Abstract
To address the adverse effects of harmful algal blooms, there are increased demands over the implementation of ozone coupled with biologically active carbon (BAC) filters in the drinking water treatment plants. Although the microbial biofilms are vital elements to support the proper performance of BAC filters, except for taxonomic affiliations, little is known about the assembly mechanisms of microbial communities in the full-scale BAC filters. This study aimed to examine how the assembly processes and their associated factors (e.g., influent characteristics, biological interactions) drive the temporal dynamics of bacterial communities in full-scale BAC filters, which underwent ozone implementation (five consecutive seasons from 2017 to 2018). The results revealed that along with the increase of bacterial taxonomic richness and evenness, stochastic processes became more crucial to determine the bacterial community assembly in the summer and autumn after ozone implementation (relative contribution: 61.23% and 83.75%, respectively). Moreover, their corresponding networks possessed simple network structures with lower modularity than other seasons, which implied lesser biological interactions among bacterial populations. The correlation between taxonomic and predicted functional diversities using functional redundancy index indicated that relatively high levels of bacterial functional redundancy (>0.83) were generally present in BAC filters. However, compared to other seasons, significantly higher degrees of functional redundancy existed in the summer and autumn after ozone implementation (0.85 ± 0.01 and 0.86 ± 0.01, respectively). Overall, this work improves our understanding of the microbial ecology of full-scale BAC filters by providing a conceptual framework that characterizes bacterial biofilm assembly processes relevant to performance optimization of full-scale BAC filters.
Keywords: Drinking water treatment, Biologically active carbon filters, Bacterial community assembly processes, Ecological network structures, Functional potentials
GRAPHICAL ABSTRACT
1. Introduction
The increasing occurrence and severity of cyanobacterial harmful algal blooms (HABs) in freshwater have challenged the quality and safety of drinking water supplies worldwide (Laszakovits and MacKay, 2019; Li et al., 2020; Pivokonsky et al., 2016). To address emerging issues (e.g., the detection of various kinds of cyanotoxins in the finished water) and provide a reliable treatment barrier for cyanobacteria-impacted source water (Moosova et al., 2019), many water utilities with conventional treatment trains are examining innovative approaches and/or upgrading their existing drinking water treatment plants (DWTPs).
Ozonation, an advanced oxidation process, followed by biologically active carbon (BAC) filters (O3-BAC) has recently attracted much attention, especially in upgrading DWTPs, as it can remove a wide range of natural organic matter (NOM) and micropollutants while reducing the formation of disinfection byproducts (Chien et al., 2018; Ross et al., 2019; Zhang et al., 2018). For successful implementation for O3-BAC, it is widely accepted that the attached biofilms in the granular activated carbon (GAC) play a critical role to degrade the upstream ozonation byproducts (Boon et al., 2011; Gibert et al., 2013) and such biodegradation processes are highly impacted by biofilm biomass concentration, activity, and biomolecular compositions (Gibert et al., 2013; Ross et al., 2019; Zheng et al., 2018). Moreover, recent studies using 16S rRNA gene sequencing analyses have indicated that the filter performance and the biostability of waters in drinking water distribution systems (DWDSs) are significantly impacted by the temporal dynamics within BAC microbial community (Boon et al., 2011; Gerrity et al., 2018; Pinto et al., 2012; Zheng et al., 2018). However, these studies have mainly focused on the composition and structure of the microbial community, and consequently the underlying mechanisms of microbial community assembly in BAC filters remain largely unknown.
Based on ecological principles, it has been suggested that microbial communities primarily assemble through two distinctive ecological processes: (1) stochastic and/or (2) deterministic processes (Zhou and Ning, 2017). Stochastic processes involve immigration (also referred to as dispersal) and drift (e.g., birth, death, and reproduction), which claim that all the species are ecologically equivalent and they are assembled by random chance (Sloan et al., 2006). In contrast, deterministic processes feed on the ecological pressure imposed by abiotic or/and biotic factors, impacting organismal fitness and as a result, promoting the selection of certain species or groups under specific environmental conditions (Li et al., 2019b; Tilman, 2004). To date, although considerable efforts have been made to unravel the relative importance of each process in determining microbial community assemblages in various ecosystems (Li et al., 2019b; Yu et al., 2019; Zhang et al., 2020; Zhang et al., 2019), only one controlled lab-scale column study for BAC filters reported that the short-term assembly (56 days) of bacterial community was governed by deterministic processes (Vignola et al., 2018). Nevertheless, this conclusion may not be held to full-scale BAC filters, where the conditions are far more complex than laboratory tests. In real DWTPs, environmental conditions impacting microbial communities in BAC filters vary depending on the characteristics of upstream filter influents, types of filter media, and unit process configurations. Among them, the influent characteristics have been shown to significantly affect microbial community composition and structure (Ma et al., 2020). It is well known that ozonation can alter the chemical structures of complex organic compounds in the filter influent, subsequently changing its bioavailability (Hammes et al., 2006; Huang et al., 2005). However, little is known about how this change by ozonation affects microbial community composition, assembly processes, and functional traits in BAC filters for a consecutive period, although there is urgent need over the implementation of O3-BAC for many water utilities to handle the adverse effects of HABs. Moreover, the biological interactions among the filter communities may result in the enrichment of certain species (Oh et al., 2018). At a temporal scale, how factors such as biological interactions and filter influent properties mediate the balance between stochastic and deterministic processes for the microbial community assembly in BAC filters have not been studied yet.
The objectives of the study are to address the following questions: (1) What are the driving processes and their related factors (e.g., filter influent characteristics and species interaction) that regulate the temporal dynamics of bacterial biofilm community assembly in full-scale BAC filters? (2) How do the different assembly processes influence the potential bacterial functions in BAC filters? To address these questions, we studied a typical DWTP with conventional treatment processes that underwent ozone implementation to better handle cyanobacteria-laden source water. Data collected from five consecutive seasons were analyzed using the null model to quantify the relative contribution of each assembly process. Network analysis was also conducted to examine the interspecies interactions among the bacterial communities in BAC filters. Additionally, the 16S rRNA gene sequencing data was used to predict the corresponding changes of bacterial functions. The approach herein described provides a conceptual framework that better characterizes biofilm assembly processes relevant to performance optimization of full-scale O3-BAC systems in water utilities.
2. Materials and methods
2.1. Study site of the treatment plant
The studied DWTP is located in the City of Oregon (Ohio, USA). The plant has a 16 million gallon treatment capacity per day and receives its source water from Lake Erie, which experiences chronic HAB. The DWTP was selected for our study as it utilizes conventional drinking water treatment processes and recently implemented O3-BAC. Around this area, there are seven more conventional DWTPs which are planning to (e.g., Toledo Collins Park DWTP, Ohio), or have adopted (e.g., Carroll Township DWTP, Ohio) O3-BAC systems. Potassium permanganate is applied to limit the growth of zebra mussels in water intake conduits, followed by the addition of powdered activated carbon to address taste and odor issues. The treatment train consists of a series of unit processes, including coagulation-flocculation with aluminum sulfate, lime softening, sedimentation, recarbonation, BAC filtration, and chlorine disinfection. The DWTP replaced the old anthracite sand filter media with new GAC and sand in May 2017 and the use of the new filters began in July 2017. Afterwards, ozone pretreatment was implemented in December 2017. The applied ozone doses ranged between 0.5 and 4 mg/L to ensure that the residual ozone concentration at the outlet of the ozone contactor was zero, preventing any potential damage to the downstream filters. The upgraded DWTP system incorporated eight dual media filters comprising a layer of granular activated carbon (GAC, Calgon Carbon, Filtrasord 300 M, effective size: 0.8–1.0 mm) on sand (effective size: 0.35–0.45 mm) at a bed height ratio of 5.5:1. The details for the GAC specification are available in Table S1. During the study period, all the filters were operated with an average empty bed contact time of ~20 min and backwashed using finished water every 6–8 days. Free chlorine was added to the filter effluent and stored in two underground reservoirs before pumping to the DWDS.
2.2. Sample collection, processing, and chemical analyses
Samples were collected over 15 months (August 2017 to November 2018), and included sampling campaigns before ozone implementation in autumn 2017 (September–November) and after ozone implementation in the following four seasons: winter (December 2017–February 2018), spring (March–May 2018), summer (June–August 2018), and autumn (September–November 2018). Because of the O3-BAC implementation schedule of the DWTP (i.e., filter replacement, testing, BAC operation followed by ozone installation and integration with BAC), we were not able to collect more GAC samples before ozone implementation. This limited the number of samples analyzed before ozone installation. For sampling of the filter media, the filter was drained and the GAC media samples were collected every month before filter backwashing from the same filter bed at depths of 2 and 6 in. (5 and 15 cm) and transferred to sterile bags. In addition, the filter influent samples (i.e., water samples collected after ozonation/before BAC filtration) were collected in the pre-sterilized 4 L sampling bottles, which were prepared by rinsing them with 70% ethanol (Decon Labs, USA) solution three times for 3 min. The collected samples were immediately transported to the laboratory. Upon arrival, a portion of the filter media samples was used to quantify the total active biomass attached on the filter media using an ATP kit (LuminUltra, NB, Canada). The remaining subset of the media samples was transferred to sterile microcentrifuge tubes and stored at −80 °C for future use. Temperature, pH, turbidity, total organic carbon (TOC), and total nitrogen (TN) of filter influents were also measured. TOC and TN were analyzed in triplicates using the TOC/TN mode of a TOC analyzer (TOC-VCSH, Shimadzu, Japan) without pre-acidification and filtration steps for samples as the turbidity of filter influent was low (2.53 ± 0.98). Additionally, to assess the bioavailable carbon concentration in filter influents, the assimilable organic carbon (AOC) concentration was measured and details are described in the Supplemental section (Text S1).
