on several defined processes starting with active cell invasion, formation of the PV, replication and egress (Black and Boothroyd, 2000). In this context, ezetimibe pre-treated extracellular tachyzoites showed impaired infection capacities, thereby hampering the replication process at the starting point. Of note, B. besnoiti tachyzoites appeared more sensitive to this treatment than T. gondii and N. caninum tachyzoites, the latter of which were hardly affected in their host cell invasion capacity.
A more detailed analysis of parasite intracellular development revealed an altered morphology of meronts in the case of all three parasites in treated host cells, suggesting that prolonged ezetimibe exposition indeed affected tachyzoite physiology, thereby reducing or hampering their ability to proliferate. Residual effects of ezetimibe on in vitro tachyzoite replication were evaluated by drug-withdrawal experiments. Notably, T. gondii and N. caninum tachyzoites recovered within 24 h post withdrawal and proliferated at normal replication rates thereby showing that ezetimibe mainly induced developmental arrest but did not kill the parasites. In contrast, B. besnoit tachyzoites suffered more profoundly from ezetimibe treatments since drug removal did not result in full recovery of parasite replication. These reactions indeed indicated species-specific sensitivity towards ezetimibe.
Overall, it is challenging to dissect if ezetimibe exclusively affects the parasites and/or the host cells, or if cumulative effects are to be considered. However, the current data showed that pre-treatments of host cells did not affect infection permissiveness since infection rates were similar in treated and control cells, when using non-treated tachyzoites for infection. Nevertheless, ezetimibe treatment of tachyzoites before host cell invasion led to a significant reduction of infection rates, suggesting that ezetimibe also directly acts on tachyzoite invasive capacity in a rather species-dependent manner. Besides this mode of action, anti-parasitic activity of ezetimibe was also associated with inhibition of parasite replication within PV. By using live cell 3D holotomographic microscopy as a reliable tool for 3D cell visualization in vivo (Silva et al., 2019; Velásquez et al., 2019), we showed that ezetimibe-treated host cells infected with T. gondii, N. caninum and B. besnoiti presented a reduced number of tachyzoites per meront. Summarizing these data, ezetimibe might act on both, extra- and intracellular tachyzoites.
Hypolipidaemic/cholesterol-lowering properties of ezetimibe have previously been reported for humans as well as animals (Bays et al., 2001; van Heek et al., 2001; Knopp et al., 2003). In vivo, this compound undergoes phase II metabolism to form a glucuronide conjugate, thereby improving NPC1L1-specific affinity and binding capacities (Garcia-Calvo et al., 2005). Besides being the major metabolite detected in plasma (Garcia-Calvo et al., 2005), ezetimibe-glucuronide is therefore considered as main active metabolite in ezetimibe treatments in vivo. To parallel in vivo situation, additional studies on the effect of ezetimibe-glucuronide on T. gondii, B. besnoiti and N. caninum tachyzoite proliferation in vitro were performed. Unexpectedly, treatments with ezetimibe-glucuronide failed to hamper intracellular tachyzoite replication thereby implicating that ezetimibe-mediated anti-parasitic effects might be NPC1L1-independent. In line, we were not able to demonstrate a consistent infection-driven induction of NPC1L1 mRNAs since these gene transcripts could hardly be detected in infected BUVEC or control cells even though intestinal control tissues gave good PCR signals. Consequently, we here assume a very low expression of this transporter in BUVEC. Likewise, the presence of NPC1L1 protein expression by Western blotting was not achieved, so far (unpublished data). Noteworthy, ezetimibe was originally identified as an ACAT II inhibitor (Clader, 2004) and therefore as acting on other potential targets besides NPC1L1. Irrespective of this, it is well documented that NPC1L1 incorporates cholesterol through an ezetimibe-sensitive pathway, however, the binding mechanism between ezetimibe and NPC1L1 remains unknown (Betters and Yu, 2010). Recently, it has been reported that ezetimibe, but not ezetimibe-glucuronide, reduces the cellular content of cholesteryl esters in a NPC1L1-independent manner in human monocytes, implying an inhibition of ACAT II (Orso et al., 2019). Likewise, we here propose that the current ezetimibe-mediated anti-coccidian effects may rather be linked to an inhibition of cholesterol esterification. In agreement, the importance of functional cholesterol esterification for coccidian replication was already confirmed for T. gondii (Sonda et al., 2001), B. besnoiti (Silva et al., 2019) and E. bovis (Hamid et al., 2014). However, the actual role of ezetimibe in cholesterol esterification and its cytosolic targets in primary bovine endothelial host cells should be further addressed in future studies. Even though in vitro studies have been published reporting anti-parasitic activities of ezetimibe treatments, in vivo evidence still needs to be addressed. Nevertheless, administration of ezetimibe to L. amazonensis-infected mice led to reduced parasite burden and boosted anti-leishmanial activity of ketoconazole (Andrade-Neto et al., 2016). However, given that ezetimibe treatments of P. yoelii-infected mice failed to affect parasitaemia (Kume et al., 2016) phylum-derived differences have to be assumed.
