Abstract
IDO1 inhibitors have shown promise as immunotherapies for the treatment of a variety of cancers, including metastatic melanoma and renal cell carcinoma. We recently reported the identification of several novel heme-displacing IDO1 inhibitors, including the clinical molecules linrodostat (BMS-986205) and BMS-986242. Both molecules contain quinolines that, while being present in successful medicines, are known to be potentially susceptible to oxidative metabolism. Efforts to swap this quinoline with an alternative aromatic system led to the discovery of 2,3-disubstituted pyridines as suitable replacements. Further optimization, which included lowering ClogP in combination with strategic fluorine incorporation, led to the discovery of compound 29, a potent, selective IDO1 inhibitor with robust pharmacodynamic activity in a mouse xenograft model.
Keywords: Indoleamine 2,3-dioxygenase; IDO1; immuno-oncology; cancer immunotherapy; quinoline; metabolism
Over the past several decades, the immune system’s role in cancer recognition and killing has been a source of debate. In recent years, as mechanisms of tumoral immune escape have been elucidated and proven in the clinic with checkpoint inhibitors such as Opdivo, Keytruda, and Yervoy, the field has gained mainstream acceptance.1 Further understanding of the impact of amino acid metabolism on the tumor microenvironment (TME) has led to the identification of immuno-oncology (IO) targets capable of small-molecule perturbation.2 One such target, indoleamine 2,3-dioxygenase-1 (IDO1), is a cytosolic enzyme expressed in tumors and tumor-associated cells such as dendritic cells (DCs) and endothelial cells. High IDO1 expression in the tumor or tumor-draining lymph nodes has been associated with poor prognosis.3 Checkpoint inhibitors activate T cells within the tumor and cause antitumor inflammation, which can increase IDO1 expression in the TME. Preclinically, IDO1 inhibition has been shown to work synergistically with both conventional cancer therapies and checkpoint immunotherapy.4−6 This combination of attributes makes IDO1 a promising target for cancer immunotherapy.
IDO1 catalyzes the oxidative cleavage of the 2,3-double bond in d- or l-tryptophan. This oxidative cleavage event is the initial and rate-limiting step of tryptophan catabolism. IDO1 is a monomeric heme-containing dioxygenase, meaning that both atoms of molecular oxygen are incorporated into the tryptophan-derived oxidative cleavage product, N-formylkynurenine, which can then undergo deformylation with kynurenine formamidase to form kynurenine.7 The closely related enzymes IDO2 and tryptophan 2,3-dioxygenase (TDO) are also capable of effecting this transformation. The physiological role of IDO2 has not been fully elucidated, and although it is primarily expressed in hepatocytes, it is not critical for systemic tryptophan homeostasis, as is the case with TDO.8 IDO2 possesses only 43% sequence homology with IDO1 and weaker affinity for l-tryptophan.9 TDO (sometimes called TDO2) is tetrameric as opposed to monomeric, enantiospecific for the catabolism of l-tryptophan, and expressed mainly in the liver.10,11 TDO is primarily responsible for maintaining homeostasis of dietary l-tryptophan, suggesting a role distinct from that of IDO1.12
IDO1 expression can be induced in a variety of ways, including type I and type II interferons (including IFNγ), reverse signaling from inhibitory T cell receptors such as binding of CTLA4 to CD80, and COX-2 via PGE2 production.13 High levels of IDO1 expression in the TME lead to localized l-Trp depletion and high kynurenine levels. Low l-Trp levels can activate stress response kinase GCN2, leading to biased differentiation of naïve T cells into regulatory T (Treg) cells, T cell anergy, and inhibition of T cell proliferation.14,15 Additionally, activation of GCN2 can alter DCs to a more immunosuppressive phenotype that produces TGFβ and IL-10.16 Kynurenine can activate the aryl hydrocarbon receptor (AhR) upon binding, which can also cause differentiation of naïve T cells into Treg cells and lead to the formation of immunosuppressive DCs.17 These myriad effector pathways can ultimately lead to immune tolerance toward tumoral antigens.18
Encouraged by the promising preclinical and clinical data available for IDO1, many companies targeted small-molecule inhibitors for cancer immunotherapy. Several candidates have entered the clinic in combination with chemotherapy, vaccines, and checkpoint immunotherapy.19 More specifically, Bristol Myers Squibb advanced linrodostat (1)20 and closely related BMS-986242 (2)21 into clinical studies (Figure 1a). The unique binding mode of these inhibitors to apo-IDO1 (3 in Figure 1) and mechanistic studies were previously disclosed.22,23
Figure 1.
