Abstract
Objectives:
To determine the association between mean airway pressure and 90-day mortality in patients with acute respiratory failure requiring mechanical ventilation and to compare the predictive ability of mean airway pressure compared with inspiratory plateau pressure and driving pressure.
Design:
Prospective observational cohort.
Setting:
Five ICUs in Lima, Peru.
Subjects:
Adults requiring invasive mechanical ventilation via endotracheal tube for acute respiratory failure.
Interventions:
None.
Measurements and Main Results:
Of potentially eligible participants (n = 1,500), 65 (4%) were missing baseline mean airway pressure, while 352 (23.5%) were missing baseline plateau pressure and driving pressure. Ultimately, 1,429 participants were included in the analysis with an average age of 59 ± 19 years, 45% female, and a mean Pao2/Fio2 ratio of 248 ± 147 mm Hg at baseline. Overall, 90-day mortality was 50.4%. Median baseline mean airway pressure was 13 cm H2O (interquartile range, 10–16 cm H2O) in participants who died compared to a median mean airway pressure of 12 cm H2O (interquartile range, 10–14 cm H2O) in participants who survived greater than 90 days (p < 0.001). Mean airway pressure was independently associated with 90-day mortality (odds ratio, 1.38 for difference comparing the 75th to the 25th percentile for mean airway pressure; 95% CI, 1.10–1.74) after adjusting for age, sex, baseline Acute Physiology and Chronic Health Evaluation III, baseline Pao2/Fio2 (modeled with restricted cubic spline), baseline positive end-expiratory pressure, baseline tidal volume, and hospital site. In predicting 90-day mortality, baseline mean airway pressure demonstrated similar discriminative ability (adjusted area under the curve = 0.69) and calibration characteristics as baseline plateau pressure and driving pressure.
Conclusions:
In a multicenter prospective cohort, baseline mean airway pressure was independently associated with 90-day mortality in mechanically ventilated participants and predicts mortality similarly to plateau pressure and driving pressure. Because mean airway pressure is readily available on all mechanically ventilated patients and all ventilator modes, it is a potentially more useful predictor of mortality in acute respiratory failure.
Keywords: acute respiratory distress syndrome, mean airway pressure, mechanical ventilation, respiratory failure
Approximately 40% of all patients admitted to ICUs require mechanical ventilation for acute respiratory failure (ARF) during their hospitalization (1, 2). As the population ages, the frequency of respiratory failure is projected to rise by 80% by 2026 (3). Ventilation guidelines for ARF are frequently extrapolated from evidence-based recommendations for acute respiratory distress syndrome (ARDS) that center on reducing tidal volumes (Vts) and inspiratory airway pressures (4). Plateau pressure (Pplat), the airway pressure during a brief inspiratory pause, is one of the primary measurements that guide ventilator management. Recent evidence also suggests that driving pressure (Pdriv), measured as the Pplat minus positive end-expiratory pressure (PEEP), may be a more useful target (5). However, recent large observational studies suggest that clinicians fail to measure Pplat, and subsequently Pdriv, in over 50% of ARDS patients on controlled modes of ventilation and 60% of ARF patients receiving mechanical ventilation (1, 2).
Mean airway pressure (Pmean) is determined by the peak inspiratory pressure (PIP), PEEP, and the inspiratory to expiratory time ratio (e-Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F323; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). It is a commonly available pressure measurement that has received relatively little attention in the literature. In contrast to Pplat and Pdriv, Pmean is measured and reported by most mechanical ventilators automatically with every breath and is thus more easily obtained (6). Under normal conditions, Pmean closely correlates with mean alveolar pressure (7) and thus may represent the stresses applied to the lung parenchyma with ventilation. Furthermore, unlike Pplat and Pdriv, Pmean is affected by minute ventilation and therefore may reflect dead space, acidosis, and other aspects of critical illness severity. We hypothesize that elevations in Pmean are associated with higher mortality and that Pmean will perform similarly to Pplat and Pdriv to predict mortality in ARF. To evaluate this hypothesis, we analyzed a prospective observational cohort of critically ill participants with ARF requiring mechanical ventilation admitted to ICUs in Lima, Peru.
MATERIALS AND METHODS
Study Design, Patient Selection, Data Collection
The INTENSIVOS (meaning critical in Spanish) study was a prospective longitudinal cohort of mechanically ventilated patients in Lima, Peru (8). We received ethics approval and permission to conduct this study in each of the participating institutions: Hospital Nacional Edgardo Rebagliati Martins (Reba), Hospital Nacional Guillermo Almenara Irigoyen (Almenara), Hospital Nacional Arzobispo Loayza (Loayza), and Hospital de Emergencias Casimiro Ulloa (Casimiro Ulloa). Ethics approvals were obtained from the institutional review boards of A.B. PRISMA and ESSALUD Hospital Nacional Edgardo Rebagliati Martins in Lima, Peru, and the Johns Hopkins School of Medicine, in Baltimore, MD. We obtained a waiver of written informed consent to conduct this observational study. Eligible participants were enrolled in the cohort between December 2010 and October 2013 in five ICUs of four public hospitals. Eligibility criteria included adults over age 18 years with at least 24 hours of invasive mechanical ventilation via endotracheal tube in one of the participating ICUs. All participants were enrolled within 48 hours of mechanical ventilation initiation. Mechanical ventilation data was recorded as data from the first 24 hours of mechanical ventilation initiation (day 0) and then once daily thereafter. Recorded data was collected using values obtained as close as possible to 8:00 AM. Demographics, chronic disease states, and acute physiologic data were obtained at enrollment for all participants. Participants were followed daily to monitor clinical and ventilator management, acute physiology, and vital status during their ICU stay for up to 28 days in the ICU, until ICU discharge, or death. Participants were contacted at 90 days after enrollment to assess vital status.
