Abstract
Background:
Ovarian cancer is the most lethal gynecological malignancy, and chemotherapy remains the cornerstone for ovarian cancer management. Due to the unsatisfactory prognosis, a better understanding of the underlying molecular carcinogenesis is urgently required.
Methods:
Assays for determining cell growth, cell motility, and apoptosis were employed to evaluate the potential antitumor effects of metformin against ovarian cancer cells. Molecular biological methods were employed to explore the underlying mechanism. Human ovarian cancer samples and Gene Expression Profiling Interactive Analysis (GEPIA) dataset were used for uncovering the clinical significances of mesothelin (MSLN) on ovarian cancer.
Results:
In the present work, we found that metformin treatment led to cell growth and cell migration inhibition, and induced cell apoptosis. Metformin administration also impaired cancer cell stemness and the capillary-like structure formation capacity of SKOV3 cells. On mechanism, metformin treatment remarkably reduced mesothelin (MSLN) expression, downregulated IL-6/STAT3 signaling activity, subsequently resulted in VEGF and TGFβ1 expression. We also observed an oncogenic function of MSLN on ovarian cancer.
Conclusions:
Collectively, our findings suggested that metformin exerts anticancer effects by suppressing ovarian cancer cell malignancy, which attributed to MSLN inhibition mediated IL6/STAT3 signaling and VEGF and TGFβ1 downregulation.
Keywords: metformin, mesothelin, angiogenesis, stemness, IL-6/STAT3, ovarian cancer
Introduction
Ovarian cancer is the fifth leading cause of death in women and the most lethal of gynecological malignancies 1 –3 . Currently, chemotherapy remains the cornerstone for ovarian cancer management 1 –5 . Despite ovarian cancer chemotherapies have improved substantially, the prognosis for patients remains unsatisfactory. The 5-year survival rate after initial diagnosis for patients with stage III and IV ovarian cancer is still <20% 3 . Thus, a better understanding of the underlying molecular carcinogenesis of ovarian cancer is urgently required for developing more reliable therapy for ovarian cancer.
In addition to being widely accepted as the first-line antidiabetic agent, metformin (1,1-dimethylbiguanide hydrochloride) is currently attracting considerable attention as an antineoplastic agent. In intrahepatic cholangiocarcinoma (ICC) cells, glioblastoma cells, cervical carcinoma cells, endometrial cancer cells, and melanoma cells, metformin was demonstrated to inhibit proliferation, migration and facilitate apoptosis 6 –14 . Metformin was also reported inhibited TGFβ1-induced EMT via PKM2 relative-mTOR/p70s6 k signaling pathway 7 . Furthermore, metformin treatment possessed an inhibitory effect on growth and motility through modulation of microRNA expression 11 . Numerous epidemiological investigations and preclinical studies have demonstrated diverse mechanisms underlying the antitumor action of metformin. However, the exact function of metformin on ovarian cancer remains unclear, though metformin intake was reported associated with better survival and metformin also was found to inhibit the development and metastasis of ovarian cancer.
Mesothelin (MSLN) is a cell surface glycoprotein that is overexpressed in various cancer, such as malignant mesothelioma, pancreatic cancer, and lung adenocarcinoma 15 –22 . MSLN overexpression has been shown correlated with poor prognosis in esophageal and gastric cancer 16,18,19,21,22 . In vitro, overexpression of MSLN significantly promotes cell proliferation, cell cycle progression, and resistance to TNF-a induced growth inhibition and apoptosis in pancreatic cancer cell lines 15,23 . In human breast cancer cell lines (MCF-7, T47D, and MDA-MB-231), overexpression of MSLN promoted anchorage-independent growth in soft agar 24 . In lung cancer, MSLN was found to be highly upregulated in non-small cell lung cancer (NSCLC) patient tissues and lung carcinoma, and MSLN genetic deficiency significantly reduced anchorage-independent cell growth, tumorsphere formation, cell adhesion, migration, and invasion in vitro, as well as tumor formation and metastasis in vivo 17,18 , suggested an oncogenic function of MSLN on cancer. On mechanism, MSLN was found to participate in IL6/STAT3 signaling regulation. MSLN overexpression leads to constitutive activation of the transcription factor Stat3, which results in enhanced expression of cyclin E and increased G1-S transition 23 . Also, MSLN overexpressed pancreatic cancer cell line produced increased IL-6 and resistance to TNF-α-induced apoptosis 15,25 . The exact function of MSLN on ovarian cancer remains unclear.
In the present study, we explored the effect of metformin on ovarian cancer and revealed a functional axis that metformin through down-regulating MSLN expression and subsequently inhibiting IL6/STAT3-TGFβ signaling to suppress ovarian cancer cells malignancy.
Materials and Methods
Cell Culture
Characterized ovarian cancer cell line SKOV3 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, St. Louis, MO, USA) with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 1% streptomycin (v/v) (Sigma) and 1% penicillin (v/v) (Sigma) in an incubator at 37°C in 5% CO2.
Cell Viability Assay
Cell viability was evaluated using cell counting kit-8 (CCK-8) (Dojindo, Mashiki-machi, Japan), according to the manufacturer’s instruction. Briefly, cells (2 × 103 per well) were plated in 96-well plates and treated with increasing doses of metformin (0, 0.5, 1, and 5 mM) (Sigma-Aldrich). To determine the percentage of viability, the absorbance was measured at 450 nm using a microplate reader (MQX 200, BioTek Instruments, Winooski, VT, USA) following treatment with CCK-8 for 2 h at 37°C.