In conclusion, the current study shows that ezetimibe effectively inhibits T. gondii, N. caninum and B. besnoiti tachyzoite replication in BUVEC in a time-sustained but reversible manner. Apparently, the anti-coccidian effect of ezetimibe is associated with both impairment of tachyzoite infectivity and intracellular replication blockage. Of note, we additionally observed that ezetimibe-glucuronide does not interfere with parasite replication thereby suggesting an NPC1L1-independent anti-parasitic mechanism.
Acknowledgements
The authors would like to thank Christine Henrich, Dr Christin Ritter and Hannah Salecker for their outstanding technical support. We also are very thankful to Prof. Dr A. Wehrend (Clinic for Obstetrics, Gynaecology and Andrology of Large and Small Animals, Justus Liebig University, Giessen, Germany) for the continuous supply of bovine umbilical cords. Further, we thank Oliver Bender (Ubl butcher shop, Langsdorf, Germany) for the supply of bovine small intestine samples.
Author contributions
AT, CH, CL and LS conceived and designed the experiments. CL and LS performed the experiments. All authors performed analyses and interpretation of the data, preparation of the manuscript and approved final version of manuscript.
Financial support
CL was funded by the National Agency for Research and Development [(ANID), DOCTORADO BECAS CHILE/2017–72180349].
Ethical standards
Not applicable
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021000822.
click here to view supplementary material
Data
All data are available in the manuscript and Supplementary data files.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Altmann SW, Davis HR Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N and Graziano MP (2004) Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science (New York, N.Y.) 303, 1201–1204. [DOI] [PubMed] [Google Scholar]
- Alvarez-Garcia G, Frey CF, Mora LM and Schares G (2013) A century of bovine besnoitiosis: an unknown disease re-emerging in Europe. Trends in Parasitology 29, 407–415. [DOI] [PubMed] [Google Scholar]
- Andrade-Neto VV, Cunha-Junior EF, Canto-Cavalheiro MM, Atella GC, Fernandes TA, Costa PR and Torres-Santos EC (2016) Antileishmanial activity of ezetimibe: inhibition of sterol biosynthesis, in vitro synergy with azoles, and efficacy in experimental cutaneous leishmaniasis. Antimicrobial Agents and Chemotherapy 60, 6844–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Neto VV, Rebello KM, Pereira TM and Torres-Santos EC (2021) Effect of itraconazole-ezetimibe-miltefosine ternary therapy in murine visceral leishmaniasis. Antimicrobial Agents and Chemotherapy 65, e02676–20, /aac/65/5/AAC.02676-20.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter PJ and Rye KA (2016) New era of lipid-lowering drugs. Pharmacological Reviews 68, 458–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, Lipka LJ, Lebeaut AP, Yang B, Mellars LE, Cuffie-Jackson C, Veltri EP and Ezetimibe Study G (2001) Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clinical Therapeutics 23, 1209–1230. [DOI] [PubMed] [Google Scholar]
- Benavides J, Fernandez M, Castano P, Ferreras MC, Ortega-Mora L and Perez V (2017) Ovine toxoplasmosis: a new look at its pathogenesis. Journal of Comparative Pathology 157, 34–38. [DOI] [PubMed] [Google Scholar]
- Betters JL and Yu L (2010) NPC1L1 And cholesterol transport. FEBS Letters 584, 2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MW and Boothroyd JC (2000) Lytic cycle of Toxoplasma gondii. Microbiology and Molecular Biology Reviews 64, 607–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Valencia ME, Hermosilla C, Alcalá-Canto Y, Tapia G, Taubert A and Silva LMR (2019) Antiparasitic efficacy of curcumin against Besnoitia besnoiti tachyzoites in vitro. Frontiers in Veterinary Science 5, 333. doi: 10.3389/fvets.2018.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clader JW (2004) The discovery of ezetimibe: a view from outside the receptor. Journal of Medicinal Chemistry 47, 1–9. [DOI] [PubMed] [Google Scholar]