(a) IDO1 inhibitors either clinically investigated by Bristol Myers Squibb (1, 2) or crystallized with hIDO1 protein (3). (b) X-ray cocrystal of human IDO1 and compound 3 (in green), a closely related analogue of linrodostat (1). Heme (in orange) has been added to show that 3 occupies the heme binding pocket.
While our (and others’) initial efforts toward identifying an IDO1 inhibitor that was differentiated from 1 focused primarily on structure–activity relationship (SAR) exploration in the amide portion of the molecule,21,24a,24c we subsequently sought to modify the quinoline present in both 1 and 2. Quinolines, while found in many marketed drugs, including antimalarials25 and anticancer drugs,26 are known sites of metabolic activation.27 Indeed, in vitro biotransformation studies of 2 in hepatocytes of preclinical species revealed that the quinoline was susceptible to metabolic oxidation. For this reason, efforts were initiated to improve the metabolic stability of this chemotype by either adding electron-withdrawing groups to the quinoline to reduce the N-oxidation potential24a or replacing it entirely while simultaneously lowering the lipophilicity, which is the focus of this work.
Quinoline replacements were designed to maintain the π-stacking interaction with Phe270 and probe whether hydrogen bonding with Arg343 was necessary (Figure 1b). Additionally, computer-aided drug design (CADD) conformational studies revealed that the dihedral angle between the quinoline and the cyclohexane in the X-ray cocrystal of 3 resided very close to the dihedral angle of the calculated solution-state intrinsic torsional energy minimum. During analogue design, intrinsic torsional energy analysis was used to prioritize compounds biased to reside in an active conformation.28
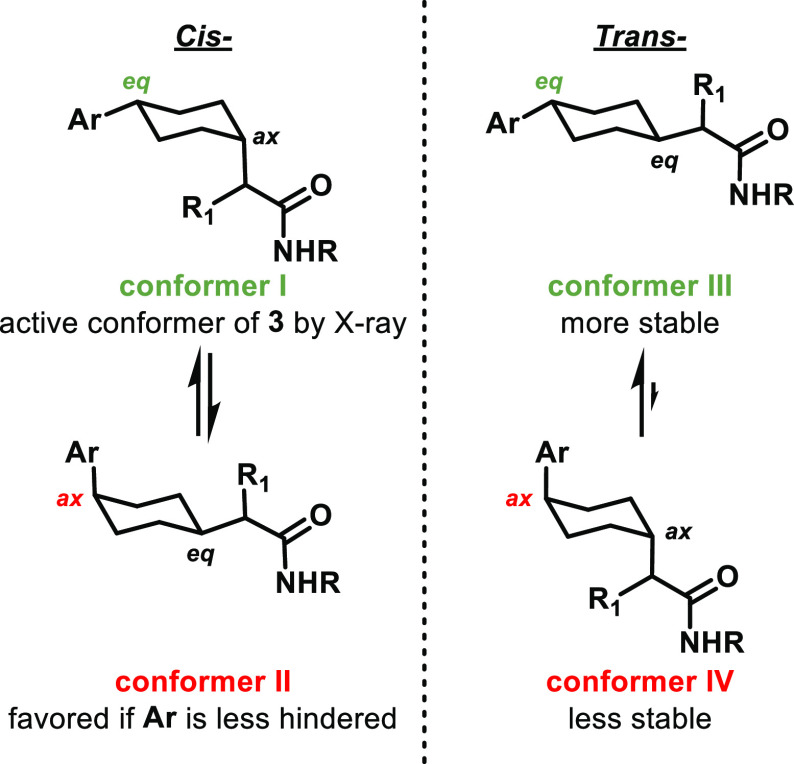
Docking studies showed that both cis-cyclohexanes in conformer I (the cocrystal conformer of 3) and trans-cyclohexanes in conformer III were predicted to bind well (see Figure 2). Modeling also predicted that cis-cyclohexanes in conformer II and trans-cyclohexanes in conformer IV would not bind. We worried that replacing the cis-cyclohexylquinoline in 3 with smaller aryl groups could bias new analogues toward disfavored conformer II. We were also wary of only targeting cis-cyclohexanes and potentially overlooking active trans-cyclohexanes, which have a strong energetic bias to reside in conformer III. Therefore, both cis- and trans-cyclohexanes were pursued simultaneously via a stereodivergent route during initial SAR exploration (Scheme 1).
