Abstract
Background
Gut is a crucial organ for the host’s defense system due to its filtering action of the intestinal membrane from hazardous foreign substances. One strategy to strengthen the gut epithelial barrier function is to upregulate beneficial microflora populations and their metabolites. Sophorolipid (SPL), which is a glycolipid bio-surfactant, could increase beneficial microflora and decrease pathogenic bacteria in the gastrointestinal tract. Therefore, herein, we conducted an experiment with broiler chickens to investigate the fortifying effects of SPL on the host’s gut defense system by modulating the microbiota population.
Methods
A total of 540 1-day-old chicks (Ross 308) were used, and they were immediately allotted into three treatment groups (6 replications with 30 chicks/pen) according to their initial body weight. The dietary treatments consisted of CON (basal diet), BAM (10 mg/kg bambermycin), and SPL (10 mg/kg SPL). During the experiment, birds freely accessed feed and water, and body weight and feed intake were measured at the end of each phase. On d 35, birds (one bird/pen) were sacrificed to collect jejunum and cecum samples.
Results
Dietary SPL and BAM supplementation significantly accelerated birds’ growth and also significantly improved feed efficiency compared to CON. Intestinal microbial community was significantly separated by dietary SPL supplementation from that of CON, and dietary SPL supplementation significantly increased Lactobacillus spp. and Akkermansia muciniphila. Moreover, birds fed with dietary SPL also showed the highest concentration of cecal butyrate among all treatment groups. Gut morphological analysis showed that dietary SPL significantly increased villus height, ratio of villus height to crypt depth, goblet cell numbers, and the gene expression levels of claudin-1 and mucin 2. Additionally, dietary SPL significantly decreased the mRNA expression level of pro-inflammatory cytokine, interleukin-6, and increased that of anti-inflammatory cytokine, interleukin-10, compared to other treatments.
Conclusions
Dietary SPL increases the beneficial bacterial population and butyrate concentration, which leads to a strengthened gut barrier function. In addition, the intestinal inflammation was also downregulated by dietary SPL supplementation.
Keywords: Broilers, Gut microbiota, Gut morphology, Local inflammation, Mucus barrier, Sophorolipids
Introduction
In the livestock industry, antibiotics have been widely used as growth promoters with sub-therapeutic dosage due to their outstanding efficacy in feed conversion and animal growth [1]. Nonetheless, the use of antibiotic growth promoters has been banned because the livestock fed with antibiotic growth promoter could serve as a reservoir of antibiotic-resistant bacteria [2]. Hence, it could threaten human life by transmitting antibiotic-resistant bacteria to humans by direct animal contact or indirect environmental contact [3].
Consequently, the development of novel and eco-friendly materials (e.g., probiotics, prebiotics, organic acids, essential oils, and enzymes) to replace antibiotic growth promoters is needed [4]. Additionally, various bio-surfactants have been investigated because of their antibacterial property [5]. Among bio-surfactants, sophorolipid (SPL) has received much attention in various industrial fields, such as medical, hygiene, and pharmaco-dermatological areas, due to their relatively less toxicity and more biodegradability [6].
SPL is a glycolipid-type amphiphilic compound similar to bambermycin, and it is produced by non-pathogenic yeast species, including Candida bombicola [7]. It consists of a nonpolar fatty acid tail of 16 or 18 carbon atoms and a polar dimetric carbohydrate head, which is linked by a glycosidic bond and binding of non-polar fatty acid and polar carbohydrate determines structure of SPL, acidic and lactonic forms [8]. Both forms of SPL are able to be produced by Candida bombicola, and they exert diverse biological properties according to their forms [9]. In acidic form, SPL shows high solubility with foam-forming ability by its higher esterification capacity, however, lactonic form of SPL exhibits better surface tension reducing activity and biological activity [10].
Collectively, SPL exerts diverse biological properties, including antibacterial activity, immunomodulation capacity, and stimulation of dermal fibroblasts and collagen production, [11, 12]. These properties imply that SPL has a potential to be applied in the animal feed industry for improving animal health and growth however, there are few reports on the application of this compound in this field. Hence, we conducted an experiment to evaluate the efficacy of SPL on gut microbial population and their metabolites could lead to improvement of growth performance in in broiler chickens.
Materials and methods
We conducted all of the studies on the birds in accordance with the guidelines and regulations of the Animal Ethics Committee approved by Korea University (Seoul, Republic of Korea), and it was carried out at a research farm in Cheonan, Republic of Korea (Approval number: KUIACUC-2020-0097).
Birds and diets
A total of 540 1-day-old male chicks (Ross 308) were used, and they were allotted into three experimental treatment groups according to their body weight (BW; initial BW: 40.1 g). Each treatment had six replicates with 30 chicks per pen. The dietary treatments consisted of CON (basal diet), BAM (10 mg/kg of bambermycin-supplemented diet), and SPL (10 mg/kg SPL supplemented diet). The feed composition is shown in Table 1, and the feed and SPL were supported by EASY BIO Inc. (Seoul, Republic of Korea). Birds freely accessed feed and water during the experiment, and their BW and feed intake were measured at the end of each phase (Phase 1: d 0–10; phase 2: d 11–20; phase 3: d 21–35) after 8 h of feed deprivation to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (FE). Birds were raised in a controlled experimental room with a rice hull with an average relative humidity of 60%. Temperature was maintained for 3 d at 30 °C and daily reduced by 0.5 °C to 24 °C, and lightening was provided by artificial light for 24 h/d.