Colony Formation Assay
For colony formation assay, SKOV3 cells at the exponential growth phase were seeded at 200 cells per well, incubated until cells attached, and then different doses (0, 1, and 5 mM) of metformin was added to the cell culture. After the treatment, the cells were again kept for incubation for 14 days, Colonies were fixed and stained with crystal violet and counted under the light microscope. All assays were done in triplicate, and the results were presented as the percentage (%) of the untreated group.
Annexin V Staining and Flow Cytometry
Cells were stained with an Annexin V PE Apoptosis Detection Kit (BD, USA) according to the manufacturer’s instructions. The flow cytometry assay to analyze the percentage of apoptotic cells was performed on BD FACSCalibur (Becton, Dickinson and Company, Lake Franklin, New Jersey, USA).
Cell Scratch and TUNEL Assay
Cell scratch assay was used to detect the migration of SKOV3 cells. For the scratch assay, SKOV3 cells were cultured until confluency. Following serum starvation for 24 h, a linear wound was made by scratching the bottom of the dish. The wound images were captured after scratch 24 h using the Motic AE31 Photometry and Dimensioning microscope (Milton, MA, USA). Cell apoptosis was detected using TUNEL (Roche Applied Science) assays according to instructions of the manufacturer. For quantification, the numbers of TUNEL-positive cells were counted in at least five randomly with three independent samples.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA of SKOV3 cells was extracted by Trizol reagent according to the manufacturer’s instruction (Takara, Dalian, China), and then quantified by NanoDrop Spectrophotometer. Equal amount RNAs were subjected to reverse transcription for cDNAs with a Takara PrimeScript RT-PCR Kit (Takara, Dalian, China). Real-time PCR was done using the TB Green Fast qPCR Mix (Takara, Dalian, China), and the reference gene GAPDH was used as the internal control. The primers used in the present study were listed as follows:
TGFB1 forward, 5′-GGCCAGATCCTGTCCAAGC-3′;
TGFB1 reverse: 5′-GTGGGTTTCCACCATTAGCAC-3′;
VEGFA forward: 5′-AGGGCAGAATCATCACGAAGT-3′;
VEGFA reverse: 5′-AGGGTCTCGATTGGATGGCA-3′;
SOX2 forward: 5′-GCCGAGTGGAAACTTTTGTCG-3′;
SOX2 reverse: 5′-GGCAGCGTGTACTTATCCTTCT-3′;
OCT4 forward: 5′-CGGAAGAGAAAGCGAACTAGC-3′;
OCT4 reverse: 5′-ATTGGCGATGTGAGTGATCTG-3′;
ZEB1 forward: 5′-ACTCTGATTCTACACCGC-3′;
ZEB1 reverse: 5′-TGTCACATTGATAGGGCTT-3′;
Snail forward: 5′- TCGGAAGCCTAACTACAGCGA-3′;
Snail reverse: 5′- AGATGAGCATTGGCAGCGAG-3′;
Vimentin forward: 5′- AAACTTAGGGGCGCTCTTGT-3′;
Vimentin reverse: 5′-CGCTGCTAGTTCTCAGTGCT-3′;
FN1 forward: 5′-CCATCGCAAACCGCTGCCAT-3′;
FN1 reverse: 5′-CCATCGCAAACCGCTGCCAT-3′;
E-cadherin forward: 5′-GCGTCCTGGCAGAGTGAATTTT -3′;
E-cadherin reverse: 5′-GGCCTTTTGACTGTAATCACAAA-3′;
N-cadherin forward: 5′-TCAGGCGTCTGTAGAGGCTT-3′;
N-cadherin reverse: 5′- ATGCACATCCTTCGATAAGACTG-3′;
GAPDH forward: 5′-CTGGGCTACACTGAGCACC-3′;
GAPDH reverse: 5′-AAGTGGTCGTTGAGGGCAATG-3′.
Western Blot Analysis
Western blot analysis was performed to determine the protein expression and phosphorylation levels. Cellular proteins were extracted using RIPA lysis buffer (Beyotime Institute of biotechnology). 20 µg lysate was loaded and separated in each lane of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrophoresed for 2 h at 100 V in the buffer, and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking in 5% fat-free dry milk, antibodies for MSLN, IL-6, p-STAT3, STAT3, TGFβ1, p-Smad2, Smad2, SOX2, OCT4 and VEGF were employed. Target proteins and phosphorylation were detected by chemiluminescence with a Tanon-5500 Imaging System (Tanon Science&Techology Ltd., Shanghai, China) and quantified with the ImageJ software.
Tube Structure Formation and BrdU Assay
SKOV3 cells were treated with the indicated dose of metformin (0, and 5 mM). Tube structure formation experiment was performed as previously described 6 . Briefly, cells (2 × 104 per well) were cultured in a 96-well plate coated with 50 µl of Matrigel (356234; BD Biosciences). Tube-like SKOV3 cell structure formation was imaged after 6 hours in five random microscopic fields with an inverted phase-contrast microscope. The number of loops/meshes was quantified and used to calculate the tube formation. Corresponding BrdU assay was performed according to the manufacturer’s instruction (Roche Diagnostics, Penzberg, Germany).
MSLN knock out by CRISPR/Cas9 Gene Editing and STAT3 knockdown
In the present study, the MSLN gene was knocked out by the CRISPR/Cas9 mediated gene editing. In brief, sgRNA oligo targeted MSLN sequence 5′-GGTTGGCCAGGACTCCGTCC-3′ was synthesized and cloned into the PX459 vector (Addgene plasmid #62988). Plasmid construction was performed according to protocol and confirmed by sequencing. To obtain MSLN knocking out SKOV3 cell lines, MSLN knocking out plasmids were transfected by Lipofectamine 3000 transfection reagent (ThermoFisher) for 27 h, and then were selected with puromycin (concentration 2 ng/μl). Single-cell cloning was performed in a 96-well plate to grow single clones, and MSLN knock out was detected by western blot and sequencing.