Figure 2.
Potential chair conformational isomers of cis- and trans-cyclohexane analogues: conformers I and III bind to hIDO1 in a docking model, whereas conformers II and IV do not.
Scheme 1. General Synthetic Scheme for Compounds 14–25 and 27–29.

Reagents and conditions: (a) NaH, THF, 0 °C (H = 96% yield, Me = 78% yield); (b) Pd–C, MeOH, H2 (balloon to 50 PSI) (H = 98% yield, Me = 89% yield); (c) 4 N HCl, acetone, 45 °C (H = 73% yield, Me = 79% yield); (d) NaHMDS (2 M in THF), N-phenyltrifluoromethanesulfonimide, MTBE, −78 °C (H = 76% yield, Me = 96% yield); (e) B2Pin2, KOAc, NaBr, Pd(dppf)Cl2, dioxane, 110 °C (H = 50% yield, Me = 60% yield); (f) LDA, DMPU; EtI, −78 °C (95% yield); (g) LDA, MOMCl, −78 °C (61% yield); (h) Pd(PPh3)4, K2CO3, dioxane, H2O, 100 °C (27−99% yield); (i) Pd–C, MeOH, HCOONH4, 80 °C or Pd–C, MeOH, H2 (30–99% yield); (j) LiOH, H2O, THF, 70 °C, 72 h (58% to ca. quant. yield); (k) DPPA, TEA, toluene, 70 °C; LiOH, THF (51–78% yield); (l) EDCI, HOBt, DIPEA, DMF, rt or HATU, DIPEA, DMF (3–39% yield).
Compounds 13–24 and 27–29 can be synthesized as mixtures of diastereomers from intermediate 7a or 7b starting from cyclohexanedione monoketal 4 following a previously reported five-step sequence.29 Horner–Wadsworth–Emmons olefination followed by reduction and ketal hydrolysis gave ketones 6a or 6b. Formation of the vinyl triflate followed by Miyaura borylation provided 7a or 7b. For 7c or 7d, 7a was alkylated with either ethyl iodide or methoxylmethyl chloride, respectively. Aryl halides could be installed via Suzuki coupling to give 8a–c. Reduction of the styrenyl olefin via transfer hydrogenation led to a mixture of cis and trans diastereomers. In some cases, diastereomers were separated at this point. Hydrolysis of the ester yielded acids 9a–c. Curtius rearrangement provided amines 10a–c, which were then coupled to the necessary acids to give compounds 13–24 and 27–29 as mixtures of four diastereomers. For the final compounds 25 and 26, an additional displacement with sodium methoxide was performed on advanced intermediates to provide 2-methoxypyridines (see the Supporting Information). For each of the final compounds reported, chiral resolution was performed, and the most potent enantiomer is reported in Tables 1 and 2. While absolute stereochemistry was not routinely determined, 2D NMR techniques were employed to assign relative cis/trans stereochemistry for all of the final compounds (see the Supporting Information).
Table 1. Quinoline Replacements: Broad Structure–Activity Relationships and Metabolic Stability.