Table 1.
Ingredients and nutritional values of basal diets
| Ingredients, % | Starter | Grower | Finisher |
|---|---|---|---|
| Corn | 60.92 | 56.29 | 62.07 |
| Soybean meal | 26.53 | 18.25 | 10.63 |
| Fermented soybean meal | 5.00 | 0.00 | 0.00 |
| DDGS | 0.00 | 5.00 | 5.00 |
| Unpolished rice | 0.00 | 4.00 | 3.00 |
| Rice bran polish | 0.00 | 1.00 | 1.50 |
| Rapeseed mineral | 0.00 | 4.00 | 3.00 |
| Sesameseed meal | 0.00 | 0.00 | 0.50 |
| Poultry meal | 2.50 | 5.50 | 8.00 |
| Animal fat | 0.00 | 1.72 | 2.00 |
| L-Lysine sulfate (55%) | 0.62 | 0.72 | 0.75 |
| L-Methionine (90%) | 0.47 | 0.35 | 0.31 |
| Threonine (98%) | 0.22 | 0.19 | 0.19 |
| L-Tryptophan (99%) | 0.01 | 0.03 | 0.03 |
| Choline chloride (50%) | 0.10 | 0.11 | 0.14 |
| MCP | 1.54 | 1.08 | 0.81 |
| Limestone | 1.45 | 1.23 | 1.55 |
| Salt | 0.25 | 0.25 | 0.25 |
| NaHCO3 | 0.05 | 0.05 | 0.05 |
| Vitamin premixa | 0.20 | 0.14 | 0.11 |
| Mineral premixb | 0.15 | 0.12 | 0.12 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated value | |||
| ME, kcal/kg | 2851.00 | 2945.00 | 3040.00 |
| CP, % | 21.85 | 20.40 | 19.00 |
| Ca, % | 1.00 | 0.90 | 1.04 |
| P, % | 0.77 | 0.70 | 0.64 |
| Lysine, % | 1.49 | 1.32 | 1.19 |
| Methionine, % | 0.75 | 0.62 | 0.56 |
| Threonine, % | 1.02 | 0.94 | 0.89 |
| Tryptophan, % | 0.25 | 0.23 | 0.20 |
aProvided per kilogram of complete diet: vitamin A, 6300 IU; vitamin D, 2,800 IU; vitamin E, 35 mg; vitamin K3, 1.75 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B6, 3 mg; vitamin B12, 13 μg; biotin, 0.1 mg; calcium pantothenic acid, 15 mg; folic acid, 1.5 mg; niacin, 50 mg
bProvided per kilogram of complete diet: Mn, 100 mg; Cu, 17 mg; Zn, 92 mg; Fe, 50 mg; I, 1.5 mg; Co, 0.15 mg; Se, 0.3 mg
Sample processing
At the end of the experiment, 18 birds (one bird per pen, randomly selected) were sacrificed, and the weight and length of the small intestine of the birds were measured. Jejunum and cecum samples were collected, immediately frozen, and stored at − 80 °C until further analysis. Parts of jejunum samples were fixed in 4% formalin solution for histological analysis.
Next generation sequencing
Quick-DNA™ Fecal/Soil Microbe Microprep Kit (Zymo Research, CA, USA) was used to extract total genomic DNA from the cecal samples of chicks according to the manufacturer’s protocol. For the microbial community structure analysis, V3-V4 regions of 16S rDNA were amplified using the following universal primer set (forward 5′-CCTACGGGNGGCWGCAG-3′, reverse 5′- GACTACHVGGGTATCTAATCC-3′), synthesized by Integrated DNA Technologies (IDT, Singapore). PCR conditions for amplifying the V3-V4 regions of 16S rDNA were as follows; 5-min initial denaturation at 98 °C, followed by 20 amplification cycles (30 s at 98 °C, 30 s at 56 °C, 1 min 30 s at 72 °C), 5 min final extension at 72 °C. The resulting DNA amplicons were purified using magnetic beads (TopQ XSEP MagBead, CELLMICS, USA). A second PCR was performed to attach the Illumina universal p5/p7 overhang sequence and sample-specific barcodes. Second PCR conditions were as follows; 1-min initial denaturation at 98 °C, followed by 10 amplification cycles (30 s at 98 °C, 30 s at 60 °C, 1 min at 72 °C), final extension at 72 °C 3 min. The sequencing-ready libraries were purified using magnetic beads (TopQ XSEP MagBead, CELLMICS, USA). Size distribution of the sequencing ready libraries were determined using the QSep fragment analyzer (Qsep Inc., Taiwan). DNA quantification is performed using the Quit dsDNA HS assay kit (Thermo Scientific, USA). Finally, all the libraries were pooled in equimolar quantities and diluted as necessary. Sequencing was performed on the Illumina MiSeq platform (Illumina, USA) using MiSeq Reagent Kit V3 (2 × 300 PE) (Illumina, USA). Mock DNA libraries were prepared with the ZymoBiomics microbial community DNA standard (Zymo Research, USA) using the amplicon library preparation procedure described above and sequenced together. This control library allowed us to evaluate potential biases and errors associated with the amplification and sequencing steps. Taxonomy profiling was performed using the open software program Quantitative Insights Into Microbial Ecology (QIIME) version 1.9.0 with NCBI 16S DB and BLASTN v2.3.0 [13]. We also performed a closed-reference operational taxonomic unit picking process to assign taxonomy using NCBI 16S DB and BLASTN (v2.3.0).
