Abstract
Osteoporosis causes bones to become weak, porous and fracture more easily. While a vertebral fracture is the archetypal fracture of osteoporosis, it is also the most difficult to diagnose clinically. Patients often suffer further spine or other fractures, deformity, height loss and pain before diagnosis. There were an estimated 520,000 fragility fractures in the United Kingdom (UK) in 2017 (costing £4.5 billion), a figure set to increase 30% by 2030. One way to improve both vertebral fracture identification and the diagnosis of osteoporosis is to assess a patient’s spine or hips during routine computed tomography (CT) scans. Patients attend routine CT for diagnosis and monitoring of various medical conditions, but the skeleton can be overlooked as radiologists concentrate on the primary reason for scanning. More than half a million CT scans done each year in the National Health Service (NHS) could potentially be screened for osteoporosis (increasing 5% annually). If CT-based screening became embedded in practice, then the technique could have a positive clinical impact in the identification of fragility fracture and/or low bone density. Several companies have developed software methods to diagnose osteoporosis/fragile bone strength and/or identify vertebral fractures in CT datasets, using various methods that include image processing, computational modelling, artificial intelligence and biomechanical engineering concepts. Technology to evaluate Hounsfield units is used to calculate bone density, but not necessarily bone strength. In this rapid evidence review, we summarise the current literature underpinning approved technologies for opportunistic screening of routine CT images to identify fractures, bone density or strength information. We highlight how other new software technologies have become embedded in NHS clinical practice (having overcome barriers to implementation) and highlight how the novel osteoporosis technologies could follow suit. We define the key unanswered questions where further research is needed to enable the adoption of these technologies for maximal patient benefit.
Keywords: artificial intelligence, computed tomography, epidemiology, fragility fracture, innovation, Osteoporosis, QCT, screening, technology, vertebral fracture
Introduction
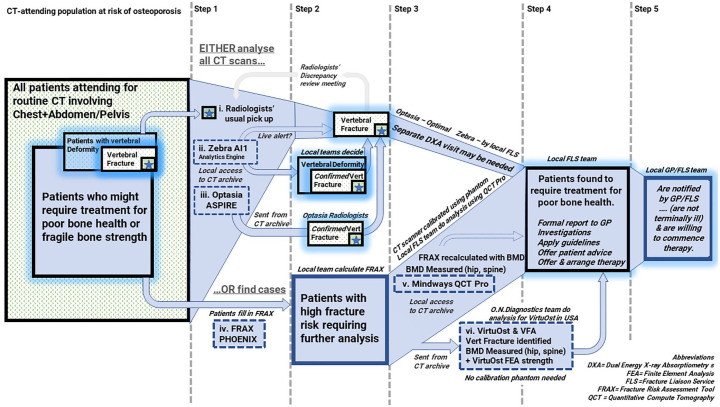
With modern computed tomography (CT) scans, some portion of the patients’ spine is visualised in detail during ordinary chest, abdomen and pelvis scanning, giving ample opportunity for diagnosing osteoporosis and for various methods of vertebral fracture assessment (VFA) technologies. These range from manual identification right through to semi-automated and fully automated methods, some of which are accepted for diagnosis by international specialist societies. A summary of products and services available to measure bone health in the CT-attending population is provided in Figure 1, highlighting the niches they occupy in typical primary and secondary osteoporosis screening strategies. This review focus not only on the technologies, but also on the barriers to their adoption.
Figure 1.
Comparison of available products and services (i–vi) to measure bone health in the CT-attending population, their place in screening and the barriers to adoption in a health service (dashed grey horizontal lines). A large proportion of older patients have previously undiagnosed osteoporosis (left panel), and some even have previously undiagnosed vertebral fractures (with or without osteoporosis). Starting with all older patients attending for routine CT, there are tools to screen all scans (Optasia ASPIRE and Zebra AI1) to identify possible vertebral fractures. Other tools (Mindways QCT Pro and VirtuOst) are best suited to some form of fracture risk assessment, with higher-risk individual scans being selected for analysis of density, strength and vertebral fracture (depending on the system).
CT, computed tomography; DXA, dual energy X-ray absorptiometry; GP, general practitioner; FEA, finite element analysis; FLS, fracture liaison service; FRAX, fracture risk assessment tool; QCT, quantitative CT.
Artificial intelligence (AI), along with its sub-disciplines of machine learning (ML) and deep learning (DL) are emerging as key technologies with the potential to improve patient outcomes. ML is a set of software algorithms and statistical models used to perform a specific task, without using explicit instructions. This approach is different from the other types of software we review, where products have emerged from coding done intentionally (based on what developers already know about proven osteoporosis predictors). With AI, large data sets of CT images are coupled with knowledge of eventual fracture outcomes and prevalence to ‘learn’ which imaging features predict the outcome of interest.
This Rapid Review aims to provide a comprehensive review of the topic but is not a full systematic review of all related literature. Cochrane guidance on Rapid Review methodology was published recently (https://tinyurl.com/y6ce5g4v). For in-depth evaluation of the technical CT methodologies, we recommend two recent review papers.1,2
Definitions and searches for the rapid evidence review
Definition of ‘approved’ software and services
This review considers all technologies that have either received United States (US) Food and Drug Administration (FDA) approval, ISO 13485 certification (in the case of Medical Devices that involve a phantom), a European CE mark for diagnosis, or are National Health Service (NHS) Care Quality Commission (CQC) regulated technology services. We also evaluated studies showing the cost effectiveness of the use of CT technologies. We consider each technology, its mechanism, integration within clinical systems and the evidence for its efficacy.
Patient and public involvement
The Patient and Public team of the Royal Osteoporosis Society conducted a survey of members seeking their views on different research questions through their Bone Academy Patient Insight Group (December 2019–January 2020). In total, 2313 patient responders with osteoporosis (from 7237 mailed) graded priorities and expectations from 13 key areas across the domains of Osteoporosis Causes, Technology and Service Effectiveness. Opportunistic detection of osteoporosis and vertebral fractures from CT data was the highest ranking priority, with 70% of patients thinking that patients were ‘extremely likely to benefit’ from the idea and a further 22% ‘very likely to benefit’ (92% score in total).
Data sources
Data sources searched include:
NICE Evidence library portal;
Systematic reviews via: Cochrane Library;
Electronic bibliographic databases: Embase, Medline; Tripdatabase; Web of Science;
Websites: NICE;
Search engines: Google Scholar and Google;
Theses and dissertations;
Individual companies: Mindways; Optasia Medical; ON Diagnostics; Zebra Medical were all contacted for supporting research literature relevant to their technology.
Grey literature, such as research studies carried out by charities and research institutes, reports, commentaries and review papers from government, policy bodies and professional organisations, was reviewed in support of the academic literature. In particular, the Grand Challenge AI for Radiology engine for CT products relating to osteoporosis and fracture terms was reviewed.
Search strategy
A Boolean search was performed using the operators AND, OR, NOT in combination with the following keywords, index headings and free text: Computed tomography; biomechanical computed tomography; computed axial tomography; computer assisted tomography; CT; computed tomography x-ray absorptiometry; CTXA; finite element analysis; FEA; dual energy X-ray absorptiometry; DXA; DEXA; osteoporosis; bone density; bone mineral density; fracture; screening; diagnosis; diagnostic; opportunistic; Mindways; Optasia; O.N. Diagnostics; Zebra Medical. Truncation techniques using asterisks and wildcard techniques using question marks were employed when free text searching. Additionally, reference lists of key relevant primary research, systematic reviews and meta- analyses and grey literature were examined to identify further studies. Citation searches of key relevant articles were undertaken. Targeted searches for publications by key academic researchers were made. Searches were limited to the English language.
Osteoporosis, vertebral fragility fractures, fracture liaison services and case-finding during CT
Osteoporosis and vertebral fragility fractures
Osteoporosis is a disease that causes bones to become weak and fragile. It is a major cause of disability, loss of quality of life and early death in the older population and poses a significant public health problem in a globally ageing population. The condition is usually asymptomatic until a fracture occurs, and patient perception of fracture risk is often underestimated. 3 Vertebral fragility fractures occur either spontaneously, as a result of normal activities such as lifting or coughing, or from mild trauma. These spinal fractures are the most common of all osteoporotic fragility fractures, occurring in 25% of men and post-menopausal women. 4 Under-diagnosis is a particular issue for vertebral fractures as only a minority result from a fall and symptoms may be attributed by both patients and clinicians to another cause. 5 Nearly all fractures are associated with an increased risk of future fracture, regardless of age, bone mineral density (BMD) and fracture location. 6 According to the International Osteoporosis Foundation (IOF), 520,000 fragility fractures occurred in 2017 in the United Kingdom (UK), costing £4.5 billion. This expenditure is set to increase by 30% by 2030 due to the ageing population.7–10
Treatment and behavioural interventions for people diagnosed with osteoporosis and vertebral fractures have been shown to reduce hip and other fracture rates by 40–70%. 11 A recent comprehensive review has found that secondary prevention strategies appear to be better developed and more successful than primary prevention strategies. 12 However, currently less than half of patients with a fragility fracture undergo secondary osteoporosis screening. 13 This is a missed opportunity, since the ‘lowest hanging fruit’ in secondary prevention of fractures are those people presenting to secondary care with a first fragility fracture. This group includes people found to have an incidental vertebral fracture after a CT scan, which is the subject of this evidence review.