- Coppens I (2013) Targeting lipid biosynthesis and salvage in apicomplexan parasites for improved chemotherapies. Nature Reviews Microbiology 11, 823–835. [DOI] [PubMed] [Google Scholar]
- Davis HR Jr., Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP and Altmann SW (2004) Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. Journal of Biological Chemistry 279, 33586–33592. [DOI] [PubMed] [Google Scholar]
- Ehrenman K, Wanyiri JW, Bhat N, Ward HD and Coppens I (2013) Cryptosporidium parvum scavenges LDL-derived cholesterol and micellar cholesterol internalized into enterocytes. Cellular Microbiology 15, 1182–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr., Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill K A, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT and Thornberry NA (2005) The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1). Proceedings of the National Academy of Sciences of the United States of America 102, 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL and Song BL (2008) The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metabolism 7, 508–519. [DOI] [PubMed] [Google Scholar]
- Hamid PH, Hirzmann J, Hermosilla C and Taubert A (2014) Differential inhibition of host cell cholesterol de novo biosynthesis and processing abrogates Eimeria bovis intracellular development. Parasitology Research 113, 4165–4176. [DOI] [PubMed] [Google Scholar]
- Hamid PH, Hirzmann J, Kerner K, Gimpl G, Lochnit G, Hermosilla CR and Taubert A (2015) Eimeria bovis infection modulates endothelial host cell cholesterol metabolism for successful replication. Veterinary Research 46, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa EH, Yamaguchi K, Mori M and Nardone G (2020) Real-time cholesterol sorting in Plasmodium falciparum-erythrocytes as revealed by 3D label-free imaging. Scientific Reports 10, 2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa EH, Kato H, Nardone GA and Usukura J (2021) A prospective mechanism and source of cholesterol uptake by Plasmodium falciparum-infected erythrocytes co-cultured with HepG2 cells. Parasitology International 80, 102179. [DOI] [PubMed] [Google Scholar]
- Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP and Ezetimibe Study G (2003) Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. European Heart Journal 24, 729–741. [DOI] [PubMed] [Google Scholar]
- Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB and Hunter CA (2016) Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nature Microbiology 1, 16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jähne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orsó E and Schmitz G (2005) Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. Journal of Biological Chemistry 280, 1306–1320. [DOI] [PubMed] [Google Scholar]
- Kume A, Herbas MS, Shichiri M, Ishida N and Suzuki H (2016) Effect of anti-hyperlipidemia drugs on the alpha-tocopherol concentration and their potential for murine malaria infection. Parasitology Research 115, 69–75. [DOI] [PubMed] [Google Scholar]
- Labaied M, Jayabalasingham B, Bano N, Cha SJ, Sandoval J, Guan G and Coppens I (2011) Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cellular Microbiology 13, 569–586. [DOI] [PubMed] [Google Scholar]
- Labonté ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA and Hui DY (2007) Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochimica et Biophysica Acta 1771, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Yang H and Song B-L (2020) Mechanisms and regulation of cholesterol homeostasis. Nature Reviews Molecular Cell Biology 21, 225–245. [DOI] [PubMed] [Google Scholar]