Gas chromatography–mass spectrometry
The concentration of short-chain fatty acids (SCFA) in the cecum contents was determined by gas chromatography–mass spectrometry (GC–MS). Briefly, 10 mg of cecal contents were homogenized with extraction solution consisting of 100 μL of internal standard (100 μmol/L crotonic acid), 100 μL hydrochloric acid, and 200 μL of ether. After vigorous vortexing for 10 min, the homogenates were centrifuged at 1,000×g for 10 min, and 80 μL of supernatants were transferred into new glass vials. Aliquots were mixed with 16 μL of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) and sealed tightly. The glass vials were heated at 80 °C for 20 min in a water bath, and then left at room temperature for 48 h for derivatization. The derivatized samples were run through a 6890 N Network GC System with an HP-5MS column and 5973 N network mass selective detector. Pure helium was used as carrier gas and delivered at a 1.2 mL/min flow rate. The head pressure was set at 97 kPa with a 20:1 split. The inlet temperature was 250 °C, and the transfer line temperature was 260 °C. The temperature program was as follows: 60 °C for 3 min, 60–120 °C (5 °C per min), and 120–300 °C (20 °C per min). The run time was 30 min, and SCFA concentrations were quantified by comparing their peak areas with standards.
Gut histological assay
Jejunum samples, fixed in formalin, were embedded into paraffin blocks to prepare 5-μm cross sections using a Rotary Microtome CUT 5062 (SLEE MAINZ, Mainz, Germany). These sections were stained with hematoxylin and eosin and Alican blue staining methods. Total 10 villi and 10 crypts were randomly selected per experimental unit. A single observer measured villus height (VH) and crypt depth (CD) and counted the goblet cells numbers.
qRT-PCR analysis
Total RNA from jejunum samples was extracted using Trizol® (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s procedure, and the concentration and purity of RNA were determined using Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Subsequently, cDNA samples were synthesized with the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. Target gene expression levels were determined using RealHelix™ Premier qPCR kit (NanoHelix, Daejun, Korea) with a StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Primers for the target genes are listed in Table 2, and the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is used as a house-keeping gene. The 2-ΔΔCT method was used to quantify relative mRNA expression levels.
Table 2.
Oligonucleotide primers used in jejunal qRT-PCR analysisa
| Gene name | Sequence (forward, reverse) | Reference |
|---|---|---|
| GAPDH | 5′-GAGGGTAGTGAAGGCTGCTG-3’ | [14] |
| 5′-CCACAACACGGTTGCTGTAT-3’ | ||
| CLDN1 | 5′-TGGAGGATGACCAGGTGAAGA-3’ | [15] |
| 5′-CGAGCCACTCTGTTGCCATA-3’ | ||
| MUC2 | 5′-TTCATGATGCCTGCTCTTGTG-3’ | [15] |
| 5′-CCTGAGCCTTGGTACATTCTTGT-3’ | ||
| IL-6 | 5′-GACGAGGAGAAATGCCTGACG-3’ | [16] |
| 5′-CCGAGTCTGGGATGACCACTTC-3’ | ||
| IL-10 | 5′-TCTACACAGATGAGGTCCTGCC-3’ | [16] |
| 5′-AGGTGAAGAAGCGGTGACAG-3’ |
aAbbreviations: CLDN1 claudin-1, GAPDH glyceraldehyde-3-phosphate dehydrogenase, IL-6 interleukin-6, IL-10 interleukin-10, MUC2 mucin 2, OCLD occludin, ZO-1 zonula occludens-1
Statistical analysis
All data were analyzed using analysis of variance (ANOVA) with Statistical System 9.4 (SAS Institute, Cary, NC, USA). Significant differences between treatments were determined using Duncan’s multiple-range tests and were defined at the P < 0.05 level.
Results
Growth performance
As indicated in Table 3, birds in the BAM and SPL groups showed significantly higher BW and ADG (P < 0.05) compared to those in the CON group. There were no significant differences in average feed intake between treatments, however, feed efficiency was significantly higher (P < 0.05) in the BAM group compared to CON group.
Table 3.
