Abstract
Purpose:
The high fatality-to-case ratio of hepatocellular carcinoma is directly related to metastasis. The signal transducer and activator of transcription-3 is a key mediator of the cytokine and growth factor signaling pathways and drives the transcription of genes responsible for cancer-associated phenotypes. However, so far, no specific inhibitor for signal transducer and activator of transcription-3 has been used in clinical practice. Therefore, targeting the signal transducer and activator of transcription-3 for cancer therapy is highly desired to improve outcomes in patients with hepatocellular carcinoma.
Experimental Design:
Using the small-molecule inhibitor NT157, the effect of signal transducer and activator of transcription-3 inhibition on cell migration was tested in hepatocellular carcinoma cell lines and a lung metastasis model of the disease.
Results:
NT157 significantly inhibited the migration of hepatocellular carcinoma cell lines in vitro and lung metastasis of hepatocellular carcinoma in vivo. Mechanistically, it inhibited the phospho-signal transducer and activator of transcription-3 in a dose- and time-dependent manner. Furthermore, NT157 treatment suppressed the c-Jun activation domain-binding protein-1 levels in the nucleus but no significant decrease was observed in its expression in the cytoplasm. Finally, high mRNA expression levels of signal transducer and activator of transcription-3 and c-Jun activation domain-binding protein-1 in hepatocellular carcinoma were associated with significantly low survival rates.
Conclusion:
NT157 inhibits hepatocellular carcinoma migration and metastasis by downregulating the signal transducer and activator of transcription-3/c-Jun activation domain-binding protein-1 signaling pathway and targeting it may serve as a novel therapeutic strategy for the clinical management of hepatocellular carcinoma in the future.
Keywords: NT157, hepatocellular carcinoma, migration, metastasis, STAT3, Jab1
Introduction
Hepatocellular carcinoma (HCC) is 1 of the most common malignant tumors and ranks 4th in incidence and 3rd in mortality rates worldwide among all types of cancers. 1 Although some treatment options, such as surgical intervention, radiotherapy, and chemotherapy are available, the rates of metastasis and relapse remain high in patients with HCC. 2 Recently, several novel molecular inhibitors have been approved for the treatment of advanced liver cancer, but the overall survival rate of patients has not improved significantly, and the efficacy of these drugs is not promising. Tumor progression induced by invasiveness and metastasis leads to high causes high mortality in HCC patients. 3 Therefore, there is an urgent need to improve the treatment options in clinical practice.
Signal transducer and activator of transcription-3 (STAT3) is a member of a family of 7 cytoplasmic proteins that function as key mediators of cytokine and growth factor signaling pathways. 4,5 Following activation by tyrosine phosphorylation, STAT3 dimerizes and enters the nucleus, where it drives the transcription of genes responsible for cancer-associated phenotypes such as survival, angiogenesis, metastasis, and immune evasion. 4,5 Studies have demonstrated that 1 STAT3 based molecular mechanism leads to HCC migration. 6 STAT3 is constitutively active in many human tumors, including melanoma, nasopharyngeal cancer, and myeloma. 7 -9 Enhanced STAT3 activation is associated with increased cell-cell contact, suggesting that it serves as a sensor of tumor cell contact and upregulates the expression of genes necessary for cell invasion and migration. 10 In particular, the STAT3 signaling pathway is involved in tumor angiogenesis and promotes migration by interacting with the vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF). 11,12 In addition, matrix metalloproteinase-2 (MMP-2) and matrix metallopeptidase-9 (MMP-9) are the targets of STAT3, and the active STAT3 binds to the promoters of MMPs to upregulate their expression. 13,14 However, to date, no specific STAT3 inhibitor has been used in clinical practice, although targeting STAT3 for cancer therapy is highly desired. 15,16 Dephosphorylation of phospho-STAT3 (p-STAT3) is expected to result in the abrogation of multiple pro-tumor events, such as tumor cell survival, invasion, angiogenesis, and immune evasion.