Data are reported as averages of ≥2 test results. See the Supporting Information for a description of the assay conditions.
Percentage of the parent compound (0.5 μM) remaining after a 10 min incubation with 1 mg/mL HLM or MLM.
The absolute stereochemistry of R1 is R.
Table 2. 2,3-Disubstituted Pyridines as Quinoline Replacements.

Data are reported as averages of ≥3 test results. See the Supporting Information for a description of the assay conditions.
Data are reported as averages of ≥2 test results. See the Supporting Information for a description of the assay conditions.
Percentage of the parent compound (0.5 μM) remaining after a 10 min incubation with 1 mg/mL HLM, RLM, or MLM.
As stated above, while absolute stereochemistry was not routinely determined, all of the mixtures were chirally resolved, and the relative stereochemistry was assigned by 2D NMR analysis. The most potent enantiomer is reported below in Table 1. On the basis of intrinsic torsional energy analysis, aromatic or heteroaromatic rings containing substitution at positions either ortho or meta to the cyclohexyl ring were prioritized. Pyridone 13 and benzodioxole 14 were prioritized on the basis of this strategy. Interestingly, compounds 13 and 14 maintained double-digit nanomolar activity in human cellular assays without containing a hydrogen-bond acceptor to interact with Arg343. Unfortunately, both were significantly less potent than BMS-986242 (2), and 14 suffered from very poor metabolic stability. Contrary to our original hypothesis, para-substituted arene 15 demonstrated that neither ortho nor meta substitution is required to maintain double-digit nanomolar human IDO1 potency. Also, the trans isomer of 15 was superior to the cis isomer (data not shown). This trans preference was observed in several compounds where ortho substitution was absent (15, 16, 17, 19, and 20), which could be due to a preference for cis isomers with these aryl groups to adopt putatively disfavored conformer II (Figure 2). Compound 21 was an exception to this trend, perhaps because the 4-pyridyl nitrogen can interact with Arg343 to bias 21 to adopt conformer I in the binding pocket. Conversely, when ortho substitution was present, the cis isomer was usually preferred (compounds 14, 18, 22, and 23), which could be due to a preference for these aryl groups to adopt known active conformer I (Figure 2). 4-Pyridyl compounds such as 21, 22, and 23 not only had torsional energy minima corresponding to the active conformation of the quinoline but also retained the nitrogen to potentially interact with Arg343. As expected, 21, 22, and 23 were more potent in cellular assays, with 22 and 23 achieving single-digit nanomolar activity. However, they suffered from very poor metabolic stability.
Our focus then turned to improving the metabolic stability of the 4-pyridyl analogues (Table 2). Analogue 24, with 2-fluoro in place of the 2-methyl group in 23, performed in the same fashion as 23, displaying poor metabolic stability. However, swapping for a 2-methoxy group (25) led to a significant improvement in not only human whole-blood activity but also metabolic stability. The 3-methyl group in 25 was a potential metabolic soft spot, so it was replaced with a fluorine (26) or a difluoromethyl group (27) in an attempt to bolster the metabolic stability.30 Gratifyingly, 27 with the difluoromethyl group performed comparably to 25 in cellular and whole-blood assays with further improved metabolic stability. Attempts to introduce more polarity into the molecule and lower ClogP to improve the metabolic stability included adding a MOM group at R1 (28) and a methoxypyridine at R3 (29). SAR around the amide portion of the molecule has been discussed in greater detail previously.21,24 Modification of the cyclohexyl core to more polar, saturated heterocycles (not shown) did not yield superior leads. While the MOM group in 28 was unsuccessful in improving the metabolic stability, methoxypyridine-containing 29 showed an overall superior profile in terms of human cellular and whole-blood potency as well as metabolic stability across species. Compound 29 has a lower ClogP than prior candidates 1 and 2 (6.39 for 1 vs 5.94 for 2 vs 4.46 for 29) and other closely related analogues shown in Table 2.
