Abstract
Objectives:
To compare the characteristics and outcomes of patients presenting to hospital with alcohol-induced and gallstone-induced acute pancreatitis.
Methods:
Retrospective study of all patients with alcohol-induced or gallstone-induced pancreatitis during the period 1 June 2012 to 31 May 2016. The primary outcome measure was hospital mortality. Secondary outcome measures included hospital length of stay, requirements for intensive care unit admission, intensive care unit mortality, mechanical ventilation, renal replacement therapy, requirement of inotropes and total parenteral nutrition.
Results:
A total of 642 consecutive patients (49% alcohol; 51% gallstone) were included. No statistically significant differences were found between alcohol-induced and gallstone-induced acute pancreatitis with respect to hospital mortality, requirement for intensive care unit admission, intensive care unit mortality and requirement for mechanical ventilation, renal replacement therapy, inotropes or total parenteral nutrition. There was significant difference in hospital length of stay (3.07 versus 4.84; p < 0.0001). On multivariable regression analysis, Bedside Index of Severity in Acute Pancreatitis score (estimate: 0.393; standard error: 0.058; p < 0.0001) and admission haematocrit (estimate: 0.025; standard error: 0.008; p = 0.002) were found to be independently associated with prolonged hospital length of stay.
Conclusion:
Hospital mortality did not differ between patients with alcohol-induced and gallstone-induced acute pancreatitis. The duration of hospital stay was longer with gallstone-induced pancreatitis. Bedside Index of Severity in Acute Pancreatitis score and admission haematocrit were independently associated with hospital length of stay.
Keywords: Pancreatitis, gallstone, alcohol, intensive care, mortality
Introduction
Acute pancreatitis (AP) is an unpredictable disease with the potential for significant morbidity, mortality, prolonged hospital admissions and healthcare costs. 1 It is known that approximately 15% of patients with AP will develop severe acute pancreatitis (SAP) which has historically been associated with a mortality of approximately 15% 2 but as high as 25% in some recent studies3,4 and which is characterised by the presence of severe multi-organ dysfunction. 5 Epidemiological data have also demonstrated that alcohol and gallstones account for the large majority of cases of AP, some studies suggesting up to 70%–80% of AP cases.6–8
At present, clinicians are able to assess the risk of patients developing SAP using scoring systems such as the Glasgow Score, Ranson Score, Atlanta Score, Bedside Index of Severity in Acute Pancreatitis (BISAP) score, sequential organ failure assessment (SOFA) score, Chinese Simple Scoring System (CSSS) and Acute Physiology and Chronic Health Evaluation (APACHE) score.9–12 These scoring systems are relatively complex and involve multiple individual pieces of biochemical and clinical data collected at different periods of time from admission which are not always collected by treating clinicians. Importantly, none of the commonly used scoring systems consider the underlying cause of AP – such as alcohol-induced or gallstone-induced AP in risk stratifying. The ability to risk stratify patients based on the cause of their AP would likely be useful for clinicians and potentially alter patient management.
Some studies have shown that there is increased mortality in gallstone pancreatitis patients,13,14 some others show increased complications15,16 and mortality 16 in alcohol-induced pancreatitis, while others show no difference in mortality.1,17,18 It is possible that outcomes are likely to be determined by the characteristics of the patients and the aetiology of pancreatitis. There is no published literature to the best of our knowledge that compared the outcomes of gallstone-induced and alcohol-induced pancreatitis in an Australian population. The objective of this study was to compare the characteristics and outcomes of patients presenting to hospital with alcohol-induced or gallstone-induced AP.