NT157, a new class of anticancer agents that affect both cancer cells and their supportive microenvironment, has been verified to be effective in a variety of cancer cell lines in vitro, including those derived from colon cancer, prostate cancer, and melanoma. 17 -19 NT157 has been shown to inhibit both insulin receptor substrate 1/insulin receptor substrate 2 (IRS1/2) and STAT3 pathways. Nevertheless, the efficacy and action mechanism of NT157 in HCC, especially its impact on the STAT3 pathway, remains to be seen.
In this study, we aimed to investigate the role of NT157 in HCC. We found that inhibition of p-STAT3 and Jab1 by NT157 suppressed the migration of HCC cells in vitro and in vivo. Furthermore, we assessed the expression levels of STAT3 and the fifth component of the COP9 signalosome complex (CSN5 or COPS5, more commonly known as Jab1, an oncogenic transcription factor) in tissue samples from HCC tissues and adjacent normal tissues. We found that STAT3 and Jab1 were overexpressed in HCC specimens and correlated with poor prognosis in HCC patients, while overexpression of STAT3 was associated with high expression of Jab1. HCC patients with the highest expression of both STAT3 and Jab1 had the poorest prognosis. Our findings show that NT157 may serve as a novel therapeutic strategy for the clinical management of hepatocellular carcinoma in the future.
Materials and Methods
HCC Cell Lines
Human HCC cell lines, HuH-7, MHCC-97 H, SMMC-7721, MHCC-97 L, and HepG2 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were incubated at 37°C in a 5% CO2 atmosphere. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and 1% penicillin-streptomycin. Interleukin 6 (IL-6) (50 ng/mL; PeproTech, Rocky Hill, CT, USA) was added to the culture medium to activate the STAT3 pathway. The in vitro experiments were repeated at least 3 times.
Reagents and Antibodies
Antibodies specific to the following proteins were used in this study: JAB1 (Santa Cruz, USA); Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), p44/42 MAPK (Erk1/2), Phospho-STAT3 (Tyr705), and STAT3 from Cell Signaling Technology (Danvers, MA, USA); Lamin B, horseradish peroxidase (HRP)-conjugated beta Actin, HRP-conjugated goat anti-rabbit IgG (H + L), and HRP-conjugated goat anti-mouse IgG (H+L) were from ProteinTech; NT157 and PLX4720 were from Selleck; IL-6 was from PeproTech. The detail of dilutions and catalog number are included in supplementary materials and methods. NT157 was diluted in 20% 2-hydroxypropyl-β-cyclodextrin (2-HP-β-CD) (Selleck) for in vivo administration (100 mg/kg, intraperitoneal injection). NT157 was diluted in dimethyl sulfoxide (DMSO) for in vitro use at a concentration of 2 μM or as shown in details in the figures.
Wound Healing Assay
HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L cells (1 × 106 cells/well) were seeded into six-well plates (Millipore, USA). Three longitudinal scratches were made using sterile 10 µL pipette tips 24 h after treatment. The floating cell debris was removed by washing 3 times with phosphate-buffered saline (PBS). Subsequently, the cells were cultured in a serum-free medium or in presence of 2 μM NT157. Wound healing was observed and images were acquired at 0 and 24 h under an inverted microscope.
Cell Migration Assay
Cell migration ability was assessed using 24-well transwell plates. Twenty-four hours after treatment, the cells were trypsinized and counted. Approximately 1 × 105 cells resuspended in 200 µL serum-free DMEM were seeded into the upper chambers, whereas the bottom chamber was filled with 600 µL of 10% FBS medium or medium containing 2 μM NT157. After 24 h, non-migrated cells were wiped off with a cotton bud, and the cell migrated underneath the chamber were fixed with 95% ethyl alcohol and stained with 0.1% crystal violet. The cells were counted and photographed at 200 × magnification in 5 randomly selected fields.
Western Blotting
The cells were lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with 1 × proteinase and phosphatase inhibitor cocktail (Heart Biological, Xi’an, China). Total protein was quantified using a bicinchoninic acid (BCA) assay kit (Bioss, Beijing, China). The protein lysate was separated via electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Danvers, MA, USA). After blocking with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20, membrane was incubated with primary antibodies against β-actin, p-STAT3, STAT3, and Jab1 (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. After incubation with a secondary antibody, chemiluminescence was detected using ECL kit (Millipore, USA) to analyze the protein levels. Each sample was analyzed 3 times. ImageJ software was used to quantify the protein expression.