2.3. DNA extraction, high-throughput amplicon sequencing, and data analysis
To examine the bacterial communities in BAC filters, DNA was first extracted from the filter media samples (~0.25 g of each) according to the manufacturer’s protocol (PowerSoil DNA isolation kit; Mo-Bio, CA, USA). The V4 region of bacterial 16S rRNA genes were amplified from the DNA extracts using the 515F and 806R primers (Caporaso et al., 2012). The metabarcoded sequencing libraries were generated using the Illumina Miseq and PE250 kits (Kapoor et al., 2016). The obtained raw sequence data was analyzed using the QIIME2 (v2019.1) (Bolyen et al., 2019). Briefly, primer sequences were first trimmed from the paired-end demultiplexed sequences using the Cutadapt plugin (Martin, 2011). Then, the obtained sequences were processed using DADA2 (Callahan et al., 2016), including quality filtering, denoising, merging, and chimera filtering, which subsequently resulted in a feature table artifact and a feature data artifact including a list of representative sequences with unique amplicon sequence variants (ASVs). The representative sequences were taxonomically classified using a scikit-learn naive Bayesian classifier trained on SILVA 16S rRNA gene reference database version 132 (Bokulich et al., 2018; Quast et al., 2012). The taxonomic alpha and beta diversity were estimated at the lowest sequencing depth of 11,952 using q2-diversity. Additionally, the functional traits were predicted using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) (Douglas et al., 2019). Using the feature table and representative sequences from the 16S rRNA gene data, the predicted gene family functions (here we used Enzyme Classification (EC) numbers) were generated by the script PICRUSt2 (picrust2_pipeline.py) with default options. The alpha and beta diversity of the predicted functions were estimated by subsampling the functional feature table to a depth of 9,529,784 reads. The EC numbers were collapsed into the corresponding MetaCyc pathway and the relative abundance of each pathway was calculated.
2.4. Network construction
To reveal the possible interactions among species, the phylogenetic molecular ecological networks (pMEN) of bacterial communities in BAC filters were constructed using a molecular ecological network analysis pipeline (MENA, http://ieg4.rccc.ou.edu/mena) (Deng et al., 2012) and visualized using Gephi (0.9.2) (Bastian et al., 2009). Only those ASVs detected in more than 50% of the samples were selected for network construction to reduce the network complexity. Briefly, the pairwise associations between ASVs were calculated based on Spearman correlation. Then, an appropriate threshold determined by the random matrix theory algorithm was applied to the association matrix, and only the correlation coefficients with absolute values above the selected threshold were retained for empirical network constructions. For comparison purposes their corresponding random networks were also generated using the Maslov-Sneppen method. To calculate the modular structure of pMENs, the fast-greedy optimization method was applied. A module in the network is a group of ASVs (nodes) that are highly interconnected themselves but only have a few connections outside the group. Modularity is a topological index describing how well a network could be divided into modules, where higher modularity usually implies a greater extent of niche differentiation (Faust and Raes, 2012; Shi et al., 2016). Other topological properties of the obtained networks were also calculated using the MENA pipeline. The average connectivity and clustering coefficient can be used to infer the complexity of networks, while the harmonic geodesic distance may imply the transfer efficiency of information within networks (Deng et al., 2012). Generally, shorter harmonic geodesic distance and complex network structures imply strong microbial interactions (Faust and Raes, 2012). Additionally, among-module connectivity (Zi) and within-module connectivity (Pi) were calculated to understand the topological role of bacterial ASVs in the obtained networks. According to the values of Z and P, nodes can be classified into four types: (1) peripherals (interconnected nodes inside a module with only a few connections outside the module, Zi ≤ 2.5 and Pi ≤ 0.62); (2) connectors (nodes linking to different modules, Zi ≤ 2.5, Pi > 0.62); (3) module hubs (highly connected nodes inside a module, Zi > 2.5, Pi ≤ 0.62); and (4) network hubs (acting as both module hubs and connectors, Zi > 2.5, Pi > 0.62) (Olesen et al., 2007).
2.5. Statistical analysis
Principal coordinate analysis (PCoA) based on the Bray-Curtis distance was applied to represent the temporal changes of bacterial community and functional structures. Multivariate statistical tests, including permutational multivariate analysis of variance (PERMANOVA, N = 999) and analysis of similarity (ANOSIM, N = 999), were subsequently conducted to determine the differences of bacterial community and functional structures. Kruskal-Wallis tests were performed to test the differences between the compared groups (e.g., the difference of taxonomic alpha diversity between two seasons) and the P values for the multiple comparisons were adjusted using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995). Spearman correlation tests were applied to determine whether a statistically significant relationship existed between two results or not (e.g., alpha diversity and water temperature). To quantify the relationship between taxonomic diversity and functional diversity among different seasons, functional redundancy indices were calculated (Ricotta et al., 2016). This index considers the differences between taxonomic diversity (Simpson’s index) and trait diversity (Rao’s quadratic entropy), where a community with higher taxonomic diversity or more species with similar traits may possess higher redundancy. To assess the relative importance of stochastic processes in bacterial community assembly in BAC filters, a general mathematical framework was adopted to calculate the normalized stochasticity ratio (NST) based on the Bray-Curtis distance (Ning et al., 2019). The differences of NST values between the compared groups were determined using the PERMANOVA test (N = 999).
3. Results
3.1. Physicochemical characteristics of filter influents
Table 1 shows the summary of physicochemical characteristics of filter influents. The temperature dramatically changed during the study, ranging from approximately 1 to 23 °C. The pH and TOC concentrations of filter influent were quite similar regardless of the seasons. The TN concentrations were relatively low in summer and autumn because of the excessive growth cyanobacteria in the lake, which can utilize different nitrogen sources (e.g., nitrate, ammonium) (Herrero et al., 2001; Smith and Schindler, 2009). A dramatical increase (more than two times higher) for the AOC concentration was observed after ozone implementation (winter 2017–2018).
Table 1.
Summary of the physicochemical characteristics of filter influents.
| Before ozone implementation |
After ozone implementation |
||||
|---|---|---|---|---|---|
| Autumn 2017 | Winter 2017–2018 | Spring 2018 | Summer 2018 | Autumn 2018 | |
| Temperature | 15.19 ± 6.63 | 1.64 ± 0.76 | 9.06 ± 7.65 | 23.62 ± 1.73 | 14.62 ± 8.15 |
| pH | 9.43 ± 0.16 | 9.37 ± 0.15 | 9.20 ± 0.01 | 9.27 ± 0.06 | 9.30 ± 0.10 |
| TOC (mg/L) | 1.81 ± 0.56 | 1.66 ± 0.47 | 1.99 ± 0.20 | 1.73 ± 0.68 | 1.61 ± 0.06 |
| TN (mg/L) | 0.64 ± 0.13 | 1.75 ± 0.71 | 1.22 ± 0.08 | 0.73 ± 0.22 | 0.60 ± 0.16 |
| AOC (ug/L) | 82.75 ± 15.26 | 207.36 ± 50.67 | 232.49 ± 71.40 | 225.36 ± 30.64 | 224.41 ± 52.60 |
3.2. Temporal dynamics of bacterial diversity and composition in BAC filters
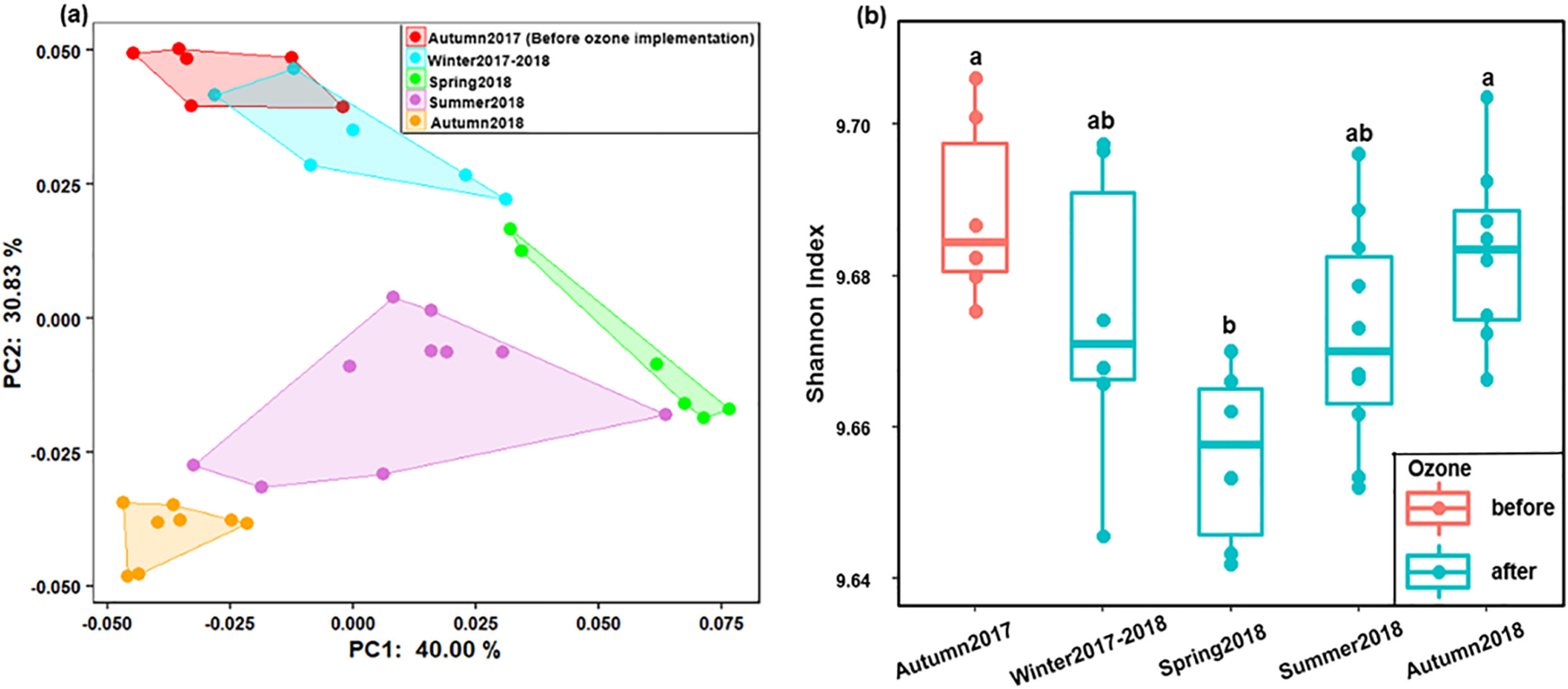
A total of 731,744 high-quality filtered sequences were retrieved from 36 samples, resulting in 1100 ASVs. The Good’s coverages for all the tested samples were higher than 99% when sequences were rarefied at the library size of 11,952, indicating that the selected sequencing depth was enough to capture the major diversity patterns. PCoA based on Bray-Curtis distances indicated that the bacterial community structures in BAC filters shifted in the studied period (Fig. 1a). Most of the samples in autumn 2017 and winter 2017–2018 were distributed along the PC2 (values ranging from 0 to 0.36) with similar PC1 values (~−0.34). In contrast, in autumn 2018, all samples were clustered together in the region with positive PC1 and PC2 values. Although overlaps between some seasons were observed, two dissimilarity test results (PERMANOVA and ANOSIM) revealed that bacterial community structure was distinctive to each season (Table S2), where the most pronounced differences were observed between autumn 2017 and 2018 samples (ANOSIM, R = 1, P <0.001). The alpha diversity also showed strong seasonal trends during the studied period. More diverse bacterial communities were found in the summer and autumn of 2018 samples (i.e., average Shannon indices of 6.03 ± 0.34 and 6.38 ± 0.23, respectively) than the other three seasons (Fig. 1b & Table S3, Kruskal-Wallis tests, P <0.05). Similar patterns were also found for the richness and evenness (Fig. S1 & Table S3, Kruskal-Wallis tests, P <0.05). Among the monitored physicochemical characteristics of filter influents, only temperature showed significantly positive correlations with the three alpha diversity indices (Spearman correlation, r = 0.53–0.57, P <0.05). Notably, these correlations increased when we excluded the autumn 2017 data (Spearman correlation, r = 0.62–0.73, P <0.05), demonstrating that the alpha diversity indices were better correlated with temperature during 2018.