Effects of sophorolipid on growth performance of broilers1,2,3
| Treatment | CON | BAM | SPL | SEM | P-value |
|---|---|---|---|---|---|
| Initial BW, g | 40.16 | 40.12 | 40.19 | 0.045 | 0.853 |
| Final BW, g | 1911.8b | 2011.6a | 2010.3a | 18.587 | 0.035 |
| ADG, g/d | 53.48b | 56.33a | 56.29a | 0.531 | 0.035 |
| ADFI, g/d | 88.62 | 87.89 | 90.45 | 0.719 | 0.344 |
| FE | 0.60b | 0.64a | 0.62ab | 0.014 | 0.010 |
1Treatments: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid supplemented diet
2Abbreviations: ADFI average daily feed intake, ADG average daily gain, BW body weight, FE feed efficiency, SEM standard error of means
3A pen is an experimental unit; 6 replications (pens) per treatment; 30 chicks per pen
a, b Mean values within a row have different superscript letters were significantly different (P < 0.05)
Intestinal microbial population
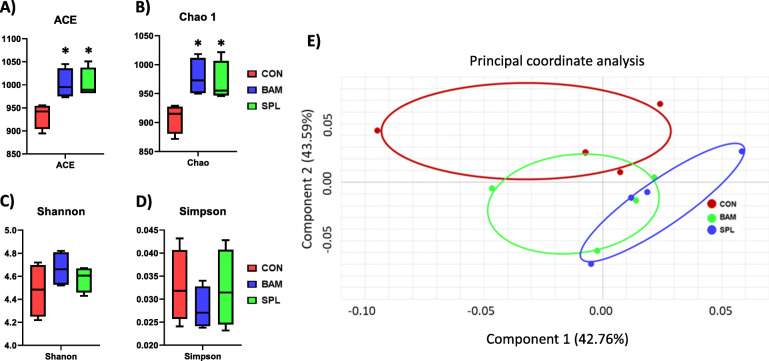
Dietary BAM and SPL supplementation significantly increased ACE and Chao 1 indexes compared to CON group (P < 0.05), however, Shannon and Simpson indexes were not different among treatments (Fig. 1a–d). Cecal microbial communities of BAM and SPL groups were partially and completely separated from that of CON group (Fig. 1e).
Fig. 1.
Cecal microbial community of broiler chickens fed experimental diets. a-b Dietary effects of bambermycin and sophorolipid on species richness indexes (ACE and Chao1); c-d Dietary effects of bambermycin and sophorolipid on diversity indexes (Shannon and Simpson); e Principal component Analysis ordination plots of microbial communities in the CON, BAM, and SPL groups based on the Jensen-Shannon distance metric. *P < 0.05 compared with CON group. Treatment groups: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid-supplemented diet
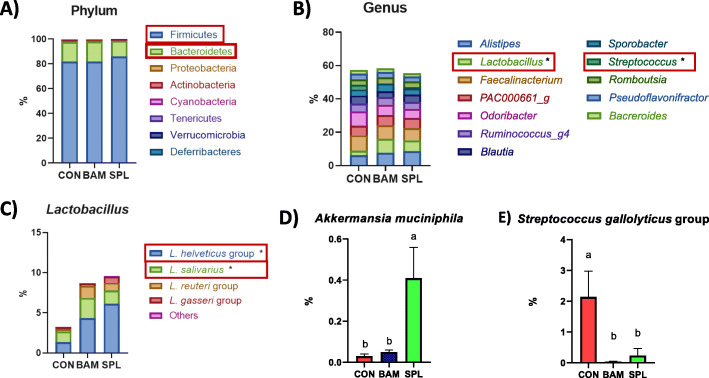
At phylum level, dietary SPL supplementation increased Firmicutes population and decreased Bacteroidetes population (Fig. 2a). Additionally, dietary SPL supplementation significantly increased (P < 0.05) the genus level of Lactobacillus and decreased (P < 0.05) that of Streptococcs (Fig. 2b). At the species level, dietary SPL supplementation significantly increased (P < 0.05) the populations of Lactobacillus helveticus and Lactobacillus salivarius in the SPL group compared to their populations in the CON group (Fig. 2c), and the SPL group also showed a significantly higher (P < 0.05) level of Akkermansia muciniphila compared to the other treatment groups (Fig. 2d). The population of the Streptococcus gallolyticus group was significantly decreased (P < 0.05) by dietary BAM and SPL supplementation (Fig. 2e).
Fig. 2.
Gut microbiota population of broilers fed with experimental diets. a Intestinal microflora at phylum; b Intestinal microflora at genus level; b–d Specific bacterial populations at the species level in the cecum of birds (Lactobacillus family, Akkermansia muciniphila, and Streptococcus gallolyticus group). *P < 0.05 compared with CON group. a, bMean values within a row have different superscript letters were significantly different (P < 0.05). Treatment groups: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid-supplemented diet
Cecal short-chain fatty acid concentration
As listed in Table 4, dietary SPL supplementation significantly increased (P < 0.05) the total concentration of SCFA, and those of acetate and butyrate in the SPL group compared to that in the BAM group. In addition, the ratio of propionate was significantly lowered (P < 0.05) in SPL group compared to BAM group.
