Abstract
Background:
In 2016, a UK vessel health and preservation (VHP) framework was developed to support healthcare staff to select the most appropriate vascular access device for patients requiring intravenous therapy. The VHP framework was based on available evidence and expert consensus. The VHP was based on available evidence and expert consensus.
Development of the VHP 2020 Framework:
A multidisciplinary team reviewed the original UK VHP framework and considered new published evidence, national and international guidelines and expert opinion. A literature search was performed using Cinahl and Medline, incorporating a variety of terms linked to vascular access devices, assessment and selection. Articles published in and after 2014 in English were included. Twelve articles were found to be relevant including three evidence-based guidelines, two randomised control trials and one systematic review.
Findings:
Three main studies provided the evidence for the update: the MAGIC study that assessed the appropriateness of peripherally inserted central catheters in patients; a study that utilised the ‘A-DIVA scale’ to predict the likelihood of difficult venous access; and a study that incorporated an ‘I-DECIDED tool’ for peripheral intravenous catheter assessment and decision-making for device removal. In addition, published guidelines provided evidence that the original advice on appropriate osmolarity of medicines for peripheral administration needed updating.
Conclusion:
The 2020 UK VHP framework reflects latest evidence-based research and guidelines, providing healthcare staff updated guidance to assist in maintaining good practice in vascular access assessment and device selection and patient safety.
Keywords: Vessel health, vessel health and preservation, vascular access device selection, vascular access device assessment, peripheral vein assessment
Introduction
The evidence-based Vessel Health & Preservation (VHP) concept of vascular access device (VAD) management was originally introduced in the United States in 2012 (Moureau et al., 2012). The essence of VHP is to assist healthcare practitioners (HCPs) in providing an appropriate, timely, intentional, proactive selection of VADs to achieve minimal damage to the patient’s veins. VHP also incorporates initial and continued assessment of the necessity of the device as well as ongoing maintenance (Fiorini et al., 2018; Moureau et al., 2012). The UK VHP Framework was adapted and developed from the US model to support practitioners to undertake vessel assessment and decision making based on individual need and risk assessment (Hallam et al., 2016). New evidence has necessitated an update of the original UK VHP framework. This paper describes the process and presents the results of this update.
Background
It is important for patients that the selection of VAD is appropriate and timely. Poor selection may be due to an absence of clear guidelines on which device to select in different clinical situations and a lack of understanding of the rationale for specific device selection (Jackson et al., 2014). In two studies, poor selection resulted in a failure rate for first-time insertion of peripheral intravenous catheters (PIVCs) of 17% in surgical patients (van Loon et al., 2016) and 19% in medical and surgical patients (van Loon et al., 2019). There is also evidence that subsequent attempts cause extra discomfort to patients with fragile veins and in particular elderly and frail patients (Oliver, 2015). PIVCs have also been found to fail on delivery of courses of treatment in 35%–50% of cases resulting in negative cycle of re-cannulation (Gorski et al., 2016; Helm et al., 2015). In an international study, 10% of PIVCs were reported by patients as being painful and/or had signs of phlebitis and a further 10% had signs of malfunction, including leaking, and dislodgement due to poor placement or visible blood in the tubing resulting in delayed treatments including analgesia, antibiotics and IV fluids (Alexandrou et al., 2018). These findings support the need for up-to-date guidance on the assessment and selection of VAD.