The role of fracture liaison services
The failure to treat osteoporosis even after knowledge of a fragility fracture is known as the ‘osteoporosis treatment gap’. The percentage of women who did not receive treatment after a fracture was estimated by the IOF to be 49%.9,10 Despite proven efficacy of osteoporosis therapy, simple guidelines and multiple simple therapeutic options, treatment prescription rates remain sub-optimal. 14 Clinical care systems have been slow to incorporate secondary prevention. The usual care following a low-trauma fracture (including hip and vertebral) can still lack a simple evaluation and/or treatment of the osteoporosis that contributed to the fracture. 15 A multidisciplinary fracture liaison service (FLS) can facilitate case identification, investigation and intervention, 16 reducing the osteoporosis treatment gap and preventing fractures.17–20 Their effectiveness at reducing the risk of subsequent fracture is supported by level 1 evidence from systematic reviews with meta-analyses.21,22 Currently, it is unusual for FLS to follow up patients whose vertebral fractures are identified ‘opportunistically’ during CT scanning for other reasons.
Osteoporosis case-finding during CT
Patients attending hospital for routine CT are a group of patients who might be suitable for targeted case-finding. The Royal Osteoporosis Society and the Royal College of Radiologists (UK) have recently overseen education and audit initiatives focused on improving the identification of osteoporotic vertebral fractures in imaging done for other reasons, including CT. Their recent audit of UK radiology departments found that only 26% of vertebral fractures visualised incidentally on CT images were reported accurately, and less than 3% of patients were referred onwards for appropriate management (see Figure 1).
This review focusses on approved technology systems and their potential for adoption in health services, by which we mean systems that have received either FDA approval, ISO 13485 certification (in the case of Medical Devices that involve a phantom), a European CE mark for diagnosis, or are NHS CQC regulated technology services. This does not currently include the substantial body of research literature investigating the utility of direct Hounsfield unit (HU) estimation of bone health. In the UK fracture risk assessment is generally recommended by the National Institute of Health and Care Excellence (NICE) when patients have specified co-morbidities – a so-called targeted case-finding approach.11,23 In the case of patients attending hospital for routine CT, these patients will often fulfil the NICE criteria that recommend a fracture risk assessment due to age and co-morbidities. However, CT-attenders are not currently targeted for risk assessment. Fracture risk assessment using the online FRAX tool to input simple questionnaire answers gives a person-specific 10-year risk of osteoporotic fracture. A ‘FRAX 10-year risk’ of major osteoporotic or hip fracture is therefore the commonest method used to identify individuals at high risk of fracture in both primary and secondary prevention. The electronic output of FRAX in the UK is matched with nationally agreed osteoporosis guideline thresholds (National Osteoporosis Guideline Group: NOGG) that indicate the need for drug treatment. However, the current prevalence of high-risk patients (by FRAX) attending CT units is not known. Enthusiasm to screen all CT attenders with FRAX (or for that matter to investigate every single CT image for osteoporosis) must be tempered by the clear advice concerning primary osteoporosis screening programmes in the UK; the National Screening Committee (https://legacyscreening.phe.org.uk/osteoporosis) do not recommend population screening, citing a lack of effectiveness criteria. Nevertheless emerging data from the US on screening with CT bone strength analysis are encouraging. 24
Diagnosing osteoporosis using DXA
Dual energy X-ray absorptiometry (DXA) remains the traditional imaging technique in osteoporosis and gold standard for diagnosis. It uses an X-ray and detector system to measure the mineral content of bone and is especially well suited to the average lumbar spine (usually lumbar vertebrae 1 and 2 or 1, 2, 3 and 4) as well as the proximal femur [femoral neck (FN) and ‘total hip’]. The Word Health Organisation (WHO) definition of osteoporosis is based on a DXA measurement of BMD, deriving from evidence showing a clear link between lower BMD and increased fracture risk. 25 Diagnostic criteria use standard deviation (SD) scores of BMD related to peak bone mass in healthy young women, with osteoporosis being defined as a BMD T score of −2.5 or less and low bone mass (osteopenia) as a BMD T-score between −1 and −2.5. 26 DXA BMD values, particularly derived from the FN, are a very good indicator of future fracture risk and have long been incorporated into modern fracture risk estimating tools such as FRAX. DXA is subject to the limitations of a planar two-dimensional (2D) technology to represent a three-dimensional (3D) bone, and availability is patchy. Another limitation of DXA spine measurements are the inaccuracies in the setting of degenerative spinal pathology, and that this measurement is limited only to the lumbar spine. Each T-score unit decrease in BMD confers approximately a doubling of fracture risk. However, most osteoporotic fractures occur in individuals who do not have an ‘osteoporotic range’ BMD. In addition, other risk factors (e.g. age, sex, previous fracture) are associated with fracture risk independently of BMD.27,28 DXA has a very low radiation dose.
Diagnosing osteoporosis and vertebral fractures using CT; technology and services overview
Quantitative CT (QCT) is an established 3D imaging technique having been used in clinical practice since the 1970s. For a clinician seeking a diagnosis of osteoporosis in their patient, a CT scan is requested far less frequently than DXA due to the higher radiation dose with CT. Spine QCT measures the true volumetric BMD (vBMD) of trabecular bone within vertebrae, usually the average of lumbar vertebrae 1 and 2 (or 1, 2 and 3) with low values being an excellent risk marker for prevalent vertebral fracture.29–31 QCT BMD measurements of the spine do not give the same values as DXA (QCT Pro Spine, Software Mindways, Austin, TX, USA and VirtuOst Spine, O.N. Diagnostics, Berkeley, CA, USA). Software can also ‘project’ areal BMD (aBMD) of the hip from CT scans, more akin to DXA imaging. Like DXA, this areal density (g/cm2) is measured at the ‘FN’ and ‘total hip’ regions (QCT Pro CTXA and VirtuOst Hip) – the measurements are directly comparable with DXA and can be entered specifically into the FRAX online tool to give patient-specific 10-year fracture risk and UK treatment thresholds using either the ‘Mindways QCT’ drop-down option (CTXA) or the ‘T-score’ option (VirtuOst).
Developed as an adjunct to DXA and performed on the same machine, ‘vertebral morphometry’ images [also called VFA] are used to visualise a patient’s entire lateral spine encompassing key areas at risk of fracture, from the thoracic to the lumbosacral region. Fractures are diagnosed by reference to various shape criteria in the lateral projection of the wedged, crushed or biconcave-appearing vertebra. Software technology-based services are now emerging that automate or semi-automate the process of identifying vertebral fractures from CT data, where scans are done for other medical reasons and not for the primary purpose of osteoporosis assessment. In this emerging field, CT scans can be sent to an external company (ASPIRE service, Optasia Medical, Manchester, UK or AI1 Solutions using Zebra Bone Health algorithm, Zebra Medical Vision Ltd, Shefayim, Israel). In other scenarios, the automatic identification of vertebral fractures is integrated with other point of care AI tools visible to the radiologist reviewing the original CT scan (AI1 Integrations, using the Zebra Bone Health Algorithm).
Opportunistic ancillary screening for osteoporosis, low bone strength and vertebral fractures in CT scans done for other indications
Practical aspects of ancillary screening of CT data
According to the latest data from NHS England, almost 6 million CT scans were performed October 2019–October 2020 for patients in England. 32 Of these, over 1 million were estimated to include the chest and/or abdomen. If opportunistic ancillary screening was performed on these CT scans, earlier treatment for those with previously undetected osteoporosis might have saved and improved lives, and potentially saved significant costs to healthcare systems. Opportunistic CT-based screening methods have the potential to be light-touch (in terms of cost, time and inconvenience to stakeholders) and to prevent unnecessary hospital visits and further irradiation. 33
Academic researchers, software companies and service providers have realised the potential to diagnose osteoporosis and identify vertebral fractures as an ‘added extra’ service applied to CT scan images that have already been taken for other clinical reasons. A single clinical CT scan consists of a batch of hundreds of consecutive 2D slices (axial sections) through a person (the number of slices depending on the predetermined slice thickness and the amount of the body covered by the scan). The software and services for screening for osteoporosis and vertebral fractures are not usually installed on the radiographers’ CT scanning personal computer (PC) that drives the CT scanner. Instead the bone analysis software can be located either on nearby ‘standalone’ PCs or on the radiologists’ analysis terminal [called a picture archive and communication system (PACS) diagnostic workstation] or even at a totally distant site. In the latter case, analysis can be done by technical staff rather than radiographers, and sometimes away from the hospital, as long as the organisation providing the service is CQC-approved by the NHS. Using computer software to diagnose osteoporosis or vertebral fractures can be done any time; from minutes to hours after the patient has left the CT department, up to many months after the original scan. Here, the extra radiation dose is zero and the patient may be spared additional DXA imaging visits.