Fig. 1.
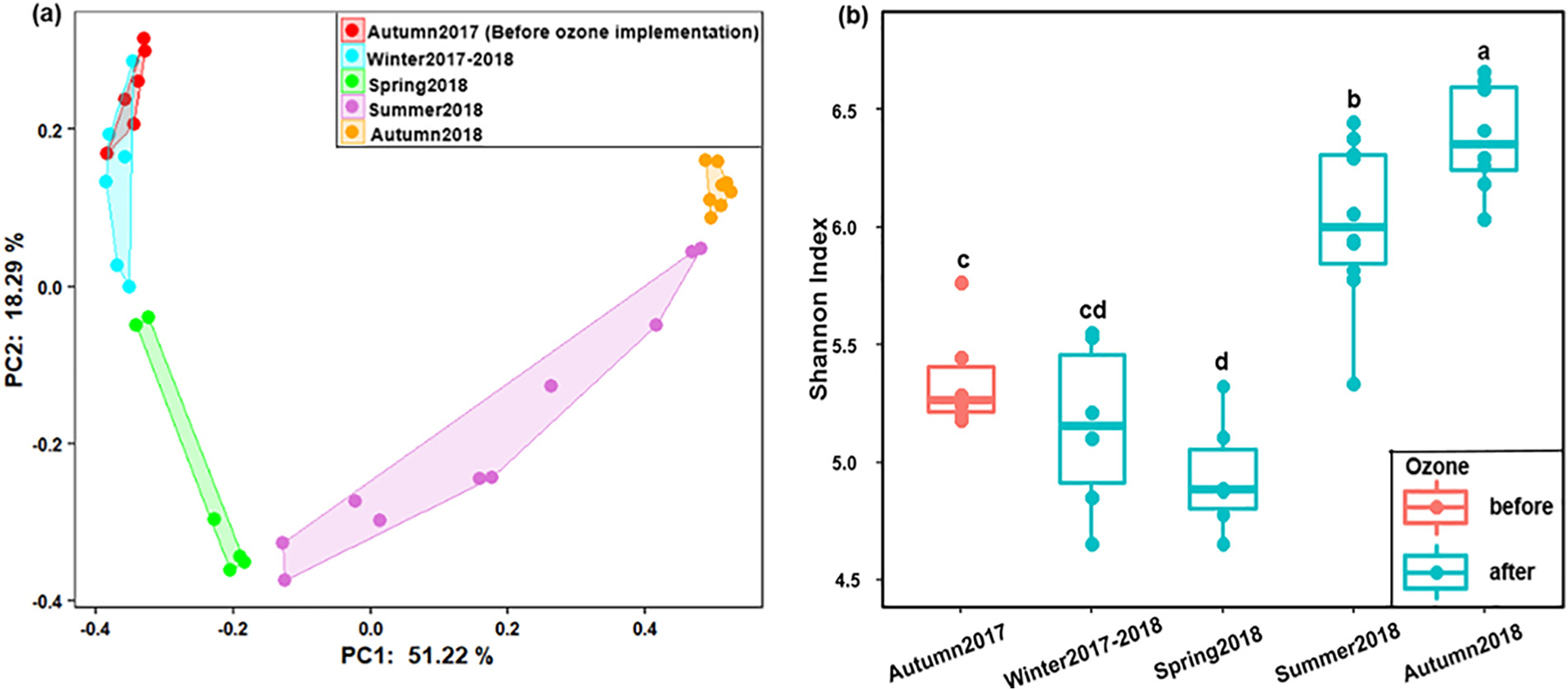
Temporal dynamics of bacterial community in BAC filters (a) Ordination of bacterial community compositions (at ASV level) by PCoA based on the Bray-Curtis dissimilarity matrix, (b) Species diversity characterized by the Shannon index. Kruskal-Wallis pairwise tests were performed to compare the differences of Shannon index values between seasons. Different lowercase letters above the boxes show significant differences in Shannon index values between seasons.
The most abundant phylum in BAC filters was Proteobacteria, accounting for 71.23–94.84% of bacterial sequences. Within Proteobacteria, changes in the relative abundance of Gammaproteobacteria were similar to a quadratic curve which had a highest value in spring 2018 (59.70 ± 10.91%), while opposite trends were observed for the relative abundance of Alphaproteobacteria (lowest relative abundance of 31.43 ± 8.62% in spring 2018, Fig. S2). At the genus level, several genera were predominant in autumn 2017, winter 2017–2018, and spring 2018 (e.g., Rhodanobacteraceae (family), SD04E11, Rhodobacteraceae, Methylotenera, and) (Fig. 2). In contrast, no extremely predominant genus was found in 2018 summer and autumn samples, which reflected the higher evenness in this period (Fig. S1b). The genus Bradyrhizobium associated with the degradation of aromatic compounds was frequently found and enriched in the BAC filters that receive pre-ozonated waters (Oh et al., 2018). However, this genus was only detected in summer and autumn 2018 after ozone installation in the winter of 2017.
Fig. 2.
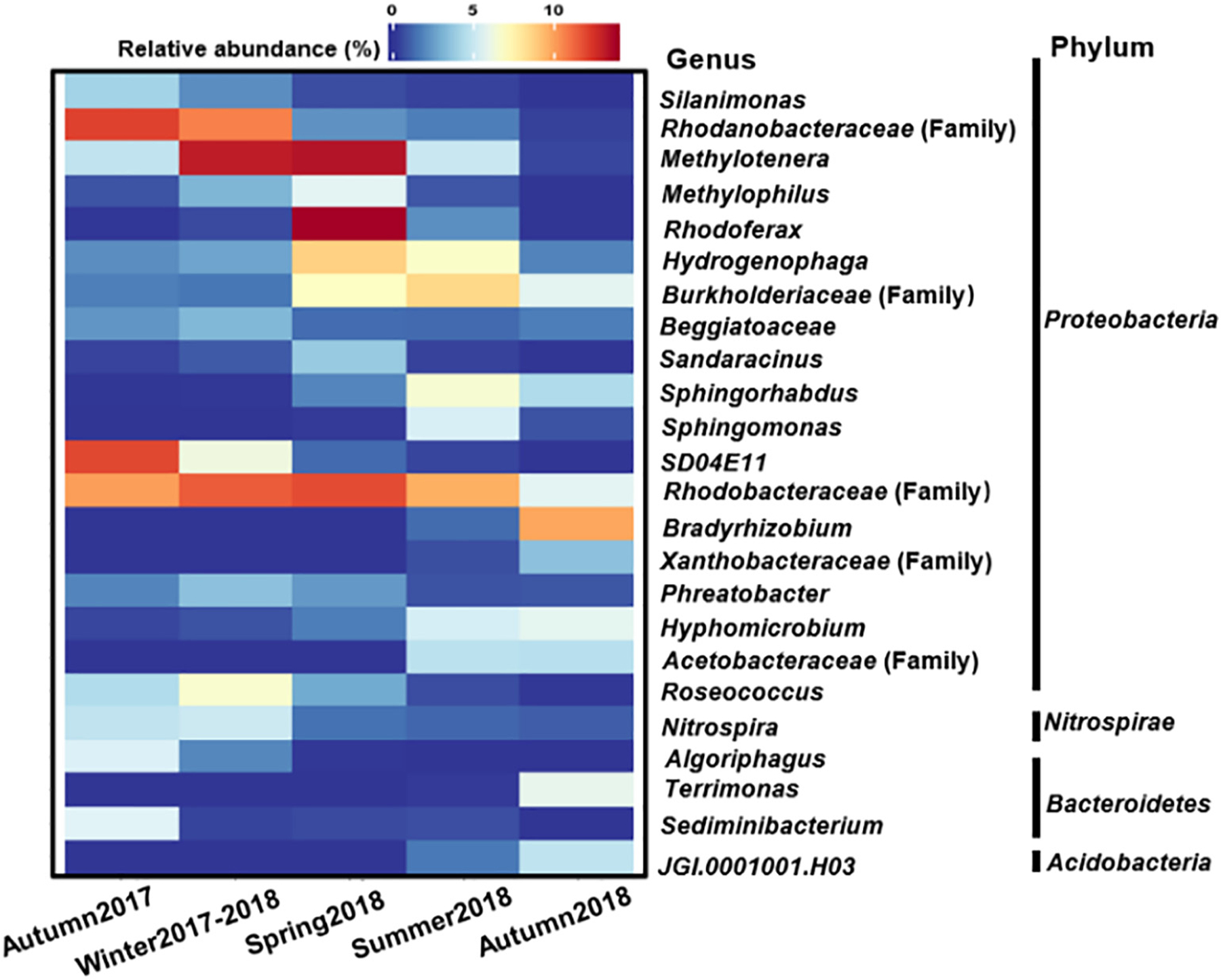
Heatmap of bacterial community compositions at the genus level in the BAC filters in different seasons (present at a relative abundance >3% in at least one season).