Variables and Primary Outcome
We analyzed baseline data from the first 24 hours of mechanical ventilation initiation. The primary exposure variable was baseline Pmean measured at day 0 (i.e., day of mechanical ventilation initiation). Participants were excluded from the analysis if they were on a ventilation mode that may not allow for accurate measurements of Pplat, such as pressure support ventilation, bi-level ventilation, or high-frequency oscillatory ventilation. ARDS at baseline was identified as present if the participant met all of the Berlin criteria (9).
The primary outcome of interest was 90-day mortality. If participants were discharged to a care facility or to home prior to 90 days, they were contacted by phone by the data coordinating center at 90 days to assess vital status. Pplat and Pdriv at baseline were used as comparisons to baseline Pmean. Pdriv was calculated as Pplat minus PEEP.
Biostatistical Methods
The primary objective was to evaluate the relationship between baseline Pmean and 90-day mortality. To test for an independent association between Pmean and mortality, we performed simple and multivariable logistic regressions. We then repeated the analysis separately using Pplat and Pdriv in place of Pmean. Multivariable logistic regressions were used to control for age, sex, Pao2/Fio2, Acute Physiology and Chronic Health Evaluation (APACHE) III, PEEP, Vt per kilogram of predicted body weight (PBW) (10), and ICU site as a categorical variable. For the multivariable analysis, we identified variables a priori that were associated with mortality, could influence ventilator management, and were not collinear with Pmean. These variables were identified a priori based on scientific and biologic importance. We used baseline values at time of enrollment for all time-varying covariates (i.e., Pao2/Fio2, APACHE III, PEEP, Vt). Collinearity for all variables was tested using the variance inflation factor and subsequent sensitivity analyses (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). We did not include Vt in regression models containing Pdriv given concerns for collinearity.
In exploratory analyses, we evaluated the shape of the associations of Pmean, Pao2/Fio2, and their interaction with mortality (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Since Pao2/Fio2 (a proxy for oxygenation) is a well-established predictor of clinical outcomes (11), we explored extensively for potential interactions between Pmean and Pao2/Fio2 on mortality and for nonlinear associations in Pmean and Pao2/Fio2 with knots at the 5th, 50th, and 95th percentiles, and mortality (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Our final multivariable logistic regression model for mortality included the following variables: Pmean, a restricted cubic spline for Pao2/Fio2, age, sex, APACHE III, PEEP, Vt per kg PBW, and ICU site as a categorical variable. Since Pmean was treated as a continuous variable in the final model, we scaled the odds ratio (OR) of mortality to interquartile range (IQR) increments (i.e., odds of mortality comparing the 75th percentile to the 25th percentile for Pmean).
Receiver operating characteristic curves were used to compare the prognostic value of Pmean, Pplat, and Pdriv for predicting 90-day mortality in our cohort. We evaluated discrimination of Pmean as a predictor using C-statistics as a proxy for the area under the curve (AUC). We evaluated the calibration of Pmean as a predictor of mortality using the Hosmer-Lemeshow goodness of fit test. We validated results internally by using leave-one-out cross validation.
We performed several prespecified sensitivity analyses to evaluate the robustness of our findings (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Specifically, we evaluated the effect of missingness on the data using multiple imputation techniques. We also tested the effect of spontaneous breathing efforts by identifying participants with a total respiratory rate greater than the set respiratory rate. In these participants, Pmean may be different from values that would be obtained without spontaneous respiratory efforts. Therefore, we used an interaction term to evaluate whether spontaneous breathing modified the association between airway pressure and mortality.
For other analyses, continuous variables are presented as mean ± sd if normally distributed and median (IQR) if non-normally distributed. Categorical variables are presented as counts and percentages. Comparisons between variables were conducted using the Student t test for continuous variables and the Pearson chi-square or Fisher exact test for categorical variables. Statistical analysis was performed by using STATA Version 14.0 (StataCorp., College Station, TX) and R (www.r-project.org). We analyzed and reported this study according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
RESULTS
Participant Characteristics
From the cohort of 1,657 participants with ARF on mechanical ventilation, 1,429 were ultimately eligible for inclusion into our study (e-Fig. 2, Supplemental Digital Content 3, http://links.lww.com/CCM/F325; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Individuals were excluded if they were on a mode of ventilation that prevented accurate measurement of Pplat (n = 157, 9.5%), for example, pressure support, bi-level or airway pressure release ventilation, and high-frequency oscillatory ventilation. Of the remaining participants, we also excluded participants missing the primary exposure Pmean (n = 65, 4.3%) or the primary outcome of 90-day mortality (n = 6, 0.4%). Of those participants on a controlled mode of ventilation that allows for accurate measurement of Pplat (n = 1500), 352 participants did not have baseline Pplat recorded (23.5% of the potential cohort) while only 65 participants did not have baseline Pmean documented (4.3% of the potential cohort). From the final cohort of 1,429 participants, 322 participants (22.5% of final cohort) did not have a baseline Pplat recorded.
Overall, participants had an average age of 59.5 ± 19.0 years, 45% were female, and the mean Pao2/Fio2 ratio was 248 ± 147 mm Hg at baseline. Median Vt at baseline was 8.3 mL/kg PBW (7.2–9.6 mL/kg PBW). Of the 1,429 participants included in our analysis, 258 participants (18.1%) had ARDS. Overall 90-day mortality was 50.4%. Pressure control was the mode most commonly used (62%), while 34% participants were maintained on a volume control mode, 2.3% participants on synchronized intermittent mandatory ventilation, and 0.2% participants on pressure-regulated volume control mode at baseline. Survivors were typically younger, with lower severity of illness scores, higher Pao2/Fio2 ratios, and were less likely to have ARDS. Baseline Pmean, Pplat, and Pdriv were higher in nonsurvivors compared with survivors (Table 1).
TABLE 1.