Methods
Ethics Approval: Peninsula Health Human Research Ethics Committee approved this study as a retrospective audit for quality assurance (ref. QA/16PH17). Consent was not required from the participants as the study was a retrospective audit of data routinely collected for patient care and not experimental research. Reporting of this study adheres to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patients
All patients with AP presenting to an Australian metropolitan teaching hospital were retrospectively identified using hospital database records during the period of 1 June 2012 to 31 May 2016. Patients were included in the study if they were identified as having AP due to either alcohol or gallstones. Alcohol-induced pancreatitis was diagnosed as the aetiology when a clear history of abnormal alcohol intake before the attack of pancreatitis was present and when other aetiology excluded. Gallstone-induced pancreatitis was diagnosed as aetiology when biliary sludge or gallstones were detected ultrasonographically or on computer tomography accompanied by elevation of serum aspartate aminotransferase and/or bilirubin. All patients were treated with recommended management of AP including pain management, fluid replacement, enteral nutrition support where possible and judicious use of antibiotics. In patients with gallstone-induced pancreatitis, endoscopic retrograde cholangio-pancreatography (ERCP) was performed early when common bile duct obstruction was noted radiologically or when the patients had cholangitis. Patients were excluded from the study if they had AP due to an alternative cause or their details were incomplete, duplicate or contradictory.
Study design
A retrospective observational study design was utilised including all consecutive patients admitted to the hospital with a diagnosis of alcohol-induced or gallstone-induced pancreatitis during the study period. Patient details including age, sex, comorbidities, cause of AP, hospital mortality, hospital length of stay (LOS), requirement for intensive care unit (ICU) stay, ICU mortality, mechanical ventilation, renal replacement therapy, ERCP and surgery were collected. BISAP score was calculated for each patient as a marker of illness severity which is a simple and well validated score for AP.19,20
Outcome measures
The primary outcome was hospital mortality. Secondary outcomes included hospital LOS, ICU mortality and requirement for ICU admission, mechanical ventilation, renal replacement therapy, inotropes and total parenteral nutrition (TPN).
Statistical analysis
Continuous variables were summarised using mean (standard deviation) or median (interquartile range) according to data type and distribution. Categorical variables were summarised using frequency tables presenting the subject counts and percentages. Comparisons between groups (alcohol versus gallstone) were made using the Student’s t test for normally distributed continuous variables, Wilcoxon rank-sum test for non-normally distributed continuous variables and chi-square or Fisher’s exact test as appropriate for categorical variables. Multivariable analysis for hospital LOS was performed using multiple linear regression with results presented as parameter estimates and standard errors. As hospital LOS had a positively skewed distribution, logarithmic transformation was applied prior to the analysis. Variables with p < 0.05 on univariable analysis or those deemed to be clinically important were entered into a hierarchical regression model to identify the factors independently associated with LOS. To test the robustness of the outcome comparisons, a sensitivity analysis was also performed in a subgroup of patients with BISAP score 2 and above. A two-sided p value less than 0.05 was chosen to indicate statistical significance. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
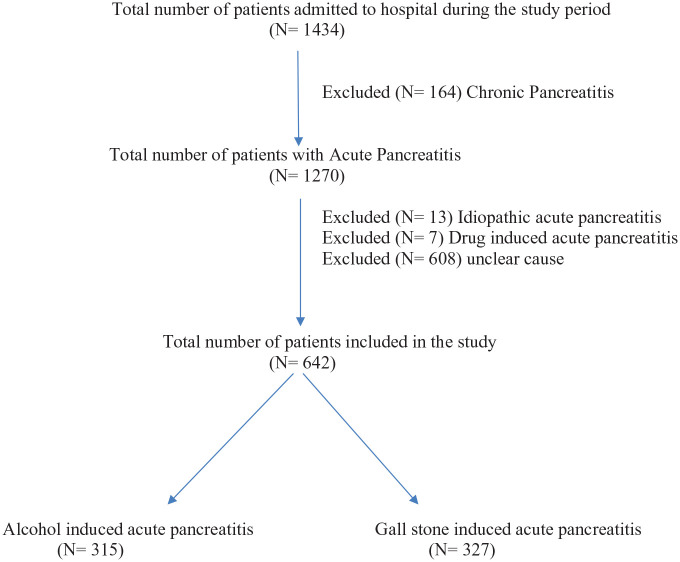
A total of 642 patients were identified in the study period to have either alcohol-induced or gallstone-induced pancreatitis at admission to hospital (Figure 1).
Figure 1.
Flow diagram of the patients included in the study.