Table 4.
Effects of sophorolipid on cecal SCFA concentration of broilers1,2,3
| Treatment | CON | BAM | SPL | SEM | P-value |
|---|---|---|---|---|---|
| Absolute concentration of SCFA, μmol/g | |||||
| Total SCFA | 226.43a | 179.47b | 249.61a | 10.802 | 0.012 |
| Acetate | 185.91a | 143.14b | 206.06a | 9.984 | 0.017 |
| Propionate | 17.62 | 15.73 | 17.18 | 0.676 | 0.526 |
| iso-butyrate | 3.69 | 3.84 | 4.01 | 0.208 | 0.850 |
| Butyrate | 19.20ab | 16.76b | 22.36a | 0.978 | 0.049 |
| Percentage of SCFA, % | |||||
| Acetate | 82.11 | 78.98 | 82.48 | 0.852 | 0.187 |
| Propionate | 7.76ab | 8.91a | 6.88b | 0.322 | 0.021 |
| iso-butyrate | 1.66 | 2.17 | 1.60 | 0.130 | 0.140 |
| Butyrate | 8.47 | 9.95 | 9.04 | 0.615 | 0.647 |
1Treatments: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid supplemented diet
2Abbreviations: SCFA short-chain fatty acid, SEM standard error of means
3A chick is an experimental unit; 6 replications per treatment
a, b Mean values within a row have different superscript letters were significantly different (P < 0.05)
Intestinal characteristics and histological analysis
As shown in Table 5, birds fed with the SPL-supplemented diet (SPL group) showed significantly reduced (P < 0.05) intestinal weight compared to those of the other treatment groups; however, gut weight per length was not changed by SPL supplementation. Hence, dietary SPL supplementation significantly increased (P < 0.05) VH compared to that of birds in the other treatment groups without affecting CD (Table 5). The ratio of VH to CD in the birds fed with the SPL-supplemented diet was the highest (P < 0.05) compared to that of birds in CON group. Moreover, the goblet cell numbers per 1 μm of villus were also significantly increased (P < 0.05) in the SPL treatment group compared to that in the other groups.
Table 5.
Effects of sophorolipid on gut characteristic and histological analysis of broilers1,2,3
| Treatment | CON | BAM | SPL | SEM | P-value |
|---|---|---|---|---|---|
| Intestinal, g/100 g body weight | 3.18a | 3.08ab | 2.96b | 0.037 | 0.038 |
| Intestinal weight/length, g/m | 30.60 | 31.12 | 30.54 | 0.319 | 0.735 |
| Villus height, μm | 371.27b | 398.17ab | 422.50a | 7.483 | 0.010 |
| Crypt depth, μm | 110.58 | 104.40 | 105.50 | 1.366 | 0.142 |
| Villus height/crypt depth | 3.37b | 3.82a | 4.01a | 0.099 | 0.013 |
| Goblet cells/villus height, /μm | 0.22b | 0.21b | 0.34a | 0.016 | < 0.001 |
1Treatments: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid supplemented diet
2Abbreviation: SEM standard error of means
3A chick is an experimental unit; 6 replications per treatment
a, b Mean values within a row have different superscript letters were significantly different (P < 0.05)
Gene expression levels related to inflammation, tight junction, and mucin secretion
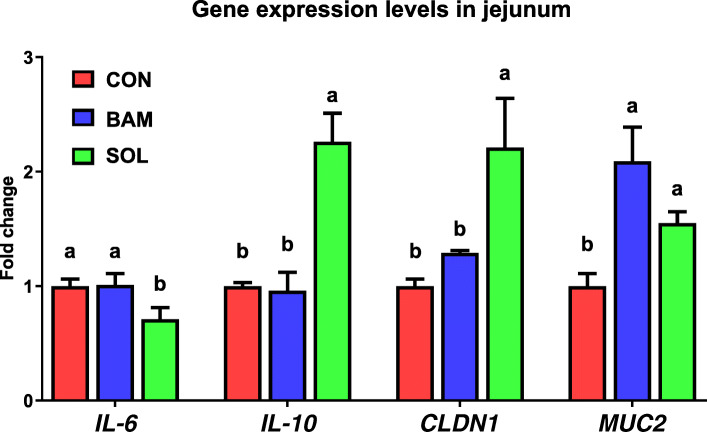
As presented in Fig. 3, dietary SPL supplementation significantly downregulated (P < 0.05) the expression level of interleukin-6 (IL-6) and upregulated (P < 0.05) that of interleukin-10 (IL-10) compared to the other treatments. The expression level of claudin-1 (CLDN1) significantly increased (P < 0.05) in the SPL group compared to the other groups. Moreover, the BAM and SPL groups showed significantly increased (P < 0.05) expression level of mucin 2 (MUC2) compared to the CON group.