Evaluation of the original UK VHP Framework (2016)
Since its introduction in 2016, the original UK VHP framework has been evaluated by two studies. One study involved the Infection Prevention Society (IPS) and National Infusion and Vascular Access Society (NIVAS) members (Burnett et al., 2018). That study utilised an outcome logic model to evaluate the short- and medium-term outcomes of the VHP Framework. The findings indicated that respondents were aware of the framework, found it easy to understand and it was being used in a variety of ways, for example as a teaching aid and decision-making tool around device choice and peripheral vein assessment. Benefits included improvements in clinical practice, increased knowledge and confidence around device selection, improved patient experience and reduced rates of infection (Burnett et al., 2018). The second was a small-scale pilot study to evaluate the impact of the framework on the insertion and management of VADs (Weston et al., 2017). In that study, the VHP framework was tested in a haematology ward using the Model for Improvement approach (Taylor et al., 2014), which included the following measurements: the knowledge of HCPs before and after implementation; and the number of days from admission to referral for alternative VAD and cannula usage. Weston et al. (2017) noted that the framework had empowered frontline staff to escalate to an alternative device. The time from patient admission to line insertion also decreased. Although there was increase in appropriate alternative device placement, there was a significant decrease in PIVC placement (approximately 30%) (Weston at al., 2017).
These evaluations show the framework to be a valuable and practical tool, but since evidence supporting the framework was gathered over four years ago, an update was required before seeking to publicise its wider availability and usage.
Development of the VHP 2020 Framework
The review and update of the UK VHP 2020 framework was undertaken by a working group of experts, comprising infection control nurses, a vascular access nurse, an intensivist and a pharmacist led by the IPS in collaboration with the Royal College of Nursing (RCN) and the NIVAS. In addition, a representative from the Medusa Advisory Board joined the working group to provide expert guidance on injectable medicines.
A literature search was undertaken to identify studies and guidelines relevant to the assessment and selection of VAD. The search was performed using Cinahl and Medline including the following terms: vascular access devices; vascular access device selection; vascular access assessment; vascular access complications; peripheral intravascular catheter; peripheral cannula; midline catheter; peripherally inserted central catheter; central venous catheter; tunnelled central venous catheter; totally implanted port; central vascular access device; and vessel health and preservation. Articles were restricted to those published in or after 2014 and in English.
Findings
Twelve articles were found that were reviewed by the whole working group. These included three evidence-based guidelines, two randomised control trials, one systematic review and 10 further articles including observational, prospective and validation studies. After the review of these papers, updates of all four of the main sections of the 2016 VHP Framework were deemed necessary. The changes are presented in each of the individual sections detailing the new evidence with discussion on how this evidence has been utilised in the VHP 2020.
Vein assessment
The updated UK VHP 2020 framework now incorporates consideration of patients with difficult intravenous access (DIVA) and the need to identify these patients at the earliest opportunity (Smith-Ehrhardt et al., 2018; van Loon et al., 2019). Van Loon et al. (2019) found that even the most experienced healthcare staff undertaking cannulation on a regular basis experienced difficulty in cannulation, with a failure rate up to 19% on the first attempt. Patients with DIVA, traditionally, are identified after numerous failed attempts at PIVC insertion but identification of these patients prospectively can reduce the failure rate of cannulation and improve the patient experience (van Loon et al., 2019). The UK VHP 2020 Framework recommends known patients with difficult IV access be referred to an IV specialist and require an individualised pathway.
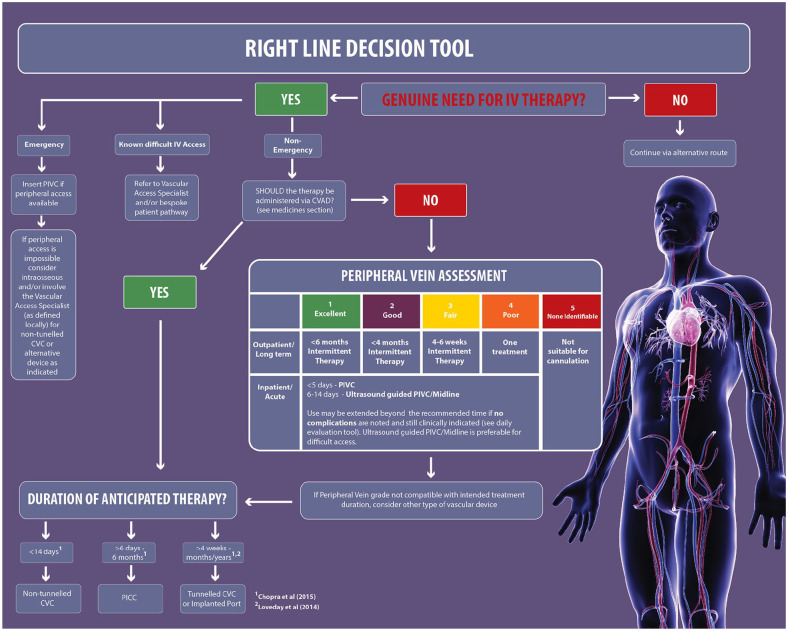
Figure 1 demonstrates the peripheral vein assessment section of the framework incorporating vein size and difficult IV access.