Diagnosis and fracture-prediction from QCT imaging technologies; landmark studies, diagnostic criteria and regulatory aspects
Traditionally, QCT measurements of vBMD have been made with specialised software and the patient lying on an ergonomic, slim bone calibration phantom. Phantoms are manufactured with materials of known density, usually calcium/potassium hydroxyapatite and are placed under the patient’s lower back and hips in order to mitigate for the variability in CT scanners by converting CT attenuation (measured in HU) to vBMD. 34 This is known as synchronous calibration. Bone density measurements made using synchronous calibration have been in clinical practice for many years and are usually reported by reference to the American College of Radiology criteria, where spine BMD values below 80 mg/cm3 are considered osteoporotic. Age- and sex-specific reference ranges of spine QCT BMD have long been available for adults and diagnostic test data are also published.29,30 While age-related reference ranges are used to generate Z-scores, to avoid confusion, T-scores (the diagnostic WHO criteria for DXA) are not generally used. A T-score is rather a DXA-specific concept and probably best kept linked to that particular planar, summative imaging method.
In 2014, a suitably designed and powered prospective study of healthy adult men and women was published confirming diagnostic accuracy of the 80 mg/cm3 threshold (i.e. ‘ACR standardised’ phantom-calibrated spine vBMD) in predicting (a) vertebral fracture (with complete 5-year follow-up spine imaging for coverage of all vertebral fractures occurring in the cohort) and (b) incident hip fracture (using ICD hospital codes). Average spine vBMD (L1 and L2) measured by QCT was highly statistically significantly associated with incident vertebral fracture; age-adjusted odds ratio (OR) for vertebral fracture was 3.1 [95% confidence interval (CI) 2.2–4.7] for every one SD lower spine vBMD, giving a typical 75-year old female or male with baseline vBMD of 80 mg/cm3 a 14.6% (11.1, 19.3) predicted probability of vertebral fracture. 31 A clinical study recently found that spine QCT was superior to DXA in predicting incident vertebral fracture in clinical practice, but caution is needed when evaluating the study. 35 Data from dedicated healthy ageing cohorts that match baseline high-quality CT imaging to contemporaneous modern DXA methods are needed.
More recent technological advances have opened the possibility of calculating the BMD of a patient without the phantom being present at the time of scan, known as ‘phantom-less’ or ‘asynchronous’ approaches. The various methods of achieving this are listed in Table 1. The application of this nascent technology is highlighted below.
Table 1.
| Method | Notes |
|---|---|
| Traditional phantom-based synchronous calibration | • Patient lies on an ergonomic phantom with materials of known densities (usually 2–5 rods of different human tissue density equivalents) |
| • CT attenuation values of the hip or spine are converted to BMD by reference to the known density values (QCT Pro) | |
| • Hip scans can be adapted to derive areal BMD, suitable for use in FRAX (CTXA) | |
| Phantom-less synchronous internal calibration | • No external calibration phantom scanned |
| • CT attenuation of adjacent internal tissues (e.g. blood or fat) used to calibrate attenuation measurements (VirtuOst) | |
| • Can be adapted to derive areal BMD, suitable for use in FRAX (VirtuOst Hip, T-score) | |
| Asynchronous external calibration | • Phantom scanned regularly. |
| • Simple, single-material phantom (Mindways Model 4 phantom, CliniQCT) | |
| • Hounsfield numbers of bone are then compared with phantom | |
| • Asynchronous CT of proximal femur can be adapted to derive areal BMD, suitable for use in FRAX (CliniQCT CTXA) | |
| Asynchronous external calibration with the ACRad phantom | • Routine calibration using ACRad phantom |
| • Direct CT attenuation values (HUs) are used to determine trabecular radiodensity without a BMD-specific calibration phantom | |
| • Does not require specialised software – can be performed on PACS workstation or any computer with standard tools used for viewing CT images |
ACRad, American College of Radiology; BMD, bone mineral density; CT, computed tomography; CTXA, CT X-ray absorptiometry; FRAX, fracture risk assessment tool; HU, Hounsfield units; PACS, picture archive and communication system; QCT, quantitative CT.
Commercially available methods can also identify individuals at high risk of fracture using CT combined with FEA.1,42 Initially introduced 40 years ago, FEA is a non-destructive computer simulation method that estimates the stiffness of a structure by dividing it into a number of simple parts, termed finite elements, that are connected by points termed nodes. Combinations of FEA and in vivo bone imaging data have significantly improved the estimation of bone mechanical behaviour compared with imaging alone.43,44 This combined ‘biomechanical CT’ (BCT, VirtuOst software, O.N. Diagnostics) approach provides non-invasive estimates of the breaking strength of the hip and spine. Combining that measurement with a CT-based measurement of a DXA-equivalent hip BMD T-score, BCT provides a more comprehensive diagnostic assessment of osteoporosis than bone strength or BMD alone.36,45–47 In the aforementioned diagnostic accuracy study, the age-adjusted BCT OR for incident vertebral fracture was 4.3 (2.4, 7.6). A 75-year old woman whose L1 vertebral strength lies exactly on the Fragile Bone Strength threshold for a female (4500N) has a predicted probability of vertebral of 22.2% (18.5, 26.4). The predicted probability of vertebral fracture increased more steeply with declining L1 Strength than for vBMD. 31
From a regulatory perspective, the CQC is the UK’s independent regulator of health and social care. Their report from March 2020 highlighted a range of observations and recommendations. 48 They emphasised the need for good governance of clinical, information, technical and human aspects of any ML tools in diagnostic services. They stated that most suppliers of ML applications in diagnostics will not need to register with CQC, only those that deliver clinical activity themselves. These few will need to be regulated and assessed by national standards to ensure safety and efficacy. The report emphasised that there is need for more assurance about the clinical aspects of algorithms in ML and clarity on how they can be implemented to ensure high-quality clinical care. There is also the need for technology suppliers to be clear what their products, solutions and devices do and how they perform, as suppliers do not always accurately state whether their products use ML, which makes it harder to implement devices safely.
Clinical effectiveness of currently available tools and services to diagnose osteoporosis, low bone strength and vertebral fractures in CT scans done for other indications
VirtuOst software, including FE (BCT)
VirtuOst fracture risk assessment service using strength-based classifications is referenced by the International Society of Clinical Densitometry (ISCD) guidelines as suitable for osteoporosis identification, fracture risk assessment and therapy monitoring. This technology has been solely used in the Mayo Clinic (Rochester, MN, USA) for the last 4–5 years, but increased cover and reimbursement by Medicare (as an official screening test for osteoporosis) may lead to wider adoption. The patient’s CT scan is sent to the company electronically, the analysis performed by the company and the results sent back to the ordering physician. Regulatory approval anywhere outside the US has not yet been applied for.
VirtuOst identifies osteoporosis on the basis of BMD, bone strength measurement, or both, at the hip and spine using synchronous internal calibration (Table 1).31,49 The results are of diagnostic quality and do not need verification by DXA or any other tests. DXA-equivalent areal BMD T-scores are obtained for the FN, which can therefore be used with FRAX (using the ‘T-score’ drop-down box on the FRAX website).50–53 A typical report from VirtuOst has areal hip BMD (in g/cm2), the associated T-score, plus L1 vertebral volumetric BMD in mg/cm3, as well as a measure of strength of the hip and vertebra calculated from 3D FEA. The latter (measured in Newtons) is reported by reference to a threshold of ‘fragile bone strength’ against age-specific expected values. Finally, VirtuOst encompasses a VFA covering as much of the spine as is captured in an individual’s scan. Each of these components of the VirtuOst clinical report has been verified independently in large fracture prediction studies for hip and spine fractures so that a ‘high risk’ individual might achieve that through one or more components of their analysis.