Fig. 3.
mRNA expression levels of genes related to inflammation, tight junction, and mucin in the jejunum of birds fed with experimental diets. a, bMean values within a row have different superscript letters were significantly different (P < 0.05). Treatment groups: CON, control group fed with basal diet; BAM, group fed with 10 mg/kg of bambermycin supplemented diet; SPL, group fed with 10 mg/kg of sophorolipid-supplemented diet. Abbreviations: IL-6, interleukin-6; IL-10, interleukin-10; CLDN1, claudin-1; MUC2, mucin 2
Discussion
First, the results of this study indicated that SPL could accelerate the growth of broilers as much as bambermycin, which is in agreement with the reports of Boontiam et al. [17] which demonstrated that surfactant supplementation with a low energy and crude protein diet could improve average daily gain and feed efficiency during the overall experimental period without affecting average daily feed intake. Furthermore, both glycolipid-type antibiotics and biosurfactant increased the beneficial microbiota L. heliveticus and L. salivarius and decreased the pathogenic bacteria S. gallolyticus. In accordance with our results, Abu Hafsa and Ibrahim [18] also demonstrated that similar modulation of gut microbiota (increased Lactobacillus and decreased Sterptococcus) by dietary probiotic supplementation significantly improved the growth of birds. Various reports related Lacobacillus species have suggested that L. heliveticus is known as the beneficial microbes in bone and mental health and L. salivarius has a positive relationship with growth of birds [19–21]. Collectively, microflora communities modulated by bambermycin and SPL were similar, however SPL have seemed to accelerate the shift of the community compared to that of bambermycin. Also, these results suggested that this microbial shift has a potential to improve the growth and health of birds by modulation of their metabolites.
On the other hand, SCFA are the main metabolites of microbial fermentation, and they have been widely studied to elucidate the specific mechanism of antibiotic growth promoter linking the host and its intestinal microbiota [22]. In 2019, Guinan et al. demonstrated that water supply with antibiotic could downregulated the SCFA concentration in cecal contents by increased colonization of Candida albicans [23]. In agreement with this study, the results of our study demonstrated that dietary bambermycin supplementation significantly reduced the concentration of total SCFA compared to birds fed control diet and SPL supplemented diet. Additionally, both acetate and butyrate were increased in the SPL group than BAM group, which might be due to the increased populations of butyrate-producing bacteria, L. helveticus and mucin-degrading bacteria, A. muciniphila [24, 25]. An in vitro study with L. helveticus showed that this probiotics strain significantly increased butyrate concentration, and it may be due to the upregulated conversion ratio of lactate to butyrate by lactating-utilizing bacteria [26]. And A. muciniphila supplemented with mucin could produce acetate and ethanol from mucin fermentation [27]. Collectively, these results suggest that dietary SPL treatment maintain the cecal SCFA concentration by intestinal colonization of SCFA-producing bacteria unlike bambermycin supplementation.
The gastrointestinal tract is the main region that plays a role in a protective system because it is the first organ to meet external substances with a large contact area [28]. Hence, the gut defense strategy employs various physiological factors (e.g., mucus barrier, tight junctions, and immune cytokines) to maintain the intestinal homeostatic balance [29]. Our results suggest that dietary SPL supplementation could enhance mucin-presenting capacity and villus turnover balance by increasing beneficial bacterial populations. At phylum level, there were higher portion of Firmicutes, and lower that of Bacteroidetes in SPL treatment group compared to the other groups. Bacteroides, known as the LPS-generating bacteria group when they are lysed, may play a critical role to destruct intestinal integrity and morphology by lowering intestinal permeability [30], resulted in the lower Firmicutes/Bacteroidetes ratio is considered as the favorable index of weight loss [31]. At species level, Akkermansia muciniphila, has received attention because of its specific biological properties including immune modulation, wound healing, and SCFA production [32]. Moreover, various studies have demonstrated that A. muciniphila has the potential to act as a probiotic because it could exert a glucose-lowering effect by regulating gut barrier integrity through increased expression levels of tight junction proteins [33, 34]. Similar to the results of these studies, we also found an increased population of A. muciniphila and strengthened gut epithelial integrity and mucus secretion capacity.
Additionally, our results demonstrated that SPL could relieve the local gut immune response and strengthen the intestinal epithelial barrier by modulating the gut microbiota population. S. gallolyticus, a gram-positive pathogenic bacterium, which is commonly found in various animals and humans, and it is a potential transmission bacterium with antibiotic resistance [35, 36]. In addition, Li et al. [37] demonstrated that inoculation of S. gallolyticus in duckling induced macrophage necroptosis in spleens by bacterial infection and increased the expression levels of IL-6, and that decreased population of S. gallolyticus achieved in response to dietary oregano powder has a negative relationship with the increase of anti-inflammatory cytokine IL-10 [38]. In accordance with the results of these previous studies, decreased population of pathogenic bacteria (S. gallolyticus) by sophorolipid supplementation has the potential to alleviate immune responses by improving pro- (IL-6) and anti-inflammatory (IL-10) cytokine production. On the other hands, our results also found that the higher population of Lactobacillus genus in BAM and SPL groups compared to CON group. Lactobacillus is the genera of gram positive and facultative anaerobes and it can inhibit the pathogen growth by establishment of low pH environment through lactic acid producing capacity [39]. Therefore, our results demonstrated that microbial shift by SPL might be able to modulate immune response in broilers’ intestine.