Figure 1.
Peripheral vein assessment.
Suitability of medicines
When considering if a medicine is suitable for administration via a PIVC, there is a potentially complex relationship between the physical properties of the medicine, the anticipated duration of therapy and, most importantly, the quality of the patient’s veins (Jackson et al., 2014).
All IV medicines potentially pose a threat to vessel health. Risk factors associated with the likelihood of vessel damage include pH, osmolarity, viscosity, concentration, speed of infusion, vesicant or irritant properties and vasoactivity of the medicine. Published guidelines suggest peripheral cannulae and midlines are generally unsuitable for the following infusions:
Continuous vesicant chemotherapy
Parenteral nutrition solutions
Solutions with osmolarity > 900 mOsmol/L (RCN, 2016)
Previous guidelines suggested that solutions with a pH < 5 or > 9 were unsuitable for peripheral administration. There is little evidence to support this and the recommendation is not included in recent iterations of national and international guidance (Gorski et al., 2016; RCN, 2016). However, the consensus of IV experts at the NIVAS Conference in Manchester in 2019 was that the advice remains valid when considering the appropriate type of device. The Medusa Injectable Medicine Advisory Board endorsed the position that administration via a central venous access device is preferable for medicines with extreme pH (< 5 and > 9) after appropriate dilution. For these medicines, the Injectable Medicines Guide gives the following advice: ‘This medicine has a low/high pH and may cause venous irritation and tissue damage in cases of extravasation. If a central venous access device is unavailable, administer via a large peripheral vein monitoring insertion site closely using a recognised phlebitis scoring tool. Re-site cannula at first signs of inflammation’ (Medusa Injectable Medicines Guide, 2020).
General guidance should be available locally in all clinical settings, particularly for specific medicines in common use or those considered to pose particular danger to vessel health. The Medusa Injectable Medicines Guide website (https://medusa.wales.nhs.uk/Home.asp), which contains up-to-date information on pH and osmolarity of injectable medicines, should be used alongside local guidance and policies.
The previous VHP Framework included a list of medicines that should be administered by a centrally placed catheter. This has been removed from the VHP 2020 update as it was considered to be falsely reassuring as it does not allow consideration of wider factors.
Right line decision tool
The right line decision tool is presented as an algorithm to aid clinician decision-making when selecting VADs for patients. This assists in optimising device selection thus reducing avoidable complications, e.g. multiple stabs, phlebitis, thrombophlebitis, insertion site infection, and bloodstream infection (Hallam et al., 2016). Therefore, its use has the potential to improve efficiency and reduces costs. The algorithm enables the clinician to combine assessments with the intended medicines, the peripheral vein condition and the duration of treatment when making a decision for VAD selection. If the choice of medication and duration of treatment is suitable for peripheral administration then a PIVC may be appropriate depending on the peripheral vein assessment (see the vein assessment section for information on assessment, use of technology and experience required for cannulation).
