Abstract
A recently discovered coronavirus, SARS-CoV-2, caused a global respiratory disease pandemic called COVID-19. Many studies have shown the excessive activation of the innate immune response that leads to the adverse outcomes of COVID-19, and anti-inflammatory drugs are very useful in the treatment and management of this infection. The activities of Colchicine, one of the anti-inflammatory drugs, target several pathways related to excessive inflammation of COVID-19. This study aimed to evaluate the efficacy of Colchicine in the treatment of COVID-19 using a meta-analysis approach. Scopus, Pubmed, Google scholars, Web of Science, and Science direct were used to search all the randomized controlled trials, case-control, and cross-sectional studies that have evaluated the efficacy of Colchicine as a treatment for COVID-19 (up to 28 May 2021). The overall effect of Colchicine versus the control group was determined using a random-effects model meta-analysis where we compared changes (i.e. mean differences—Colchicine group vs Control group) between the two conditions in test scores indicative of hospitalization time (day) and mortality rate. The results illustrated Colchicine therapy is associated with a decreased mortality rate in COVID-19 patients and associated with a decrease in hospitalization time (day) in COVID-19 patients. Present preliminary data shows that Colchicine has a beneficial effect on coronavirus disease care in 2019. Therefore, Colchicine can be a good suggestion in the management of COVID-19.
Keywords: Colchicine, COVID-19, hospitalization time, mortality rate, anti-inflammatory
Introduction
Coronavirus Disease 2019 (COVID-19) infection displayed mild symptoms but in 15% of overall contributes to acute respiratory distress syndrome (ARDS) with pulmonary damage and systemic inflammation.1,2 After infection with COVID-19, the immune system recognized virus particles through a variety of different receptors on and within immune cells, including Toll-like receptors (TLRs) and NOD-like receptors (NLRs). Stimulation of these receptors triggers intracellular signaling cascades, leading to the release of pro-inflammatory cytokines and chemokines that are necessary for the development of adaptive immunity.2,3 But swarming internal stimulation leads to pyroptosis cell death regulated by the Nod-like receptor family, pyrin-containing domain 3 (NLRP3) through inflammasome activation; these events induce cytokine release syndrome (CRS) in severe COVID-19 patients.4,5
For this reason, in treating and managing COVID-19, anti-inflammatory drugs are very important. 6 One of these medications is Colchicine, which is used to treat autoinflammatory diseases such as Mediterranean fever, Behcet’s disease, and other rheumatic diseases7,8 and displays its immunomodulatory effect through various actions (Figure 1).9–11
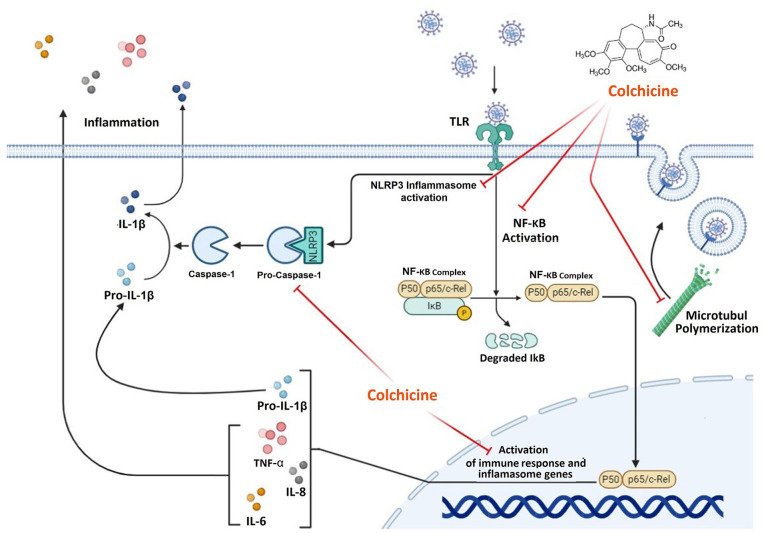
Figure 1.
Anti-inflammatory mechanisms of action of Colchicine. Colchicine interferes with many inflammatory pathways, including adhesion, superoxide production, inflammatory activation, and proinflammatory cytokine release. The effective mechanisms of action include: colchicine reduced NF-KB complex activity and decreased immune response and inflammatory genes such as NALRP3, IL-1, Pro-IL-1β, IL-6, and TNF. Colchicine inhibits the activation of NALRP3 and CASPASE-1. Colchicine also inhibits the assembly, development, and elongation of microtubules and can prevent membrane-dependent functions necessary for SARS-CoV-2 intake, transport, and replication. Red lines refer to the inhibitory action of Colchicine (Figure was constructed using Biorender (https://biorender.com)).