The relative stereochemistry around the cyclohexane in 29 was confirmed to be cisvia 2D NMR experiments. The absolute stereochemistry at R1 was confirmed to be R, which is consistent with the stereochemistry in 1 and 2 (see the structure in Table 3).31 Further in vitro profiling revealed that lower ClogP correlated with improved in vitro oxidative metabolism in most species (CYP t1/2 for 2 = 14 (H), 4 (M), 10 (R), 10 (D), 2 (C)) and lower protein binding (0.8/1% free for H/M for 29 vs <0.1% free for both H/M for 1 vs <0.3/<0.2% free for H/M for 2). Compound 29 had good intrinsic permeability and only modest PXR activation. Like compound 2, 29 did show CYP inhibition in some isoforms, but it was less potent (human CYP IC50(μM) for 2: 0.74 for 2C9; 1.39 for 2C8; 3.37 for 2C19). Compound 29 showed some hERG inhibition at higher concentrations as well as sodium and calcium channel inhibition, but the hERG inhibition was still attenuated compared with 2 (87% inhibition at 10 μM).
Table 3. In Vitro Profile of Compound 29.
| met. stability CYP (t1/2 min) | 32 (H), 11 (M), 20 (R), 7 (D), 3 (C) |
| met. stability UGT (t1/2 min) | >120 (H, R, M, D, C) |
| PAMPA Papp (pH 7.4) | 1070 nm/s |
| Caco Papp (a–b:b–a) | 122:56 nm/s |
| PXR-TA EC50 (μM) | 1.9 (22% Ymax) |
| human CYP IC50 (μM) | 2C9, 1.89; 2C8, 4.01; 2C19, 9.50; others, >10 |
| hERG IC50 (μM) | 17.4 (61% inh. at 30 μM) |
| Na+ patch clamp | <18% inh. at 10 μM (1 and 4 Hz) |
| Ca2+ patch clamp IC50 (μM) | 0.76 |
| protein binding (% free) | H 0.8, M 1 |
In a PK–PD study in a nu/nu mouse xenograft model implanted with human SKOV3 cells (ovarian cancer cell line), compound 29 had lower tumor exposures than 1 at doses of 60, 120, and 180 mg/kg, and the exposures were not dose-proportional for doses from 20 to 180 mg/kg (Table 4). Despite the lower exposure, the 20 mg/kg dose of 29 still caused a significant reduction in tumor kynurenine (58%), possibly due to lower protein binding (vide supra). While the 180 mg/kg dose displays a deeper PD effect (80% reduction), it is unclear why the 120 mg/kg dose, despite reaching similar exposure in the tumor, reached only 63% kynurenine reduction. Pharmacokinetic studies in preclinical species (Table 5) showed moderate to low clearance in mouse and rat, respectively, but higher clearance in dog and particularly cyno, which also showed very low oral bioavailability. High volumes of distribution were observed across species. Additionally, liver accumulation was seen in rats (24 h liver/plasma ratio = 8.3).
Table 4. PK–PD study of Compound 29 vs Linrodostat in a Human SKOV3 Xenograft Tumor Mouse Model.
QD po dosing.
PK is AUC (0–24 h) in μM·h measured in the tumor.
PD is percent kynurenine AUEC (0–24 h) reduction measured in the tumor. %Kyn reduction was measured at steady state after the fifth dose (calculated as the area under the Kyn concentration–time curve from 0 to 24 h) and compared with that of the vehicle control.
Table 5. PK Parameters for Compound 29.
| parameter | mousea | rata | doga | cynoa |
|---|---|---|---|---|
| dose (mg/kg) iv/po | 1b/10c | 0.5b/10c | 1d/5e | 1d/5e |
| CL (mL/min/kg) iv | 33 | 15 | 18 | 29 |
| Vss (L/kg) iv | 6.2 | 4.0 | 9.0 | 4.5 |
| AUCtotal (μM·h) iv/po | 1.2/3.9 | 1.3/8.7 | 2.3/3.2 | 1.3/0.029 |
| t1/2 (h) iv | 5.0 | 7.1 | 14 | 11 |
| Fpo (%) | 33 | 34 | 28 | 4.5 |
Data are reported as averages over ≥3 animals.