The updated UK VHP 2020 Framework includes the use of extended length cannula (placed using ultrasound guidance into deeper veins) based on evidence from the following studies. A single-site, non-blinded, randomised trial of catheter survival in adult patients presenting to the Emergency Department (ED) found an increased median survival time of 136 h with the extended length PIVC compared to 92 h with standard length PIVC (Bahl et al., 2019). The use of ultrasound-guided insertion to locate veins was found to increase success of cannulation and dwell time and reduce insertion-related complications in a prospective, observational single-site study of adult patients in the ED (Pandurangadu et al., 2018). Furthermore, a recent prospective comparator single-centre clinical superiority design study suggests that it is possible to extend the dwell time of a cannula using a bundle approach, a specialist team and use of ultrasound-guided cannulation using the forearm (Steele et al., 2019)
A systematic review of the literature was undertaken by Chopra et al. (2015) in response to the increasing use of peripherally inserted central catheters (PICC) and recognition of the additional risk of thrombosis and infection. An international panel reviewed 665 PICC-related scenarios in the literature and assessed the appropriateness of the PICC in each case. Following the findings of this study, ‘The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC)’ initiative was published (Chopra et al., 2015; Woller et al., 2015). The study concluded that PIVCs were suitable for continuous administration of low irritant drugs with consideration given to using ultrasound-guided insertion of a PIVC to locate larger veins for treatment duration of more than five days and up to 14 days (Chopra et al., 2015).
Chopra et al. (2015) also found that midline catheters can be used as an alternative choice for peripheral administration. Midline catheters are usually in the range of 7.5–20 cm in length and are inserted into the upper arm with the tip of the catheter not extending beyond the subclavian vein (Simonov et al., 2015). The Infusion Nurses Society (INS) Infusion Therapy Standards of Practice evidence-based guidelines state that midlines are suitable for the administration of medications such as antimicrobials, analgesics and fluid replacements that are normally tolerated by peripheral veins (Gorski et al., 2016).
Following an in-depth summary of the relevant anatomical considerations, physical characteristics, advantages, and disadvantages of VADs commonly used in the hospital setting, it was suggested that midlines provide an advantage over PIVCs due to the location being in a larger and faster-flowing vessel (Simonov et al., 2015). Guidance suggests the suitability of midlines for treatment duration up to 14 days (Chopra et al., 2015) and they should not be used for the administration of vesicant drugs, parental nutrition and infusates with an osmolarity > 900 mOsm/L (Gorski et al., 2016). Further information regarding drug suitability and device selection is included in the ‘Suitability of drugs’ section of the VHP 2020 Framework. As with the ultrasound-guided PIVC, the midline dwell time could potentially be extended beyond the 14 days when controls for insertion, maintenance and daily checks for complications are in place.
A PICC is a vascular access catheter inserted into the upper arm and advanced so that the tip is located in the superior vena cava (Gorski et al., 2016). PICCs are usually recommended for treatment over six days up to six months (Chopra et al., 2015). The MAGIC guidelines state that a PICC is preferred to a midline if the proposed duration of treatment is 15 days or more even if the drugs are compatible with peripheral infusion (Chopra et al., 2015).
MAGIC guidelines (Chopra et al., 2015) suggest the appropriateness of non-tunnelled central venous access devices (CVAD) for short-term treatment for up to 14 days involving drugs and solutions that are not suitable for peripheral administration. Non-tunnelled CVADs are usually placed in the internal jugular or subclavian vein but can also be placed in the femoral vein with the catheter tip located in the superior vena cava vein. According to the evidence-based guidelines developed by the Association of Anaesthetists of Great Britain and Ireland, a non-tunnelled CVAD is preferable to a PICC when a greater number of lumens is required than available with a PICC, such as in critical care patients (Bodenham et al., 2016).
There was no new evidence found relating to the treatment duration when selecting long-term VADs, including tunnelled CVAD and totally implanted vascular access device (TIVAD). A tunnelled CVAD is surgically placed with a subcutaneous tunnel before entering the central vein with a cuff embedded into the tissue to provide additional protection from catheter associated infections (RCN, 2016). TIVADs are tunnelled beneath the skin and have a subcutaneous port accessed with a needle and are suitable for long-term treatment where access is infrequent (Loveday et al., 2014). The guidance to long-term VAD selection remains the same and should be chosen for patients requiring intravenous treatment for longer than four months (Loveday et al., 2014).