- Maley SW, Buxton D, Rae AG, Wright SE, Schock A, Bartley PM, Esteban-Redondo I, Swales C, Hamilton CM, Sales J and Innes EA (2003) The pathogenesis of neosporosis in pregnant cattle: inoculation at mid-gestation. Journal of Comparative Pathology 129, 186–195. [DOI] [PubMed] [Google Scholar]
- Nayeri T, Sarvi S, Moosazadeh M, Amouei A, Hosseininejad Z and Daryani A (2020) The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: a systematic review and meta-analysis. PLoS Neglected Tropical Diseases 14, e0008103. doi: 10.1371/journal.pntd.0008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan SJ, Romano JD, Luechtefeld T and Coppens I (2015) Neospora caninum recruits host cell structures to its parasitophorous vacuole and salvages lipids from organelles. Eukaryotic Cell 14, 454–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso E, Robenek H, Boettcher A, Wolf Z, Liebisch G, Kramer W and Schmitz G (2019) Nonglucuronidated ezetimibe disrupts CD13- and CD64-coassembly in membrane microdomains and decreases cellular cholesterol content in human monocytes/macrophages. Cytometry. Part A: The Journal of the International Society for Analytical Cytology 95, 869–884. [DOI] [PubMed] [Google Scholar]
- Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LF and Ellis JT (2013) What is the global economic impact of Neospora caninum in cattle – the billion dollar question. International Journal for Parasitology 43, 133–142. [DOI] [PubMed] [Google Scholar]
- Silva LMR, Lutjohann D, Hamid P, Velasquez ZD, Kerner K, Larrazabal C, Failing K, Hermosilla C and Taubert A (2019) Besnoitia besnoiti infection alters both endogenous cholesterol de novo synthesis and exogenous LDL uptake in host endothelial cells. Scientific Reports 9, 6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonda S, Ting LM, Novak S, Kim K, Maher JJ, Farese RV Jr. and Ernst JD (2001) Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. Journal of Biological Chemistry, 276, 34434–34440. [DOI] [PubMed] [Google Scholar]
- Taubert A, Zahner H and Hermosilla C (2006) Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Veterinary Parasitology 142, 214–222. [DOI] [PubMed] [Google Scholar]
- Taubert A, Hermosilla C, Silva LM, Wieck A, Failing K and Mazurek S (2016) Metabolic signatures of Besnoitia besnoiti-infected endothelial host cells and blockage of key metabolic pathways indicate high glycolytic and glutaminolytic needs of the parasite. Parasitology Research 115, 2023–2034. [DOI] [PubMed] [Google Scholar]
- Taubert A, Silva LMR, Velásquez ZD, Larrazabal C, Lütjohann D and Hermosilla C (2018) Modulation of cholesterol-related sterols during Eimeria bovis macromeront formation and impact of selected oxysterols on parasite development. Molecular and Biochemical Parasitology 223, 1–12. [DOI] [PubMed] [Google Scholar]
- van Heek M, Farley C, Compton DS, Hoos L and Davis HR (2001) Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. British Journal of Pharmacology 134, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez ZD, Conejeros I, Larrazabal C, Kerner K, Hermosilla C and Taubert A (2019) Toxoplasma gondii-induced host cellular cell cycle dysregulation is linked to chromosome missegregation and cytokinesis failure in primary endothelial host cells. Scientific Reports 9, 12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez ZD, Lopez-Osorio S, Pervizaj-Oruqaj L, Herold S, Hermosilla C and Taubert A (2020) Besnoitia besnoiti-driven endothelial host cell cycle alteration. Parasitology Research 119, 2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chu BB, Ge L, Li BL, Yan Y and Song BL (2009) Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. Journal of Lipid Research 50, 1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021000822.
click here to view supplementary material
Data Availability Statement
All data are available in the manuscript and Supplementary data files.