Characteristics of Patients With Acute Respiratory Failure by Survival
| Characteristica | All Participants, n = 1,429 | Survivors, n = 709 | Nonsurvivors, n = 720 | p |
|---|---|---|---|---|
| Age, yr | 59.5 (19.0) | 56.8 (18.6) | 62.2 (19.0) | < 0.001 |
| Female sex | 638 (44.6) | 321 (45.3) | 317 (44.0) | 0.64 |
| Weight, kg | 70.2 (16.0) | 71.1 (16.8) | 69.2 (15.1) | 0.03 |
| Acute Physiology and Chronic Health Evaluation III | 83.4 (28.4) | 75.4 (26.8) | 91.4 (27.7) | < 0.001 |
| Pao2/Fio2 ratio | 248.8 (146.6) | 266.1 (154.1) | 231.8 (137.0) | < 0.001 |
| Minute ventilation, L/min | 8.3 (7.2–9.9) | 8.2 (7.1–9.9) | 8.5 (7.2–9.9) | 0.66 |
| Static compliance, mL/cm H2O | 30 (24.1–38.8) | 31.8 (25.4–40.7) | 28.9 (22.2–37.5) | < 0.001 |
| Tidal volume, mL | 475 (410–510) | 480 (423–520) | 461 (400–502) | < 0.001 |
| Tidal volume, mL/kg predicted body weight | 8.3 (7.2–9.6) | 8.4 (7.3–9.7) | 8.2 (7.1–9.6) | 0.035 |
| Positive end-expiratory pressure, cm H2O | 6 (5–10) | 6 (5–10) | 7 (5–10) | 0.006 |
| Mean airway pressure, cm H2O | 12 (10–15) | 12 (10–15) | 13 (10–16) | < 0.001 |
| Plateau pressure, cm H2O | 23 (20–28) | 22 (19–27) | 24 (20–30) | < 0.001 |
| Driving pressure, cm H2O | 16 (13–20) | 15 (13–19) | 17 (13–20) | < 0.001 |
| Reason for hospital admission | < 0.001 | |||
| Medical | 1,038 (72.7) | 482 (68.0) | 556 (77.4) | |
| Trauma | 155 (10.9) | 98 (13.8) | 57 (7.9) | |
| Surgical, scheduled | 41 (2.9) | 22 (3.1) | 19 (2.6) | |
| Surgical, nonscheduled | 149 (10.4) | 79 (11.1) | 70 (9.7) | |
| ARDS | 258 (18.1) | 109 (15.4) | 149 (20.8) | 0.008 |
| Risk factor for ARDSb | 0.041 | |||
| Pneumonia | 224 (15.8) | 90 (12.8) | 134 (18.8) | |
| Sepsis | 94 (6.6) | 42 (6.0) | 52 (7.3) | |
| Trauma | 20 (1.4) | 11 (1.6) | 9 (1.3) | |
| Aspiration | 19 (1.3) | 10 (1.4) | 9 (1.3) | |
| Multiple transfusions | 2 (0.1) | 0 (0.0) | 2 (0.1) | |
| Pancreatitis | 14 (1.0) | 9 (1.3) | 5 (0.7) | |
| Other | 24 (1.7) | 13 (1.8) | 11 (1.5) | |
| No risk factor | 1,022 (72.0) | 530 (75.2) | 492 (68.9) | |
| Time on ventilator, d | 7 (4–12) | 7 (4–12) | 7 (4–11) | 0.41 |
| ICU length of stay, d | 12 (7–21) | 12 (7–21) | 14 (8–23) | 0.26 |
ARDS = acute respiratory distress syndrome.
Mean (sd), median (interquartile range), and n (%).
Comparisons for multiple transfusions and pancreatitis categories by Fisher exact test, all other categorical variables Pearson χ2 test.
For all participants included in our analysis, baseline median (IQR) Pmean was 12 cm H2O (10–16 cm H2O), Pdriv was 15 cm H2O (13–19 cm H2O), and Pplat was 23 cm H2O (20–28 cm H2O) (Fig. 1). Pmean demonstrated a strong positive correlation with Pplat (r = 0.74; p < 0.0001), but only a weak positive correlation with Pdriv (r = 0.32; p < 0.0001) (Fig. 2). Participants with ARDS compared with non-ARDS had higher median baseline inspiratory pressures (i.e., Pmean, Pplat, and Pdriv) and PEEP, despite similar Vts (e-Table 1, Supplemental Digital Content 4, http://links.lww.com/CCM/F326).
Figure 1.