In addition to assessing the duration of treatment and drug suitability, there are a number of factors that should be considered that assist in refining device choice in individual patients (Gorski et al., 2016; Hallam et al., 2016). The risk benefits of individual device choice are starting to be challenged in large clinical trials, with one randomised control trial of patients with cancer suggesting that TIVAD might be associated with fewer adverse events than a PICC and should be considered especially in patients with solid tumours, suggesting that the VHP 2020 Framework may require adaption for specific groups of patients (Taxbro et al., 2019).
Figure 2 shows the right line decision algorithm.
Figure 2.
Right line decision tool.
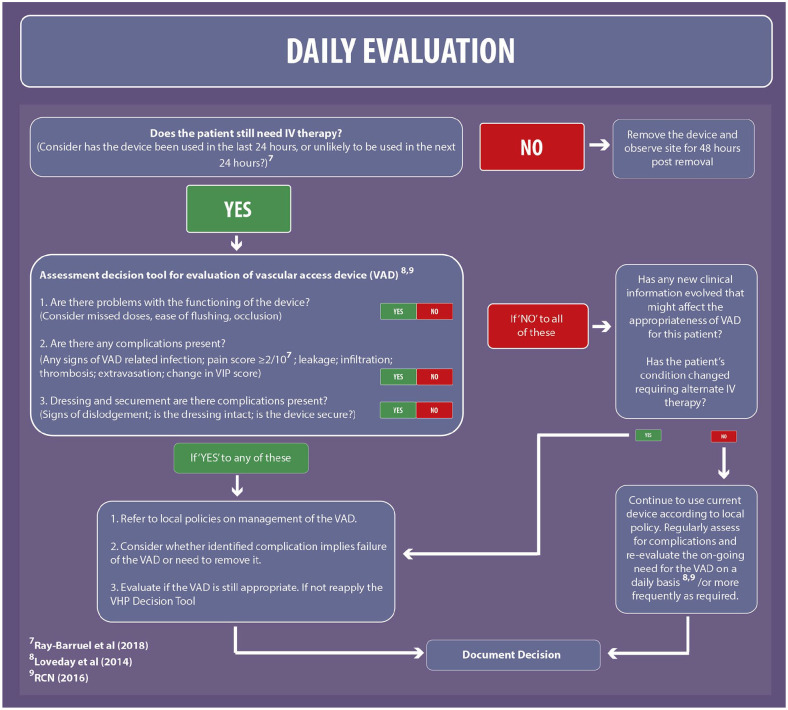
Daily evaluation
Assessment and visual observation of all VADs is essential for the early detection of complications of the VAD (Gorski et al., 2016). The updated framework considers the I-DECIDED tool, which was developed from international vascular access guidelines into a structured mnemonic for device assessment and decision-making to prompt timely removal of the PIVC and early detection of complications (Ray-Barruel et al., 2018). The I-DECIDED tool was validated by a group of international vascular access experts via an online survey followed by an inter-rater reliability study across adult wards in three Australian hospitals that demonstrated high-level reliability (Ray-Barruel et al., 2020).
The I-DECIDED tool includes pain as an indicator of potential complications when pain is reported as ⩾ 2 out of a score of 10; this has been included in the evaluation section of the VHP 2020. Complications or problems with the functioning of the device may indicate that it is not the most appropriate device to deliver the intended treatment for the patient; therefore, the evaluation section supports the assessment of whether the VAD is still appropriate and, if not, prompts the need to reapply the VHP decision tool.
The evaluation section of the VHP 2020 has been modified, starting with the simple question ‘Does the patient still need IV therapy?’ and to consider ‘Has the device been used in the last 24 hours, or unlikely to be used in the next 24 hours?’ with the aim to prompt timely removal. The following questions to assess the VAD have been simplified in the VHP 2020 Framework as below.