The various components of the VirtuOst service were validated in nine fracture-outcome studies, mostly conducted in the US and Iceland. The BCT technique showed BMD scores obtained from DXA and CT colonography had a high degree of agreement (R2 = 0.84). 54 In a cohort of 136 patients undergoing CT enterography (CTE), this technique also demonstrated a high degree of sensitivity and specificity for confirming osteoporosis (85.7% and 98.5%, respectively) or osteopenia (85.1% and 85.4%, respectively). 55 In another cohort of 136 women undergoing CT enterography, BCT analysis identified osteoporosis (as defined by DXA) with 100% specificity in 8 out of 8 patients, and 98.4% specificity in 126 of 128 patients (95% CI: 94.5%, 99.6%). 56 These data are further validated by a US retrospective case-cohort study of 4000 participants, in which accuracy of the BMD T-score as measured by VirtuOst analysis was consistent with DXA for all fracture-risk metrics and both sexes. 57 Importantly, the use of VirtuOst could be vital in inflammatory bowel disease monitoring, where a study of 257 patients who underwent CTE and BCT showed 54.5% of patients had high/increased fracture risk, of which 40.3% did not meet any of the Cornerstone screening criteria (IBD checklist for monitoring and prevention in bone health). 58
The prospective diagnostic accuracy of 2D measurements of FN BMD (using QCT) for incident hip fracture was established recently using the VirtuOst method. 31 Average FN aBMD measured by QCT was highly statistically significantly associated with incident hip fracture; age-adjusted OR for incident hip fracture was 3.5 (2.5–5.0) for every one SD lower FN BMD using QCT, giving a 75-year old female with baseline FN BMD T-score of −2.5 a predicted probability of hip fracture of 21.8% (17.0, 27.5). For men these figures were slightly higher at OR 3.7 (2.5, 5.6) and a 33.4% (23.3, 45.4) probability of hip fracture.
Mindways QCT Pro software (QCT Pro, CliniQCT, CTXA hip)
Mindways QCT software calibrates HU measured by any CT machine against a bone-density equivalent phantom to give consistent hip and spine bone density measurements across devices. There are two main products; QCT Pro and CliniQCT; the difference being that CliniQCT permits opportunistic osteoporosis hip and/or spine assessment from abdomino-pelvic scans in any CT scanner that has been calibrated with the supplied Model 4 cylinder phantom (a small cylinder of uniform material that calibrates using the CT beam hardening effect, monthly). CliniQCT is the system most relevant to this review. BMD measurements can be made from a wide range of scans such as abdomen/pelvis/spine CT, CT urography and cancer related positron emission tomography (PET-CT): the only requirements are that the scan covers an appropriate skeletal region. This requirement for a local user/analyst is one aspect that separates Mindways software from the other technologies in this review. There are clinical distributors in 12 countries spanning the US, Europe and Asia who assist with installation/training. 59
CliniQCT involves the FDA-approved and ISO 13485-approved ‘asynchronous QCT calibration’ method to analyse bone density in CT images from any scanner that has been calibrated by the Model 4 cylinder phantom. This enables the opportunistic use of CT data sets acquired for other purposes that did not include a CT calibration phantom in the patient images; a technical obstacle that is also overcome (albeit using a different method) by VirtuOst. Like VirtuOst, Mindways’ DXA-equivalent CTXA hip module gives areal bone density values (in g/cm2) and T-scores that are approved for diagnosis by the ISCD, as well as 3D volumetric analysis of BMD in the spine (in g/cm3). Phantom scans maintain precision and account for drift. Mindways’ CTXA data analysed in both a conventional and asynchronous manner confirmed diagnostic accuracy with excellent intra-and inter-reader reliability and correlation with DXA (r2 = 0.907, r2 = 0.82).38,60–63 QCT Pro is different insofar it requires a Model 3 flattened, curved phantom to be placed under the patient during a dedicated hip and or spine scan, plus a separate QA phantom is fitted onto the Model 3 for monthly quality scans (or after a CT X-ray tube change).
Mindways software can be run on a standard PC and does not require radiology-specific monitors or computers. Unlike the other systems in section 4 of this review, end-users of CliniQCT or QCT Pro typically retrieve eligible CT scans into their standard PC workstation from any PACS archive (at any time), perform the hip/spine analysis on a local copy of the CT scans and create a compliant clinical report. Alternatively, radiographers may decide to send CT scans from the actual CT scanning console to the Mindways DICOM server (i.e. both ‘push’ and ‘pull’ of CT images is supported). The software connects directly with any hospital PACS infrastructure to facilitate these retrieval and send/archive steps. Mindways software features a simple graphical user interface (GUI), guiding the user through bone analysis, creation of the report, printing and, if required, exporting the results back to the PACS archive to sit alongside the CT slices for all PACS users to see; analysis takes 2–3 min. The current Slicepick module displays anterior–posterior (AP) and lateral flattened composite spine images and contains basic measurement tools for identifying and confirming vertebral fracture by morphometry. The QCT Pro measurement of spine BMD (typically L1–L3 but supported from lower thoracic to lower lumbar spine with age and sex-matched reference data) has FDA approval, as does the CTXA method for diagnosing osteoporosis. As outlined above, the ACR threshold for spinal osteoporosis <80 mg/cm3) is very strongly associated with prevalent and incident vertebral fracture.
Even more important than the diagnostic accuracy and utility of CTXA measurements of FN BMD are their clinical utility when imported into the FRAX tool. Thus patient-specific 10-year major and hip osteoporotic fracture risk augmented by FN BMD (the gold standard recommended by most national guidelines) can be achieved if patients first fill in the FRAX questionnaire before CT. Indeed, The FRAX tool BMD entry has a specific ‘Mindways’ category reflecting the acceptance of this way of measuring bone density by the ISCD and FRAX; inputting CTXA density to FRAX is possible in 66 countries worldwide at the time of writing. The feasibility of opportunistic screening for osteoporosis and vertebral fractures using CliniQCT and CTXA with FRAX is currently being tested in the PHOENIX study (ISCRTN 14722819, https://doi.org/10.1186/ISRCTN14722819.)
Optasia medical
Optasia Medical specialises in software powered by ML algorithms that support the opportunistic case-finding of vertebral fracture patients. The Optasia Medical ASPIRE service out-sources the reporting of vertebral fractures visualised incidentally on CT, using a high degree of automation, combined with oversight from an in-house radiologist to improve the accuracy and efficiency of VF reporting. The service is already regulated by the CQC in the UK, and the technology has achieved CE-marking. Their technology, developed together with academic partners in University of Manchester, UK, provides a semi-automated quantitative vertebral morphometry devised from shape-based statistical modeling.64–69 These are used to identify and grade vertebral fractures using output measurements including vertebral height measurements and ratios and vertebral fracture classifications. In a real-world test of the software capability on CT scout views, their earlier SpineAnalyzer software, applied to CT lateral scout views, provided good-excellent agreement with the standard radiologist grading for prevalent vertebral fractures, with excellent intra and inter-reader reliability (coefficients 0.96–0.98). 67
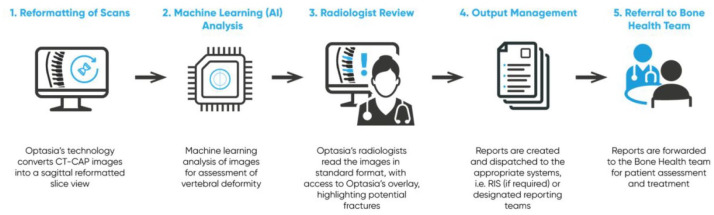
The ASPIRE software is designed to interface to a hospital PACS via a virtual machine running on a remote network. The software searches PACS for any relevant CT scans that include the spine and fulfil other criteria for example, patients >50 years of age. Identified scans are analysed and the output is reviewed by the radiologist who confirms or refutes the diagnosis, following which a report is automatically generated and returned to the requesting hospital site, the patients GP, and their local FLS or bone health team (Figure 2).
Figure 2.
Optasia medical service provision (CQC approved).
CQC, Care Quality Commission.
Retrospective feasibility studies involved a random sample of 1638 scans from five UK NHS Hospitals (Croydon, Cambridge, East Lancashire, Oxford and Salford). 70 Vertebral fractures were identified in 237 patients (14.2% ± 2.0). Only 67.7% of patients with vertebral fracture identified by the service had been found in the original radiology report, and only 13.3% of patients had been referred for appropriate management. In other feasibility studies of two different NHS sites (n = 7103), vertebral fractures were found in 20% of cases, of which 34% had been identified in radiology reports and 5.2% had been referred for appropriate management. As a result of the study, 1205 patients were referred by the service. These data were used to change practice in Croydon, where local physicians implemented a new reporting system to alert referrers so that when incidental fractures are found on CT they undertake a bone health review according to local pathways, ensuring timely assessment and treatment as appropriate. 71
Zebra Medical Vision
Clinicians are keen to explore ‘point of review’ tools that alert the specialist radiologist that the CT scan they are reviewing has a prevalent vertebral fracture, ‘red flags’ for eventual future vertebral fracture or prevalent osteoporosis. Zebra Medical Vision (Zebra-Med), focuses on AI in medical imaging.72–74 In May 2020, their software was FDA approved for opportunistic detection in osteoporosis. Zebra-Med analyses chest and abdominal CT scans using deep neural network technology: a combination of convolutional neural network and recurrent neural network technology.75,76 These analyse data from the spine to analyse bone density and detect vertebral fractures. The software uses statistical and machine learning methods to identify vertebral fractures, to measure the minimal L1–L4 vertebral spine density or to emulate a lumbar spine DXA T-score. The latter DXA emulation approach is different to those listed in 4a and 4b and cannot be imputed to FRAX at present. The DXA emulation method could be run on 96.5% of CT scans in a very large cohort, whereas the method approximating L1–L4 minimal trabecular density could be run successfully on 62.3% of CT scans. 77
In addition, Zebra-Med has released another bone health application, based on DL, to automatically identify fractures. 78 The fracture-identifying component of Zebra’s AI1 software could be run successfully on 84.3% of CT scans in the aforementioned cohort. The software extracts a virtual sagittal section visualising the spinal mid-plane and identifies VFs using ML algorithms. It outputs the probability that the volume contains a VF, and a heat map indicating the probable location of the VF in the sagittal image. In a single-site ‘real world’ clinical implementation study involving thoracic CT scans from 1696 patients with a VF prevalence of 24%, the system achieved a sensitivity of 54%, specificity of 92% and accuracy of 83%. The radiologist or other clinician is tasked with confirming whether the algorithmic output is correct and, if so, to grade the fracture. 79 From 48,227 individuals (51.8% women) age 50–90, the Zebra-Med algorithms applied together showed non-inferiority to basic FRAX in assessing 5-year fracture risk, and slightly better sensitivity and positive predictive value (+2.4%, +0.7% respectively). A shortcoming is that the study used only the most basic FRAX from charts rather than the online calculator to derive FRAX estimates; this is therefore based on the number of risk factors rather than the actual individually weighted risk factors.