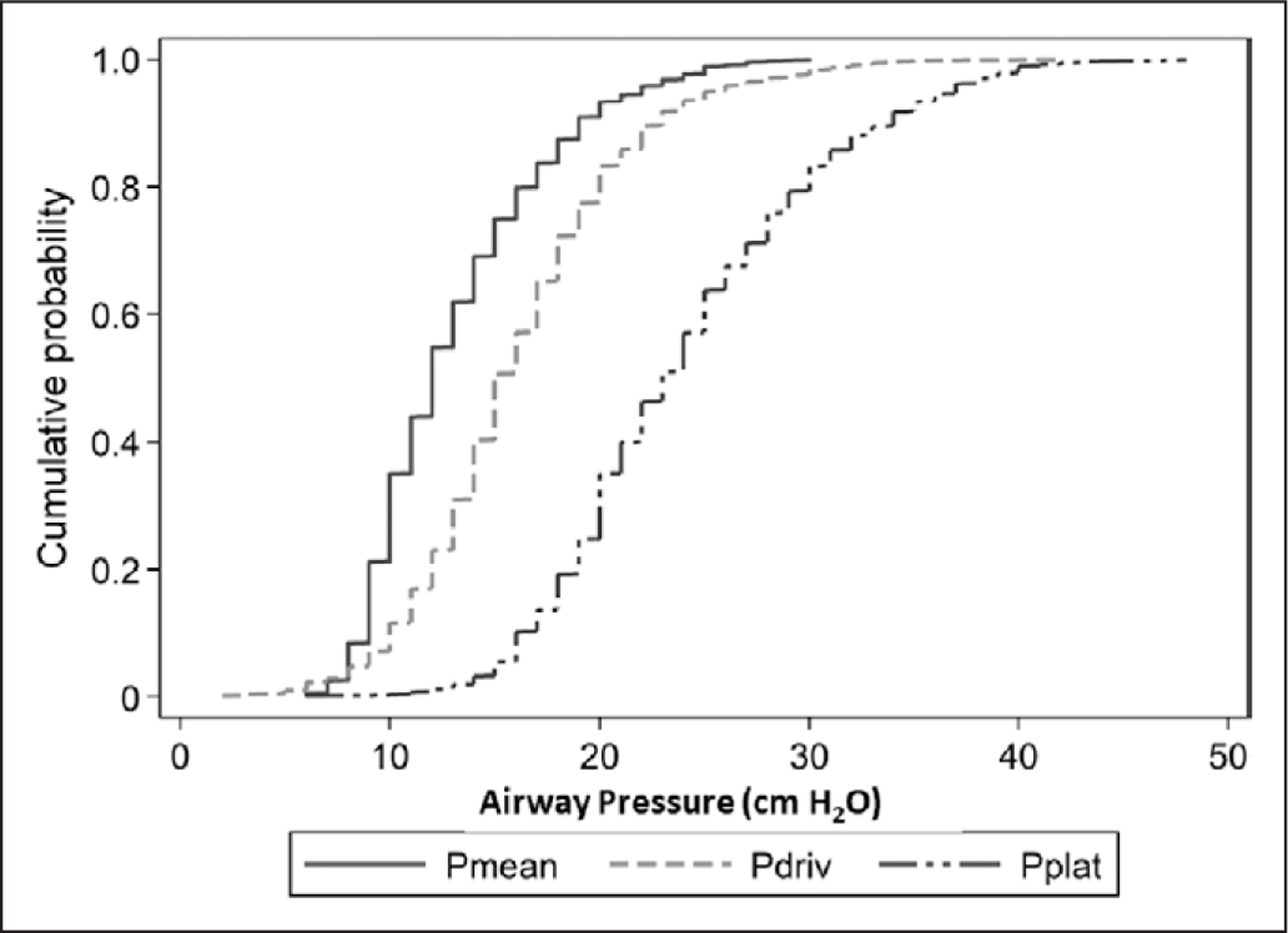
Cumulative distribution of inspiratory airway pressures. Cumulative frequency distribution for mean airway pressure (Pmean), driving pressure (Pdriv), and plateau pressure (Pplat) at baseline.
Figure 2.
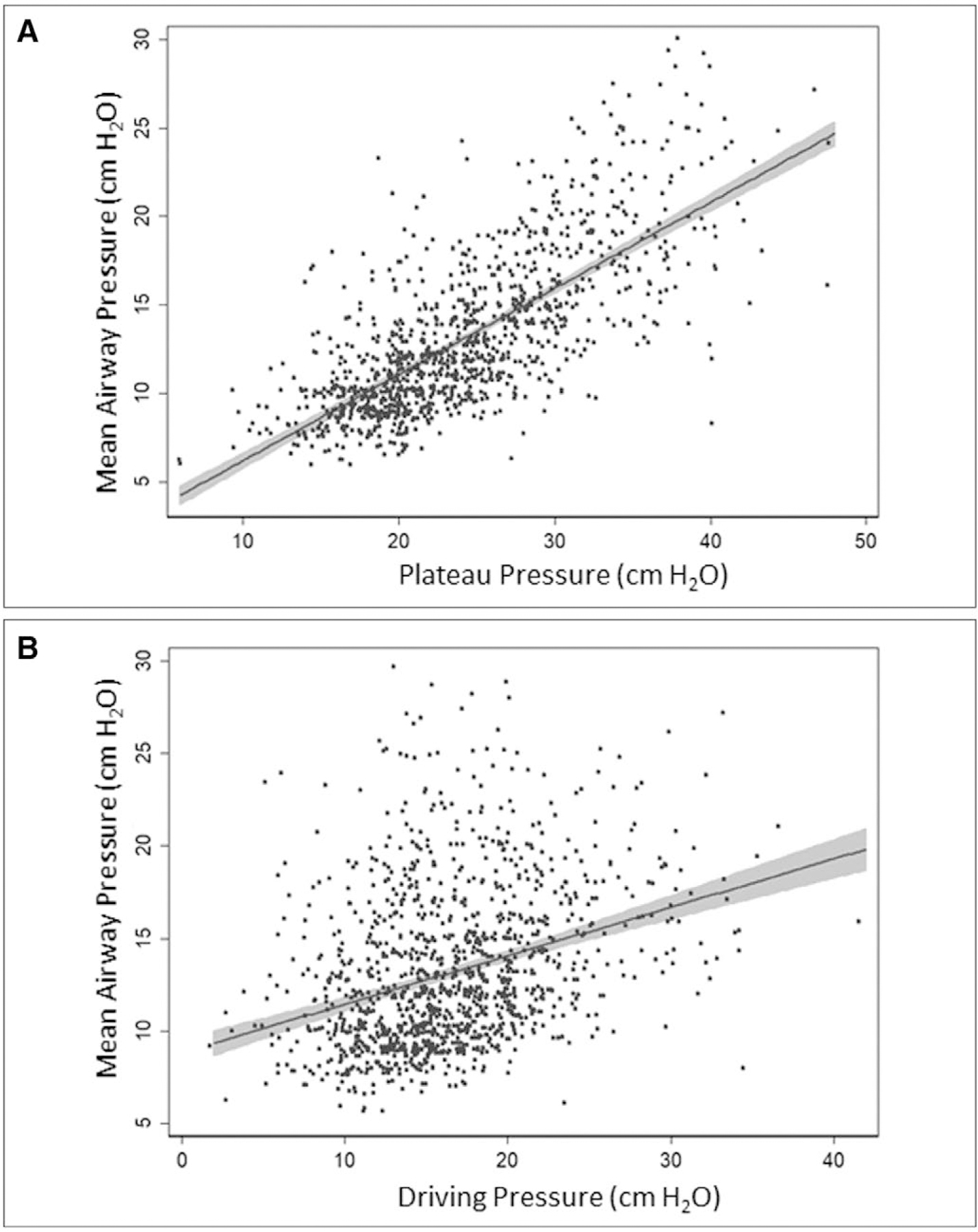
Correlation among three inspiratory airway pressures. Solid lines represent linear prediction line with 95% CIs (gray zones). A, Correlation between mean airway pressure and plateau pressure (r = 0.7350; p < 0.001). B, Correlation between mean airway pressure and driving pressure (r = 0.3247; p < 0.001).