Are there problems with the functioning of the device?
Are there any complications present?
Dressing and securement: are there complications present?
Figure 3 shows the daily evaluation tool.
Figure 3.
Daily evaluation tool.
Limitations of the UK VHP 2020 Framework
It is acknowledged that both the original UK VHP framework and the updated VHP 2020 framework do not include insertion techniques incorporating site selection for PIVC or device selection including gauge size or number of lumens for CVADs. However, it is important to acknowledge the following practice considerations:
PIVCs should also be selected using the smallest gauge that will accommodate the prescribed therapy and patient need, ideally 20–24 G (Gorski et al., 2016).
VADs that accommodate more than one-third of the blood vessel will reduce the blood flow and increase the risk of thrombus formation (Sharp et al., 2016).
Placement of the PIVC should avoid areas of flexion where possible (RCN, 2016), e.g. the antecubital fossa. When the time is taken to place the PIVC away from joints and movement will results in lower rates of complications (Marsh et al., 2017).
Selection of CVAD should consider the minimum number of ports or lumens essential for management of the patient (Loveday et al., 2014).
Local policy guidance should be followed for the insertion of all PIVCs, midlines and CVADs.
In addition, the revised UK VHP 2020 Framework is intended for adult vascular access in acute or planned settings. While the principles of VHP should be incorporated into any emergency situation, it is recognised that other issues may take priority depending on the condition of the patient and availability vascular access expertise; therefore, other immediate routes of access may be more appropriate, e.g. intraosseous access.
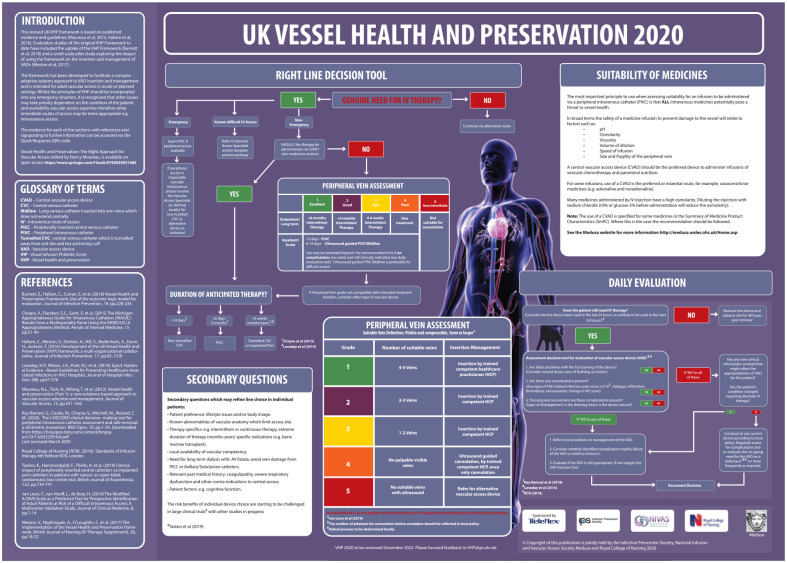
Figure 4 shows the UK VHP 2020 Framework.
Figure 4.
UK VHP 2020 Framework.