STAT3 knockdown SKOV3 cell line was constructed by infecting cells with lentivirus vectors that expressing a specific small hairpin RNA targeted STAT3 (5′-CTTCAGACCCGTCAACAAA-3′).
Ovarian Cancer Tissues and Immunohistochemical Staining
Forty-three paired ovarian cancer tissue and corresponding adjacent samples were obtained from patients histopathologically and clinically diagnosed with ovarian cancer at the Chengdu fifth people’s hospital.
Informed consent was obtained from each patient, and all experiments were performed with the approval of the Clinical Research Ethics Committee of Chengdu fifth people’s hospital. Anti-MSLN (1:500; Cell Signaling Technology, USA, 99966) were used as an antibody to detect protein expression levels based on the standard immunohistochemical staining procedure. Immunohistochemical staining scores were generated by multiplying the percentage of stained positive cells (0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%) and the staining intensity (0, negative; 1, weak; 2, moderate; and 3, strong).
Statistical Analysis
All values were presented as the means ± SEM. Statistical analysis was performed using a one-way ANOVA to compare between multiple groups and Student’s t-test to compare between two groups. A value of P < .05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics software (SPSS16.0).
Results
Metformin Administration Suppressed Ovarian Cancer Cells Malignancy
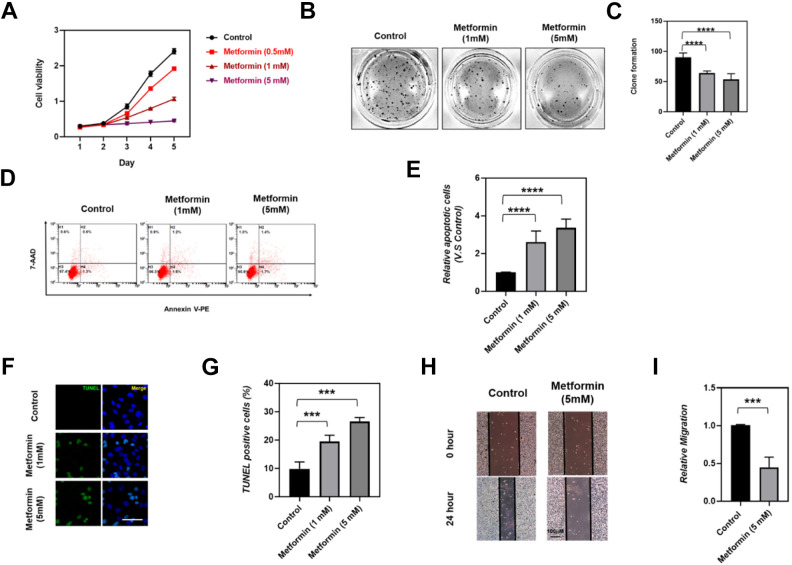
To explore the effects of Metformin administration on ovarian tumor malignancy, SKOV3 cells were treated with the indicated concentrations of metformin and cell viability was determined by CCK-8 assay. Our results illustrated that, compared to the untreated group, SKOV3 cells treated with metformin displayed a dose-dependent decrease in cell viability (Fig. 1A). In addition, this inhibition was also reflected in the colony formation ability of SKOV3. As shown in Fig. 1B, C, the colony formation was observed dramatically inhibited in SKOV3 cells that were administrated with 1 mM or 5 mM metformin. Furthermore, we determined the effect of Metformin treatment on cell apoptosis by Annexin V staining and TUNEL assay, and found that SKOV3 cells apoptosis was significantly induced by 1 mM or 5 mM metformin administration (Fig. 1D–G). Similarly, SKOV3 cells wound healing was detected remarkably inhibited by 5 mM metformin treatment (Fig. 1H, I), which suggested an inhibitory effect of metformin on SKOV3 cell migration. Epithelial mesenchymal transition plays a prominent role in tumoral cells migration and tumor metastasis, thus we supposed that metformin treatment may affect the epithelial mesenchymal transition process of ovarian cancer cells. As expected, we detected a significant facilitation in epithelial marker expression and a dramatically reduction in mesenchymal markers expression (Supplemental Fig. S1), indicated an inhibitory effect of metformin on epithelial mesenchymal transition in ovarian cancer cells. Collectively, our results indicated that metformin possesses an inhibitory function on ovarian cancer malignancy by suppressing ovarian cancer cell growth, cell migration, and inducing SKOV3 cells apoptosis.
Figure 1.
Effects of metformin on cell viability, colony formation, apoptosis, and migration of SKOV3 cells. (A) SKOV3 cells were treated with the indicated concentration of metformin (0, 0.5, 1, and 5 mM), cell viability was determined by CCK-8 at indicated time point. (B) Colony formation assays were performed to determine the proliferation of SKOV3 cells treated with 0, 1, and 5 mM metformin respectively. (C) Quantitative analysis of colony formation in control, 1 mM and 5 mM metformin treatment SKOV3 cells; N = 5 per group, Data are mean ± SEM,****P < .0001. (D-E) Quantitative analysis of the effect of Metformin on apoptosis of SKOV3 cells by flow cytometry assessed FITC-Annexin V staining, N = 5 per group, Data are mean ± SEM, ****P < .0001. (F, G) Representative TUNEL staining and quantitative analysis of the effect of Metformin on apoptosis of SKOV3 cells in control, 1 mM and 5 mM metformin treatment, N = 5 per group, Data are mean ± SEM, ***P < .001. (H, I) Representative cell scratch assay and quantitative analysis of the effect of metformin on cell migration of SKOV3 cells in control and 5 mM metformin treatment, N = 5, data were showed in mean ± SEM, ***P < .001.