The mean age was 52.9 years and 364 (56.7%) were male. Alcohol was the causative agent in 315 (49%) of cases and gallstones were the causative agent in 327 (51%) of cases.
A comparison of patient demographics and comorbidities, laboratory data and illness severity scores at presentation to hospital is shown in Table 1. Patients with gallstone pancreatitis were older, had a higher proportion of females and had higher cardiovascular, endocrine and other comorbidities (Table 1). The laboratory data showed a significant difference in blood glucose, white cell count, haematocrit, lipase, urea and creatinine between both the groups (Table 1). BISAP score on admission was also higher in patients with gallstone pancreatitis (Table 1). A comparison of laboratory and physiological data at 24 and 48 h of hospital admission is presented in Table 2. The gallstone-induced group was more likely to require surgical intervention (3 (0.97%) versus 110 (34.4%) p < 0.0001) and to undergo ERCP (5 (1.6%) versus 61 (19.1%) p < 0.0001).
Table 1.
Comparison of patient demographics, comorbidities, laboratory data and illness severity scores at the time of hospital admission.
| All patients (n = 642) | Alcohol (n = 315) | Gallstone (n = 327) | p value | |
|---|---|---|---|---|
| Age (mean ± SD) | 52.9 ± 18.2 | 46.3 ± 13.5 | 59.3 ± 19.7 | <0.0001 |
| Gender, n (%) | ||||
| Male | 364 (56.7%) | 225 (71.4%) | 139 (42.5%) | <0.0001 |
| Female | 278 (43.3%) | 90 (28.6%) | 188 (57.5%) | <0.0001 |
| Prior admission for AP | 232 (36.3%) | 160 (51%) | 72 (22.2%) | <0.0001 |
| Comorbidities, n (%) | ||||
| Cardiovascular | 241 (38.1%) | 87 (27.7%) | 154 (48.4%) | <0.0001 |
| Respiratory | 92 (14.6%) | 46 (14.6%) | 46 (14.5%) | 0.95 |
| Endocrine | 123 (19.5%) | 39 (12.4%) | 84 (26.4%) | <0.0001 |
| Renal | 15 (2.4%) | 4 (1.3%) | 11 (3.5%) | 0.07 |
| Other | 481 (76.2%) | 253 (80.0%) | 228 (71.7%) | 0.007 |
| Smoking history | ||||
| Current smoker | 244 (46.8%) | 181 (73.3%) | 63 (23%) | <0.0001 |
| Ex-smoker | 106 (20%) | 20 (8.1%) | 86 (31.4%) | <0.0001 |
| Never smoked | 171 (32.8%) | 46 (18.6%) | 125 (45.6%) | <0.0001 |
| Laboratory data | ||||
| Blood glucose, median (IQR) | 6.3 [5.4–7.9] (n = 482) | 6.2 [5.25–7.55] (n = 298) | 6.45 [5.5–8.1] (n = 234) | 0.041 |
| WCC, median (IQR) | 10.9 [8.2–14.1] (n = 631) | 10.6 [7.8–14] (n = 314) | 11.4 [8.7–14.4] (n = 317) | 0.008 |
| Haematocrit, median (IQR) | 42.2 [38.4–45.3] (n = 574) | 42.9 [39.7–45.7] (n = 281) | 41 [37.8–44.9] (n = 293) | <0.0001 |
| Serum AST, median (IQR) | 67 [30–187] (n = 618) | 46 [25–107] (n = 305) | 122 [44–305] (n = 313) | <0.0001 |
| Lipase, median (IQR) | 1055 [391–3082] (n = 628) | 673 [279–1420] (n = 311) | 1994 [647–5213] (n = 317) | <0.0001 |
| Urea, median (IQR) | 5.2 [3.7–6.9] (n = 623) | 4.5 [3.1–5.8] (n = 309) | 5.9 [4.4–7.7] (n = 314) | <0.0001 |
| Creatinine, median (IQR) | 75 [61–89] (n = 625) | 72 [59–86] (n = 310) | 77 [62–94] (n = 315) | <0.0001 |
| Pleural effusion detected, n (%) | 43 (6.8%) | 22 (7%) | 21 (6.6%) | 0.85 |
| Illness severity scores | ||||
| SIRS, median (IQR) | 1 [1–2] (n = 642) | 1 [1–2] (n = 315) | 1 [1–2] (n = 327) | 0.91 |
| BISAP, median (IQR) | 1 [0–2] (n = 642) | 1 [0–1] (n = 315) | 1 [1–2] (n = 327) | <0.0001 |
AP: acute pancreatitis; BISAP: Bedside Index of Severity in Acute Pancreatitis; IQR: interquartile range; SIRS: systemic inflammatory response syndrome; AST: aspartate aminotransferase; WCC: white cell count.
