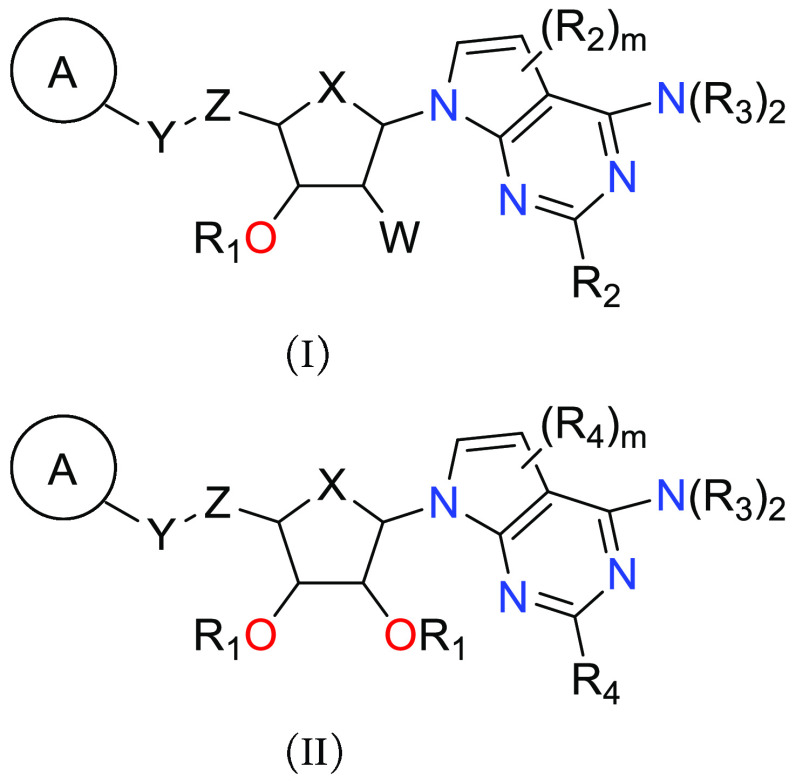
Important Compound Classes
Title
METTL3 Modulators
Patent Publication Number
WO 2021/079196 A2
Publication Date
April 29, 2021
Priority Application
US 62/923,936
Priority Date
October 21, 2019
Inventors
Tasker, A. S.; Daniels, M. H.; Duncan, K. W.; Sparling, B. A.; Wynn, T. A.; Hodous, B. L.; Boriack-Sjodin, P. A.; Sickmier, E. A.; Mills, J. E. J.; Copeland, R. A.
Assignee Company
Accent Therapeutics Inc., USA
Disease Area
Cancer, AML
Biological Target
METTL3
Summary
Among all RNA modifications, N6-methyladenosine (m6A) is the most abundant mRNA internal modification. It plays important roles in the biogenesis and functions of RNA. m6A deposition on mRNA is regulated by the dynamic interplay between RNA specific methylase (“writers”) and binding proteins (“readers”), and demethylases (“erasers”). m6A methylation is controlled by a large RNA methyltransferase complex (MTase), composed of the methyltransferase-like 3 and 14 (METTL3 and METTL14) proteins and their cofactors, Wilms’ tumor 1-assocated protein (WTAP). METTL3 is the catalytic component that forms a heterodimer with METTL14, which facilitates the interaction with its target mRNA.
METTL3 has been demonstrated to modulate embryonic development, cell reprogramming, spermatogenesis, regulation of T cell homeostasis, and endothelial-to-hematopoietic transition via methylation of specific target transcripts. Aberrant METTL3 expression has been associated with various pathophysiology, such as cancer, obesity, infection, inflammation, and immune response. AML is one of the cancers with the highest expression of both METTL3 and METTL14. Both genes were found upregulated in all subtypes of AML compared to normal hematopoietic cells.
The present application describes a series of novel METTL3 modulators for the treatment of cancer, especially, acute myeloid leukemia (AML) and viral infections. Further, the application discloses compounds, their preparation, use, pharmaceutical composition, and treatment.
Definitions
X = O and CH2;
R1 = H, C1–6alkyl, and −C(=O)–C1–6alkyl;
W = H, halo, C1–6alkyl, and NH2;
Y = O, S, C(Ra)2, and NRb;
Z = C1–6alkyl, C2–6alkenyl, and C2–6alkynyl, each of which is optionally substituted with 1–3 halo;
ring A = benzene, naphthalene, 4–7-membered monocyclic heterocycloalkyl, 5–6-membered monocyclic heteroaromatic ring, and 8–10-membered bicyclic heteroaromatic ring, each of which is optionally substituted with 1–4 independently selected R5;
R2 = H, C1–6alkyl, C2–6alkenyl, C2–6alkynyl, C3–8cycloalkyl, C5–8cycloalkenyl, 4–7-membered heterocycloalkyl, 4–7-membered heterocycloalkenyl, phenyl, 5–6-membered heteroaryl, halo, −CN, −OR2a, −N(R2a)2, −C(=O)OR2a, −C(=O)R2a, and −C(=O)N(R2a)2;
R3 = H or C1–6alkyl optionally substituted with 1–3 substituents selected from C3–6cycloalkyl, phenyl, and halo;
R4 = H, C1–6alkyl, C2–6alkenyl, C2–6alkynyl, C3–8cycloalkyl, C5–8cycloalkenyl, 4–7-membered heterocycloalkyl, 4–7-membered heterocycloalkenyl, phenyl, 5–6-membered heteroaryl, halo, −CN, −OR2a, −N(R2a)2, and −C(=O)N(R2a)2; and
m = 1 or 2.
Key Structures
Biological Assay
The METTL3 standard enzyme assay and m6A-mRNA LC-MS/MS assay was performed. The compounds described in this application were tested for their ability to inhibit METTL3. The METTL3 IC50 (nM) and m6A IC50 (μM) are shown in the following table.
Biological Data
The table below shows representative compounds were tested for METTL3 inhibition. The biological data obtained from testing representative examples are listed in the following table.
For METTL3 IC50 (nM): “A” means <10.0 nM; “B” means ≥10.0 to <100.00 nM; “C” means ≥100.0 to <1000.00 nM; and “D” means ≥1000.0 nM.
For m6A IC50 (μM):
“*”
means ≥10.0 μM; “**” means ≥1.0
to <10.00 μM; and “***” means <1.00 μM.
Claims
Total claims: 55
Compound claims: 48
Pharmaceutical composition claims: 1
Method of treatment claims: 6
Recent Review Articles
-
1.
Dong S.; Wu Y.; Liu Y.; Weng H.; Huang H.. Cancer Commun. 2021, in press.
-
2.
He P. C.; He C.. EMBO J. 2021, 40, e105977.
-
3.
Huang J.; Chen Z.; Chen X.; Chen J.; Cheng Z.; Wang Z.. Mol. Ther. Nucleic Acids 2021, 23, 887.
-
4.
Cai Y.; Feng R.; Lu T.; Chen X.; Zhou X.; Wang X.. Biomarker Res. 2021, 9, 27.
-
5.
Zeng C.; Huang W.; Li Y.; Weng H.. J. Hematol. Oncol. 2020, 13, 117.
-
6.
Huo F.; Zhu Z.; Pei D.. Cell Prolif. 2020, 53, e12921.
The author declares no competing financial interest.