80% PEG 400, 20% water.
5% ethanol; 55% PEG 400; 20% propylene glycol, 20% TPGS.
70% PEG 400; 30% water.
90% PEG 400; 10% solutol.
In vitro biotransformation studies in hepatocytes (H/R/D/C) and in vivo metabolite identification studies in nu/nu mice from the PK–PD study confirmed demethylation of the difluoromethylpyridine as the primary route of metabolism. The demethylated metabolite of 29 was synthesized to confirm its structure. While the metabolite was much less active against IDO1 (IDO1 HWB IC50 > 1000 nM), it was still liable to oxidative metabolism (CYP t1/2 = 24 (H), 36 (M), 26 (R), 38 (D), 7 (C)). The −OCD3 analogue of 29 was synthesized in an attempt to attenuate the demethylation, but the in vitro oxidative metabolic stability was not substantially improved (CYP t1/2 = 15 (H), 11 (M), 18 (R), 29 (D), 8 (C)).
In summary, structure-based drug design, torsional angle energy minima, and the potential for conformational isomerization of cyclohexane were considered in designing replacements for the quinolines found in linrodostat (1) and BMS-986242 (2). A stereodivergent synthesis was pursued that identified a variety of successful quinoline replacements with both cis and trans stereochemistry around the cyclohexane. Strategic incorporation of fluorine and polarity into 2,3-disubstituted 4-pyridyl analogues led to the identification of compound 29. Despite lower exposures, compound 29 achieved similar or even superior (180 mg/kg dose) kynurenine reduction in tumors in a human SKOV3 xenograft tumor mouse model compared with 1.
Glossary
Abbreviations
- TEA
triethylamine
- THF
tetrahydrofuran
- DPPA
diphenylphosphoryl azide
- DMPU
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
- LDA
lithium diisopropylamide
- MTBE
methyl tert-butyl ether
- B2Pin2
bis(pinocolato)diboron
- EDCI
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- HOBT
hydroxybenzotriazole
- DIPEA
N,N-diisopropylethylamine
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
- DMF
N,N-dimethylformamide
- EtI
ethyl iodide
- MOMCl
methoxymethyl chloride
- NaOMe
sodium methoxide
- H
human
- M
mouse
- R
rat
- D
dog
- C
cyno
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00236.
Biological assay protocols, in vivo pharmacokinetic–pharmacodynamic study protocols, experimental procedures, and analytical data for all final compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Prendergast G. C. Immunological thought in the mainstream of cancer research. Oncoimmunology 2012, 1, 793–797. 10.4161/onci.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. L.; Smothers J.; Srinivasan R.; Hoos A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discovery 2015, 14, 603–622. 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- Godin-Ethier J.; Hanafi L. A.; Piccirillo C. A.; Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunological perspectives. Clin. Cancer Res. 2011, 17, 6985–6991. 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- Muller A. J.; DuHadaway J. B.; Donover P. S.; Sutanto-Ward E.; Prendergast G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target for the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- Monjazeb A. M.; Kent M. S.; Grossenbacher S. K.; Mall C.; Zamora A. E.; Mirsoian A.; et al. Blocking indoleamine 2,3-dioxygenase rebound immuno suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin. Cancer Res. 2016, 22, 4328–4340. 10.1158/1078-0432.CCR-15-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard R. B.; Zamarin D.; Munn D. H.; Wolchok J. D.; Allison J. P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402. 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H.; Oda S.