Conclusions
Dietary sophorolipid supplementation in broiler feed could modulate the intestinal microbiota population and short-chain fatty acid levels. Hence, the promoted gut environment could improve gut defense integrity, including intensified mucus layer and tight junction, and alleviate local inflammation, resulting in the acceleration of chick growth. Further studies are required to elucidate the precise relationship between sophorolipids and their effects on intestinal health.
Acknowledgements
Not applicable.
Abbreviations
- ADFI
Average daily feed intake
- ADG
Average daily gain
- ANOVA
Analysis of variations
- BW
Body weight
- CD
Crypt depth
- CLDN1
Claudin-1
- FE
Feed efficiency
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GC–MS
Gas chromatography–mass spectrometry
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- MTBSTFA
N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide
- MUC2
Mucin 2
- QIIME
Quantitative Insights Into Microbial Ecology
- SPL
Sophorolipid
- SCFA
Short-chain fatty acid
- VH
Villus height
Authors’ contributions
MJK, MYP, JGK and KYW conceived and designed the experiments; MJK and MYP mainly performed the experiments; MJK and YSC analyzed the data; JC, DP, HBL and IGC contributed reagents/materials/analysis tools; MJK wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by EASYBIO Inc. and Korea University.
Availability of data and materials
Not applicable.
Declarations
Ethics approval
All of works related to animal was conducted in accordance with the guidelines and regulations for the care and the use of experimental animals was approved by the Korea University Institutional Animal Care & Use Committee (Permission No. KUIACUC-2020-0097).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Min-Jin Kwak and Min-Young Park contributed equally to this work.
Contributor Information
Min-Jin Kwak, Email: ics1051@korea.ac.kr.
Min-Young Park, Email: bennjerrry@korea.ac.kr.
Yong-Soon Choi, Email: dj1235@korea.ac.kr.
Junghwan Cho, Email: junghwancho@korea.ac.kr.
Duleepa Pathiraja, Email: pmduleepa@gmail.com.
Jonggun Kim, Email: ketone@korea.ac.kr.
Hanbae Lee, Email: hanbae.lee@pathway-intermediates.com.
In-Geol Choi, Email: igchoi@korea.ac.kr.
Kwang-Youn Whang, Email: kwhang@korea.ac.kr.
References
- 1.Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6(11):e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates J, Jordens JZ, Griffiths DT. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antibicrob Chemother. 1994;34(4):507–514. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 3.Munk P, Knudsen BE, Lukjacenko O, Duarte ASR, Luiken RE, Van Gompel L, et al. Abundance and diversity of the fecal resistome in slaughter pigs and broilers in nine European countries. Nature. 2017;3:898–908. doi: 10.1038/s41564-018-0192-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang JP, Lee JH, Yoo JS, Cho JH, Kim HJ, Kim IH. Effects of phenyllactic acid on growth performance, intestinal microbiota, relative organ weight, blood characteristics, and meat quality of broiler chicks. Poult Sci. 2010;89(7):1549–1555. doi: 10.3382/ps.2009-00235. [DOI] [PubMed] [Google Scholar]
- 5.Roy A, Haldar S, Mondal S, Ghosh TP. Effects of supplemental exogenous emulsifier on performance, nutrient metabolism, and serum lipid profile in broiler chickens. Vet Med Int. 2010;11:262604. doi: 10.4061/2010/262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Jung B, Kim WK. Effects of lysophospholipid on growth performance, carcass yield, intestinal development, and bone quality in broilers. Poult Sci. 2019;98(9):3902–3923. doi: 10.3382/ps/pez111. [DOI] [PubMed] [Google Scholar]
- 7.Cho KJ, Kim YB, Kim EK. Production and application of sophorolipid, a microbial surfactant. Korean Soc Biotechnol Bioeng J. 1999;14:747–753. [Google Scholar]
- 8.Develter DW, Lauryssen LM. Properties and industrial applications of sophorolipid. Eur J Lipid Sci Technol. 2010;112(6):628–638. doi: 10.1002/ejlt.200900153. [DOI] [Google Scholar]
- 9.Borsaniyiova M, Patil A, Mukherji R, Prabhune A, Bopegamage S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2016;61(1):85–89. doi: 10.1007/s12223-015-0413-z. [DOI] [PubMed] [Google Scholar]
- 10.Kwak MJ, Park MY, Kim J, Lee H, Whang KY. Curative effects of sophorolipid on physical wounds: in vitro and in vivo studies. Vet Med Sci. 2021;00:1–9. 10.1002/vms3.481. [DOI] [PMC free article] [PubMed]
- 11.Hardin R, Pierre J, Schulze R, Mueller CM, Fu SL, Wallner SR, et al. Sophorolipids improve sepsis survival: effects of dosing and derivatives. J Surg Res. 2008;142:314–319. doi: 10.1016/j.jss.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Concaix FB. Use of sophorolipids comprising diacetyl lactones as agent for stimulating skin fibroblast metabolism. 2003. [Google Scholar]