3.3. Topological features of bacterial networks in BAC filters
Since the temporal dynamics of bacterial community in BAC filters showed strong seasonal trends, five individual pMENs with the same applied threshold (0.948) for each season were constructed to examine the co-occurrence patterns of bacterial communities in BAC filters [Fig. 3 (a–e) & Table 2]. The modularity and average clustering coefficients of empirical networks were significantly higher than those of their corresponding random networks, suggesting that the networks appeared to be modular (Deng et al., 2016). The positive links (associations) were predominant in all the networks, accounting for 63.64% to 92.31% of the species-species associations in different seasons (Table 2). When we focused on the four seasons following ozone implementation, less connected and complex network structures with higher modularity were observed in the summer and autumn of 2018 than those of winter and spring as indicated by the lower average clustering coefficient, less average connectivity, and longer harmonic geodesic distance (Deng et al., 2016; Kara et al., 2013). On the other hand, when we compared the two autumn seasons (i.e., before and after ozone implementation), the results indicated that the pMEN in autumn 2017 (before ozone implementation) possessed several large modules, higher average connectivity and clustering coefficient, and shorter harmonic geodesic distance, resulting in the formation of more complex network structures with lower modularity. The Z-P plot shows that all nodes in the five obtained networks belonged to peripherals, demonstrating that overall, the module structures of networks in BAC filters did not have extreme reliance on particular bacterial species (Fig. 3f) (Zhang et al., 2020).
Fig. 3.
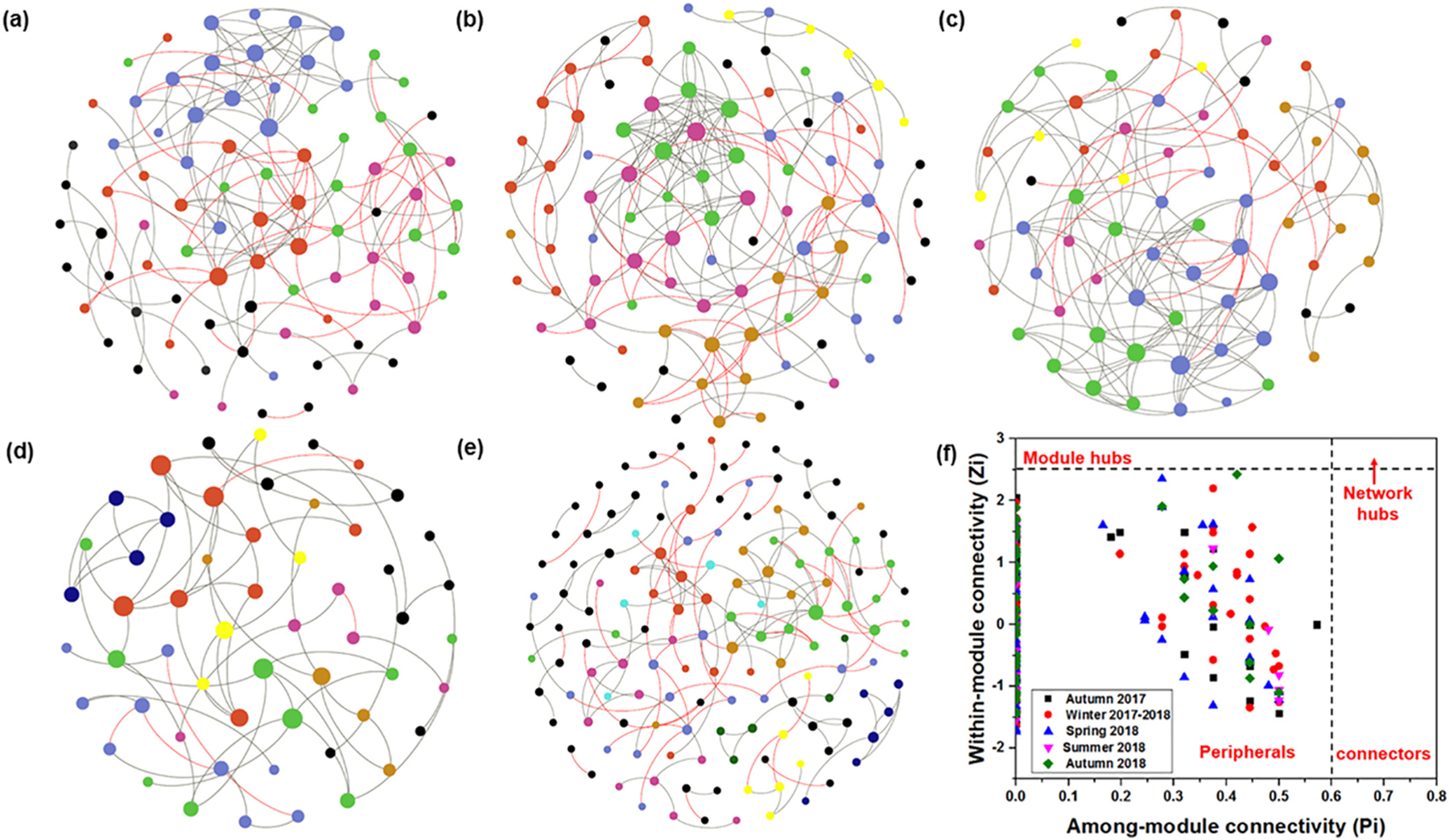
Networks in BAC filters in different seasons. (a–e) Visualization of co-occurrence networks for autumn 2017, winter 2017–2018, spring 2018, summer 2018, and autumn 2018. Nodes represent ASVs, and links between nodes indicate significant correlations. Positive links between nodes are colored grey and negative links are colored red. Modules are randomly colored at each season, and nodes in modules with less than five nodes are colored black. (f) Z-P plot based on the topological roles of each node in different seasons. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Topological properties of the empirical pMENs of bacterial communities in each season and their associated random pMENs.
| Empirical networks |
Random networks |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| network size | total links | similarity threshold | R2 of power law | Average connectivity | Harmonic geodesic distance | Average clustering coefficient | Modularity | Harmonic geodesic distance | Average clustering coefficient | Modularity | |
| Autumn 2017 | 86 | 149 | 0.948 | 0.863 | 3.465 | 5.419 | 0.417 | 0.717 | 3.257 ± 0.151 | 0.069 ± 0.024 | 0.489 ± 0.015 |
| Winter 2017–2018 | 95 | 185 | 0.948 | 0.810 | 3.895 | 6.731 | 0.514 | 0.658 | 3.258 ± 0.180 | 0.113 ± 0.025 | 0.456 ± 0.016 |
| Spring 2018 | 70 | 149 | 0.948 | 0.724 | 4.257 | 4.235 | 0.547 | 0.625 | 2.612 ± 0.074 | 0.110 ± 0.031 | 0.412 ± 0.017 |
| Summer 2018 | 55 | 65 | 0.948 | 0.679 | 2.364 | 10.452 | 0.359 | 0.791 | 3.876 ± 0.238 | 0.030 ± 0.029 | 0.629 ± 0.019 |
| Autumn 2018 | 139 | 154 | 0.948 | 0.918 | 2.216 | 17.911 | 0.347 | 0.850 | 6.213 ± 0.477 | 0.024 ± 0.016 | 0.730 ± 0.014 |
3.4. Variations of ecological processes in the bacterial community assembly in BAC filters
The null model analysis was performed to disentangle the relative importance of stochastic and deterministic processes in bacterial community assembly. Here the metacommunity was defined as all ASVs observed in the collected samples. A statistical index, NST (i.e., ranging from 0 to 1) was used to assess the contribution of stochastic processes in bacterial community assembly. Meanwhile, serving as the complement of NST, the normalized deterministic ratio was also calculated. Stochastic and deterministic processes happened simultaneously in determining the assembly patterns of bacterial communities in BAC filters across all the seasons, while stochasticity played a more dominant role (over than 60%) in 2018 summer and autumn samples (Fig. 4). The PERMANOVA tests further demonstrated that the stochastic processes in these two seasons were significantly higher than those of the other three seasons (Table S4). The increased stochasticity inferred that compared to the deterministic processes, stochastic events, such as neutral immigration from the filter influents and/or local random birth/death, predominated bacterial assembly in BAC filters in the summer and autumn of 2018.
Fig. 4.