-I.; Otsuki T.; Hino T.; Yoshida T.; Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 2611–2616. 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusof F. F.; Bakmiwewa S. M.; Weiser S.; Too L. K.; Metz R.; Prendergast G. C.; Fraser S. T.; Hunt N. H.; Ball H. J. Investigation of the tissue distribution and physiological roles of indoleamine 2,3-dioxygenase-2. Int. J. Tryptophan Res. 2017, 10, 1178646917735098. 10.1177/1178646917735098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantouris G.; Serys M.; Yuasa H. J.; Ball J. J.; Mowat C. G. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids 2014, 46, 2155–2163. 10.1007/s00726-014-1766-3. [DOI] [PubMed] [Google Scholar]
- Shimizu T.; Nomiyama S.; Hirata F.; Hayaishi O. Indoleamine 2,3-dioxygenase. purification and some properties. J. Biol. Chem. 1978, 253, 4700–4706. 10.1016/S0021-9258(17)30447-7. [DOI] [PubMed] [Google Scholar]
- Haber R.; Bessette D.; Hulihan-Giblin B.; Durcan M. J.; Goldman D. Identification of tryptophan 2,3-dioxygenase RNA in rodent brain. J. Neurochem. 1993, 60, 1159–1162. 10.1111/j.1471-4159.1993.tb03269.x. [DOI] [PubMed] [Google Scholar]
- Zhai L.; Spranger S.; Binder D. C.; Gritsina G.; Lauing K. L.; Giles F. J.; Wainwright D. A. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 5427–5433. 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaha T. L.; Huang L.; Lemos H.; Metz R.; Mautino M.; Prendergast G. C. L.; Mellor A. L. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunology Reviews 2012, 249, 135–157. 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H.; Sharma M. D.; Baban B.; Harding H. P.; Zhang Y.; Ron D.; Mellor A. L. GCN2 Kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005, 22, 633–642. 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fallarino F.; Grohmann U.; You S.; McGrath B. C.; Cavener D. R.; Vacca C.; Orabona C.; Bianchi R.; Belladonna M. L.; Volpi C.; Santamaria P.; Fioretti M. C.; Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006, 176, 6752–6761. 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Liu H.; Huang L.; Bradley J.; Liu K.; Bardhan K.; Ron D.; Mellor A. L.; Munn D. H.; McGaha T. L. GCN2-dependent metabolic stress is essential for endotoxemic cytokine induction and pathology. Mol. Cell. Biol. 2014, 34, 428–438. 10.1128/MCB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R.; Rust S.; DuHadaway J. B.; Mautino M. R.; Munn D. H.; Vahanian N. N.; Link C. J.; Prendergast G. C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012, 1, 1460–1468. 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J.; Prendergast G. C. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr. Cancer Drug Targets 2007, 7, 31–40. 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C.; Malachowski W. P.; DuHadaway J. B.; Muller A. J. Discovery of IDO1 inhibitors: from bed to benchside. Cancer Res. 2017, 77, 6795–6811. 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog A.; Lin T.-a.; Maley D.; Gullo-Brown J.; Kandoussi E. H.; Zeng J.; Hunt J. T. Preclinical characterization of linrodostat mesylate, a novel, potent, and selective oral indoleamine 2,3-dioxygenase 1 inhibitor. Mol. Cancer Ther. 2021, 20, 467–476. 10.1158/1535-7163.MCT-20-0251. [DOI] [PubMed] [Google Scholar]