In some case reports and randomized control trials, the use of Colchicine in SARS-CoV-2 infection has been confirmed.12–16 These studies examine the beneficial effects of Colchicine in infected patients with covid-19, such as reducing the incidence of hospitalization, reducing the risk of mechanical ventilation, and death rate. Based on the findings of recent clinical trials, the question is whether Colchicine is an effective therapeutic medication for the treatment of COVID-19 infection. For this reason, in this study, the findings of studies using Colchicine in the treatment of patients with COVID-19 disease have been meta-analyzed.
Material and methods
Search strategy
A literature search was conducted using accessible international online databases, including Scopus, Pubmed, Google scholars, Web of Science, and Science direct up to 28 May 2021. The relevant keywords have been used: “Colchicine,” “Colcrys,” “Mitigare,” “Gloperba,” “SARS-CoV-2,” “COVID-19,” “2019 novel coronavirus infection,” “2019-nCoV disease,” “novel coronavirus,” “ randomized controlled trial,” “controlled clinical trial,” “clinical trials as topic,” “Case and control,” “Cross-sectional,” and “RCT” which were combined with and/or/not. Two researchers evaluated the search method separately and verified that all relevant studies had been found. The scanning of documents was carried out in three phases. First, delete duplicate posts. Second, the collection of related papers based on title and abstract. Third, a thorough analysis of the full text of all the papers chosen. Finally, the sources of all the publications included have been checked.
Inclusion and exclusion criteria
All studies with the following inclusion criteria have been included: (1) All English studies; (2) studies in human populations with a confirmed diagnosis of Covid-19; (3) studies reporting on the adjusted effect estimates of the association between Colchicine use in COVID-19 patients; (4) reporting one of the following outcomes: in-hospital mortality and hospitalization; and (5) RCTs, Case-Control, and cross-sectional studies. Criteria for the exclusion of research: (1) papers with no access to full text; (2) non-original publications, including systematic analyses, meta-analysis, reviews, comments, conference abstracts, and letters; (3) case reports studies; (4) duplicative publications; and (5) non-English.
Quality assessment and data extraction
The quality evaluation has been carried out using the Jadad scale for RCTs. The Jadad scale consists of three categories: randomization (0–2 scores), blinding (0–2 scores), and an account of all patients (0–1 score). 17 Depending on the quality analysis, we graded the quality of the RCTs as good (4–5 scores), fair (3 scores), or poor (0–2 scores). Also, the modified versions of the Newcastle-Ottawa Scales (NOS) were used for Case-Control and cross-sectional studies. 18 After the quality evaluation, the following information is collected from each of the selected studies: first author’s name, year of publication, Study area, Total patients, Patients Colchicine community, Hospitalized (day), and death. The data were extracted and entered into a Microsoft Excel sheet. The Flow chart of the selected studies were showed in the Figure 1.
Statistical analysis
The primary outcomes were the effect estimates of Colchicine on the death rate and hospitalization of patients with Covid-19. In our study, the heterogeneity between the primary studies’ outcomes was calculated using the Cochran Q test statistic and the I2 index. To consider substantial heterogeneity, a P-value of less than 0.1 was used. The heterogeneity and homogeneity of the suspected factor were performed using random and fixed-effect models, and more than 50% were considered high heterogeneity. According to the analysis results, the measurement of odds ratios (OR) was performed by forest plots with 95% confidence intervals (crossed lines). Every box in the forest plot indicated the weight of the sample. All statistical analyses were conducted using the Comprehensive Meta-Analysis V.2 program.