Animal Models
Five-week-old male BALB/c nude mice were purchased from the Shanghai Experimental Animal Center and housed in a pathogen-free facility at the Animal Center of Xi’an Jiaotong University. Animal experiments were approved by the Animal Experiment Administration Committee of Xi’an Jiao Tong University. SMMC-7721 cells (1 × 106 in 100 µL PBS) were injected into the tail vein of each mouse. Mice were cared for by trained staff. Mice were treated with NT157 (100 mg/kg, intraperitoneal injection) once every 2 days. After 4 weeks, the mice were sacrificed, and lung tissue was obtained.
Data Processing
The raw data and clinical information of HCC patients were downloaded from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) and The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/). Gene expression profiling of the GSE36376 datasets was performed using the GPL10558 platform, which included 47,000 unique probes and tested more than 31,000 genes. The GSE36376 series consisted of 240 HCC and 193 non-tumor samples. From TCGA website, we downloaded information related to 371 HCC and 50 non-tumor samples. The mRNA-seq data were preprocessed and submitted for analysis as upper quantile-normalized fragments per kilobase of transcript per million mapped reads (FPKM) values.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7 or the SPSS software. For all experiments, P-values were determined using a two-tailed Student’s t-test. The Chi-square or Fisher’s exact tests were performed to compare categorical variables. Correlations between STAT3 and Jab1 expression were analyzed. The Kaplan-Meier method was used to evaluate the overall survival (OS), and the log-rank test was used for survival comparisons. Results are expressed as mean ± S.D. from an appropriate number of experiments (3 to 6 biological replicates, and statistical significance was set at *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
NT157 Inhibits HCC Migration and Metastasis
HCC is a malignant tumor that still lacks effective treatment. NT157 has been shown to inhibit various cancers, including colon cancer and melanoma. 17,19
In this study, we investigated the anticancer efficacy of NT157 in HCC. The antitumor effects of 2 μM NT157 were detected for 24 h in 4 different HCC cell lines (HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L). As shown in Figure 1A, the results of wounding healing showed that the wound width was wide in the NT157 treatment groups, thereby indicating that the migration ability of HCC cells treated with NT157 decreased. Furthermore, we performed transwell assay to investigate the ability of NT157 to inhibit cancer cell migration. After NT157 treatment, the number of cells that crossed the membrane was lower than that in the control group, suggesting that NT157 had a significant inhibitory effect on the migration of HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L cells (Figure 1B).
Figure 1.
NT157 inhibits HCC cell line migration in vitro and their lung metastasis in vivo. A, NT157 inhibits migration of HCC cells as shown by wound healing. Serum-starved HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L cells were treated with or without 2 μM NT157, and wound healing of these cells was detected after 24 hours. B, NT157 inhibits migration of HCC cells as shown by transwell assay. Serum-starved HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L cells were seeded in the upper chambers of transwell system with or without 24 h 2 μM NT157 treatment. The numbers of migrated cells were counted. C, NT157 inhibits HCC metastasis in vivo. The nude mice injected with SMMC-7721 cells (1 × 106 in 100 µL PBS) were sacrificed after 4 weeks with or without NT157 treatment (100 mg/kg, intraperitoneal injection). The incidences of lung metastasis were counted.
These results demonstrate the migration suppressive function of NT157 in vitro. A lung metastasis HCC model in nude mice was then constructed to validate this conclusion in vivo. SMMC-7721 cells (1 × 106 in 100 µL PBS) were injected into the tail vein of nude mice to investigate the antitumor effect of NT157 in vivo. Ten tumor-bearing mice were divided into 2 groups: vehicle control (n = 5) and NT157-treated (n = 5). As expected, 4 weeks after injection, the incidence of lung metastases was significantly lower in mice treated with NT157 than that in control mice (Figure 1C). This in vivo result suggests that NT157 inhibits HCC cell metastasis, which is concordant with the results obtained in vitro.