Association Between Pmean and Mortality
Median baseline Pmean was 13 cm H2O (10–16 cm H2O) in participants who died compared with a median Pmean of 12 cm H2O (10–14 cm H2O) in participants who survived 90 days (p < 0.001) (Table 1). Exploratory analyses pointed to a nonlinear relationship between either Pao2/Fio2 or Pmean and mortality (Fig. 3). We did not final strong evidence for an interaction between Pmean and Pao2/Fio2 on mortality based on visual examination of the probability of death by looking at all possible combinations of quintiles of these two variables (e-Fig. 3, Supplemental Digital Content 5, http://links.lww.com/CCM/F327; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Specifically, the odds of death were higher for the same degree of change in Pao2/Fio2 at lower values than at higher values. There appeared to be a Pmean threshold around 10 cm H2O, where the odds of death were linearly associated with Pmean. When fitting smoothing splines to Pmean, Pao2/Fio2 and a smooth surface to the interaction between Pmean and Pao2/Fio2 (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324), we found that there was no interaction between these factors (p = 0.10; Wald test; e-Fig. 4, Supplemental Digital Content 6, http://links.lww.com/CCM/F328 [legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324]). The relationship between Pao2/Fio2 and mortality remained nonlinear whereas the relationship between Pmean and mortality was linear at any value of Pao2/Fio2 when adjusted for a priori confounders (Fig. 4). These findings informed our final model using a multivariable logistic regression with a restricted cubic spline for Pao2/Fio2 to model its nonlinear relationship with mortality and a single regression parameter for Pmean to model its linear relationship with mortality.
Figure 3.
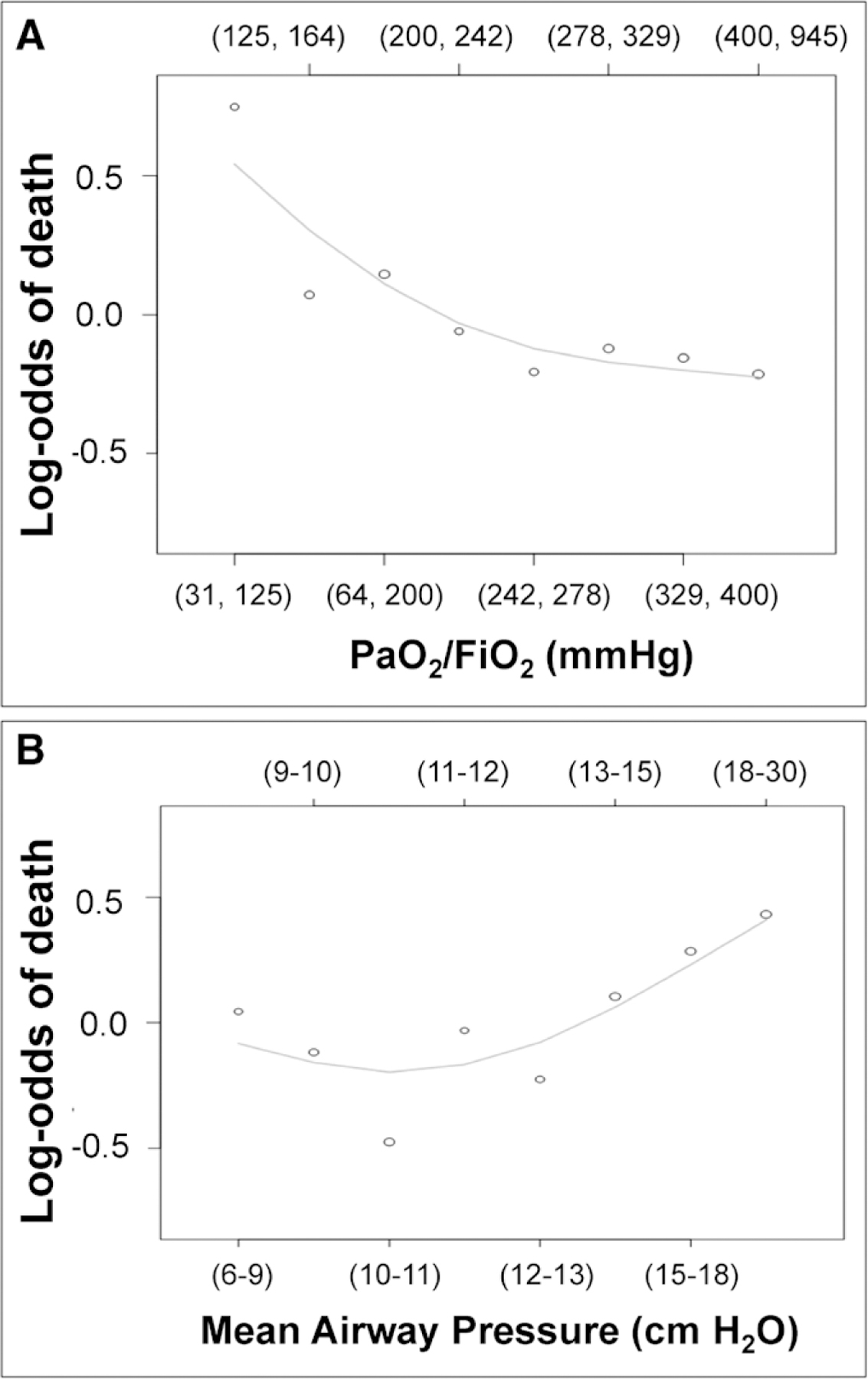
Unadjusted log-odds of mortality as a function of Pao2/Fio2 and mean airway pressure. Unadjusted association between octiles of Pao2/Fio2 (A) and mean airway pressure (B) and log odds of death at 90 d.