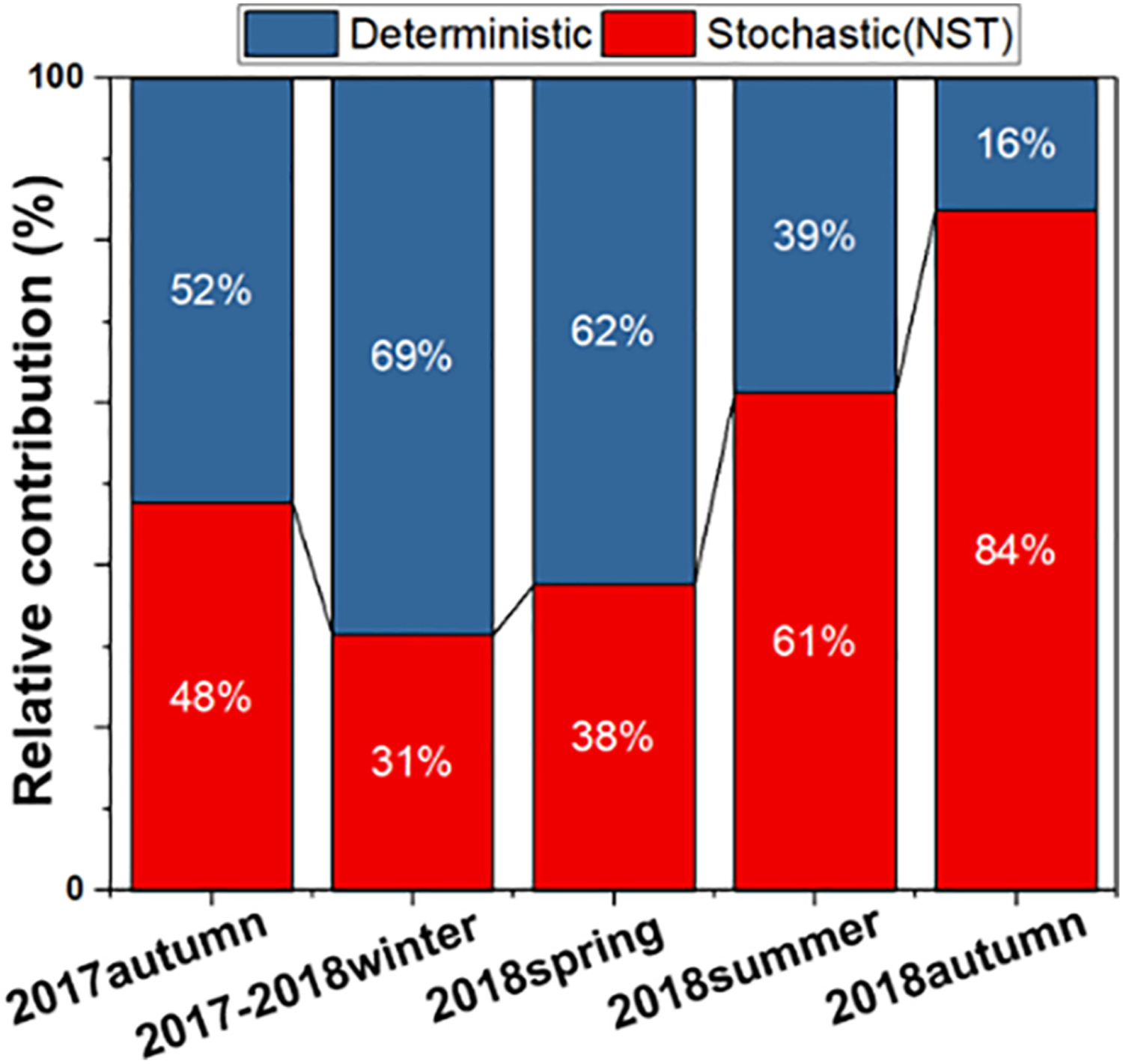
The relative contributions of deterministic and stochastic processes underlying the changes of bacterial community assembly in BAC filters in different seasons.
3.5. Prediction of bacterial functions in BAC filters using PICRUST2
In total, 1982 different functional traits (enzymatic numbers) were identified. The predicted functional traits using PICRUST2 were further annotated to the MetaCyc pathway six major categories: biosynthesis; detoxification; degradation/utilization/assimilation; generation of precursor metabolites and energy; metabolic clusters; and activation/inactivation/interconversion (Caspi et al., 2007). Aerobic respiration I (cytochrome c) was the most dominant pathway across different seasons with the relative abundance of 1.60 ± 0.02% (Fig. S3). This pathway is related to electron transfer processes. The electrons obtained from the oxidation process of cytochrome c can flow through the membrane-bound electron transfer chain to the terminal acceptors, which includes various kinds of organic/inorganic compounds (Caspi et al., 2011).
In addition, we rarefied all the samples at the depths of 9,529,784 (Good’s coverages >99%) to assess the core diversity metrics of potential functional traits. For the beta diversity, like the dynamics of bacterial communities, the functional profiles displayed seasonal associations, where the highest dissimilarities were observed between autumns 2017 and 2018 (Fig. 5a & Table S5). For the alpha diversity indices, although the richness of functional traits slightly increased in the summer and autumn of 2018, the evenness indices of these two seasons were the lowest (Fig. S4 & Table S6). Consequently, the bacterial functional diversity (Shannon indices) was similar in each season (Fig. 5b). These results demonstrated that the variation trends of bacterial functional and taxonomic diversities in BAC filters were different. Previous ecological studies have used several scenarios to describe the relationships between functional and taxonomic diversities (Guillemot et al., 2011; Micheli and Halpern, 2005). These studies suggest that when correlations followed a linear regression trend, if the slope equals one, each species may have unique functional traits. However, if the slope is less than one, some functional redundancy may exist as multiple species conduct similar/same functions. In this study, we found that the taxonomic and functional diversities (Shannon indices) had positive correlations with a slope equal to 0.01 (Fig. 6a), which implied that some functional redundancy might exist in the BAC bacterial communities in this study. To further quantify and compare the functional redundancy levels in different seasons, the functional redundancy indices were calculated (Fig. 6b). All the functional redundancy indices were higher than 0.83, further suggesting that overall high levels of functional redundancy exist in the BAC filters. Moreover, the results indicated that significantly greater extents of functional redundancy were present in the summer and autumn of 2018 than the other three seasons (Fig. 6b & Table S7, Kruskal-Wallis tests, P <0.05).
Fig. 5.
Temporal dynamics of bacterial functional traits in BAC filters (a) Ordination of bacterial functional traits (at Enzymatic number level) by PCoA based on the Bray-Curtis dissimilarity matrix, (b) Diversity of functional traits characterized by the Shannon index. Kruskal-Wallis pairwise tests were performed to compare the differences of Shannon index values between seasons. Different lowercase letters above the boxes show significant differences in Shannon index values between seasons.
Fig. 6.
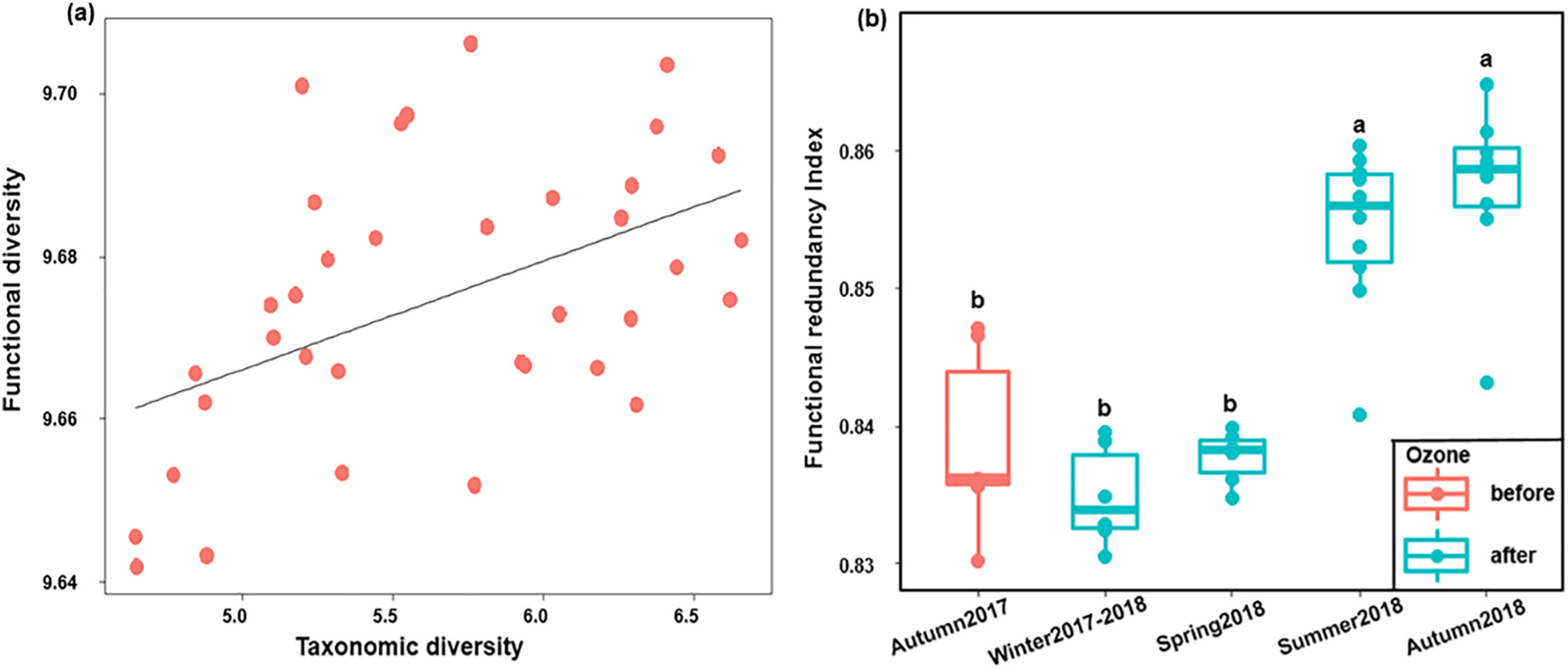
Relationships between bacterial taxonomic and functional traits in BAC filters (a) Linear regressions between taxonomic and functional diversities (the black line represents the fitted linear regression curve), (b) Functional redundancy index of bacterial communities. Kruskal-Wallis pairwise tests were performed to compare the differences of functional redundancy index values between seasons. Different lowercase letters above the boxes show significant differences in functional redundancy index values between seasons.