Table 2.
Laboratory and physiological data at first 24 and 48 h of hospital admission.
| All patients (n = 642) | Alcohol (n = 315) | Gallstone (n = 327) | p value | |
|---|---|---|---|---|
| First 24 h | ||||
| Highest urea, median (IQR) | 5.5 [4.0–7.1] | 4.8 [3.5–6.4] | 6.05 [4.6–7.9] | <0.0001 |
| Highest creatinine, median (IQR) | 78 [64.0–93.5] | 45 [62–88] | 82.5 [67.0–97.0] | 0.022 |
| Highest body temperature, mean (SD) | 37.4 (0.799) | 37.3 (0.813) | 37.4 (0.796) | 0.66 |
| Lowest body temperature, mean (SD) | 36.1 ± 0.466 | 36.1 ± 0.501 | 36.1 ± 0.428 | 0.23 |
| Highest respiratory rate, mean (SD) | 18.4 ± 3.09 | 18.5 ± 2.96 | 18.4 ± 3.22 | 0.54 |
| Highest heart rate, mean (SD) | 91.3 ± 19.1 | 94.1 ± 20.2 | 88.6 ± 17.6 | <0.0001 |
| Highest WCC, median (IQR) | 11.5 [8.6–14.9] | 10.7 [7.9–14.1] | 12.3 [9.1–15.8] | <0.0001 |
| Lowest WCC, mean (SD) | 10.6 (9.61) | 10.2 (4.91) | 10.9 (12.6) | 0.36 |
| At 48 h | ||||
| Highest urea, mean (SD) | 6.03 ± 3.21 | 5.22 ± 2.48 | 6.89 ± 3.64 | <0.0001 |
| Highest creatinine, median (IQR) | 78 [65–94] | 76 [64–90] | 83 [67–97] | 0.05 |
| Highest WCC, mean (SD) | 12.9 ± 5.74 | 12.5 ± 5.4 | 13.3 ± 6.08 | 0.91 |
| Lowest serum albumin, median (IQR) | 38.8 [34.0–42.0] | 38.5 [34.0–43.0] | 37.0 [34.0–40.0] | 0.022 |
| Lowest PaO2, mean (SD) | 83.5 ± 27.5 | 75.2 ± 27.5 | 88.6 ± 27.1 | 0.23 |
| Lowest serum Ca, mean (SD) | 2.18 ± 0.235 | 2.19 ± 0.191 | 2.17 ± 0.273 | 0.46 |
| Highest glucose, mean (SD) | 8.13 ± 2.8 | 7.88 ± 2.69 | 8.4 ± 2.89 | 0.11 |
| Highest serum LDH, median (IQR) | 470 [378–676] | 433 [365–520] | 537 [403–704] | 0.003 |
IQR: interquartile range; WCC: white cell count; LDH: lactate dehydrogenase.
Primary and secondary outcomes are displayed in Table 3. No statistically significant differences were found between alcohol-induced and gallstone-induced AP with respect to hospital mortality, requirement for ICU admission, ICU mortality, requirement for mechanical ventilation, renal replacement therapy, inotrope requirement or need for TPN. However, there was a statistically significant difference in hospital LOS (Table 3). A subgroup analysis was conducted (Table 4) to compare the outcomes with more severe pancreatitis (BISAP score 2 and above). There were 176 patients with a BISAP score ⩾2 and, irrespective of their aetiology, had comparable outcomes between alcohol and gallstone induced AP (Table 4). On multivariable regression analysis, BISAP score (estimate: 0.393; standard error: 0.058; p < 0.0001) and admission haematocrit (estimate: 0.025; standard error: 0.008; p = 0.002) were independently associated with prolonged hospital LOS after adjusting for age, sex, cardiovascular and endocrine comorbidities, smoking status, prior admissions to hospital with pancreatitis, admission lipase, serum urea, serum creatinine and white blood cell count (Table 5).