NT157 Inhibits Migration by Decreasing the Activation of STAT3/Jab1 Signaling Pathway
As described above, NT157 effectively inhibited migration of HCC cell lines. This, together with the role of NT157 in colon cancer metastasis and its potential as a novel drug, led us to explore the mechanisms of NT157-induced inhibition of cell migration.
First, we assessed the activation of STAT3 pathways, as they were previously reported to be involved in HCC metastasis. After treatment with NT157, a decrease in phosphorylation of STAT3 was observed in a dose- and time-dependent manner, whereas the levels of total STAT3 remained unchanged (Figure 2A and B). STAT3 functionally interacts with Jab1 during progression of nasopharyngeal carcinoma. 20 It is normally located in the cytosol, but the activated STAT3 complex translocates to the nucleus to initiate the transcription of STAT3 target genes, including the genes encoding Bcl-XL, 7 MCL1, 21 survival, 22 and VEGF. 11 Therefore, we investigated the Jab1 expression in the nucleus and cytoplasm following NT157 treatment. Our results showed that after NT157 treatment, phosphorylated STAT3 levels decreased along with Jab1 suppression in the nucleus (Figure 2C), whereas no significant decrease was observed in the Jab1 expression in cytoplasm (Figure 2D), indicating that NT157 inhibited p-STAT3 and decreased the Jab1 levels in the nucleus.
Figure 2.
NT157 inhibits activation of STAT3/Jab1 signaling. A, NT157 inhibited p-STAT3 in HCC cells in a dose-dependent manner. Serum-starved HepG2 and SMMC-7721 cells were treated with NT157 as indicated and either stimulated with IL-6 (50 ng/mL) or not, before lysis. B, NT157 inhibited p-STAT3 in HCC cells in a time-dependent manner. Serum-starved HepG2 and SMMC-7721 cells were treated with NT157 as indicated and either stimulated with IL-6 (50 ng/mL) or not before lysis. C, NT157 inhibited p-STAT3 and Jab1 in nucleus. Serum-starved HepG2 cells were treated with NT157 (2 μM) for 8 h, and nucleus protein and plasma protein were extracted and analyzed. D, Jab1 expression was slightly decreased in plasma protein. E, The effect of NT157 against p-STAT3 was not entirely ERK-dependent. Serum-starved HepG2 cells were treated with PLX4720 for 30 min followed by NT157 (2 μM) treatment for 8 h. The cells were then lysed and analyzed by western blotting.
Signaling pathways are usually inter-connected in many cancers. To verify the pathways related to NT157-induced inhibition in HCC, we treated HCC cells with the Raf inhibitor PLX4720, as NT157 has been demonstrated to activate extracellular signal-regulated kinase (ERK) in melanoma. 19 The results showed that after PLX4720 treatment, expression of ERK, the downstream of Raf, was inhibited, whereas the NT157-induced inhibition of p-STAT3 was sustained (Figure 2E). This indicated that the effect of NT157 on p-STAT3 was not entirely dependent on ERK and also highlighted the potential of combination therapy for some patients. Thus, NT157 inhibited p-STAT3 and nuclear Jab1 expression in HCC.