Metformin Impaired Ovarian Cancer Cells Stemness and Angiogenesis
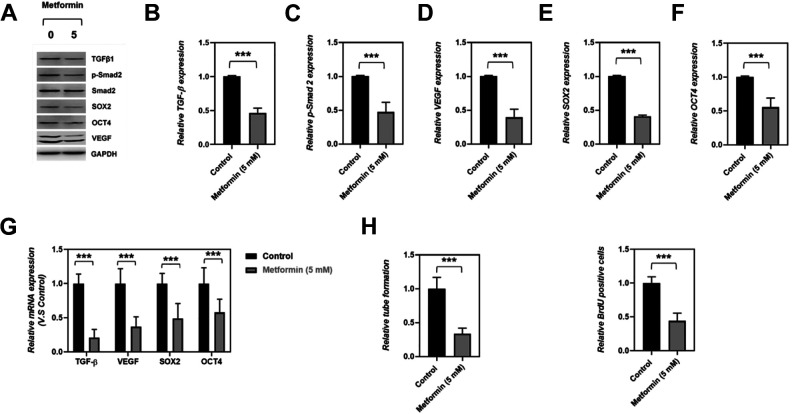
To further determine the effects of metformin on tumor-initiating (stemness) capacity and angiogenesis of SKOV3 cells, the levels of angiogenic factors (TGFβ1 and VEGF), stemness-related genes (SOX2 and OCT4), and Smad2 expressions were determined by western blot assay and qRT-PCR. Our data clearly showed that the cancer cell stemness was significantly inhibited by metformin treatment, as reflected by down-regulated SOX2 and OCT4 expression on mRNA and protein level in 5 mM metformin-treated cells (Fig. 2A–G). Consistently, metformin treatment reduced the proportion of BrdU-positive cells of SKOV3 (Supplemental Fig. S2A and Fig. 2I), which further demonstrated Metformin impaired ovarian cancer cell stemness. Furthermore, we detected a down-regulation on angiogenic factors (TGFβ1 and VEGF) expression and a reduction of Smad-2 phosphorylation in ovarian cancer cells SKOV3 that administrated with 5 mM metformin (Fig. 2A–G), suggested that metformin possessed a suppressive effect on ovarian cancer cell angiogenesis, which was further demonstrated by the observation that metformin treatment led to a decreased capacity of tube formation in SKOV3 cells (Supplemental Fig. S2B, Fig. 2H). Taken together, our data indicated that metformin possessed suppressive function on ovarian cancer cell stemness and angiogenesis.
Figure 2.
Effects of metformin on TGFβ1-mediated angiogenesis and stemness. (A–F) Representative western blot and quantitative analysis of the effects of metformin on TGFβ1, pSMAD2, VEGF, SOX2. and OCT4 expression, N = 5, data were showed in mean ± SEM, ***P < .001. (G) Q-RT-PCR determined the mRNA expression of TGFβ1, VEGF, SOX2, and OCT4 in control and 5 mM metformin-treated SKOV3 cells, data were showed in mean ± SEM, N = 5, ***P < .001. (H-I) Quantitative analysis of capillary-like structure formation and BrdU staining in control, with 5 mM metformin treatment SKOV3 cells; N = 5 per group, Data are mean ± SEM, ***P < .001.
MSLN Mediated the Effects of Metformin on Inhibiting the Malignancy of Ovarian Cancer
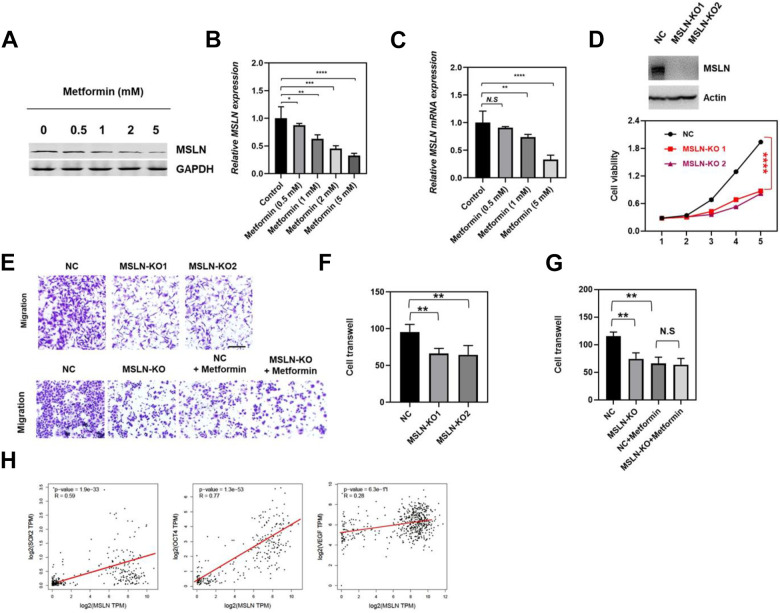
Given previous studies implicating that aberrant expression of MSLN was involved in various types of cancers 16,19 , we determined whether the anti-cancer effects of metformin in ovarian cancer cell malignancy was dependent on MSLN regulation. Thus, SKOV3 cells were treated with the indicated concentrations of metformin and MSLN mRNA and protein level were detected by western blot and qRT-PCR. As illustrated in Fig. 3A–C, the expression of MSLN was remarkably reduced in metformin treatment groups on mRNA and protein level in SKOV3 cells, which was confirmed in OV90 cells (Supplemental Fig. S3). In addition, cell viability and cell migration were strikingly suppressed by MSLN knock-out, indicated MSLN has a promoting function on ovarian cancer cell SKOV3 malignancy (Fig. 3D, F). Furthermore, we found that metformin treatment no longer decreased SKOV3 cell migration while MSLN was knock out (Fig. 3E, G), indicated that metformin inhibiting SKOV3 cell migration via down-regulating MSLN expression. Next, we determined if MSLN mediated the inhibitory effects of metformin on ovarian cancer cell stemness and angiogenesis. By analyzing the ovarian cancer data from TCGA, a positive correlation was observed between MSLN and SOX2, OCT4 and VEGF (Fig. 3H), suggested that MSLN could promote function on ovarian cancer cell stemness and angiogenesis. Given that metformin administration significantly suppressed MSLN expression and ovarian cancer cell stemness and angiogenesis, we suggested that MSLN mediated the inhibitory effects of metformin on ovarian cancer cell stemness and angiogenesis. Collectively, our data demonstrated that MSLN mediated the function of metformin on inhibiting the malignancy of OV cancer.