Figure 4.
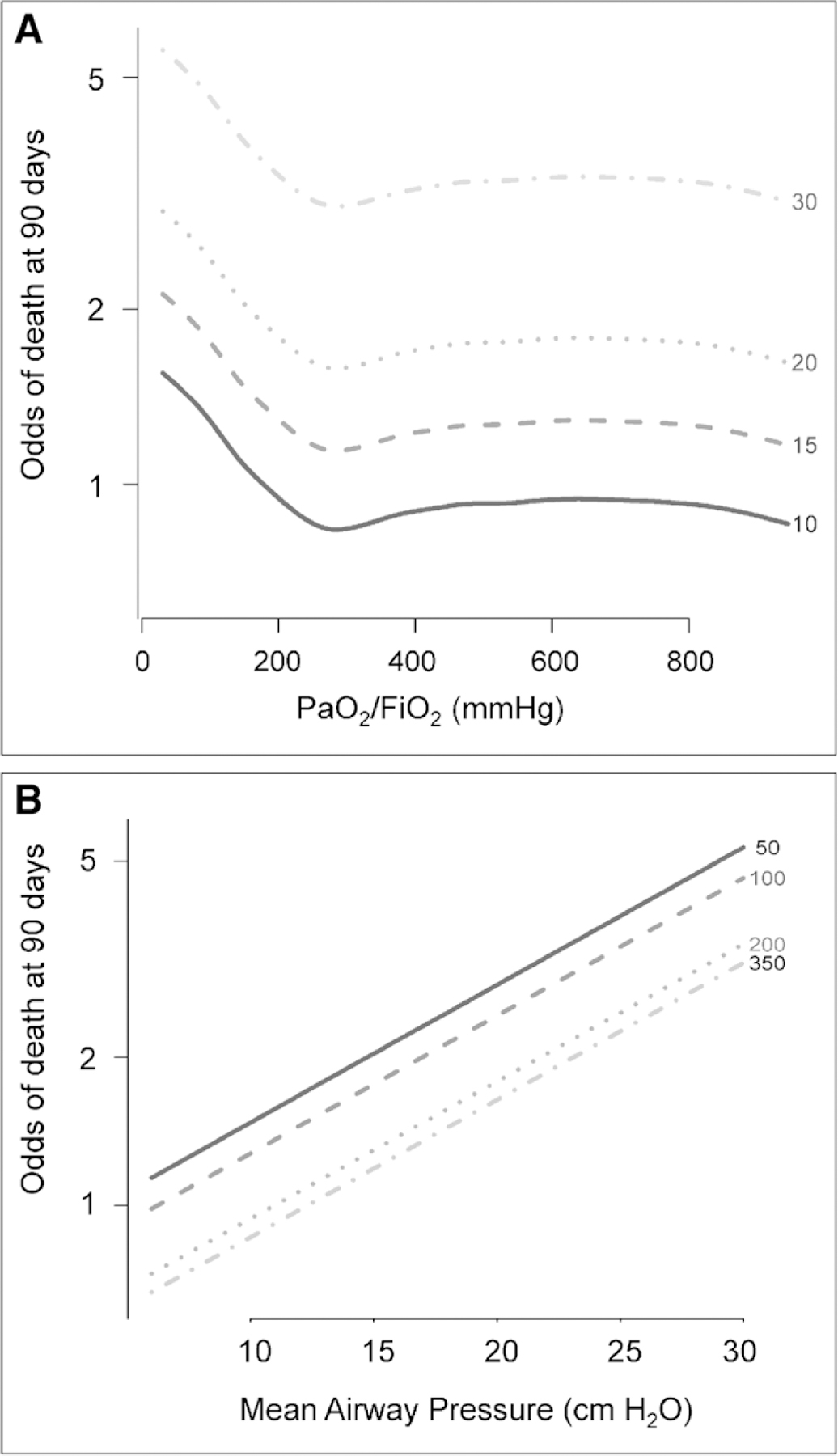
Adjusted odds of mortality as a function of Pao2/Fio2 and mean airway pressure (Pmean) (B). At higher Pmean and at lower Pao2/Fio2, the odds of mortality are independently higher. A, In adjusted analysis, the relationship between Pao2/Fio2 and mortality is nonlinear. Nevertheless, lower Pao2/Fio2 is independently associated with mortality over a range of Pmean with an inflection near 300 mm Hg. B, In adjusted analysis, the relationship between Pmean and mortality is linear at any value of Pao2/Fio2 (i.e., 50, 100, 200, 350 mm Hg).
In unadjusted analysis, higher baseline Pmean was associated with a greater odds of death (IQR OR, 1.32; 95% CI, 1.16–1.50; p < 0.001). In multivariable analysis, Pmean remained independently associated with 90-day mortality in mechanically ventilated participants (IQR OR, 1.38; 95% CI, 1.10–1.74) (e-Table 2, Supplemental Digital Content 7, http://links.lww.com/CCM/F329). Older age and higher APACHE III were also independently associated with a greater odds of mortality. However, higher PEEP was associated with a lower odds of 90-day mortality (IQR OR, 0.75 for each 5 cm H2O; 95% CI, 0.58–0.98; p = 0.036).