Table 3.
Comparison of primary and secondary outcomes.
| All patients (n = 642) | Alcohol (n = 315) | Gallstone (n = 327) | p value | |
|---|---|---|---|---|
| Hospital mortality, n (%) | 5 (0.786%) | 2 (0.637%) | 3 (0.932%) | 1.00 |
| Hospital LOS, median (IQR) | 3.9 [2.19–6.62] | 3.07 [2.01–5.59] | 4.84 [2.59–7.69] | <0.0001 |
| ICU admission and outcomes in ICU | ||||
| ICU admission, n (%) | 47 (7.4%) | 19 (6.1%) | 28 (8.7%) | 0.2 |
| ICU LOS, median (IQR) | 2.05 [1.16–8.63] | 3.92 [1.44–8.64] | 1.72 [1.13–7.52] | 0.38 |
| ICU mortality, n (%) | 5 (10.6%) | 2 (10.5%) | 3 (10.7%) | 1.00 |
| Required mechanical ventilation, n (%) | 19 (3%) | 8 (2.5%) | 11 (3.4%) | 0.52 |
| Required dialysis (RRT), n (%) | 8 (1.3%) | 4 (1.3%) | 4 (1.3%) | 1.00 |
| Inotropes, n (%) | 19 (40.4 %) | 9(47.4%) | 10(37.5%) | 0.42 |
| TPN, n (%) | 16 (27.8%) | 9(47.4%) | 7 (25%) | 0.11 |
LOS: length of stay; ICU: intensive care unit; TPN: total parenteral nutrition; RRT: renal replacement therapy; IQR: interquartile range.
Table 4.
Comparative primary and secondary outcomes in patients with BISAP score 2 and above.
| All patients (n = 176) | Alcohol (n = 46) | Gallstone (n = 130) | p value | |
|---|---|---|---|---|
| Hospital mortality, n (%) | 4 (2.3%) | 2 (4.3%) | 2 (1.5%) | 0.28 |
| Hospital LOS, median (IQR) | 6.07 [3.21–10.5] | 4.6 [2.55–8.66] | 6.4 [3.81–10.8] | 0.1 |
| ICU admission and outcomes in ICU | ||||
| Required ICU admission, n (%) | 36 (20.5%) | 12 (26.1%) | 24 (18.05%) | 0.27 |
| ICU LOS, median (IQR) | 2.02 [1.08–7.09] | 2.96 [0.969–6.15] | 1.83 [1.13–7.52] | 0.96 |
| ICU mortality, n (%) | 4 (11.1%) | 2 (17.6%) | 2 (8.3%) | 0.59 |
| Required mechanical ventilation, n (%) | 13 (7.4%) | 4 (8.7%) | 9 (6.9%) | 0.69 |
| Required RRT, n (%) | 5 (2.8%) | 2 (4.3%) | 3 (2.3%) | 0.61 |
| Inotropes, n (%) | 14 (38.9%) | 5 (41.7%) | 9 (37.5%) | 0.81 |
| TPN, n (%) | 10 (27.8%) | 4 (33.3%) | 6 (25%) | 0.6 |
LOS: length of stay; ICU: intensive care unit; TPN: total parenteral nutrition; RRT: renal replacement therapy; IQR: interquartile range.
Table 5.