STAT3 and Jab1 Overexpression Are Correlated With Decreased OS
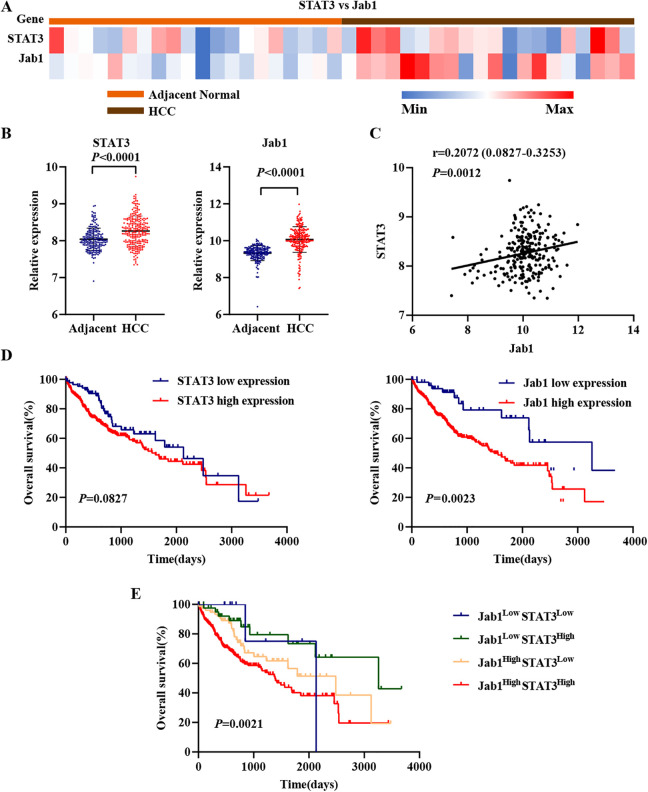
To have a better understanding regarding the potential of STAT3 and Jab1 as therapeutic targets in HCC, we analyzed their expression in HCC tissues and adjacent normal tissues (https://www.ncbi.nlm.nih.gov/geo/) (Figure 3A). The results showed that compared with the normal tissues, HCC tissues had significantly higher expression of STAT3 (P < 0.0001; Figure 3B) and Jab1 (P < 0.0001; Figure 3B). Furthermore, we found that STAT3 expression correlated with Jab1 expression in HCC tissues (r = 0.2072, P = 0.0012; Figure 3C). We investigated 371 cases from TCGA (https://cancergenome.nih.gov/) that had associated RNASeq data. We used the Kaplan-Meier plotter to further explore the association between STAT3 and Jab1 expression and survival. After gaining the best cutoff value of gene expression by the “surv_cutpoint” function of the “survminer” R package, Jab1 overexpression was associated with poor OS (P = 0.0023; Figure 3D), whereas STAT3 overexpression was not significantly associated with OS (P = 0.0827; Figure 3D). In our previous study, increased Jab1 expression was significantly associated with poor OS in HCC. 23 STAT3 contributes to nasopharyngeal carcinoma progression by regulating Jab1 expression. 20 Therefore, in the present study, we analyzed STAT3 and Jab1 expression phenotypes in combination. We divided the HCC patients into 4 groups with optimal cutoff: low expression of STAT3 and Jab1, low expression of STAT3, and high expression of Jab1, high expression of STAT3, and low expression of Jab1, and high expression of STAT3 and Jab1. The results showed that patients with high expression of STAT3 and Jab1 had the shortest mean survival duration (P = 0.0021; Figure 3E). To further identify STAT3 and Jab1 expression phenotypes in combination with potential prognostic value, we assessed the differences in clinicopathological factors in the high expression group (high expression of STAT3 and Jab1) and low expression group (low expression of STAT3 and Jab1, low expression of STAT3, and high expression of Jab1, high expression of STAT3, and low expression of Jab1) in HCC. The results showed that the HCC patients in the 2 groups had significant differences in the grade and tumor cell vascular infiltration (Table 1). HCC patients in the high expression group had higher grade and more tumor cell vascular infiltration, suggesting that they had a poor prognosis.
Figure 3.
Expression patterns of STAT3 and Jab1 in normal liver tissues and HCC tissues. The clinical data were downloaded from TCGA. A, Heat-map of STAT3 and Jab1 expression. B, STAT3 and Jab1 expression in normal liver tissues and HCC tissues. C, Correlation of STAT3 and Jab1 expression. D, Kaplan-Meier analyses of the association between STAT3 or Jab1 protein expression and survival. The patients were stratified into high expression group and low expression group according to the optimal cutoff value of mRNA. E, Kaplan-Meier analyses of the association between combined STAT3 and Jab1 protein expression and survival.
Table 1.
Relationship Between STAT3/Jab1 mRNA Expression and Clinicopathological Features in Hepatocellular Carcinoma Patients.
| STAT3 + Jab1 | χ2 | P value | |||
|---|---|---|---|---|---|
| Low expression | High expression | ||||
| Sex | Male | 53 | 144 | 0.000 | 0.986 |
| Female | 22 | 77 | |||
| Stage | Ⅰ + Ⅱ | 63 | 172 | 0.260 | 0.612 |
| Ⅲ + Ⅳ | 12 | 49 | |||
| Grade | G1 + G2 | 49 | 128 | 4.045 | 0.044 |
| G3 + G4 | 26 | 93 | |||
| Vascular infiltration | Yes | 18 | 84 | 4.913 | 0.027 |
| No | 57 | 137 | |||
Abbreviations: Low expression, Jab1LowSTAT3Low + Jab1LowSTAT3High + Jab1HighSTAT3Low; High expression, Jab1HighSTAT3High.