In a different study using chest and abdominal CT scans from 1000 patients, sensitivity, specificity and accuracy were 84%, 73% and 82% respectively. 80 Simulated T-scores for 1693 CT studies compared with DXA showed few false positives (n = 92) relative to true positives (n = 1444) but more false negatives (n = 212) compared with true negatives (n = 245.) Clinical applications have been implemented in Europe and the US and, in partnership with tele-diagnostic company Tererad Tech, have expanded Zebra’s cloud-based DL analytics engine to more than 20 countries and 150 hospitals and healthcare organisations in India, Africa and Asia.
CT measurements of Hounsfield units
While there are many research studies evaluating CT HU for osteoporosis screening applications, the HU thresholds for consistent application across devices vary both with device and with protocol so it is not considered feasible to undertake a calibration exercise for each combination of device and protocol, meaning that such methods have not achieved clinical adoption and are unlikely to ever fulfil ‘approved technology’ status (see above for definitions). They are not dealt with further in this review.
Cost-effectiveness, futility and acceptability studies
For the implementation of opportunistic screening of CT for osteoporosis in a healthcare setting, understanding of its cost-effectiveness is vital, especially given the amount of work generated downstream for FLS and prescribers. It is also important to know in which patients screening would be futile. This is particularly true for opportunistic evaluation of CT; many routinely acquired CT scans are for patients with cancer or cancer-monitoring in whom mortality is higher than the general public. Indeed, the ancillary finding of a vertebral fracture in CT reduces survival markedly; from around 60% 4-year survival to about 30% 4-year survival (in adults 75 years or older undergoing chest CT) 81 . Here, there is a large research gap.
There have been only a small number of cost-effectiveness studies published. In a state-transition simulation study of a hypothetical cohort of 1 million post-menopausal women age >55 years, a screening programme of combined DXA and QCT performed at age 55 years with subsequent QCT every 5 years, was found to be most cost effective [$2000 per quality-adjusted life year (QALY)]. 24 With this strategy, there was a 12.8% lifetime hip fracture risk, compared with 18.7% with no screening and 15.8% with DXA alone. Favourable outcomes were also seen for wrist and vertebral fractures. However, this study is specific to a US Healthcare system model, and focused mostly on White American women. In an earlier, separate analysis, Viceconti calculated that BCT could be cost effective in the UK at $14,656 per QALY, 47 if offered at a fee of $100 per patient in addition to payment for a dedicated CT examination. 82 Neither of these studies consider an opportunistic approach, and do not study men.
A more recent analysis focussed on a one-time ancillary BCT offered only to patients already undergoing abdominal CT, and who had not had a recent DXA. 83 Researchers used a one-time biomechanical CT test (VirtuOst) to assess the accuracy and cost-effectiveness of this strategy in male and female patients aged >65 years in a hypothetical cohort of 1000 patients who underwent either this BCT approach or usual care using DXA or no screening. The BCT approach proved more cost-effective and clinically beneficial. Using the biomechanical CT strategy, 90% of women were screened and 21% tested positive for osteoporosis. Using DXA, 37.4% were screened and 12.5% tested positive for osteoporosis. For women, when using the no-screening model as a reference point, biomechanical CT prevented 5.5 hip fractures while DXA prevented 2.4 hip fractures. For men, biomechanical CT prevented 2 hip fractures and DXA prevented 0.2 hip fractures. When screening was restricted to patients at a 2-fold higher risk for hip fracture, prevented hip fractures also increased 2-fold with biomechanical CT, with a proportional increase in cost savings. These studies show promising results; however, more data specific to different healthcare systems and populations are crucial in the integration of these technologies into the healthcare setting. Finally, there is no data published describing patient attitudes to being offered ‘opportunistic’ screening. While there are general support from the osteoporosis patient community, work is needed to identify concerns and expectations among CT attenders.
Barriers for implementation of novel osteoporosis screening technologies and possible solutions
Non-adoption, adoption or abandonment of osteoporosis screening technologies and the challenges to scale-up, spread and sustain such technologies in healthcare organisations and systems
It takes on average 17 years to incorporate research discoveries into the practice of healthcare providers. 84 A thorough review of this topic is beyond the scope of this rapid evidence review; therefore, we include a few highlights and key themes relevant to embedding existing technologies into the NHS. Greenhalgh et al. developed an evidence-based framework for studying the non-adoption, adoption or abandonment of technologies and the challenges to scale-up, spread, and sustain such technologies in healthcare organisations and systems (abbreviated NASSS).85,86 The NASSS framework includes seven domains: the condition/illness, the technology, the value proposition, the adopters, the organisation(s), the wider context and changes over time. Each domain can be rated from simple to complex, with more complex projects being associated with higher failure rates. The NASSS framework can be applied to technologies in health and care either prospectively, to guide design and implementation, or retrospectively, to learn from failure. A diverse range of technology-supported programmes has been tested using this framework. Failure is often linked with complexity across multiple NASSS domains, and 10 principles have been highlighted to help manage and minimise this complexity. Table 2 shows the application of these principles to the current osteoporosis challenge. Opportunistic screening for osteoporosis and vertebral fractures in CT comes up against the four well-recognised barriers to implementation of technology in the NHS. First, against poor communication and connectivity, which slows innovation across individuals and organisations due to the fragmented structure of UK health services. Second, against lack of evaluation by NICE of the complex new technology. Third, against a lack of funding to take technologies forward for implementation at scale, even after successful pilots. Finally, even well designed innovations require system changes that the NHS is simply unable to afford the time, money and staff to implement, despite clear evidence that these changes would bring major benefits in the long run. An Institute of Public Policy Research report concluded that whilst barriers can vary between different innovations, a number of common problems exist across most innovations, namely: complexity, culture and money. 87 Several organisations and reports have highlighted challenges in implementing novel technologies in the NHS and provided some guidance on how these can be overcome. These include The Nuffield Trust, The Kings Fund and the Institute for Public Policy Research.88,89
Table 2.
The 10 NASSS principles applied to opportunistic analysis for osteoporosis using clinical CT (Greenhalgh). 90
| 1. Strengthen program leadership across academic and commercial research, NHS radiology, FLS, IT, metabolic bone, patient and public involvement, NHS procurement and management departments |
| 2. Develop a vision for National opportunistic screening of CT scans for osteoporosis and fractures |
| 3. Nurture key relationships between software developers, designers, vendors, image analysis providers, NHS X, CCGs, NIHR, RCR, Society of Radiographers, image exchange portal, ROS, ISCD and other essential stakeholders |
| 4. Develop champions through the national Academy initiatives and encourage them to problem solve local problems creatively |
| 5. Make resources available via the academy and other funding organisations for creative individuals/teams to use for generating solutions to local challenges to implement image analysis |
| 6. Capture data on progress and feedback to leadership, teams and individuals |
| 7. Acknowledge and address concerns of frontline NHS staff from idea to implementation |
| 8. Work with intended users to co-design practice-ready imaging technologies and FLS integration |
| 9. Control scope of the project, for example, concentrating initially on moderate- severe vertebral fractures |
| 10. Address regulatory and policy barriers via CE marking, ISO certification, FDA approval, ISCD |
CCG, clinical commissioning groups; CT, computed tomography; FDA, United States Food and Drug Administration; FLS, fracture liaison service; ISCD, International Society for Clinical Densitometry; ISO, International Organization for Standardization; IT, information technology; NHS, National Health Service; NIHR, National Institute of Health Research; RCR, Royal College of Radiologists; ROS, Royal Osteoporosis Society.