- 13.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, McFarland DC, Velleman SG. Effect of Smad3-mediated transforming growth factor-β1 signaling on satellite cell proliferation and differentiation in chickens. Poult Sci. 2008;87(9):1823–1833. doi: 10.3382/ps.2008-00133. [DOI] [PubMed] [Google Scholar]
- 15.Song B, Li H, Wu Y, Zhen W, Wang Z, Xia Z, Guo Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim Feed Sci Technol. 2017;232:6–15. doi: 10.1016/j.anifeedsci.2017.07.009. [DOI] [Google Scholar]
- 16.Heidari M, Wang D, Delekta P, Sun S. Marek’s disease virus immunosuppression alters host cellular reponses and immune gene expression in the skin of infected chickens. Vet Immunol Immunopathol. 2016;180:21–28. doi: 10.1016/j.vetimm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Boontiam W, Hyun YK, Jung B, Kim YY. Effects of lysophospholipid supplementation to reduced energy, crude protein, and amino acid diets on growth performance, nutrient digestibility, and blood profiles in broiler chickens. Poult Sci. 2019;98(12):6693–6701. doi: 10.3382/ps/pex005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu Hafsa SH, Ibrahim SA. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J Anim Physiol Anim Nutr. 2018;102(1):268–275. doi: 10.1111/jpn.12688. [DOI] [PubMed] [Google Scholar]
- 19.Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38(9):1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Zhu NH, Zhang RJ, Wu H, Zhang B. Effects of Lactobacillus cultures on growth performance, xanthophyll deposition, and color of the meat and skin of broilers. J Appl Poult Res. 2009;18(3):570–578. doi: 10.3382/japr.2009-00012. [DOI] [Google Scholar]
- 22.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 23.Guinan J, Wang S, Hazbun TR, Yadav H, Thangamani S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-45467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acid (SCFA)-mediated gut epithelial and immune regulation and its relevance in inflammatory bowel diseases. Front Immunol. 2019;10:277–292. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taverniti V, Guglielmetti S. Health-promoting properties of Lactobacillus helveticus. Front Microbiol. 2012;3:392–404. doi: 10.3389/fmicb.2012.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali B, Ndagijimana M, Maccaferri S, Blagi E, Guerzoni ME, Brigidi P. An in vitro evaluation of the effect of probiotics and prebioitcs on the metabolic profile of human microbiota. Anaerobe. 2012;18(4):386–391. doi: 10.1016/j.anaerobe.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. Sp. Nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 28.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep. 2019;7(1):3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K, Zhao H, Shu L, Xing H, Wang C, Lu C, Song G. Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice. Int J Food Sci Nutr. 2020;71(8):965–978. doi: 10.1080/09637486.2020.1754351. [DOI] [PubMed] [Google Scholar]
- 31.Qu L, Liu Q, Zhang Q, Tuo X, Fan D, Deng J, Yang H. Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. 2019;125:85–94. doi: 10.1016/j.fct.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 32.Earley H, Lennon G, Balfe Á, Coffey JC, Winter DC, O’Connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashrafian F, Behrouzi A. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol Hepatol Bed Bench. 2019;12(2):163–168. [PMC free article] [PubMed] [Google Scholar]
- 34.Su H, Mo J, Ni J, Ke H, Bao T, Xie J, et al. Andrographolide Exerts antihyperglycemic effect through strengthening intestinal barrier function and increasing microbial composition of Akkermansia muciniphila. Oxid Med Cell. 2020;6538930:1–20. 10.1155/2020/6538930. [DOI] [PMC free article] [PubMed]
- 35.Dumke J, Hinse D, Vollmer T, Schulz J, Knabbe C, Dreier J. Potential transmission pathways of Streptococcus gallolyticus subsp gallolyticus. PLoS One. 2015;10:e0126507. doi: 10.1371/journal.pone.0126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomoto R, Le HTT, Sekizaki T, Osawa R. Antimicrobial susceptibility of Streptococcus gallolyticus isolated from humans and animals. Jpn J Infect Dis. 2013;66(4):334–336. doi: 10.7883/yoken.66.334. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Liu B, Gu C, Zhang W, Yang J, Cheng G, Liu C, Hu X. Necroptosis of splenic macrophages induced by Streptococcus gallolyticus subsp. pasteurianus. Avian Dis. 2017;61(1):115–122. doi: 10.1637/11449-061216-Reg. [DOI] [PubMed] [Google Scholar]
- 38.Bauer BW, Gangadoo S, Bajagai YS, Van TTH, Moore RJ, Stanley D. Oregano powder reduces Streptococcus and increases SCFA concentration in a mixed bacterial culture assay of chicken. PloS One. 2019;13(3):e0216853. 10.1371/journal.pone.0216853. [DOI] [PMC free article] [PubMed]
- 39.Rodriguez-Cebezas ME, Camuesco D, Arribas B, Garrido-Mesa N, Comalada M, Bailon E, et al. The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin Nutr. 2010;29(6):832–839. doi: 10.1016/j.clnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.