Note: Values in bold indicate P < 0.05.
Discussion
Our data derived from an in vitro system showed that NT157 suppressed the migration of HCC cell lines HuH-7, MHCC-97 H, SMMC-7721, and MHCC-97 L. Further, we observed that NT157 treatment decreased the lung metastasis of SMMC-7721-induced tumors in nude mice. Furthermore, we demonstrated that NT157 inhibited p-STAT3 and Jab1 expression. In addition, STAT3 was overexpressed in HCC tissues than in adjacent normal tissues. Meanwhile, STAT3 was positively correlated with Jab1 expression, and its high expression was correlated with poor OS. Finally, the patients with high expression levels of STAT3 and Jab1 had the poorest OS, and the HCC patients in this group had higher grade and more tumor cell vascular infiltration.
Distant metastasis causes HCC-related mortality. 3 An increasing number of studies have reported STAT3 activation or overexpression during the metastatic cascade of HCC. 3,24 Moreover, targeted STAT3 therapy has not yet entered the clinical practice, although it is generally accepted that targeting STAT3 for cancer therapy may be fruitful. Therefore, targeting STAT3 therapy is a promising strategy for the metastasis inhibition and treatment of HCC. NT157 exerts significant antitumor effects on various cancers, such as colon cancer, melanoma, and prostate cancer, 17 -19 indicating that it is an ideal target drug that can bring clinical benefits to patients. However, so far, no studies have evaluated the effects of NT157 in HCC. Therefore, we aimed to explore the antitumor effects of NT157 in HCC. Indeed, we observed that HCC migration and metastasis were significantly inhibited by NT157 in vitro and in vivo.
STAT3 is a critical driver of cell growth in cancer, and constitutively active STAT3 contributes to cell metastasis and invasion in human melanoma. 25 Enhanced STAT3 activation is associated with increased cell-cell contact, suggesting that STAT3 serves as a sensor of tumor cell contact to promote cell invasion and migration. 10 In the present study, we showed that NT157 inhibited HCC metastasis by inhibiting STAT3 activation. Similarly, in an earlier study, cucurbitacin I (JSI-124), a small-molecule inhibitor of the Janus kinase/signal transducers and activators of transcription (JAK/STAT3), inhibited STAT3 activation in the HONE-1-Epstein-Barr virus (HONE-1-EBV) cells and subsequently inhibited the growth and invasion of these cells. 8
Jab1 is a modulator of intracellular signaling and affects cellular proliferation, apoptosis, migration, and invasion in various cancers. 26 -28 Our previous studies have demonstrated that Jab1 is overexpressed and plays a critical role in the HCC pathogenesis. 23 In addition, STAT3 contributes to the progression of nasopharyngeal carcinoma by regulating Jab1 expression. 20 Hence, in the present study, we further analyzed Jab1 expression in the nucleus and cytoplasm and found that NT157 suppressed Jab1 levels in the nucleus.
In the present study, we also found that STAT3 was aberrantly expressed in HCC tissues compared to the non-cancerous tissues. Moreover, we found that STAT3 expression in HCC tissues positively associated with Jab1 expression. Furthermore, our analysis revealed that high expression levels of STAT3 and Jab1 correlated with poor OS, and HCC patients in the high expression group had higher tumor grade and increased tumor cell vascular infiltration. In line with our findings, studies using immunohistochemistry techniques have revealed that STAT3 and Jab1 protein levels and p-STAT3 levels were higher in HCC tissues and correlated with poor prognosis. 23,29 -32 Collectively, these results indicate the high potential of NT157 for clinical application and provide strong evidence that assessing STAT3 and Jab1 levels may help in predicting the prognosis of patients with HCC.