Several initiatives and organisations exist that try to improve the process. The NHS Accelerated Access Collaboration, NHS Innovation Accelerator and its associated programmes support fast-track of innovations from idea to adoption and spread; evaluation of this organisation has shown effectiveness in scale-up and spread of innovations.87,91–96 In April 2020, in the NHS Long Term Plan, a MedTech Funding Mandate was introduced as part of the wider strategy to accelerate the uptake of NICE-approved cost-saving MedTech products in the NHS.97–99 Evidence shows that nationally managed schemes resulted in a more rapid and complete uptake compared with devices that were not part of a national programme. 100 In February 2019, NHSX was established as a government unit that is responsible for setting consistent national policy and developing best practice for technology, digital services and data throughout the NHS.101,102 NHSX is actively looking at screening programmes for high-risk populations, but it is currently unclear whether or how this unit’s work would be relevant to this technology’s implementation.
How osteoporosis screening from clinical CT could follow a successful pathfinding CT software technology solution into routine NHS practice: HeartFlow FFRCT
An example of NHS software technology adoption is HeartFlow FFRCT – a technology that was recommended by NICE under its medical technologies guidance work stream (MTAG). 103 HeartFlowFFR is used to estimate fractional flow reserve from CT coronary angiography, and may avoid the need for invasive coronary angiography in patients with stable, recent onset chest pain. Draft recommendations based on all the evidence presented in the support of the technology were given, considering key clinical outcomes. NICE considered a total of over 69 studies comprising diagnostic accuracy, clinical effectiveness and cost-evidence. It is notable that this represents far more evidence than is available for the osteoporosis technologies. Whilst HeartFlowFFR was selected by the MTAG committee in December 2014, the final guidance was not published until February 2017 (27 months) demonstrating the long timescales often involved.
Discussion: areas for further research and development
Ancillary screening of CT data for osteoporosis and vertebral fractures is well supported by numerous academic papers focussed on software development and successful use in clinical practice. Various tools can now provide a rapid and reproducible screening method for osteoporosis and previously unidentified fractures. However, there are areas where further research is needed in order to address evidence gaps. It is currently unclear which patient groups should be included in opportunistic screening. It could be used exclusively in older adults, or also include other high-risk groups. A large proportion of routine CT attenders have specific co-morbidities, such as cancer, in comparison with the general population. Thus, while they have a higher unmet osteoporosis burden, the effects of screening, treatment and survival in these attenders needs to be understood in order to ascertain its clinical impact and cost-effectiveness.
It is yet to be determined how opportunistic CT imaging could be clinically integrated with current diagnostic methods. Determining whether it would be used in addition to, or instead of DXA, and for screening and/or definitive diagnosis, remains to be established. Further data will be required on which site(s) should be primarily used in opportunistic CT screening; for example, regions within the lumbar spine or hip and, if so, which sub-regions. The additional value of measuring hip and spine bone strength with CT FEA (to diagnose and treat patients on the basis of FBS) over simpler QCT methods needs to be quantitated. There are also no head-to-head studies providing comparative data that assess whether technologies that detect fracture are more effective than those assessing bone density/ bone strength, or comparing these technologies with any other methods of opportunistic screening.
Further understanding of the technology itself will be key to its widespread implementation. Each of the different calibration techniques has advantages and pitfalls; and additional research is necessary to characterise the sources of variation between scans using each calibration technique. In addition, the exact effect of IV contrast on the accuracy of the data is not yet known.
There are several service delivery issues. Should the services be standalone outside the NHS, embedded as ‘point of care’ or near ‘point of care’ tools and when should CT data be ‘sent’ for screening? A key emerging issue is the ability for healthcare providers to manage the higher workload resulting from increased case-finding. FLS and other healthcare providers could potentially be required to consult, administer treatment, follow up and monitor vastly increased numbers of affected patients. Local systems for service delivery would need to be established. These include logistics of how relevant diagnostic images would be stored, transmitted to healthcare providers (HCPs) and FLS teams, and how follow-up measurements, for instance, with DXA, would be comparable for the purposes of monitoring or drug-cessation.
Conclusion
Osteoporosis imposes a significant public health impact, as well as cost burden, and is increasing in prevalence. It remains under-diagnosed and under-treated. There is evidence from the literature to support multiple technologies using opportunistic screening of CT scans done for other indications, which could increase the rates of diagnosis, and therefore treatment to prevent fractures. There are still areas where further research is needed. However several barriers remain to the implementation of technologies into healthcare systems; encompassing problems with culture, complexity and funding. With further research and the use of new and existing initiatives, there may be opportunities for the implementation of these technologies into clinical practice.
Acknowledgments
This Rapid Evidence Review was commissioned by the Technology Working Group of the Royal Osteoporosis Society Osteoporosis and Bone Research Academy, to inform the Society’s 2020 Research Road Map and Cure Strategy (https://tinyurl.com/y6oaj46j). This article is drawn from an initial evidence review undertaken by CM (Health Evidence Matters Ltd), which was supported by a grant from the Royal Osteoporosis Society. KESP is supported by the NIHR Cambridge Biomedical Research Centre (BRC).The full review was summarised for scientific publication by VA and KESP. The authors are grateful to Caroline Sangan, Belinda Thompson and Francesca Thompson for their assistance in convening the Working Group, whose scientific membership comprises: KESP (Chair), EMC (Vice-Chair), RLA, PB, PAB, NC, JSG, EPK, NCH, KAW and JEC (as Academy Chair). The authors are especially grateful to the Royal Osteoporosis Society Patient Advocates for their contributions to the group; Mary Bishop, Lois Ainger, Nic Vine and Karen Whitehead.
Footnotes
Conflict of interest statement: PAB: Research collaboration with Optasia Medical (no financial interest).
EMC: Consultancy work with Optasia Medical Ltd.
NCH: Consultancy, lecture fees and honoraria from various pharmaceutical companies (outside the scope of the submitted work).
EPK: Research collaboration with Optasia Medical Ltd; (no financial interest).
KESP: Chief Investigator of the NIHR PHOENIX study (Picking up Hidden Osteoporosis Effectively during Normal CT Imaging without additional X-rays, http://www.isrctn.com/ISRCTN14722819).
All other authors state they have no conflicts of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Veena Aggarwal  https://orcid.org/0000-0002-3641-6388
https://orcid.org/0000-0002-3641-6388
Jennifer S Gregory  https://orcid.org/0000-0002-6328-4560
https://orcid.org/0000-0002-6328-4560
Kenneth E.S. Poole  https://orcid.org/0000-0003-4546-7352
https://orcid.org/0000-0003-4546-7352
Contributor Information
Veena Aggarwal, Kingston Hospital NHS Foundation Trust, Kingston Upon Thames, UK.
Christina Maslen, Health Evidence Matters, Bristol, UK.
Richard L. Abel, Imperial College London, London, UK
Pinaki Bhattacharya, The University of Sheffield, Sheffield, UK.
Paul A Bromiley, The University of Manchester, Manchester, UK.
Emma M. Clark, University of Bristol, Bristol, UK
Juliet E. Compston, Department of Medicine, UK
Nicola Crabtree, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, UK.
Jennifer S. Gregory, University of Aberdeen School of Medicine Medical Sciences and Nutrition, Aberdeen, UK
Eleni P. Kariki, The University of Manchester, Manchester, UK
Nicholas C. Harvey, University of Southampton Faculty of Medicine, Southampton, UK
Kate A. Ward, University of Southampton, Southampton, Hampshire, UK
Kenneth E. S. Poole, University of Cambridge School of Clinical Medicine, Addenbrooke’s Hospital, NIHR Cambridge Biomedical Research Centre, Cambridge, CB2 0QQ, UK.
References
- 1. Johannesdottir F, Allaire B, Bouxsein ML. Fracture prediction by computed tomography and finite element analysis: current and future perspectives. Curr Osteoporos Rep 2018; 16: 411–422. [DOI] [PubMed] [Google Scholar]
- 2. Keaveny TM, Clarke BL, Cosman F, et al. Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos Int 2020; 31: 1025–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gregson CL, Dennison EM, Compston JE, et al. Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int 2014; 25: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diacinti D, Vitali C, Gussoni G, et al. Misdiagnosis of vertebral fractures on local radiographic readings of the multicentre POINT (Prevalence of Osteoporosis in INTernal medicine) study. Bone 2017; 101: 230–235. [DOI] [PubMed] [Google Scholar]
- 5. Nuti R, Brandi ML, Checchia G, et al. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med 2019; 14: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15: 721–739. [DOI] [PubMed] [Google Scholar]
- 7. International Osteoporosis Foundation. Broken bones, broken lives: a roadmap to solve the fragility fracture crisis in the United Kingdom. Nyon, Switzerland: IOF. [Google Scholar]
- 8. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013; 8: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Office for National Statistics. Overview of the UK population, https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/august2019 (accessed 31 July 2020).
- 10. International Osteoporosis Foundation. Facts and statistics, https://www.iofbonehealth.org/facts-statistics#category-13 (accessed 31 July 2020).