Results
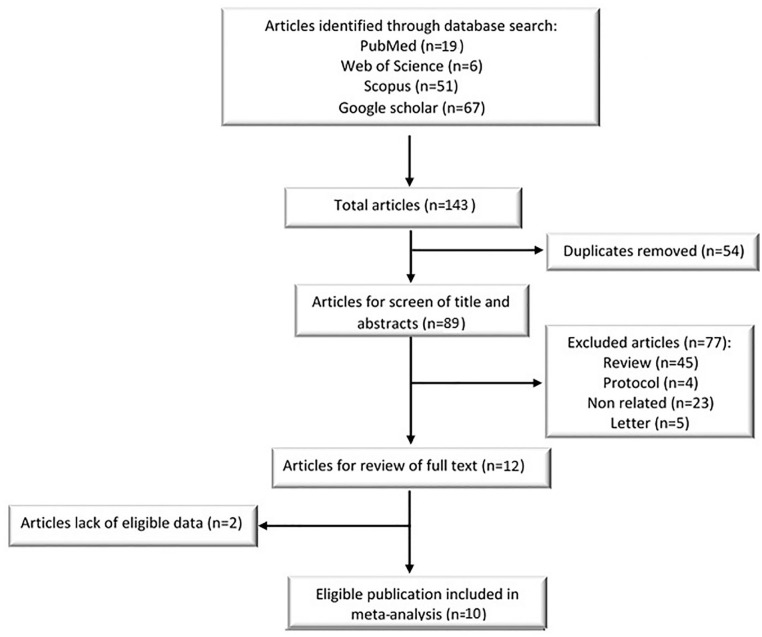
A total of 143 studies between 2019 and 2021 resulted from the database search. After removing duplicate studies, 89 articles were reviewed for titles and abstracts screening. Forty-five review articles, 23 irrelevant studies, five letters to the editor, and four protocols were omitted in the next step. Then, 10 potentially relevant documents were selected for full-text assessment, and two articles were excluded because of the lack of eligible data. Finally, 10 studies were included in this meta-analysis (Figure 2).
Figure 2.
Flow chart of the studies were included in the meta-analysis review (n: number of articles).
Accordingly, this Meta-analysis compares the mortality rate and the hospitalization time (day) between COVID-19 patients Colchicine groups, and COVID-19 patients control groups in nine selected studies (Table 1).
Table1.
Comparing of death between patients Colchicine and control groups.
| First author | Area of study | Total patients | COVID-19 patients colchicine group | Hospitalized (day) (SD) | Death | COVID-19 patients control group | Hospitalized (day) (SD) | Death | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pinzón (2020) | Colombia | 301 | 145 | N/A | 14 (9.6%) | 156 | N/A | 23 (14.74%) | Pinzón et al. 19 |
| Salehzadeh (2020) | Iran | 100 | 50 | N/A | 0 | 50 | N/A | 0 | Salehzadeh et al. 20 |
| Lopes (2021) | Brazil | 72 | 36 | 6 (3.3) | 0 | 36 | 8.5 (4.7) | 2 (5.55%) | Lopes et al. 21 |
| Brunetti (2020) | USA | 66 | 33 | N/A | 3 (9.09%) | 33 | N/A | 11 (33.33%) | Brunetti et al. 22 |
| Deftereos (2020) | Greek | 105 | 55 | 12 (9.62) | 1 (1.81%) | 50 | 13 (6.66) | 7 (14%) | Deftereos et al. 13 |
| Scarsi (2020) | Italy | 262 | 122 | 21.3 (6.8) | 19 (15.57%) | 140 | 25 (14.8) | 51 (36.42%) | Scarsi et al. 16 |
| Sandhu (2020) | USA | 112 | 34 | N/A | 16 (47.05%) | 78 | N/A | 63 (80.76%) | Sandhu et al. 15 |
| Tardif (2021) | Canada | 4488 | 2235 | N/A | 5 (0.22%) | 2253 | N/A | 9 (0.39%) | Tardif et al. 23 |
| Mahale (2020) | India | 81 | 39 | N/A | 11 (28.2 %) | 42 | N/A | 10 (23.8%) | Mahale et al. 24 |
| García-Posada (2021) | Colombia | 314 | 226 | N/A | 112 (49.55%) | 88 | N/A | 58 (65.9%) | García-Posada et al. 25 |
Analysis of mortality
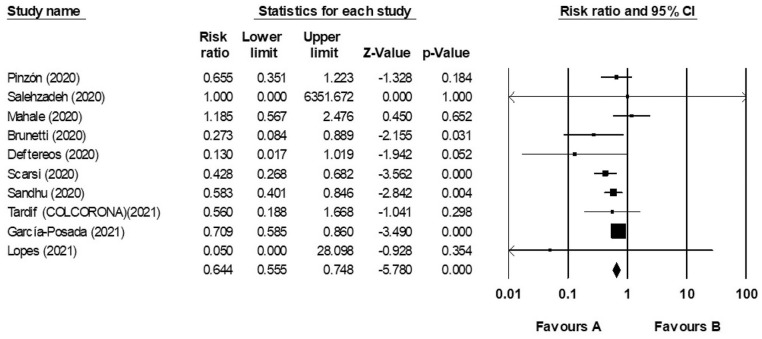
In this present study with the compounding of the results, the mortality rate between Colchicine and control groups 0.365 (95% CI: 0.555–0.748) with the confidence interval of 95% and with based on fixed effect model is (I2 = 24.149, Q = 11.86, P = 0.221) and it is indicated that heterogeneity was not observed in the result of these studies. Our meta-analysis showed that Colchicine therapy is associated with a decreased mortality rate in COVID-19 patients (Figure 3).