Figure 3.
MSLN mediated the function of Metformin on inhibiting the malignancy of OV cancer. The effect of metformin on MSLN expression was analyzed by western blot in SKOV3 cells under different treatments. GAPDH was used as an internal reference. Quantitative analysis of the effects of metformin on MSLN expression in control 0.5 mM, 1 mM, and 5 mM metformin-treated SKOV3 cells, data were showed in mean ± SEM, N = 5, ***P < .001. Q-RT-PCR determined the mRNA expression of MSLN in control 0.5 mM, 1 mM, and 5 mM metformin-treated SKOV3 cells, data were showed in mean ± SEM, N = 5, ***P < .001. MSLN was knock-out (up), and SKOV3 cell viability was determined by CCK-8 at the indicated time point (down). Cell migration was measured by transwell assay, up: NC means empty vector control, MSLN-KO1 and MSLN-KO2 indicated different MSLN knock-out clone, down: NC and MSLN-KO means control and MSLN knock-out, NC + metformin and MSLN-KO+ metformin means control cells or MSLN-KO cells treated with Metformin. (F, G) Quantitative analysis of the cell migration corresponding to the Figure 3E, data were showed in mean ± SEM, N = 5, **P < .01. (H) Correlation analysis of the expression of MSLN with the expression of SOX2, OCT4 and VEGF in ovarian cancer samples, data were from TCGA.
Metformin Inhibits IL-6/STAT3 Signaling Through Downregulating MSLN Expression
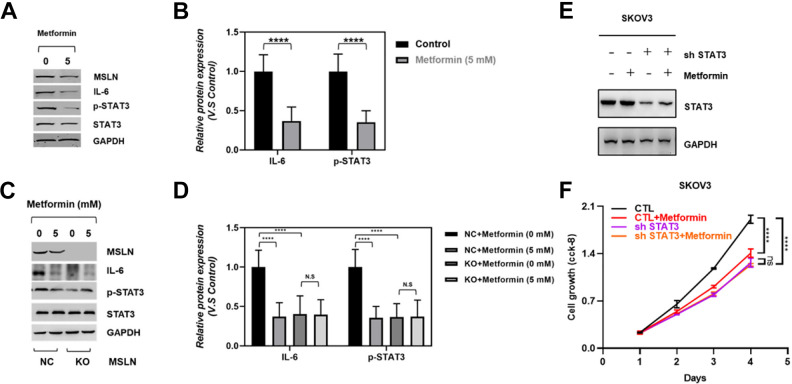
MSLN is a cell surface glycoprotein that is overexpressed in various cancer and plays crucial roles in cancer 16,18 –23 . Previous studies have shown that MSLN possesses an important promoting function on pancreatic cancer cell proliferation, cell cycle progression, and overexpression of MSLN in pancreatic cancer cells leads to constitutive activation of the transcription factor Stat3, which results in enhanced expression of cyclin E and increased G1-S transition 23,25 . In addition, MSLN overexpressed pancreatic cancer cell line MIA-PaCa2 cells produced increased IL-6 and were resistant to TNF-α-induced apoptosis 15 . Furthermore, metformin was found to target Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers, esophageal squamous cell carcinoma and bladder cancer 7,26 –28 . And based on our observation that metformin significantly inhibited ovarian cancer cell malignancy and suppressed MSLN expression, we hypothesized that IL-6/STAT3 signaling may participate in the processes that metformin exerted an inhibitory function on ovarian cancer. To examine this possibility, we determined the IL-6/STAT3 signaling in control and metformin-treated SKOV3 cells. Consistent with the decrease of MSLN expression, the IL-6 expression and STAT3 activation were dramatic reduced in the setting of 5 mM metformin administration (Fig. 4A, B). Next, we tested if metformin suppressed IL-6/STAT3 signaling by regulating MSLN. As the results show in Fig. 4C, D, in control cells, metformin remarkably suppressed MSLN expression and IL-6/STAT3 signaling, while metformin treatment has no effect on IL-6/STAT3 signaling in MSLN knock out cell line, which indicated that metformin function on inhibiting IL-6/STAT3 signaling via MSLN regulation. Next, we knockdown the expression of STAT3 in SKOV3 cell line (Fig. 4E), and test if deletion of STAT3 could reduce the observed anti-cancer effects of metformin on ovarian cancer cells. Indeed, our results illustrated that cell growth was no longer inhibited by metformin administration in STAT3 knockdown cells (Fig. 4F), indicating that the anti-cancer effects of metformin is largely dependent upon STAT3 regulation. Collectively, our observation suggested metformin function on inhibiting ovarian cancer through MSLN mediated IL-6/STAT3 signaling regulation.
Figure 4.