- Cherney E. C.; Zhang L.; Nara S.; Zhu X.; Gullo-Brown J.; Maley D.; Lin T.-A.; Hunt J. T.; Huang C.; Yang Z.; Darienzo C.; Discenza L.; Ranasinghe A.; Grubb M.; Ziemba T.; Traeger S. C.; Li X.; Johnston K.; Kopcho L.; Fereshteh M.; Foster K.; Stefanski K.; Fargnoli J.; Swanson J.; Brown J.; Delpy D.; Seitz S. P.; Borzilleri R.; Vite G.; Balog A. Discovery and preclinical evaluation of BMS-986242, a potent, selective inhibitor of indoleamine-2,3-dioxygenase 1. ACS Med. Chem. Lett. 2021, 12, 288–294. 10.1021/acsmedchemlett.0c00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelp M. T.; Kates P. A.; Hunt J. T.; Newitt J. A.; Balog A.; Maley D.; Zhu X.; Abell L.; Allentoff A.; Borzilleri R.; Lewis H. A.; Lin Z.; Seitz S. P.; Yan C.; Groves J. T. Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 3249–3254. 10.1073/pnas.1719190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K. N.; Yeh S.-R. Mapping the binding trajectory of a suicide inhibitor in human indoleamine 2,3-dioxygenase 1. J. Am. Chem. Soc. 2018, 140, 14538–14541. 10.1021/jacs.8b07994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang L.; Cherney E. C.; Zhu X.; Lin T.; Gullo-Brown J.; Maley D.; Johnston-Allegretto K.; Kopcho L.; Fereshteh M.; Huang C.; Li X.; Traeger S. C.; Dhar G.; Anandam A.; Mahankali S.; Padmanabhan S.; Rajanna P.; Murali V.; Mariappan T.; Borzilleri R.; Vite G.; Hunt J. T.; Balog A. Discovery of imidazopyridines as potent inhibitors of indoleamine 2,3-dioxygenase 1 for cancer immunotherapy. ACS Med. Chem. Lett. 2021, 12, 494–501. 10.1021/acsmedchemlett.1c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Steeneck C.; Kinzel O.; Anderhub S.; Hornberger M.; Pinto S.; Morschhaeuser B.; Albers M.; Sonnek C.; Czekanska M.; Hoffmann T. Discovery and optimization of substituted oxalamides as novel heme-displacing IDO1 inhibitors. Bioorg. Med. Chem. Lett. 2021, 33, 127744. 10.1016/j.bmcl.2020.127744. [DOI] [PubMed] [Google Scholar]; c Kinzel O.; Steeneck C.; Anderhub S.; Hornberger M.; Pinto S.; Morschhaeuser B.; Albers M.; Sonnek C.; Wang Y.; Mallinger A.; Czekanska M.; Hoffmann T. Discovery of ´highly potent heme-displacing IDO1 inhibitors based on a spirofused bicyclic scaffold. Bioorg. Med. Chem. Lett. 2021, 33, 127738. 10.1016/j.bmcl.2020.127738. [DOI] [PubMed] [Google Scholar]
- Foley M.; Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. 10.1016/S0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Jain S.; Chandra V.; Kumar Jain P.; Pathak K.; Pathak D.; Vaidya A. Comprehensive review on current developments of quinoline-based anticancer agents. Arabian J. Chem. 2019, 12, 4920–4946. 10.1016/j.arabjc.2016.10.009. [DOI] [Google Scholar]
- Reign G.; McMahon H.; Ishizaki M.; Ohara T.; Shimane K.; Esumi Y.; Green C.; Tyson C.; Ninomiya S. Cytochrome P450 species involved in the metabolism of quinoline. Carcinogenesis 1996, 17, 1989–1996. 10.1093/carcin/17.9.1989. [DOI] [PubMed] [Google Scholar]
- Potential energy surfaces of torsions were generated using conformational energetics to predict dominant species in solution. Quantum-mechanical calculations were conducted as follows: (1) initial geometry generation, (2) geometry optimization (B3LYP/6-31+G*), (3) energy calculation (RIMP2/aug-cc-pVTZ). The calculations were intrinsic and do not account for the presence of the receptor.
- a Beck H. P.; Jaen J. C.; Osipov M.; Powers J. P.; Reilly M. K.; Shunatona H. P.; Walker J. R.; Zibinsky M.; Balog J. A.; Williams D. K.; Guo W.. Preparation of substituted acetamides as immunoregulatory agents. US 20190092729 A1, March 28, 2019.; b Cherney E. C.; Shan W.; Zhang L.; Nara S. J.; Huang A.; Balog A.. Inhibitors of indoleamine 2,3-dioxygenase and methods of their use. WO 2018039512, March 1, 2018.
- Meanwell N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- To determine the absolute stereochemistry at R1, an enantioselective synthesis of compound 29 was developed and will be disclosed in due course.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.