4. Discussion
Our study combined conventional sequencing analyses, ecological null model, and network analyses to, for the first time, shed light on the temporal dynamics of complex bacterial network structures, community assembly mechanisms, and functional potential in full-scale BAC filters, which underwent ozone implementation. The results indicated that the bacterial community characteristics (i.e., taxonomic diversity and topological properties of co-occurrence networks) in BAC filters are dynamic and that they are in part capable of responding to changes in environmental conditions via different assembly processes, which may subsequently affect the functional stability and redundancy of the bacterial community. Fig. S5 summarizes the possible linkages among our major findings.
4.1. The linkage among bacterial community network structures, diversities, and assembly processes in BAC filters
Previous studies indicated that temperature plays a significant role in controlling the bacterial community diversity and network structure in lakes and marine environments (Gilbert et al., 2012; Kara et al., 2013). Similar trends were also found in our study. After ozone implementation (i.e., within the 2018 seasons), the changes of bacterial diversity and network structure (e.g., modularity, average clustering coefficient) occurred concurrently with the increase of temperature rather than other monitored physicochemical characteristics of filter influents (Table 1). Moreover, the results also revealed that the increases in bacterial community diversity (Fig. 1b) in summer and autumn of 2018 coincided with the increase of stochastic assembly, network modularity, and the decline of the network complexity (Fig. 4 & Table 2). It can be proposed that during winter and spring, the relatively low temperature may be the primary selective pressure, inhibiting the activities of most taxa. Fewer bacterial groups become dominant [e.g., Methylotenera (average relative abundance: 11.53 ± 0.27%), Rhodobacteraceae (average relative abundance: 13.01 ± 0.19%)] (Fig. 2), resulting in observed lower diversities and uneven distribution of community compositions (Figs. 1b & S1b). Under these environmental settings, more taxa tended to be tightly connected in metabolic interactions and formed more complex network structures (supported by the higher average connectivity and shorter harmonic geodesic distance in Table 2). The level of interspecies interactions and the observed uneven distribution of bacterial communities strongly support the null model results that deterministic processes played a predominant role in shaping the bacterial assemblage in BAC filters in the winter and spring seasons (Fuhrman, 2009). In contrast, in the summer and autumn of 2018, due to the lack of strong environmental filtering factors (e.g., low temperature), the growth of those dormant/inactive low abundant taxa may be triggered (Jones and Lennon, 2010), increasing the community richness and evenness (Fig. S1). Under this mild condition, microbes may not have to heavily interact to resist adverse disturbance. Instead, they are likely to be less connected, relatively independent and far apart as indicated by the lower average connectivity and longer harmonic geodesic distance (Table 2), which can lead to higher niche differentiation in BAC filters. Therefore, a lesser extent of biological interactions might be present in the bacterial networks of summer and autumn than the spring and winter of 2018 as they possessed higher modularity and less complex structures. The decrease of interactions and the relief of major selection factors could lower the influences of deterministic processes. Consequently, the temporal dynamics of bacterial assemblages in the summer and autumn of 2018 were mostly driven by stochastic processes (61% and 84%, respectively; Fig. 4).
In addition to the seasonal changes, seasonal cycling patterns in bacterial community structures have been reported in DWDSs and wastewater treatment plants, despite the variability of chemical, biological, and physical environmental parameters year to year (Flowers et al., 2013; Pinto et al., 2014; Potgieter et al., 2018). In fact, for BAC filters, the results of a previous study also inferred that the bacterial communities might exhibit some interannual cyclical trends, where samples from corresponding seasons tend to form clusters (Kim et al., 2014). Similar seasonal reoccurring patterns were also found in a decade long study that focused on the topological properties of bacterial community networks in a lake (Kara et al., 2013). While the exact mechanism of these reoccurring patterns is still not clear, the authors postulated that interannually, under the low temperature, the lake bacterial community seem to “reset”, converging to a certain level, but start to recover along with the increase of temperature.
However, this seasonal cycling trend was not found in the current study when we compared the two autumn seasons (Fig. 1), which had similar temperatures and other environment parameters except for the AOC concentrations in the filter influent. The increase of AOC concentration was the most substantial change caused by ozone implementation (Table 1), which is consistent with previous studies (Hammes et al., 2007; Ross et al., 2019). Typically, NOM is the essential organic substrate for bacterial biofilm growth in DWTPs and DWDSs. Despite the fact that the NOM concentrations in filter influents could vary interannually for the same season, the amounts of available nutrients might not be altered significantly as most fractions of NOM are recalcitrant (Hozalski et al., 1999). In contrast, although the TOC concentrations would not change drastically upon ozone treatment, ozonation can significantly alter the chemical structures of those complex organic compounds, generating more AOC, which are mainly composed of more biodegradable, low molecular weight components (Hammes et al., 2006; Huang et al., 2005). In other words, compared to the seasonal changes of the source water characteristics, which may only have limited effects on the nutrient bioavailability, ozone significantly enhances the bioavailability of the organic substrate in filter influents. This might decrease the nutrient selective pressure posed by those recalcitrant compounds, promoting the growth of a broad range of taxa rather than a few dominant ones (Figs. 2 & S1). This is in agreement with a recent groundwater microbiome study, which suggested that rather than the organic nutrient concentration, the bioavailability of these compounds play a more critical role in determining the bacterial diversities in groundwater (Yan et al., 2020). Thus, besides the increase of active biomass (indicated by ATP values, Fig. S6), a higher diversity (including Shannon index, richness, evenness) was expected in BAC filters during autumn 2018 than autumn 2017 without ozone implementation. Moreover, it was reported that under relatively benign conditions, where the physicochemical conditions did not enforce strong selections, stochastic processes became more crucial, especially with increased resource availability (Dini-Andreote et al., 2015; Zhou et al., 2014). Thus, it can be inferred that the enhancement of nutrient bioavailability in filter influents of autumn 2018 played an essential role in increasing the stochastic events of bacterial communities compared to that of autumn 2017. On the other hand, according to the network analysis results (i.e., lower average connectivity and clustering coefficient, higher modularity, and longer harmonic geodesic distance were found in autumn 2018 than autumn 2017; Table 2), some taxa relying on metabolic byproducts from the degradation of recalcitrant compounds by other groups could then function independently by utilizing easily degraded compounds, subsequently creating new niches in the BAC filters. Therefore, compared to the previous year counterpart, lesser species interactions might be found in autumn 2018. These decreases in interactions could lower the impacts of deterministic processes, and along with the increase of stochasticity due to the changes of nutrient bioavailability, overall result in the predominance of stochastic processes in governing the bacterial community assembly during autumn 2018 rather than autumn 2017 (84% and 48%, respectively; Fig. 4).
4.2. The linkage between bacterial community diversities and functional traits in BAC filters
We displayed the linear regressions between taxonomic and functional diversities as well as the functional redundancy indices of different seasons (Fig. 6). The results demonstrated that multiple species might share similar/same functional traits in the bacterial communities and quantitatively support the existence of bacterial functional redundancy in BAC filters as speculated in previous studies (Kim et al., 2014; Li et al., 2017). Functional redundancy has been shown to be positively correlated with the functional stability in microbial systems (Jurburg and Salles, 2015; Kang et al., 2015). When environmental changes occur, while sensitive taxa may die or go dormant in response to the external disturbance, the presence of functionally redundant taxa may buffer the microbial communities against these disturbances, allowing the tolerant taxa to maintain proper functions within systems (Allison and Martiny, 2008; Jurburg and Salles, 2015; Kang et al., 2015). We found significantly higher degrees of functional redundancy present in the summer and autumn of 2018 as indicated by their functional redundancy indices (0.85 ± 0.01 and 0.86 ± 0.01, respectively; Fig. 6b). Thus, it can be inferred that annually (i.e., in summer and autumn 2018) and interannually after ozone implementation (two autumn season comparison), the bacterial communities may have a higher likelihood to maintain stable community functions and tend to be more resilient upon environmental stress. These findings were also supported by the highest taxonomic evenness found in the summer (0.80 ± 0.02) and autumn (0.82 ± 0.02) of 2018 (Fig. S1b), which was in agreement with the concept that highly diverse communities with even distribution are more functionally stable than the communities primarily occupied by several dominant taxa (Jurburg and Salles, 2015; Lu et al., 2011).
4.3. The linkage between bacterial functional traits and assembly processes in BAC filters
Results in this study revealed that annually (i.e., in summer and autumn 2018) and interannually after ozone implementation (two autumn seasons comparison), the temporal dynamics of bacterial communities in BAC filters were mostly driven by stochastic processes (Fig. 4). The bacterial communities in these two seasons appeared to be more diverse and formed simpler, less connected, and higher modular network structures than in the other seasons (Fig. 1b & Table 2). These observations led to the question of how these changes and characteristics are related to the functional traits of bacterial communities. Previous studies have examined the correlations between bacterial community functions and assembly mechanisms. For example, some studies have indicated that high proportions of stochasticity might be beneficial to community functions, while others suggested that stochasticity could add some detrimental taxa to a microbial community, decreasing the community functions (Leibold et al., 2004; Zhang et al., 2020). Our results suggest that higher diversity, along with the higher level of stochasticity, might enhance the functional redundancy of bacterial communities in BAC filters (Fig. S5). This finding was supported by the results of functional redundancy indices, where higher degrees of functional redundancy appeared to be evident in the summer and autumn of 2018 than the other seasons (Fig. 6b). The network analysis results also strengthened our findings (Table 2). Specifically, from the standpoint of functional ecology, modules could be considered as functional units within a network due to niche partition (Wu et al., 2016). In a homogenous environment, higher modular structures of networks (such as the networks of summer and autumn of 2018) are proposed to be associated with the formation of some cooperative and parallel niches, and taxa between different niches potentially have some overlapping functions, improving the systems’ functional redundancy (Faust and Raes, 2012; Lin et al., 2017).