Predictors of log hospital length of stay on multivariate analysis.
| Variable | Parameter estimate | Standard error | p value |
|---|---|---|---|
| Aetiology – Biliary | 0.076 | 0.112 | 0.50 |
| Age | 0.0003 | 0.004 | 0.93 |
| Male | 0.027 | 0.103 | 0.79 |
| BISAP score | 0.393 | 0.058 | <0.0001 |
| Prior pancreatitis admission | −0.092 | 0.10 | 0.36 |
| Lipase | 0.001 | 0.001 | 0.37 |
| Urea | −0.029 | 0.139 | 0.83 |
| Creatinine | 0.004 | 0.102 | 0.97 |
| Comorbidity – Cardiovascular | −0.030 | 0.118 | 0.80 |
| Comorbidity – Endocrine | −0.02 | 0.124 | 0.87 |
| WBC count | −0.002 | 0.004 | 0.62 |
| Haematocrit | 0.025 | 0.008 | 0.002 |
| Smoker | −0.038 | 0.101 | 0.70 |
BISAP: Bedside Index of Severity in Acute Pancreatitis; WBC: White Blood Cell Count.
Discussion
This study suggests that patients presenting to hospital with alcohol-induced and gallstone-induced AP have no clinically or statistically significant difference in mortality. However, the duration of hospital stay was longer with gallstone-induced pancreatitis, and BISAP score and admission haematocrit were independently associated with the duration of hospital LOS.
The comparable hospital mortality is consistent with previous studies.17,18,21 Our study also suggests that the requirement for ICU admission, mechanical ventilation and requirement for renal replacement therapy is similar between patients with alcohol-induced and gallstone-induced AP. Moreover, there was no statistically significant difference in the requirement for inotropes or need for TPN between both groups.
This is the first study of its kind in Australasia which is significant due to the largely Western population in comparison with prior studies from South Korea 16 and India 8 which compared the characteristics and outcomes of alcohol-induced and gallstone-induced AP.
In a well-planned prospective study from South Korea by Cho et al. 16 which included 126 patients, it was demonstrated that patients with alcohol-induced AP were younger than patients with gallstone-induced AP (52.3 (±11.2) versus 67.3 years (±14.9) p = 0.104) and had a higher mortality (4/50 versus 0/76; p = 0.012). The study by Cho et al. also demonstrated a higher overall mortality than our study (3.2% versus 0.8%) despite similar BISAP scores, although this may be confounded by the smaller sample size compared to our study. Of note, the study by Cho et al. also demonstrates longer hospital stays than our study despite reporting an approximately 3-day time to alleviation of symptoms in both the alcohol-induced and gallstone-induced AP groups. Although the study by Cho et al. was well planned and prospective in design, our study had a significantly larger patient sample size (n = 642 versus n = 126).
In a retrospective study of similar size to our study (n = 759) which was conducted at a single tertiary referral hospital in northern India, Samanta et al. 8 also compared characteristics and outcomes of patients with alcohol-induced and gallstone-induced AP. The study by Samanta et al. differed significantly to the results of our study due to the overall lower patient age, higher BISAP scores and significantly higher hospital mortality and requirements for mechanical ventilation and renal replacement therapy. It is worth noting that approximately 70% of the patients in the study by Samanta et al. had a BISAP score greater than or equal to 2 and that average hospital LOS was 30 days. As such, the study by Samanta et al. 8 represents a patient sample with a higher illness severity and therefore higher predicted morbidity and mortality.
Kamal et al. 22 investigated the outcomes of pancreatitis caused by biliary, alcohol and ERCP. In their study, they noted a higher risk of death and hospital LOS in biliary pancreatitis as compared to other two aetiologies. 22 They have also noted serum urea to be independently associated with hospital LOS. Our study did show an increased hospital LOS with gallstone pancreatitis, but serum urea did not show an independent association with hospital LOS.
The hospital mortality in this study was lower in comparison with other studies.8,16,22 This may be due to the fact that we included all patients irrespective of the severity of pancreatitis who presented to hospital. It is noteworthy that none of our patients who did not require ICU admission died in hospital and all deaths occurred only in patients who required ICU admission.
While there are several scores utilised to predict organ failure, complications and mortality in patients with pancreatitis, 11 we chose to utilise BISAP score to characterise the severity of pancreatitis in our study. BISAP score has been shown to have a similar performance when compared to other well-known scoring systems such as Ranson and APACHE-II scores 11 and could be easily obtained from our data set. Our study demonstrated that BISAP score was independently associated with hospital LOS.