MSLN mediated the effects of Metformin on inhibiting the IL-6/STAT3 signaling. Western blot results showed the expression of MSLN, IL-6, p-STAT3 and STAT3 in untreated and 5 mM metformin-treated group. Quantitative analysis of the effects of metformin on IL-6 and p-STAT3 expression in control and 5 mM metformin-treated SKOV3 cells, data were showed in mean ± SEM, N = 5, ****P < .0001. All data were normalized to GAPDH and fold change was expressed in relation to the untreated group. Western blot results showed the expression of MSLN, IL-6, p-STAT3 and STAT3 in control (NC) and MSLN knock-out (KO) cells treated with 0 or 5 mM metformin. Quantitative analysis of the effects of metformin on IL-6 and p-STAT3 expression in control (NC) and MSLN knock-out (KO) SKOV3 cells treated with 0 or 5 mM metformin, data were showed in mean ± SEM, N = 5, ****P < .0001. All data were normalized to GAPDH and fold change was expressed in relation to the untreated group. Western blot results showed the expression of STAT3 in STAT3 deletion SKOV3 cells with or without metformin administration. SKOV3 cell viability was determined by CCK-8 at the indicated time point.
MSLN Functions as a Tumor-Promoting Factor in Ovarian Cancer
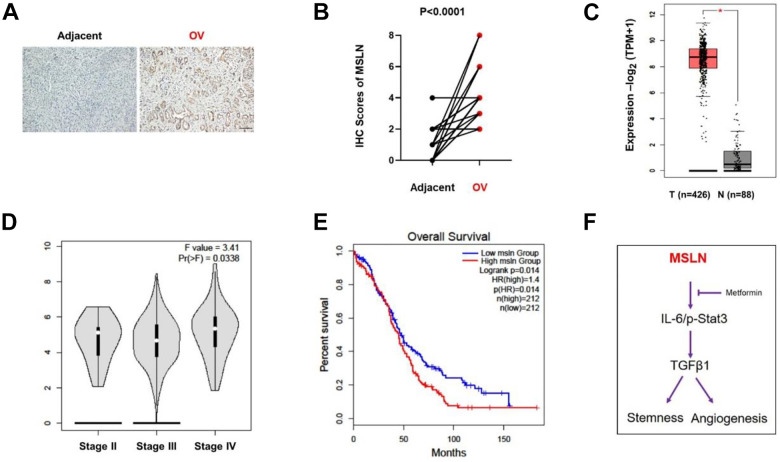
To further explore the function of MSLN on ovarian cancer, 43 paired ovarian cancer samples and adjacent tissue samples were subjected to immunohistochemistry (IHC) analysis. Higher expression of MSLN was detected in tumoral areas compared to adjacent tissues based on the IHC score (Fig. 5A, B). Furthermore, by analyzing the publicly assessable data for ovarian cancer within the Gene Expression Profiling Interactive Analysis (GEPIA) dataset, we also found that MSLN transcription was increased significantly in ovarian cancer tissues and varied in different stages (Fig. 5C, D); high mRNA expression of MSLN was associated with shorter overall survival (OS) (P = .014) (Fig. 5E). Taken together, these data strongly suggested that MSLN function as a tumor-promoting factor in ovarian cancer.
Figure 5.
MSLN function as a tumor-promoting factor on OV cancer. Representative immunohistochemical staining of MSLN in adjacent tissue (Adjacent) and ovarian cancer tissue (OV); IHC scores of MSLN in adjacent tissue (Adjacent) and ovarian cancer tissue (OV); Quantitative analysis of the expression of MSLN in normal tissue (N) and OV tissue (T) with TCGA data; *P < .05; Quantitative analysis of the expression of MSLN in stages of ovarian cancer and data were from TCGA. F value = 3.41, Pr(>F) = 0.0338; Log-rank analysis of patient overall survival in MSLN high and low group, log-rank P = .014, HR (high) = 1.4. Model of metformin functions on ovarian cancer. Metformin treatment in SKOV3 cells caused a reduction in MSLN protein expression and then inhibited IL-6 expression and STAT3 activation, which led to a reduction in TGFβ1 expression, consequently, stemness. and angiogenesis were inhibited.
Discussion
In ovarian cancer, accumulating studies has demonstrates that the abnormal activation of STAT3 signaling plays a crucial role in tumor properties. The fact that STAT3 signaling involved in regulating cell proliferation, survival, invasion, stem cell-like characteristic, angiogenesis and chemoresistancer make it to act as a promising therapeutic target in ovarian cancer 29 . Indeed, multiple natural and synthetic inhibitors targeting STAT3 signaling directly or indirectly are explored in preclinical trial or clinical trial in ovarian cancer 29 –31 . To date, some inhibitors showed significant toxicity or have synergistic effects when combined with conventional chemotherapy both in vitro and in vivo. However, quite few inhibitors exhibit minimal activity in xenografts or in patients. Thus, more effective drugs are urgently needed. In addition to as an extensively used first-line antidiabetic drug, accumulated evidence has shown that metformin has a function in protecting against cancer. In intrahepatic cholangiocarcinoma (ICC) cells 32 , metformin was demonstrated to inhibit proliferation and facilitate apoptosis. In glioblastoma cells, metformin has been found inhibited cell proliferation and migration 9 . In cervical carcinoma cells, metformin was reported to inhibit TGFβ1-induced EMT via PKM2 relative-mTOR/p70s6 k signaling pathway 7 , and metformin inhibited estrogen-dependent endometrial cancer cell growth by activating the AMPK–FOXO1 signal pathway 14 . Furthermore, metformin treatment possessed an inhibitory effect on melanoma cell growth and motility through modulation of microRNA expression 11 . In ovarian cancer, though metformin intake was reported associated with better survival and metformin also was found to inhibit the development and metastasis of ovarian cancer 8,33,34 . In our present study, our data revealed that metformin treatment showed a potential growth inhibitory activity against SKOV3 cells malignancy as indicated by suppressing ovarian cancer cell growth, cell migration and inducing SKOV3 cells apoptosis. Furthermore, consistent with the previous study, our data also showed the suppressive effect of metformin on ovarian cancer stemness in vitro. Our data clearly showed that the cancer cell stemness was significantly inhibited by metformin treatment, as reflected by down-regulated SOX2 and OCT4 expression on mRNA and protein level in 5 mM metformin-treated cells. Consistently, metformin treatment reduced the proportion of BrdU-positive cells of SKOV3. On mechanism, our observation demonstrated that metformin treatment remarkably reduced mesothelin (MSLN) expression, downregulated IL-6/STAT3 signaling activity, and the anti-cancer effects of metformin is largely dependent upon STAT3 regulation, suggested that metformin may function as a promising drug that targeting STAT3 on ovarian cancer therapy.