On the other hand, regarding functional stability, previous studies demonstrated that although diverse communities may contain more species, new species introduced by stochastic events (e.g., immigration) may have different traits than the existing species, and they may change the community functions, potentially not beneficial to the bioreactors (Zhang et al., 2020; Zhang et al., 2019). However, our results indicated that in BAC filters, although the stochastic processes were predominant, the taxonomically diverse communities (i.e., summer and autumn 2018) also possessed higher taxonomic evenness and higher extent of functional redundancy (Figs. 1b, S1b, and S5), which may subsequently enhance the stability of the community functions. Moreover, from a macroecology viewpoint, communities with simple co-occurrence patterns and higher diversities have been linked with greater systems’ stability (Kara et al., 2013; McCann, 2000). This also suggested that the bacterial communities in BAC filters, like those represented by the summer and autumn of 2018, might have a higher capability to maintain the system’s functional stability than in the other three seasons (Fig. S5).
4.4. Engineering implication and future research needs
To date, most BAC filters are still operated as “black boxes” based on a combination of trial and error and empirical experience, which makes it difficult for engineers to go beyond current practices (Curtis et al., 2013). The fundamental knowledge needed to help engineers and operators understand the BAC filtration processes is limited. Our study provides novel ecological insights for better optimizing the performance of BAC filters. First, some previous studies reported that the removal performance (e.g., turbidity, TOC, and AOC) of BAC filters, even with the upstream ozone installation were not sufficient, especially at the low temperature (Pharand et al., 2015; Stoddart and Gagnon, 2015). Our results demonstrated that stochasticity, as one of the major drivers that affect the bacterial functional redundancy in BAC filters, can be potentially engineered and enhanced by increasing nutrient bioavailability. Thus, properly adjusting the ozone dosage and increasing the contact time of waters in the ozone contactor, or biostimulation by adding desirable organic nutrients into filter influents may be possible solutions to help promote the degree of bacterial functional redundancy in BAC filters and subsequently enhance filter performance. However, further investigations are needed for optimizing preozonation (e.g., ozone dosage and bioavailability of ozone byproducts for different NOM) and biostimulation (e.g., the appropriate nutrient recipes and dosages) for biofilters. Our results also revealed that along with the seasonal changes of bacterial taxonomic compositions, network structures, and functional traits, the functional stability of bacterial communities in BAC filters may also show seasonal variations in response to perturbations (Fig. S5). In BAC filters, apart from the external environmental disturbance, the backwash step regularly applied to filters can also be a disturbance to the bacterial biofilms. After backwashing, the functional traits of bacteria in BAC filters may be slightly changed due to biofilm detachment but would recover over time (Kim et al., 2014). The observed differences of functional stability in BAC filters in different seasons imply that the recovery time of bacterial functions after backwashing might also be different. Thus, developing operational plans for BAC filters (e.g., backwash frequency, intensity, and duration) are crucial to reduce the recovery time for the bacterial communities and maintain their proper functions and performance. More studies focused on elucidating correlations between operational parameters and the functional stability of bacterial communities in BAC filters are required in the future.
To estimate the functional potential of bacterial communities in BAC filters, we utilized PICRUST2, a 16S rRNA gene-based approach. It should be noted that other long-term experimental evidence using multi-omics analyses (e.g., metagenomics, metatranscriptomics, metaproteomics, and metabolomics) are necessary to further evaluate and confirm our 16S rRNA-based predicted results (Kirisits et al., 2019; Lawson et al., 2019). Additionally, we only measured the physicochemical properties of water samples that are commonly monitored by water utilities (Table 1). Other water characteristics (e.g., chemical compositions of organic matter from different sources) may also pose certain selective pressure to the bacterial communities and affect their assembly processes (Li et al., 2019a). Thus, additional studies that take multiple water chemistry parameters into considerations are needed to unravel the relative contribution of different environmental factors that may control the bacterial community assembly in BAC filters. Lastly, we acknowledge that to make more general conclusions and evaluate the universality of our findings, future studies must include analyzing additional filter samples from a broad range of DWTPs with different unit processes and filter operational conditions (Ma et al., 2020).
5. Conclusion
This work revealed that the topological properties of bacterial networks in BAC filters were largely impacted by the ozone implementation and the seasonality. Specifically, compared to the autumn after ozone installation, the network in the previous year counterpart (before ozone installation) harbored few modules, higher average connectivity and clustering coefficient with more complex network structures. After ozone installation, one-year based results indicated that simpler and higher modular network structures were present in the bacterial community of summer and autumn than spring and winter. In addition, the null model results demonstrated that the stochastic and deterministic processes co-regulate the temporal dynamics of bacterial community assembly in BAC filters. Among the monitored physicochemical properties of filter influents, temperature, nutrient bioavailability, and biological interactions can be crucial drivers that impact the balance between these two processes and the taxonomic diversity of bacterial communities in BAC filters. Lastly, the relationships between taxonomic and functional traits of bacterial communities suggested that high levels of bacterial functional redundancy may be viable in BAC filters. Higher levels of stochasticity along with higher community diversities appeared to enhance the bacterial functional redundancy and stability in BAC filters (e.g., summer and autumn 2018).
Supplementary Material
HIGHLIGHTS.
Bacterial community assembly mechanisms in BAC filters were studied.
Temporal changes in topological properties of biofilms in BAC filters were examined.
Temperature and ozonation affect bacterial interactions in BAC filters.
Functional redundancy was found in the bacterial communities of BAC filters.
Acknowledgments
This study was supported by the National Science Foundation of United States (CBET 1605185), the Ohio Water Development Authority (7174), the University of Toledo (Interdisciplinary Research Initiation Award), and in part by the U.S. Environmental Protection Agency. Any opinions expressed do not reflect the views of the agency; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use. Authors also appreciate the staff at the City of Oregon Water Treatment Plant for helping with sampling.
Footnotes
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141409.
References
- Allison SD, Martiny JB, 2008. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci 105, 11512–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian M, Heymann S, Jacomy M, 2009. Gephi: an open source software for exploring and manipulating networks. Third International AAAI Conference on Weblogs and Social Media. [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. [Google Scholar]
- Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. , 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. , 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon N, Pycke BF, Marzorati M, Hammes F, 2011. Nutrient gradients in a granular activated carbon biofilter drives bacterial community organization and dynamics. Water Res 45, 6355–6361. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP, 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. , 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6, 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Foerster H, Fulcher CA, Kaipa P, Krummenacker M, Latendresse M, et al. , 2007. The MetaCyc Database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 36, D623–D631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Altman T, Dreher K, Fulcher CA, Subhraveti P, Keseler IM, et al. , 2011. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 40, D742–D753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien I, Wu S-P, Ke H-C, Lo S-L, Tung H. h., 2018. Comparing ozonation and biofiltration treatment of source water with high cyanobacteria-derived organic matter: the case of a water treatment plant followed by a small-scale water distribution system. Int. J. Environ. Res. Public Health 15, 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T, Polchan M, Baptista J, Davenport R, Sloan W, 2013. Microbial community assembly, theory and rare functions. Front. Microbiol 4, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Jiang Y-H, Yang Y, He Z, Luo F, Zhou J, 2012. Molecular ecological network analyses. BMC Bioinforma 13, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zhang P, Qin Y, Tu Q, Yang Y, He Z, et al. , 2016. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol 18, 205–218. [DOI] [PubMed] [Google Scholar]
- Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF, 2015. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci 112, E1326–E1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas GM, Maffei VJ, Zaneveld J, Yurgel SN, Brown JR, Taylor CM, et al. , 2019. PICRUSt2: An Improved and Extensible Approach for Metagenome Inference. BioRxiv p. 672295.