Haematocrit was also shown to be associated with increased hospital LOS in our study. Prior studies, however, did not suggest haematocrit was a good predictor of pancreatic necrosis or poor outcomes including organ failure or mortality.23–25 It is important to note that these studies did not investigate the association of haematocrit with the hospital LOS. Our hospital mortality rate was low, and we therefore could not investigate the independent association of any of the prognosticators with hospital mortality in this study.
Strengths and limitations
Our study has multiple strengths worth highlighting. In particular, our large sample size of 642 patients represented all consecutive admissions over a 4-year period to an Australian, Western culture, metropolitan health care setting which is unique compared to other similar recent studies by Cho et al., Samanta et al. and Kamal et al. Our study also included all patients with gallstone-induced and alcohol-induced pancreatitis presenting to the hospital and not just those patients with severe AP. This provides a broader insight into the disease burden as well as the outcome differences between the spectrums of illness severity noted in the current clinical practice.
Our study also includes several important limitations. By design, our study included only a single centre and data were collected retrospectively. We have not performed sample size calculation for this study but used all admissions with gallstone-induced and alcohol-induced pancreatitis. Nevertheless, our study had a larger sample size than most of the studies presenting similar comparisons between gallstone-induced and alcohol-induced pancreatitis.16,21,22,26 One of the outcome variables – length of hospital stay – may also be dependent on a number of other factors, including the age, marital status, comorbidities and characteristics of patients at admission in addition to the severity of disease requiring hospital admission. 27 However, it would be logical to expect that these factors would have been applied to all patients irrespective of whether they had gallstone-induced or alcohol-induced AP. As well as this, our description of patient’s comorbidities, was kept broad, and we did not collect data on patients’ body mass index. Another limitation to our study is that individual patient data were at time missing or incomplete for some patients. Despite this, given that we consecutively enrolled all patients over a 4-year period at our hospital, it is likely that our sample of patients does represent the current clinical experience.
Conclusion
Patients presenting to hospital in this study with alcohol-induced AP and gallstone-induced AP had no difference in mortality or the need for ICU admission, invasive mechanical ventilation, inotropes, TPN or renal replacement therapy. The hospital LOS was longer with gallstone-induced AP. Given that our study was retrospective and conducted in a single centre and has differing results to prior overseas studies, a systematic review or prospective multi-centre study may be warranted.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Peninsula Health Human Research Ethics Committee (Approval number/ ref. QA/16PH17).
Informed consent: Informed consent was not sought for this study because consent was not required from the participants as the study was a retrospective audit of data routinely collected for patient care and not experimental research, and the requirement to obtain written informed consent from the subject prior to study initiation was waived by Peninsula Health Human Research Ethics Committee.
ORCID iDs: Taha Mollah  https://orcid.org/0000-0003-3338-9633
https://orcid.org/0000-0003-3338-9633
Ravindranath Tiruvoipati  https://orcid.org/0000-0003-3800-902X
https://orcid.org/0000-0003-3800-902X
References
- 1. Andersson B, Appelgren B, Sjödin V, et al. Acute pancreatitis–costs for healthcare and loss of production. Scand J Gastroen-terol 2013; 48(12): 1459–1465. [DOI] [PubMed] [Google Scholar]
- 2. Malangoni MA, Martin AS. Outcome of severe acute pancreatitis. Am J Surg 2005; 189: 273–277. [DOI] [PubMed] [Google Scholar]
- 3. Navicharern P, Wesarachawit W, Sriussadaporn S, et al. Management and outcome of severe acute pancreatitis. J Med Assoc Thailand [Chotmaihet Thangphaet] 2006; 89(Suppl. 3): S25–S32. [PubMed] [Google Scholar]