MSLN is a cell-surface glycoprotein present on mesothelial cells and also many cancers, such as malignant mesothelioma, pancreatic cancer, ovarian cancer, and lung adenocarcinoma, and high expression of MSLN is generally associated with an unfavorable prognosis 16,19 –22,35 . Previous studies on MSLN were limited in the tumor marker identification field, however, the exact function of MLSN on ovarian cancer remains unclear. In the present study, our results demonstrated that MSLN was higher expressed in ovarian cancer, and varied in different stages, and high mRNA expression of MSLN was associated with shorter overall survival. In vitro, we found that SKOV3 cell viability and cell migration were strikingly suppressed by MSLN knock-out. Our data strongly suggested that MSLN functions as a tumor-promoting factor in ovarian cancer.
Studies have shown that MSLN possesses an important promoting function on constitutive activation of the transcription factor Stat3 and MSLN overexpression cancer cells were found producing increased IL-6 15,23,25 . In addition, metformin was found to target Stat3 to inhibit cell growth and induce apoptosis in cancer cells 7,26 –28 . Our study also showed that metformin treatment resulted in a significant decrease in MSLN expression, and its downregulation is accompanied by a decrease in IL-6 expression and low STAT3 activity. More importantly, we found that, in control cells, metformin remarkably suppressed MSLN expression and IL-6/STAT3 signaling, while metformin treatment has no effect on IL-6/STAT3 signaling in MSLN knock out cell line, indicated that metformin function on inhibiting IL-6/STAT3 signaling via MSLN regulation. Our results suggested a working model by which metformin functions as an ovarian cancer inhibitor through down-regulated MSLN expression. Then, MSLN reduction in turn inhibits IL6/STAT3 signaling and TGFβ expression, which at last suppressed ovarian cancer cells growth, migration, stemness. and angiogenesis (Fig. 5F). It has been reported that the IL-6/STAT3 signaling pathway stimulates cell proliferation and migration, and protects tumor cells against drug-induced apoptosis 36 –40 . The inhibition of STAT3 has been shown to decrease the lung metastasis rate and prolong the survival of these mice 41 . Therefore, the inhibitory effect of metformin on the IL-6/STAT3 signaling pathway may prove crucial for therapy in ovarian cancers.
Recently, in the context of epithelial-to-mesenchymal transition or fibrosis, metformin was identified as a novel TGFβ suppressor 7,12 . For example, metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of the TGFβ signaling pathway 42 . And, in cervical carcinoma cells, metformin was reported to inhibit TGFβ1-induced EMT via PKM2 relative-mTOR/p70s6 k signaling pathway 7 . In the present study, our data revealed that in administration with 5 mM metformin, the expression of TGFβ1 and VEGF were detected dramatically down-regulated, and a remarkable reduction of Smad-2 phosphorylation was also observed. Our observation demonstrated that metformin server as an inhibitor of TGFβ1/Smad signaling in ovarian cancer.
Conclusion
In summary, our findings support the use of metformin as an anti-tumorigenic and antiangiogenic drug in ovarian cancer. Furthermore, we also propose a novel mechanism by which metformin resulted in downregulation of MSLN expression in SKOV3 cells and subsequently repressed IL-6 expression, STAT3 activation and TGFβ1 expression to inhibit ovarian cancer cell malignancy.
Acknowledgments
The authors are grateful to Man for technical assistance.
Abbreviation
- (MSLN)
mesothelin
- (VEGF)
Vascular endothelial growth factor
- (ER)
Endoplasmic reticulum
- (ICC)
Intrahepatic cholangiocarcinoma
- (IL-6)
Interleukin-6
Footnotes
Author contributions: X.Y. designed the study. J.Q., S.L. L.Z. J.Z. J.C. D.J. G.Z. and C.L. performed the experiments and collected the data. X.Y. analyzed and interpreted the experimental data. X.Y. wrote the manuscript.
Data Availability: All data generated or analyzed during this study are included in this article
Ethical Approval: All the experiments and procedures were approved by the Ethics Committee of the Chengdu fifth people’s hospital, Sichuan 611130, China
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was sponsored by the Sichun Natural Science Foundation [grant No. 813807514].