Figure 3.
Forest plot. It compared the risk ratio of death between COVID-19 Colchicine and control groups according to primary studies’ results and overall estimation.
Analysis of hospitalization
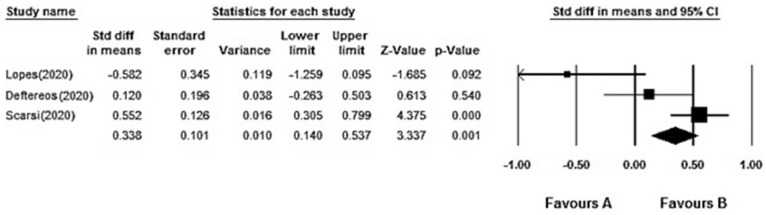
In the present study, the hospitalization time (day) of COVID-19 patients were varied from Colchicine and control groups (6–21.3 vs 8.5–25). Generally, with the compounding of the results, the hospitalization time (day) in Colchicine and control groups with the confidence interval of 95% and with based on random effect model is (I2 = 57.351%, Q = 4.689, P = 0.096) and it is shown that heterogeneity was observed among the primary results of the studies (Figure 4). Our result reported that Colchicine therapy is associated with a decreased 0.338 fold in hospitalization time (day) in COVID-19 patients (95%CI: 0.140–0.537).
Figure 4.
Forest plot. It compared the hospitalization time (day) difference between COVID-19 patients colchicine and control groups according to primary studies’ results and overall estimation.
Discussion
This research meta-analyzed the mortality rate and hospitalization time (day) between COVID-19 patients treated with Colchicine and COVID-19 patients as control groups. According to the analysis, the mortality rate in COVID-19 patients treated with Colchicine is lower than that of the COVID-19 patient’s control group. Colchicine therapy leads to a 0.365-fold decrease in the mortality rate of COVID-19 patients. Similarly, based on the random effect model, the hospitalization time (day) between COVID-19 patients, Colchicine groups showed a lower hospitalization time than COVID-19 patients control groups.
Recent evidence suggests a potential synergy of colchicine in the treatment of cytokine cascades at various levels. Colchicine inhibits a wide variety of pathways related to inflammation and works by inhibiting the NLRP3 inflammasome, microtubule-based inflammatory cell chemotaxis, production of leukotrienes and cytokines, and phagocytosis. Colchicine accumulates in white blood cells, affecting them in several ways, including motility reduction, loosening adhesion and chemotaxis, inhibition of superoxide anions formation, and disrupting the mast cell degranulation. 26 Therefore, Colchicine was reducing IL-1β/IL-18/IL-6 synthesis and inducing pro-inflammatory cytokines, and generating neutrophil extracellular traps to prevent endothelial damage. As a result, it leads to significant inhibition of the interaction between white blood cells and endothelial cells. Some studies have also shown that Colchicine has antibacterial activity against Flaviviridae by reducing virus replication by preventing the microtubule’s polymerization. Since Colchicine is a well-known anti-inflammatory drug, researchers believe its use can reduce inflammation in covid-19 patients and cure this infection. COVID-19 causes an extreme and prolonged cytokine response known as “cytokine storm,” leading to high mortality due to immunopathology. The main mechanism of Colchicine to reduce cytokine storm in COVID-19 patients is probably to inhibit IL-1, IL-6, and IL-18 production. These cytokines interfere with the inflammatory protein NLRP3, which plays an important role in the cytokine storm. NLRP3 inflammation can be triggered by various mechanisms, which play a key role in forming the cytokine storm induced by COVID-19. viroporin E, a component of the SARS-associated coronavirus (SARS-CoV), stimulates NLRP3 inflammation by forming permeable calcium ion channels. Furthermore, another viroporin 3a has been shown to stimulate the activation of NLRP3 inflammation. 