- Faust K, Raes J, 2012. Microbial interactions: from networks to models. Nat. Rev. Microbiol 10, 538. [DOI] [PubMed] [Google Scholar]
- Flowers JJ, Cadkin TA, McMahon KD, 2013. Seasonal bacterial community dynamics in a full-scale enhanced biological phosphorus removal plant. Water Res 47, 7019–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman JA, 2009. Microbial community structure and its functional implications. Nature 459, 193–199. [DOI] [PubMed] [Google Scholar]
- Gerrity D, Arnold M, Dickenson E, Moser D, Sackett JD, Wert EC, 2018. Microbial community characterization of ozone-biofiltration systems in drinking water and potable reuse applications. Water Res 135, 207–219. [DOI] [PubMed] [Google Scholar]
- Gibert O, Lefèvre B, Fernández M, Bernat X, Paraira M, Calderer M, et al. , 2013. Characterising biofilm development on granular activated carbon used for drinking water production. Water Res 47, 1101–1110. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Steele JA, Caporaso JG, Steinbrück L, Reeder J, Temperton B, et al. , 2012. Defining seasonal marine microbial community dynamics. ISME J 6, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot N, Kulbicki M, Chabanet P, Vigliola L, 2011. Functional redundancy patterns reveal non-random assembly rules in a species-rich marine assemblage. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes F, Salhi E, Köster O, Kaiser H-P, Egli T, Von Gunten U, 2006. Mechanistic and kinetic evaluation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res 40, 2275–2286. [DOI] [PubMed] [Google Scholar]
- Hammes F, Meylan S, Salhi E, Köster O, Egli T, Von Gunten U, 2007. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res 41, 1447–1454. [DOI] [PubMed] [Google Scholar]
- Herrero A, Muro-Pastor AM, Flores E, 2001. Nitrogen control in cyanobacteria. J. Bacteriol 183, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozalski RM, Bouwer EJ, Goel S, 1999. Removal of natural organic matter (NOM) from drinking water supplies by ozone-biofiltration. Water Sci. Technol 40, 157–163. [Google Scholar]
- Huang W-J, Fang G-C, Wang C-C, 2005. The determination and fate of disinfection byproducts from ozonation of polluted raw water. Sci. Total Environ 345, 261–272. [DOI] [PubMed] [Google Scholar]
- Jones SE, Lennon JT, 2010. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci 107, 5881–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurburg SD, Salles JF, 2015. Functional redundancy and ecosystem function-the soil microbiota as a case study. In: Lo YH, et al. (Eds.), Biodiversity in Ecosystems-linking Structure and Function. Intech, Rijeka, pp. 29–49. [Google Scholar]
- Kang S, Ma W, Li FY, Zhang Q, Niu J, Ding Y, et al. , 2015. Functional redundancy instead of species redundancy determines community stability in a typical steppe of Inner Mongolia. PLoS One 10, e0145605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V, Elk M, Li X, Impellitteri CA, Santo Domingo JW, 2016. Effects of Cr (III) and Cr (VI) on nitrification inhibition as determined by SOUR, function-specific gene expression and 16S rRNA sequence analysis of wastewater nitrifying enrichments. Chemosphere 147, 361–367. [DOI] [PubMed] [Google Scholar]
- Kara EL, Hanson PC, Hu YH, Winslow L, McMahon KD, 2013. A decade of seasonal dynamics and co-occurrences within freshwater bacterioplankton communities from eutrophic Lake Mendota, WI, USA. ISME J. 7, 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TG, Yun J, Hong S-H, Cho K-S, 2014. Effects of water temperature and backwashing on bacterial population and community in a biological activated carbon process at a water treatment plant. Appl. Microbiol. Biotechnol 98, 1417–1427. [DOI] [PubMed] [Google Scholar]
- Kirisits MJ, Emelko MB, Pinto AJ, 2019. Applying biotechnology for drinking water biofiltration: advancing science and practice. Curr. Opin. Biotechnol 57, 197–204. [DOI] [PubMed] [Google Scholar]
- Laszakovits JR, MacKay AA, 2019. Removal of cyanotoxins by potassium permanganate: incorporating competition from natural water constituents. Water Res 155, 86–95. [DOI] [PubMed] [Google Scholar]
- Lawson CE, Harcombe WR, Hatzenpichler R, Lindemann SR, Löffler FE, O’Malley MA, et al. , 2019. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol 1–17. [DOI] [PMC free article] [PubMed]
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, et al. , 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett 7, 601–613. [Google Scholar]
- Li Q, Yu S, Li L, Liu G, Gu Z, Liu M, et al. , 2017. Microbial communities shaped by treatment processes in a drinking water treatment plant and their contribution and threat to drinking water safety. Front. Microbiol 8, 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jeon Y, Lee S-H, Ryu H, Santo Domingo JW, Seo Y, 2019a. Dynamics of the physiochemical and community structures of biofilms under the influence of algal organic matter and humic substances. Water Res 158, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao Y, Zhang W, Wang C, Wang P, Niu L, et al. , 2019b. Homogeneous selection dominates the microbial community assembly in the sediment of the Three Gorges Reservoir. Sci. Total Environ 690, 50–60. [DOI] [PubMed] [Google Scholar]
- Li L, Jeon Y, Ryu H, Santo Domingo JW, Seo Y, 2020. Assessing the chemical compositions and disinfection byproduct formation of biofilms: application of fluorescence excitation-emission spectroscopy coupled with parallel factor analysis. Chemosphere 246, 125745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, De Vrieze J, Li C, Li J, Li J, Yao M, et al. , 2017. Temperature regulates deterministic processes and the succession of microbial interactions in anaerobic digestion process. Water Res 123, 134–143. [DOI] [PubMed] [Google Scholar]
- Lu Y, Slater F, Mohd-Zaki Z, Pratt S, Batstone D, 2011. Impact of operating history on mixed culture fermentation microbial ecology and product mixture. Water Sci. Technol 64, 760–765. [DOI] [PubMed] [Google Scholar]
- Ma B, LaPara TM, Evans AN, Hozalski RM, 2020. Effects of geographic location and water quality on bacterial communities in full-scale biofilters across North America. FEMS Microbiol. Ecol 96, fiz210. [DOI] [PubMed] [Google Scholar]
- Martin M, 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17, 10–12. [Google Scholar]
- McCann KS, 2000. The diversity–stability debate. Nature 405, 228. [DOI] [PubMed] [Google Scholar]
- Micheli F, Halpern BS, 2005. Low functional redundancy in coastal marine assemblages. Ecol. Lett 8, 391–400. [Google Scholar]
- Moosova Z, Pekarova M, Sindlerova LS, Vasicek O, Kubala L, Blaha L, et al. , 2019. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 226, 439–446. [DOI] [PubMed] [Google Scholar]
- Ning D, Deng Y, Tiedje JM, Zhou J, 2019. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci 116, 16892–16898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Hammes F, Liu W-T, 2018. Metagenomic characterization of biofilter microbial communities in a full-scale drinking water treatment plant. Water Res 128, 278–285. [DOI] [PubMed] [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P, 2007. The modularity of pollination networks. Proc. Natl. Acad. Sci 104, 19891–19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharand L, Van Dyke MI, Anderson WB, Yohannes Y, Huck PM, 2015. Full-scale ozone–biofiltration: seasonally related effects on NOM removal. J. Am. Water Works Assoc 107, E425–E435. [Google Scholar]
- Pinto AJ, Xi C, Raskin L, 2012. Bacterial community structure in the drinking water microbiome is governed by filtration processes. Environ. Sci. Technol 46, 8851–8859. [DOI] [PubMed] [Google Scholar]
- Pinto AJ, Schroeder J, Lunn M, Sloan W, Raskin L, 2014. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. MBio 5, e01135–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivokonsky M, Naceradska J, Kopecka I, Baresova M, Jefferson B, Li X, et al. , 2016. The impact of algogenic organic matter on water treatment plant operation and water quality: a review. Crit. Rev. Environ. Sci. Technol 46, 291–335. [Google Scholar]
- Potgieter S, Pinto A, Sigudu M, Du Preez H, Ncube E, Venter S, 2018. Long-term spatial and temporal microbial community dynamics in a large-scale drinking water distribution system with multiple disinfectant regimes. Water Res 139, 406–419. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. , 2012. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta C, de Bello F, Moretti M, Caccianiga M, Cerabolini BE, Pavoine S, 2016. Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecol. Evol 7, 1386–1395. [Google Scholar]
- Ross P, van der Aa L, van Dijk T, Rietveld L, 2019. Effects of water quality changes on performance of biological activated carbon (BAC) filtration. Sep. Purif. Technol 212, 676–683. [Google Scholar]
- Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK, 2016. The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol. Lett 19, 926–936. [DOI] [PubMed] [Google Scholar]
- Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP, 2006. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol 8, 732–740. [DOI] [PubMed] [Google Scholar]
- Smith VH, Schindler DW, 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol 24, 201–207. [DOI] [PubMed] [Google Scholar]
- Stoddart AK, Gagnon GA, 2015. Full-scale prechlorine removal: impact on filter performance and water quality. J. Am. Water Works Assoc 107, E638–E647. [Google Scholar]
- Tilman D, 2004. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci 101, 10854–10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola M, Werner D, Wade MJ, Meynet P, Davenport RJ, 2018. Medium shapes the microbial community of water filters with implications for effluent quality. Water Res 129, 499–508. [DOI] [PubMed] [Google Scholar]
- Wu L, Yang Y, Chen S, Zhao M, Zhu Z, Yang S, et al. , 2016. Long-term successional dynamics of microbial association networks in anaerobic digestion processes. Water Res 104, 1–10. [DOI] [PubMed] [Google Scholar]
- Yan L, Herrmann M, Kampe B, Lehmann R, Totsche KU, Küsel K, 2020. Environmental selection shapes the formation of near-surface groundwater microbiomes. Water Res 170, 115341. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wu M, Petropoulos E, Zhang J, Nie J, Liao Y, et al. , 2019. Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ 656, 625–633. [DOI] [PubMed] [Google Scholar]
- Zhang S, Courtois S, Gitungo S, Raczko RF, Dyksen JE, Li M, et al. , 2018. Microbial community analysis in biologically active filters exhibiting efficient removal of emerging contaminants and impact of operational conditions. Sci. Total Environ 640, 1455–1464. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Deng Y, Feng K, Cai W, Li S, Yin H, et al. , 2019. Deterministic assembly and diversity gradient altered the biofilm community performances of bioreactors. Environ. Sci. Technol 53, 1315–1324. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ning D, Yang Y, Van Nostrand JD, Zhou J, Wen X, 2020. Biodegradability of wastewater determines microbial assembly mechanisms in full-scale wastewater treatment plants. Water Res 169, 115276. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lin T, Chen W, Tao H, Tan Y, Ma B, 2018. Removal of precursors of typical nitrogenous disinfection byproducts in ozonation integrated with biological activated carbon (O3/BAC). Chemosphere 209, 68–77. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ning D, 2017. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev 81, e00002–e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Deng Y, Zhang P, Xue K, Liang Y, Van Nostrand JD, et al. , 2014. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci 111, E836–E845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