- 11. CG146, National Institute for Health and Care Excellence. Osteoporosis: assessing the risk of fragility fracture, https://www.nice.org.uk/guidance/cg146 (accessed August 2012). [PubMed]
- 12. Nayak S, Greenspan SL. How can we improve osteoporosis care? A systematic review and meta-analysis of the efficacy of quality improvement strategies for osteoporosis. J Bone Miner Res 2018; 33: 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith AD. Screening of bone density at CT: an overlooked opportunity. Radiology 2019; 291: 368–369. [DOI] [PubMed] [Google Scholar]
- 14. Viprey M, Caillet P, Canat G, et al. Low osteoporosis treatment initiation rate in women after distal forearm or proximal humerus fracture: a healthcare database nested cohort study. PLoS One 2015; 10: e0143842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harvey NC, McCloskey EV, Mitchell PJ, et al. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int 2017; 28: 1507–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci 2010; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012; 27: 2039–2046. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep 2013; 11: 52–60. [DOI] [PubMed] [Google Scholar]
- 19. Akesson K, Marsh D, Mitchell PJ, et al. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013; 24: 2135–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geusens P, Bours SPC, Wyers CE, et al. Fracture liaison programs. Best Pract Res Cl Rh 2019; 33: 278–289. [DOI] [PubMed] [Google Scholar]
- 21. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013; 24: 393–406. [DOI] [PubMed] [Google Scholar]
- 22. Wu C-H, Tu S-T, Chang Y-F, et al. Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone 2018; 111: 92–100. [DOI] [PubMed] [Google Scholar]
- 23. Compston J, Cooper A, Cooper C, et al.; National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agten CA, Ramme AJ, Kang S, et al. Cost-effectiveness of virtual bone strength testing in osteoporosis screening programs for postmenopausal women in the United States. Radiology 2017; 285: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet 2019; 393: 364–376. [DOI] [PubMed] [Google Scholar]
- 27. Baum T, Carballido-Gamio J, Huber MB, et al. Automated 3D trabecular bone structure analysis of the proximal femur–prediction of biomechanical strength by CT and DXA. Osteoporos Int 2010; 21: 1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Institute for Health and Care Excellence. Osteoporosis overview - NICE pathways, https://pathways.nice.org.uk/pathways/osteoporosis (accessed 31 July 2020).
- 29. Block JE, Smith R, Glueer CC, et al. Models of spinal trabecular bone loss as determined by quantitative computed tomography. J Bone Miner Res 1989; 4: 249–257. [DOI] [PubMed] [Google Scholar]
- 30. Cann CE, Genant HK, Kolb FO, et al. Quantitative computed tomography for prediction of vertebral fracture risk. Bone 1985; 6: 1–7. [DOI] [PubMed] [Google Scholar]
- 31. Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res 2014; 29: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NHS England and NHS Improvement. Diagnostic imaging dataset statistical release, https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2020/02/Provisional-Monthly-Diagnostic-Imaging-Dataset-Statistics-2020-02-20.pdf (accessed 19 February 2020).
- 33. Europe PMC. Osteoporosis: assessment by quantitative computed tomography, https://europepmc.org/article/med/3892413 (accessed 31 July 2020). [PubMed]
- 34. Link TM, Lang TF. Axial QCT: clinical applications and new developments. J Clin Densitom 2014; 17: 438–448. [DOI] [PubMed] [Google Scholar]
- 35. Löffler MT, Jacob A, Valentinitsch A, et al. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol 2019; 29: 4980–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lenchik L, Weaver AA, Ward RJ, et al. Opportunistic screening for osteoporosis using computed tomography: state of the art and argument for paradigm shift. Curr Rheumatol Rep 2018; 20: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weaver AA, Beavers KM, Hightower RC, et al. Lumbar bone mineral density phantomless computed tomography measurements and correlation with age and fracture incidence. Traffic Inj Prev 2015; 16(Suppl. 2): S153–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ziemlewicz TJ, Maciejewski A, Binkley N, et al. Opportunistic quantitative CT bone mineral density measurement at the proximal femur using routine contrast-enhanced scans: direct comparison with DXA in 355 adults. J Bone Miner Res 2016; 31: 1835–1840. [DOI] [PubMed] [Google Scholar]
- 39. Engelke K, Lang T, Khosla S, et al. Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions-part I. J Clin Densitom 2015; 18: 338–358. [DOI] [PubMed] [Google Scholar]
- 40. Pickhardt PJ, Pooler BD, Lauder T, et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 2013; 158: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Graffy PM, Lee SJ, Ziemlewicz TJ, et al. Prevalence of vertebral compression fractures on routine CT scans according to L1 trabecular attenuation: determining relevant thresholds for opportunistic osteoporosis screening. AJR Am J Roentgenol 2017; 209: 491–496. [DOI] [PubMed] [Google Scholar]
- 42. Lee Y, Ogihara N, Lee T. Assessment of finite element models for prediction of osteoporotic fracture. J Mech Behav Biomed Mater 2019; 97: 312–320. [DOI] [PubMed] [Google Scholar]
- 43. Cody DD, Gross GJ, Hou FJ, et al. Femoral strength is better predicted by finite element models than QCT and DXA. J Biomech 1999; 32: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 44. Zysset PK, Dall’Ara E, Varga P, et al. Finite element analysis for prediction of bone strength. Bonekey Rep. Epub ahead of print 7 August 2013. DOI: 10.1038/bonekey.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Löffler MT, Sollmann N, Mei K, et al. X-ray-based quantitative osteoporosis imaging at the spine. Osteoporos Int 2020; 31: 233–250. [DOI] [PubMed] [Google Scholar]
- 46. Lee SJ, Pickhardt PJ. Opportunistic screening for osteoporosis using body CT scans obtained for other indications: the UW experience. Clinic Rev Bone Miner Metab 2017; 15: 128–137. [Google Scholar]
- 47. Viceconti M. Predicting bone strength from CT data: clinical applications. Morphologie 2019; 103: 180–186. [DOI] [PubMed] [Google Scholar]
- 48. Care Quality Commission. Using machine learning in diagnostic services. A report with recommendations from CQC’s regulatory sandbox. CQC, 2020. https://www.cqc.org.uk/what-we-do/how-we-work-people/machine-learning-diagnostic-screening-services [Google Scholar]
- 49. Lee DC, Hoffmann PF, Kopperdahl DL, et al. Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision. Bone 2017; 103: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanis JA, Johnell O, Oden A, et al. The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int 2006; 17: 527–534. [DOI] [PubMed] [Google Scholar]
- 51. Melton LJ, Riggs BL, Keaveny TM, et al. Relation of vertebral deformities to bone density, structure, and strength. J Bone Miner Res 2010; 25: 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res 2012; 27: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. FRAX®. Fracture risk assessment tool, https://www.sheffield.ac.uk/FRAX/ (2008, accessed 31 July 2020).
- 54. Fidler J, Keaveny T, Murthy N, et al. Comprehensive osteoporosis assessment for the hip using ancillary CTC Radiological Society of North America; Abstract# SSJ08-03; Chicago, IL; Nov 25–30, 2012 [Google Scholar]
- 55. Weber NK, Fidler JL, Keaveny TM, et al. Validation of a CT-derived method for osteoporosis screening in IBD patients undergoing contrast-enhanced CT enterography. Am J Gastroenterol 2014; 109: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fidler JL, Murthy NS, Khosla S, et al. Comprehensive assessment of osteoporosis and bone fragility with CT colonography. Radiology 2016; 278: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adams AL, Fischer H, Kopperdahl DL, et al. Osteoporosis and hip fracture risk from routine computed tomography scans: the fracture, osteoporosis, and CT utilization study (FOCUS). J Bone Miner Res 2018; 33: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maldonado FJ, Al Bawardy BF, Nehra AK, et al. Findings of CT-derived bone strength assessment in inflammatory bowel disease patients undergoing CT enterography in clinical practice. Inflamm Bowel Dis 2019; 25: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 59. Mindways Software. Mindways QCT bone density solutions for your CT scanner, https://qct.com/index.php (accessed 3 August 2020).