Prognostic Utility of Pmean Compared to Pplat and Pdriv
The adjusted OR for mortality due to a higher baseline Pmean was larger than the adjusted ORs associated with either Pplat or Pdriv (e-Table 2, Supplemental Digital Content 7, http://links.lww.com/CCM/F329). Baseline Pmean (AUC = 0.69), Pplat (AUC = 0.69), and Pdriv (AUC = 0.69) had similar discriminative characteristics for mortality prediction (p = 0.74; e-Fig. 5, Supplemental Digital Content 8, http://links.lww.com/CCM/F330; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Baseline Pmean also demonstrated good calibration (Hosmer-Lemeshow goodness of fit: p = 0.31; e-Fig. 6, Supplemental Digital Content 9, http://links.lww.com/CCM/F331 [legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324]). Cross validation supported our observation of good performance for model calibration and discrimination (Hosmer-Lemeshow goodness of fit p = 0.21; AUC = 0.67).
Sensitivity Analyses
Multiple sensitivity analyses were performed as described in the Methods section and Supplemental Methods section (Supplemental Digital Content 2, http://links.lww.com/CCM/F324). Sensitivity analyses demonstrated no material differences in the association between Pmean and mortality (Online Supplement, Supplemental Digital Content 2, http://links.lww.com/CCM/F324).
DISCUSSION
In this study, we demonstrated that baseline Pmean was independently associated with 90-day mortality in a cohort of mechanically ventilated participants. Furthermore, we demonstrated that baseline Pmean performs equally to baseline Pplat and Pdriv as a predictor of mortality.
The results of our study demonstrate that Pmean is independently associated with mortality. As demonstrated in prior large international studies and in our own cohort, Pplat and Pdriv are useful physiologic markers of lung injury and predictors of mortality, but they are frequently not measured. In our cohort, approximately a quarter of participants did not have Pplat recorded, and these rates of missing Pplats were as high as 60% in previous studies (1, 2). In contrast, Pmean is measured and reported on every ventilator regardless of mode, with every breath. In our initial cohort, only 4% of participants were missing Pmean. Future research is necessary to understand if and how Pmean could be used in place of Pplat or Pdriv to guide ventilator management.
There are several reasons why Pmean may have a strong association with mortality in all mechanically ventilated participants. Pmean is determined by the PIP, PEEP, and the inspiratory to expiratory time ratio, while Pdriv and Pplat reflect lung stress during the inspiratory phase only. Pmean reflects the mean alveolar pressure throughout the entire respiratory cycle. Accordingly, Pmean will increase if airway resistance increases, compliance of the lung or chest wall decreases, or dead space and work of breathing increase (7). Pplat represents the stiffness in the respiratory system, which predicts mortality in patients with ARDS (12). Pmean correlates directly with Pplat, but also varies with minute ventilation, which could reflect dead space or acidosis. Previous studies have demonstrated the utility of dead space as a predictor of mortality (13). Pmean is a key component of the oxygenation index, which has also been associated with mortality in numerous studies of outcomes in both adult and pediatric respiratory failure (14–16). By taking into account the entire respiratory cycle, Pmean may better reflect the power applied to the lung and the risk for injury throughout tidal breathing (17). Thus, Pmean provides a more complete estimation of lung disease severity, respiratory compliance, and need for respiratory support than Pplat or Pdriv alone.
Elevated Pmean is additionally more likely to cause hemodynamic impairments than elevated Pplat or Pdriv (18). Increases in Pmean during high-frequency oscillation may decrease cardiac output (19, 20), while interventions that decreased Pmean reduced intrathoracic pressures and were associated with augmented hemodynamics during resuscitative efforts (21). Thus, Pmean reflects both circulatory and pulmonary impairment during mechanical ventilation that contribute to worsened outcomes.
Our study has several strengths. The INTENSIVOS cohort was prospectively enrolled with high-quality data, collected by experienced and trained research coordinators (8). Our study includes a large sample size of participants with ARF, which adds significant power to our study. Participants were enrolled within 48 hours of intubation, allowing for accurate assessment of the association between baseline Pmean and mortality. Furthermore, participants were enrolled in four different hospitals in Peru with a variety of etiologies for their ARF, which increases the generalizability of our results. Finally, several sensitivity analyses were performed to evaluate the robustness of our results and the relationship of Pmean to mortality was unchanged.
Our study also has some potential limitations. Although data were collected rigorously and prospectively, this was an observational study and there were a lack of protocols associated with ventilator management for participants. As such, average Vts were 8.3 mL/kg PBW as opposed to 6 mL/kg PBW, which may have led to higher airway pressures and higher rates of mortality. However, in a recent large epidemiologic study of ARDS frequency and management, over a third of participants received Vts greater than 8 mL/kg PBW (1). Thus, our study more truly reflects the relationship between Pmean and mortality that result from usual care mechanical ventilation practices outside the tightly controlled protocols of a clinical trial. Our patient population was further restricted to participants receiving a controlled mode of ventilation. Therefore, the relationship of Pmean to mortality should not be extrapolated to patients receiving spontaneous ventilation modes.
We only evaluated the prognostic value of Pmean at baseline within 24 hours of mechanical ventilation initiation. Thus, we did not assess if longitudinal data may provide additional predictive value. This analysis could be performed in the future to evaluate the effect of longitudinal evaluations of Pmean. However, Amato et al (5) demonstrated that including Pdriv values over the first 3 days did not significantly improve model fit compared with including only baseline values in ARDS patients. Further studies should be performed to externally validate our findings. Finally, we were unable to identify a threshold value below which further decrease in Pmean was not associated with lower odds of mortality. Although unadjusted analysis initially suggested a nonlinear relationship between Pmean and mortality (e-Fig. 3, Supplemental Digital Content 5, http://links.lww.com/CCM/F327; legend, Supplemental Digital Content 2, http://links.lww.com/CCM/F324), the adjusted analysis demonstrates a linear relationship between Pmean and mortality (Fig. 4B). Evidence suggests that Pplat and Pdriv similarly have linear relationships with mortality and targeting lower values of these airway pressures may be associated with better outcomes (5, 12, 22). Nevertheless, the existence of a therapeutic threshold for Pmean should continue to be investigated in future studies.