In conclusion, we found that STAT3 was overexpressed and correlated with Jab1 expression levels in HCC. Moreover, combined high expression of STAT3 and Jab1 resulted in poor OS. Our study not only provides the rationale for using STAT3-Jab1 as a therapeutic target in HCC, but also demonstrated that targeting STAT3-Jab1 by NT157 can inhibit HCC migration and metastasis. Our findings show that NT157 may serve as a novel therapeutic strategy for the clinical management of hepatocellular carcinoma in the future.
Supplemental Material
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211027916 for NT157 Inhibits HCC Migration via Downregulating the STAT3/Jab1 Signaling Pathway by SiZhe Yu, Yu Wang, KeJia Lv, Jia Hou, WenYuan Li, Xiao Wang, Hui Guo and WenJuan Wang in Technology in Cancer Research & Treatment
Acknowledgments
This work benefited from the Gene Expression Omnibus (GEO) database and the Cancer Genome Atlas (TCGA) database. We were grateful to the access to the resources and the efforts of the staff to expand and improve the 2 databases.
Abbreviations
- 2-HP-β-CD
2-hydroxypropyl-β-cyclodextrin
- BCA
bicinchoninic acid
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- FPKM
fragments per kilobase of transcriptper million mapped reads
- GEO
Gene Expression Omnibus
- HCC
hepatocellular carcinoma
- HIF
hypoxia-inducible factor
- HONE-1-EBV
HONE-1-Epstein-Barr virus
- HRP
horseradish peroxidase
- IRS1/2
insulin receptor substrate 1/insulin receptorsubstrate 2
- IL-6
interleukin 6
- Jab1
c-Jun activation domain-binding protein-1
- JAK/STAT3
Janus kinase/signal transducers andactivators of transcription
- MMP-2
matrix metalloproteinase-2
- MMP-9
matrix metallopeptidase-9
- OS
overall survival
- PBS
phosphate-buffered saline
- p-STAT3
phospho-STAT3
- RIPA
radioimmunoprecipitation assay
- STAT3
signal transducer andactivator of transcription-3
- TCGA
The Cancer Genome Atlas
- VEGF
vascular endothelial growth factor.
Authors’ Note: The animal experiments were approved by the Animal Experiment Administration Committee of Xi’an Jiao Tong University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by grant 81672432 from the National Natural Science Foundation of China (to H.G).
ORCID iD: Hui Guo  https://orcid.org/0000-0001-9780-0845
https://orcid.org/0000-0001-9780-0845
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12(7):408-424. [DOI] [PubMed] [Google Scholar]
- 3. Ryu SH, Jang MK, Kim WJ, Lee D, Chung YH. Metastatic tumor antigen in hepatocellular carcinoma: golden roads toward personalized medicine. Cancer Metastasis Rev. 2014;33(4):965-980. [DOI] [PubMed] [Google Scholar]
- 4. Bromberg JF, Wrzeszczynska MH, Devgan G, et al. STAT3 as an oncogene. Cell. 1999;98(3):295-303. [DOI] [PubMed] [Google Scholar]
- 5. Darnell JE, Jr. STATs and gene regulation. Science. 1997;277(5332):1630-1635. [DOI] [PubMed] [Google Scholar]
- 6. He G, Karin M. NF-kappaB and STAT3—key players in liver inflammation and cancer. Cell Res. 2011;21(1):159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of STAT3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105-115. [DOI] [PubMed] [Google Scholar]
- 8. Lui VW, Wong EY, Ho Y, et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer. 2009;125(8):1884-1893. [DOI] [PubMed] [Google Scholar]
- 9. Xie TX, Huang FJ, Aldape KD, et al. Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66(6):3188-3196. [DOI] [PubMed] [Google Scholar]
- 10. Vultur A, Cao J, Arulanandam R, et al. Cell-to-cell adhesion modulates STAT3 activity in normal and breast carcinoma cells. Oncogene. 2004;23(15):2600-2616. [DOI] [PubMed] [Google Scholar]
- 11. Niu G, Wright KL, Huang M, et al. Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000-2008. [DOI] [PubMed] [Google Scholar]