ORCID iD: Xu Yang  https://orcid.org/0000-0002-9791-5615
https://orcid.org/0000-0002-9791-5615
References
- 1. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19(1):3–10. [DOI] [PubMed] [Google Scholar]
- 2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. [DOI] [PubMed] [Google Scholar]
- 3. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho KR, Shih I-M. Ovarian cancer. Annu Rev Pathol Mechan Dis. 2009;4:287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lengyel E. Ovarian cancer development and metastasis. Ame J Pathol. 2010;177(3):1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chae YK, Arya A, Malecek M-K, Shin DS, Carneiro B, Chandra S, Kaplan J, Kalyan A, Altman JK, Platanias L. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7(26):40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng K, Hao M. Metformin inhibits TGF-beta1-induced epithelial-to-mesenchymal transition via PKM2 relative-mTOR/p70s6 k signaling pathway in cervical carcinoma cells. Int J Mol Sci. 2016;17(12):2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galdieri L, Gatla H, Vancurova I, Vancura A. Activation of AMP-activated protein kinase by metformin induces protein acetylation in prostate and ovarian cancer cells. J Biol Chem. 2016;291(48):25154–25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seliger C, Meyer AL, Renner K, Leidgens V, Moeckel S, Jachnik B, Dettmer K, Tischler U, Gerthofer V, Rauer L, Uhl M, et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of TGF-beta2. Cell Cycle. 2016;15(13):1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stambolic V, Dowling RJ. Metformin and exercise in cancer: better together. JNCI Cancer Spectrum. 2020;4(1):pkz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tseng HW, Li SC, Tsai KW. Metformin treatment suppresses melanoma cell growth and motility through modulation of microRNA expression. Cancers (Basel). 2019;11(2):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wahdan-Alaswad R, Harrell JC, Fan Z, Edgerton SM, Liu B, Thor AD. Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle. 2016;15(8):1046–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak M, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121(3):492–498. [DOI] [PubMed] [Google Scholar]
- 14. Zou J, Hong L, Luo C, Li Z, Zhu Y, Huang T, Zhang Y, Yuan H, Hu Y, Wen T, Zhuang W, et al. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK-FOXO1 signal pathway. Cancer Sci. 2016;107(12):1806–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol cancer. 2011;10(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frierson HF, Jr, Moskaluk CA, Powell SM, Zhang H, Cerilli LA, Stoler MH, Cathro H, Hampton GM. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Human pathol. 2003;34(6):605–609. [DOI] [PubMed] [Google Scholar]
- 17. He X, Wang L, Riedel H, Wang K, Yang Y, Dinu CZ, Rojanasakul Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Molecular cancer. 2017;16(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch VW. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin cancer Res. 2014;20(4):1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamberts LE, de Groot DJA, Bense RD, de Vries EG, Fehrmann RS. Functional genomic mRNA profiling of a large cancer data base demonstrates mesothelin overexpression in a broad range of tumor types. Oncotarget. 2015;6(29):28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong-Li S, Lei L, Lei S, Dan Z, De-Li D, Guo-Fen Q, Yan L, Wen-Feng C, Bao-Feng Y. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res. 2008;22(7):985–989. [DOI] [PubMed] [Google Scholar]
- 21. Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J-I, Sima C, Rusch VW, Sadelain M, Adusumilli PS. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18(9):2478–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiraishi T, Shinto E, Mochizuki S, Tsuda H, Kajiwara Y, Okamoto K, Einama T, Hase K, Ueno H. Mesothelin expression has prognostic value in stage ΙΙ/ΙΙΙ colorectal cancer. Virchows Archiv. 2019;474(3):297–307. [DOI] [PubMed] [Google Scholar]
- 23. Bharadwaj U, Li M, Chen C, Yao Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol Cancer Res. 2008;6(11):1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res. 2008;6(2):186–193. [DOI] [PubMed] [Google Scholar]
- 25. Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6 R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32(7):1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng X-S, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11(2):367–376. [DOI] [PubMed] [Google Scholar]
- 27. Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung S-C, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5(2):e1088–e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang D, Xiao H, Zhang Y, Huang Z, Yang J. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget. 2016;7(19):28235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang R, Chen X, Chen L, Wan F, Chen K, Sun Y, Zhu X. STAT3 signaling in ovarian cancer: a potential therapeutic target. J Cancer. 2020;11(4):837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JH, Chiang SY, Nam D, Chung WS, Lee J, Na YS, Sethi G, Ahn KS. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014;345(1):140–148. [DOI] [PubMed] [Google Scholar]
- 31. Binju M, Amaya-Padilla MA, Wan G, Gunosewoyo H, Suryo Rahmanto Y, Yu Y. Therapeutic inducers of apoptosis in ovarian cancer. Cancers (Basel). 2019;11(11):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ling S, Feng T, Ke Q, Fan N, Li L, Li Z, Dong C, Wang C, Xu F, Li Y. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol Rep. 2014;31(6):2611–2618. [DOI] [PubMed] [Google Scholar]
- 33. Dilokthornsakul P, Chaiyakunapruk N, Termrungruanglert W, Pratoomsoot C, Saokeaw S, Sruamsiri R. The effects of metformin on ovarian cancer: a systematic review. Int J Gynecol Cancer. 2013;23(9):1544–1551. [DOI] [PubMed] [Google Scholar]
- 34. Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, Rattan R, Cliby W, Shridhar V. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119(3):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kendrick ZW, Firpo MA, Repko RC, Scaife CL, Adler DG, Boucher KM, Mulvihill SJ. Serum IGFBP2 and MSLN as diagnostic and prognostic biomarkers for pancreatic cancer. HPB. 2014;16(7):670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grivennikov S, Karin E, Terzic J, Mucida D, Yu G-Y, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. [DOI] [PubMed] [Google Scholar]
- 39. Liu Y, Li P-K, Li C, Lin J. Inhibition of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in human liver cancer cells. J Biolog Chem. 2010;285(35):27429–27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tu B, Du L, Fan Q-M, Tang Z, Tang T-T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Letters. 2012;325(1):80–88. [DOI] [PubMed] [Google Scholar]
- 41. Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, Wu X, Zhou Q, Xu K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8(44):76116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li L, Huang W, Li K, Zhang K, Lin C, Han R, Lu C, Wang Y, Chen H, Sun F. Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway. Oncotarget. 2015;6(41):43605. [DOI] [PMC free article] [PubMed] [Google Scholar]