- 60. Brett AD, Brown JK. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J Orthop Translat 2015; 3: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown JK, Timm W, Bodeen G, et al. Asynchronously calibrated quantitative bone densitometry. J Clin Densitom 2017; 20: 216–225. [DOI] [PubMed] [Google Scholar]
- 62. Wang L, Su Y, Wang Q, et al. Validation of asynchronous quantitative bone densitometry of the spine: accuracy, short-term reproducibility, and a comparison with conventional quantitative computed tomography. Sci Rep 2017; 7: 6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pickhardt PJ, Bodeen G, Brett A, et al. Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography. J Clin Densitom 2015; 18: 5–12. [DOI] [PubMed] [Google Scholar]
- 64. Roberts MG, Pacheco EMB, Mohankumar R, et al. Detection of vertebral fractures in DXA VFA images using statistical models of appearance and a semi-automatic segmentation. Osteoporos Int 2010; 21: 2037–2046. [DOI] [PubMed] [Google Scholar]
- 65. Kim YM, Demissie S, Eisenberg R, et al. Intra-and inter-reader reliability of semi-automated quantitative morphometry measurements and vertebral fracture assessment using lateral scout views from computed tomography. Osteoporos Int 2011; 22: 2677–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roberts MG, Oh T, Pacheco EMB, et al. Semi-automatic determination of detailed vertebral shape from lumbar radiographs using active appearance models. Osteoporos Int 2012; 23: 655–664. [DOI] [PubMed] [Google Scholar]
- 67. Kim YM, Demissie S, Genant HK, et al. Identification of prevalent vertebral fractures using CT lateral scout views: a comparison of semi-automated quantitative vertebral morphometry and radiologist semi-quantitative grading. Osteoporos Int 2012; 23: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guglielmi G, Haslam J, D′Errico F, et al. Comprehensive vertebral deformity and vertebral fracture assessment in clinical practice: intra- and inter-reader agreement of a clinical workflow tool. Spine 2013; 38: E1676–E1683. [DOI] [PubMed] [Google Scholar]
- 69. Oei L, Ly F, El Saddy S, et al. Multi-functionality of computer-aided quantitative vertebral fracture morphometry analyses. Quant Imaging Med Surg 2013; 3: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bromiley P, Staal J, Kariki E. Computer-aided opportunistic identification of vertebral fragility fractures in computed tomography images: a multi-site study, https://static1.squarespace.com/static/5a6aa854d0e628e36d43761b/t/5c6c77f8c83025b969dbd842/1550612494982/qmski_program_2_reducedsize.pdf (2019, accessed 31 July 2020).
- 71. Rajak R, Patel K, Lawless R, et al. 165 Incidental capture of vertebral fragility fractures (VFFs) from CT imaging in a large district general hospital in London. Rheumatology. Epub ahead of print 1 April 2019. DOI: 10.1093/rheumatology/kez108.073. [DOI] [Google Scholar]
- 72. Greenstein S, Gulick S. Zebra medical vision. Harvard Business School Case, 2018. https://www.hbs.edu/faculty/Pages/item.aspx?num=55060 [Google Scholar]
- 73. Greenstein S. Earning stripes in medical machine learning. IEEE Micro 2019; 39: 126–128. [Google Scholar]
- 74. Businesswire. Zebra medical vision to collaborate with Google Cloud to bring a transparent all-in-one model to healthcare, https://www.businesswire.com/news/home/20171108005836/en/Zebra-Medical-Vision-Collaborate-Google-Cloud-Bring (2017, accessed 31 July 2020).
- 75. Zebra Medical Vision. Medical Imaging & AI. Computing DEXA score from CT using Deep Segmentation Networks cascade, http://www.zebra-med.com/research-publications/computing-dexa-score-from-ct-using-deep-segmentation-networks-cascade (accessed 31 July 2020).
- 76. Bar A, Wolf L, Amitai OB, et al. Compression fractures detection on CT. In: Medical imaging 2017: computer-aided diagnosis. International Society for Optics and Photonics, 2017, p. 1013440. https://patents.google.com/patent/US10716529B2/en?q=Zebra+osteoporosis&oq=Z accessed 31 July 2020ebra+osteoporosis [Google Scholar]
- 77. Dagan N, Elnekave E, Barda N, et al. Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nat Med 2020; 26: 77–82. [DOI] [PubMed] [Google Scholar]
- 78. Zebra Medical Vision. Medical imaging & AI, https://www.zebra-med.com/ (accessed 31 July 2020).
- 79. Kolanu N, Silverstone EJ, Ho BH, et al. Clinical utility of computer-aided diagnosis of vertebral fractures from computed tomography (CT) images. J Bone Miner Res 2020; 35: 2307–2312. [DOI] [PubMed] [Google Scholar]
- 80. Krishnaraj A, Barrett S, Bregman-Amitai O, et al. Simulating dual-energy X-ray absorptiometry in CT using deep-learning segmentation cascade. J Am Coll Radiol 2019; 16: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 81. Jones L, Singh S, Edwards C, et al. Prevalence of vertebral fractures in CTPA’s in adults aged 75 and older and their association with subsequent fractures and mortality. Geriatrics 2020; 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Viceconti M, Qasim M, Bhattacharya P, et al. Are CT-based finite element model predictions of femoral bone strength clinically useful? Curr Osteoporos Rep 2018; 16: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pisu M, Kopperdahl DL, Lewis CE, et al. Cost-effectiveness of osteoporosis screening using biomechanical computed tomography for patients with a previous abdominal CT. J Bone Miner Res 2019; 34: 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform 2000; 1: 65–70. [PubMed] [Google Scholar]
- 85. Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017; 19: e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Greenhalgh T. How can we make innovation programmes in health and social care work? University of Oxford. https://www.nuffieldtrust.org.uk/research/achieving-scale-and-spread-learning-for-innovators-and-policy-makers [Google Scholar]
- 87. Quilter-Pinner H, Rae K. Keeping up with the science. Institute for Public Policy Research, 2018, p. 24. https://www.ippr.org/files/2018-03/1521028372_keeping-up-with-the-science-march18.pdf [Google Scholar]
- 88. Hemmings N, Hutchings R, Castle-Clarke S, et al. Achieving scale and spread: learning for innovators and policy-makers. The Nuffield Trust. https://www.nuffieldtrust.org.uk/research/achieving-scale-and-spread-learning-for-innovators-and-policy-makers (2020, accessed 31 July 2020). [Google Scholar]
- 89. Castle-Clarke S, Edwards N, Buckingham H. Falling short: why the NHS is still struggling to make the most of new innovations. The Nuffield Trust. https://www.nuffieldtrust.org.uk/research/falling-short-why-the-nhs-is-still-struggling-to-make-the-most-of-new-innovations (2017, accessed 31 July 2020). [Google Scholar]
- 90. Greenhalgh T. How to improve success of technology projects in health and social care. Public Health Res Pract 2018; 28: 2831815. [DOI] [PubMed] [Google Scholar]
- 91. Leaver M. Impact report 2017. The AHSN Network, https://www.ahsnnetwork.com/wp-content/uploads/2018/12/AHSN-Network-Impact-Report-2017_Web_spreads.pdf (2017). (accessed 31 July 2020). [Google Scholar]
- 92. Cox A, Spiegelhalter K, Marangozov R, et al. NHS innovation accelerator evaluation. Institute for Employment Studies, https://www.employment-studies.co.uk/resource/nhs-innovation-accelerator-evaluation (2018). (accessed 31 July 2020). [Google Scholar]
- 93. Institute for Employment Studies. NHS innovation accelerator evaluation, http://www.employment-studies.co.uk/resource/nhs-innovation-accelerator-evaluation (accessed 31 July 2020).
- 94. NHS Accelerated Access Collaborative. Who we are, https://www.england.nhs.uk/aac/about-us/who-we-are/ (accessed 31 July 2020).
- 95. Making a reality of the accelerated access review . Department of Health, Department for Business, Energy & Industrial Strategy, 2017. https://www.gov.uk/government/publications/accelerated-access-review-response [Google Scholar]
- 96. Taylor H, Bell J. Review of innovative medicines and medical technologies. Accelerated access review: final report. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/565072/AAR_final.pdf
- 97. NHS England and NHS Improvement. Science in healthcare: delivering the NHS long term plan. 2020. https://www.england.nhs.uk/wp-content/uploads/2020/03/science-in-healthcare-delivering-the-nhs-long-term-plan.pdf
- 98. Musculoskeletal health: a 5 year strategic framework for prevention across the lifecourse . GW-477, June 2019. Pubic Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/810348/Musculoskeletal_Health_5_year_strategy.pdf [Google Scholar]
- 99. NHS England and NHS Improvement. Consultation on the proposals for the MedTech Funding Mandate Policy. 1910101144AP, https://www.engage.england.nhs.uk/consultation/medtech-funding-mandate/user_uploads/medtech-funding-mandate–guidance-consultation-document-v2.00.pdf (November 2019). (accessed 31 July 2020).
- 100. Leng G, Williams S, Hung I, et al. Uptake of medical devices approved by NICE. BMJ Innov 2018; 4: 178–184. [Google Scholar]
- 101. Iacobucci G. NHSX: Hancock sets up new policy unit to support technology in NHS. BMJ 2019; 364: l793. [DOI] [PubMed] [Google Scholar]
- 102. NHSX tech plan for health and care on the day briefing . NHS Providers, 28 February 2020. https://nhsproviders.org/media/689235/nhsx-tech-vision-otdb.pdf [Google Scholar]
- 103. NICE. How we develop medical technologies guidance. Medical technologies guidance. NICE guidance. Our programmes. What we do. About. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/medical-technologies-guidance/how-we-develop (accessed 31 July 2020).