- 12. Almiron BD, Havrda MC, Lee MC, et al. Secretion-mediated STAT3 activation promotes self-renewal of glioma stem-like cells during hypoxia. Oncogene. 2018;37(8):1107-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Q, Zhang JJ, Ge WL, et al. YY1 inhibits the migration and invasion of pancreatic ductal adenocarcinoma by downregulating the FER/STAT3/MMP2 signaling pathway. Cancer Lett. 2019;463:37-49. [DOI] [PubMed] [Google Scholar]
- 14. Zhang M, Xue Y, Chen H, et al. Resveratrol inhibits MMP3 and MMP9 expression and secretion by suppressing TLR4/NF-kappaB/STAT3 activation in Ox-LDL-treated HUVECs. Oxid Med Cell Longev. 2019;2019:9013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert Opin Ther Pat. 2011;21(1):65-83. [DOI] [PubMed] [Google Scholar]
- 16. Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736-746. [DOI] [PubMed] [Google Scholar]
- 17. Reuveni H, Flashner-Abramson E, Steiner L, et al. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013;73(14):4383-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibuki N, Ghaffari M, Reuveni H, et al. The tyrphostin NT157 suppresses insulin receptor substrates and augments therapeutic response of prostate cancer. Mol Cancer Ther. 2014;13(12):2827-2839. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez-Lopez E, Flashner-Abramson E, Shalapour S, et al. Targeting colorectal cancer via its microenvironment by inhibiting IGF-1 receptor-insulin receptor substrate and STAT3 signaling. Oncogene. 2016;35(20):2634-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan Y, Wang S, Su B, et al. STAT3 contributes to cancer progression by regulating Jab1/Csn5 expression. Oncogene. 2017;36(8):1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niu G, Bowman T, Huang M, et al. Roles of activated Src and STAT3 signaling in melanoma tumor cell growth. Oncogene. 2002;21(46):7001-7010. [DOI] [PubMed] [Google Scholar]
- 22. Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101(4):1535-1542. [DOI] [PubMed] [Google Scholar]
- 23. Guo H, Jing L, Cheng Y, et al. Down-regulation of the cyclin-dependent kinase inhibitor p57 is mediated by Jab1/Csn5 in hepatocarcinogenesis. Hepatology. 2016;63(3):898-913. [DOI] [PubMed] [Google Scholar]
- 24. Chen RY, Yen CJ, Liu YW, et al. CPAP promotes angiogenesis and metastasis by enhancing STAT3 activity. Cell Death Differ. 2020;27(4):1259-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res. 2005;65(13):5828-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 2010;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1). Cancer Res. 2003;63(11):2977-2981. [PubMed] [Google Scholar]
- 28. Pan Y, Zhang Q, Tian L, et al. Jab1/CSN5 negatively regulates p27 and plays a role in the pathogenesis of nasopharyngeal carcinoma. Cancer Res. 2012;72(7):1890-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang L, Liu JX, Zhang ZJ, et al. High expression of Anxa2 and Stat3 promote progression of hepatocellular carcinoma and predict poor prognosis. Pathol Res Pract. 2019;215(6):152386. [DOI] [PubMed] [Google Scholar]
- 30. Jiang LH, Hao YL, Zhu JW. Expression and prognostic value of HER-2/neu, STAT3 and SOCS3 in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2019;43(3):282-291. [DOI] [PubMed] [Google Scholar]
- 31. Liang C, Xu Y, Ge H, Li G, Wu J. Clinicopathological significance and prognostic role of p-STAT3 in patients with hepatocellular carcinoma. Onco Targets Ther. 2018;11:1203-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qin J, Wang Z, Wang Y, Ma L, Ni Q, Ke J. JAB1 expression is associated with inverse expression of p27(kip1) in hepatocellular carcinoma. Hepatogastroenterology. 2010;57(99-100):547-553. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211027916 for NT157 Inhibits HCC Migration via Downregulating the STAT3/Jab1 Signaling Pathway by SiZhe Yu, Yu Wang, KeJia Lv, Jia Hou, WenYuan Li, Xiao Wang, Hui Guo and WenJuan Wang in Technology in Cancer Research & Treatment