27 Therefore, 27 clinical trials on the use of Colchicine in COVID-19 treatment protocols have been registered in ClinicalTrials.gov until 1 January 2021. Several studies, including case reports,14,28,29 case series, 30 case-control,19,15,25 cohort, and RCTs,16,22,31,32 published their findings on the use of Colchicine in COVID-19 patients. It is worth noting that in all these trials, patients obtained hydroxychloroquine and/or azithromycin and/or tocilizumab according to the health care system guidelines. In our included studies, patients who received Colchicine had lower levels of inflammatory markers such as D-dimer and serum levels of C-reactive protein (CRP) compared to patients who did not receive Colchicine.13,15,16,19–22,23,24 Based on other clinical results, all of the included studies suggest that Colchicine could be beneficial for COVID19 treatment. This research has several limitations. The majority of the included studies were observational, and there is still a shortage of data from many RCTs’ results that have been registered in ClinicalTrials.gov up to this stage. Besides, the observational study had different inclusion and exclusion requirements, different follow-up periods, and small sample size. Some studies do not mention the SD or IQR for hospitalization time analysis, and this limitation contributed to the non-use of some study findings.
Conclusion
The present meta-analysis provides preliminary evidence of a significant impact of Colchicine treatment on mortality and hospitalization time (day) in COVID-19 patients. Due to the major limitations of the number of trials, these results only provide proof of concept for Colchicine is an effective anti-inflammatory medication. However, insufficient evidence is available at present. Future RCT outcomes are expected to more robustly assess the efficacy of Colchicine in the treatment of COVID-19 disease.
Acknowledgments
The authors gratefully acknowledge the student research committee of the Mazandaran University of Medical Science, Sari, Iran for financially supporting this research.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Monireh Golpour  https://orcid.org/0000-0003-1406-9028
https://orcid.org/0000-0003-1406-9028
References
- 1. Deftereos SG, Siasos G, Giannopoulos G, et al. (2020) The Greek study in the effects of colchicine in COVID-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic Journal of Cardiology 61(1): 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tahaghoghi-Hajghorbani S, Zafari P, Masoumi E, et al. (2020) The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Research 290: 198197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fara A, Mitrev Z, Rosalia RA, et al. (2020) Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biology 10(9): 200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman TL, Swartz TH. (2020) Targeting the NLRP3 inflammasome in severe COVID-19. Frontiers in Immunology 11: 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore JB, June CH. (2020) Cytokine release syndrome in severe COVID-19. Science 368(6490): 473–474. [DOI] [PubMed] [Google Scholar]
- 6. Deftereos S, Giannopoulos G, Vrachatis DA, et al. (2020) Colchicine as a potent anti-inflammatory treatment in COVID-19: Can we teach an old dog new tricks? European Heart Journal Cardiovascular Pharmacotherapy 6(4): 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nidorf SM, Fiolet AT, Mosterd A, et al. (2020) Colchicine in patients with chronic coronary disease. New England Journal of Medicine 383(19): 1838–1847. [DOI] [PubMed] [Google Scholar]
- 8. Slobodnick A, Shah B, Krasnokutsky S, et al. (2018) Update on colchicine, 2017. Rheumatology 57(suppl_1): i4–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angelidis C, Kotsialou Z, Kossyvakis C, et al. (2018) Colchicine pharmacokinetics and mechanism of action. Current Pharmaceutical Design 24(6): 659–663. [DOI] [PubMed] [Google Scholar]
- 10. Thompson PL, Nidorf SM. (2018) Colchicine: An affordable anti-inflammatory agent for atherosclerosis. Current Opinion in Lipidology 29(6): 467–473. [DOI] [PubMed] [Google Scholar]
- 11. Leung YY, Hui LLY, Kraus VB. (2015) Colchicine—Update on Mechanisms of Action and Therapeutic Uses. Seminars in Arthritis and Rheumatism. London: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalili N, Dalili N, Kashefizadeh A, et al. (2020) Adding colchicine to the antiretroviral medication - lopinavir/ritonavir (Kaletra) in hospitalized patients with non-severe Covid-19 pneumonia: A structured summary of a study protocol for a randomized controlled trial. Trials 21(1): 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deftereos SG, Giannopoulos G, Vrachatis DA, et al. (2020) Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial. JAMA Network Open 3(6): e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mansouri