The use of Pmean to guide mechanical ventilation should be explored further. Previously Pmean has been relatively limited to use during high-frequency oscillatory ventilation or as a component of the oxygenation index. The easy accessibility of Pmean and practical nature of measurement, however, suggest potential for more widespread use. Although we demonstrate an association between Pmean and mortality, we cannot draw conclusions regarding the causality of this association from this study. Higher Pmean may be a marker of poor outcome because it is affected by dead space and minute ventilation, which have been associated with poor outcomes as well (13). Alternatively, it may represent a potentially modifiable target for intervention to guide Vts titration or referral for advanced rescue therapies such as extracorporeal membrane oxygenation. If further studies confirm this association, future research may focus on the use of Pmean to prognosticate, stratify future trials of patients with ARF, or guide mechanical ventilation at the bedside.
CONCLUSIONS
Pmean was independently associated with 90-day mortality in a cohort of mechanically ventilated participants with ARF. Additionally, Pmean predicted mortality similarly to Pplat or Pdriv in this same cohort of patients. Future research should focus on replicating our results in cohorts of both ARDS and non-ARDS patients, utilizing Pmean as a variable for stratifying or prognostically enriching future clinical trials, and determining if targeting a lower Pmean improves clinical outcomes for critically ill patients with ARF.
Supplementary Material
Acknowledgments
Dr. Sahetya received funding support from the National Heart, Lung, and Blood Institute under award number T32HL007534 and the Pearl M. Stetler Fellowship Award. Dr. Roldan disclosed government work. Dr. Checkley was supported by a Pathway to Independence Career Award (K99HL096955). Drs. Sahetya, Herrera, Chirinos, and Checkley received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
APPENDIX
The INTENSIVOS Cohort Study are as follows:
Roy G. Brower, Francesca Capanni, Maria A. Caravedo, Jorge Cerna, William Checkley, Eduardo E. Chirinos, Long Davalos, Aldo De Ferrari, Joshua A. Denney, Augusto Dulanto, Phabiola Herrera, Amador A. Jaymez, Nicole Mongilardi, Carmen Paredes, Enrique Paz, Maria Alejandra Pereda, Jose Portugal, Rocio Quispe, Rollin Roldan, and Navid Shams.
Footnotes
The INTENSIVOS Cohort Study are listed in the Appendix.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, et al. : Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 2.Neto AS, Barbas CSV, Simonis FD, et al. ; PRoVENT; PROVE Network investigators: Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): An international, multicentre, prospective study. Lancet Respir Med 2016; 4:882–893 [DOI] [PubMed] [Google Scholar]
- 3.Needham DM, Bronskill SE, Calinawan JR, et al. : Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med 2005; 33:574–579 [DOI] [PubMed] [Google Scholar]
- 4.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 5.Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 6.Chatburn RL, Mireles-Cabodevila E: Chapter 3. Basic principles of ventilator design. In: Principles and Practice of Mechanical Ventilation, 3e. Tobin MJ (Ed). New York, NY, McGraw-Hill, 2013, pp 65–110 [Google Scholar]
- 7.Marini JJ, Ravenscraft SA: Mean airway pressure: Physiologic determinants and clinical importance–Part 1: Physiologic determinants and measurements. Crit Care Med 1992; 20:1461–1472 [PubMed] [Google Scholar]
- 8.Denney JA, Capanni F, Herrera P, et al. ; INTENSIVOS Cohort Study: Establishment of a prospective cohort of mechanically ventilated patients in five intensive care units in Lima, Peru: Protocol and organisational characteristics of participating centres. BMJ Open 2015; 5:e005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson ND, Fan E, Camporota L, et al. : The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582 [DOI] [PubMed] [Google Scholar]
- 10.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 11.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force: Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 12.Hager DN, Krishnan JA, Hayden DL, et al. ; ARDS Clinical Trials Network: Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med 2005; 172:1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuckton TJ, Alonso JA, Kallet RH, et al. : Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002; 346:1281–1286 [DOI] [PubMed] [Google Scholar]
- 14.Gajic O, Afessa B, Thompson BT, et al. ; Second International Study of Mechanical Ventilation and ARDS-net Investigators: Prediction of death and prolonged mechanical ventilation in acute lung injury. Crit Care 2007; 11:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzer F, Menk M, Ziegler J, et al. : Predictors of survival in critically ill patients with acute respiratory distress syndrome (ARDS): An observational study. BMC Anesthesiol 2016; 16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trachsel D, McCrindle BW, Nakagawa S, et al. : Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2005; 172:206–211 [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L, Tonetti T, Cressoni M, et al. : Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med 2016; 42:1567–1575 [DOI] [PubMed] [Google Scholar]
- 18.Long Y, Su L, Zhang Q, et al. : Elevated mean airway pressure and central venous pressure in the first day of mechanical ventilation indicated poor outcome. Crit Care Med 2017; 45:e485–e492 [DOI] [PubMed] [Google Scholar]
- 19.Maayan C, Eyal F, Mandelberg A, et al. : Effect of mechanical ventilation and volume loading on left ventricular performance in premature infants with respiratory distress syndrome. Crit Care Med 1986; 14:858–860 [DOI] [PubMed] [Google Scholar]
- 20.Gullberg N, Winberg P, Selldén H: Changes in mean airway pressure during HFOV influences cardiac output in neonates and infants. Acta Anaesthesiol Scand 2004; 48:218–223 [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y, Debaty G, Puertas L, et al. : Effect of regulating airway pressure on intrathoracic pressure and vital organ perfusion pressure during cardiopulmonary resuscitation: A non-randomized interventional cross-over study. Scand J Trauma Resusc Emerg Med 2015; 23:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoyama H, Pettenuzzo T, Aoyama K, et al. : Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Crit Care Med 2018; 46:300–306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