- 4. Umapathy C, Raina A, Saligram S, et al. Natural history after acute necrotizing pancreatitis: a large US tertiary care experience. J Gastrointest Surg 2016; 20(11): 1844–1853. [DOI] [PubMed] [Google Scholar]
- 5. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62(1): 102–111. [DOI] [PubMed] [Google Scholar]
- 6. Thomson SR, Hendry WS, McFarlane GA, et al. Epidemi-ology and outcome of acute pancreatitis. Brit J Surg 1987; 74: 398–401. [DOI] [PubMed] [Google Scholar]
- 7. Manrai M, Kochhar R, Gupta V, et al. Outcome of acute pancreatic and peripancreatic collections occurring in patients with acute pancreatitis. Ann Surg 2018; 267: 357–363. [DOI] [PubMed] [Google Scholar]
- 8. Samanta J, Dhaka N, Gupta P, et al. Comparative study of the outcome between alcohol and gallstone pancreatitis in a high-volume tertiary care center. JGH Open 2019; 3(4): 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao W, Yang HX, Ma CE. The Value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0130412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chatterjee R, Parab N, Sajjan B, et al. Comparison of acute physiology and chronic health evaluation II, modified computed tomography severity index, and bedside index for severity in acute pancreatitis score in predicting the severity of acute pancreatitis. Indian J Crit Care Med 2020; 24(2): 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010; 105(2): 435–441; quiz 442. [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Zeng YB, Chen JY, et al. A simple new scoring system for predicting the mortality of severe acute pancreatitis: a retrospective clinical study. Medicine 2020; 99: e20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imrie CW, Whyte AS. A prospective study of acute pancreatitis. Brit J Surg 1975; 62: 490–494. [DOI] [PubMed] [Google Scholar]
- 14. Frey CF. Gallstone pancreatitis. Surg Clin North Am 1981; 61: 923–938. [DOI] [PubMed] [Google Scholar]
- 15. Kim DB, Chung WC, Lee JM, et al. Analysis of factors associated with the severity of acute pancreatitis according to etiology. Gastroenterol Res Pract 2017; 2017: 1219464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho JH, Kim TN, Kim SB. Comparison of clinical course and outcome of acute pancreatitis according to the two main etiologies: alcohol and gallstone. BMC Gastroenterol 2015; 15: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uhl W, Isenmann R, Curti G, et al. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas 1996; 13(4): 335–343. [DOI] [PubMed] [Google Scholar]
- 18. Gullo L, Migliori M, Oláh A, et al. Acute pancreatitis in five European countries: etiology and mortality. Pancreas 2002; 24(3): 223–227. [DOI] [PubMed] [Google Scholar]
- 19. Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis – a prospective observational study. Int J Surg 2018; 54: 76–81. [DOI] [PubMed] [Google Scholar]
- 20. Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104(4): 966–971. [DOI] [PubMed] [Google Scholar]
- 21. Andersen AM, Novovic S, Ersbøll AK, et al. [Mortality and morbidity in patients with alcohol and biliary-induced acute pancreatitis]. Ugeskrift for Laeger 2007; 169: 4351–4354. [PubMed] [Google Scholar]
- 22. Kamal A, Akshintala VS, Kamal MM, et al. Does etiology of pancreatitis matter? Differences in outcomes among patients with post-endoscopic retrograde cholangiopancreatography. Pancreas 2019; 48(4): 574–578. [DOI] [PubMed] [Google Scholar]
- 23. Remes-Troche JM, Duarte-Rojo A, Morales G, et al. Hemoconcentration is a poor predictor of severity in acute pancreatitis. World J Gastroenterol 2005; 11: 7018–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lankisch PG, Mahlke R, Blum T, et al. Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am J Gastroenterol 2001; 96(7): 2081–2085. [DOI] [PubMed] [Google Scholar]
- 25. Gardner TB, Olenec CA, Chertoff JD, et al. Hemoconcentration and pancreatic necrosis: further defining the relationship. Pancreas 2006; 33(2): 169–173. [DOI] [PubMed] [Google Scholar]
- 26. Andersen AM, Novovic S, Ersbøll AK, et al. Mortality in alcohol and biliary acute pancreatitis. Pancreas 2008; 36: 432–434. [DOI] [PubMed] [Google Scholar]
- 27. Khosravizadeh O, Vatankhah S, Bastani P, et al. Factors affecting length of stay in teaching hospitals of a middle-income country. Electron Physician 2016; 8(10): 3042–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]