N, Marjani M, Tabarsi P, et al. (2020) Successful treatment of Covid-19 associated cytokine release syndrome with colchicine. A case report and review of literature. Immunological Investigations. Epub ahead of print 7 July 2020. DOI: 10.1080/08820139.2020.1789655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandhu T, Tieng A, Chilimuri S, et al. (2020) A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection. The Canadian Journal of Infectious Diseases & Medical Microbiology 2020: 8865954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scarsi M, Piantoni S, Colombo E, et al. (2020) Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Annals of the Rheumatic Diseases 79(10): 1286–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark HD, Wells GA, Huët C, et al. (1999) Assessing the quality of randomized trials: reliability of the Jadad scale. Controlled Clinical Trials 20(5): 448–452. [DOI] [PubMed] [Google Scholar]
- 18. Cullen AE, Palmer-Cooper EC, Hardwick M, et al. (2020) Influence of methodological and patient factors on serum NMDAR IgG antibody detection in psychotic disorders: a meta-analysis of cross-sectional and case-control studies. The Lancet Psychiatry 8(2): 109–120. [DOI] [PubMed] [Google Scholar]
- 19. Pinzón MA, Arango DC, Betancur JF, et al. (2020) Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in Colombia. Available at: https://clinicaltrials.gov/ct2/show/NCT04654416. [DOI] [PMC free article] [PubMed]
- 20. Salehzadeh F, Pourfarzi F, Ataei S. (2020) The impact of colchicine on the COVID-19 patients: A clinical trial study. Available at: https://www.researchsquare.com/article/rs-69374/v1. [DOI] [PMC free article] [PubMed]
- 21. Lopes MI, Bonjorno LP, Giannini MC, et al. (2021) Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 7(1): e001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brunetti L, Diawara O, Tsai A, et al. (2020) Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. Journal of Clinical Medicine 9(9): 2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tardif J-C, Bouabdallaoui N, L’Allier PL, et al. COLCORONA Investigators. (2021) Efficacy of colchicine in non-hospitalized patients with COVID-19. Medrxiv. Epub ahead of print 27 January 2021. DOI: 10.1101/2021.01.26.21250494 [Google Scholar]
- 24. Mahale N, Rajhans P, Godavarthy P, et al. (2020) A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital. Indian Journal of Critical Care Medicine 24(11): 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. García-Posada M, Aruachan-Vesga S, Mestra D, et al. (2021) Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean. Journal of Infection and Public Health 14(6): 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vitiello A, Ferrara F, Ferrara F. (2021) Colchicine and SARS-CoV-2: Management of the hyperinflammatory state. Respiratory Medicine 178: 106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vitiello A, Ferrara F, Pelliccia C, et al. (2020) Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia. Italian Journal of Medicine 14(2): 88–94. [Google Scholar]
- 28. Monti S, Montecucco C. (2020) Candidate rheumatologic treatments for COVID-19. Response to: ‘COVID-19 infection in a patient with FMF: Does colchicine have a protective effect?’ by Kobak. Annals of the Rheumatic Diseases 80: 39. [DOI] [PubMed] [Google Scholar]
- 29. Gandolfini I, Delsante M, Fiaccadori E, et al. (2020) COVID-19 in kidney transplant recipients. American Journal of Transplantation 20: 1941–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montealegre-Gómez G, Garavito E, Gómez-López A, et al. (2020) Colchicina: una herramienta terapéutica potencial frente a COVID-19. Experiencia en 5 pacientes. Reumatología Clínica. Epub ahead of print 8 May 2020. DOI: 10.1016/j.reuma.2020.05.001. [DOI] [Google Scholar]
- 31. Lopes MIF, Bonjorno LP, Giannini MC, et al. (2020) Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. MedRxiv. Epub ahead of print 1 January 2020. DOI: 10.1101/2020.08.06.20169573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salehzadeh F, Pourfarzi F, Ataei S. (2020) The impact of colchicine on the COVID-19 patients; a clinical trial study. BMC Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]