Conclusion
The 2016 UK VHP Framework required updating to remain relevant and to continue to provide IV teams and frontline staff with information and tools for appropriate and timely assessment of VAD, ongoing maintenance and evaluation. The update, which involved changes to all the sections, incorporated evidence from 12 studies. It was reviewed and approved by the IPS, NIVAS and Medusa with RCN endorsement as well as being shared with vascular access experts both in the UK and United States. Utilising the UK VHP 2020 Framework to aid decision-making and ensure that the most appropriate VAD is in situ along with daily evaluation will help to reduce the risks associated with vascular access devices. The UK VHP Framework is a live tool and will continue to be updated as new evidence becomes available.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
ORCID iD: Carole Hallam  https://orcid.org/0000-0003-3333-3250
https://orcid.org/0000-0003-3333-3250
References
- Alexandrou E, Ray-Barruel G, Carr P, Frost S, Inwood S, Higgins N, Lin F, Alberto L, Mermal L, Rickard C. and OMG Study Group. (2018) Use of Short Peripheral Intravenous Catheters: Characteristics, Management and Outcomes Worldwide. Journal of Hospital Medicine. DOI: 10.12788/jhm.3039. [DOI] [PubMed] [Google Scholar]
- Bahl A, Hang B, Brackney A, Joseph S, Karabon P, Mohammad A, Nnanabu I, Shotkin P. (2019) Standard long IV catheters versus extended dwell catheters: a randomized comparison of ultrasound-guided catheter survival. American Journal of Emergency Medicine 37(4): 715–721 [DOI] [PubMed] [Google Scholar]
- Bodenham A, Babu S, Bennett J, Binks R, Fee P, Fox B, Johnston A, Klein A, Langton J, Mclure H, Tighe S. (2016) Safe vascular access. Anaesthesia 71: 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett E, Hallam C, Curran E, Weston V. (2018) Vessel Health and Preservation Framework: Use of the outcome logic model for evaluation. Journal of Infection Prevention 19(5): 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Flanders S, Saint S, Woller S, O’Grady N, Safdar N, Trerotola S, Saran R, Moureau N, Wiseman S, Pittiruti M, Akl E, Lee A, Courey A, Swaminathan L, LeDonne J, Becker C, Krein S, Bernstein S. (2015) The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Annals of Internal Medicine 163 (Suppl. 6): S1–40. [DOI] [PubMed] [Google Scholar]
- Fiorini J, Venturini G, Conti F, Funaro E, Caruso R, Kangasniemi M, Sili A. (2018) Vessel Health and Preservation: An integrative review. Journal of Clinical Nursing 28: 1039–1047. [DOI] [PubMed] [Google Scholar]
- Gorski L, Hadaway L, Hagle M, McGoldrick, Orr M, Doellman D. (2016) Infusion Therapy Standards of Practice. Journal of Infusion Therapy 39: 1S. [Google Scholar]
- Hallam C, Weston V, Denton A, Hill S, Bodenham A, Dunn H, Jackson T. (2016) Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. Journal of Infection Prevention 17(2): 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. (2015) Accepted but unacceptable: Peripheral IV catheter failure. Journal of Infusion Nursing 38: 189–203. [DOI] [PubMed] [Google Scholar]
- Jackson T, Hallam C, Corner T, Hill S. (2014) Right line, right patient, right time: every choice matters. British Journal of Nursing 22(8): S26–28. [DOI] [PubMed] [Google Scholar]
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M. (2014) Epic3: National Evidence –Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals. Journal of Hospital Infection 86 (Suppl. 1): S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N, Webster J, Larson E, Cooke M, Mihala G, Rickard C. (2017) Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. Journal of Hospital Medicine 13(2): 83–89. [DOI] [PubMed] [Google Scholar]
- Medusa Injectable Medicines Guide. Available at: https://medusa.wales.nhs.uk/Home.asp (accessed 18 April 2020).
- Moureau NL, Trick N, Nifong T, Perry C, Kelley C, Leavett M, Gordan SM, Wallace J, Harvill M, Biggar C, Doll M, Papke L, Benton L, Phelan DA. (2012) Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. Journal of Vascular Access 13: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G. (2015) Whose line is it anyway? British Journal of Nursing 24 (Suppl. 2): S3. [Google Scholar]
- Pandurangadu AV, Tucker JM, Brackney AR, Bahl A. (2018) Ultrasound guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emergency Medicine Journal 35(9): 550–555. [DOI] [PubMed] [Google Scholar]
- Ray-Barruel G, Cooke M, Mitchell M, Chopra V, Rickard CM. (2018) Implementing the I-DECIDED clinical decision-making tool for peripheral intravenous catheter assessment and safe removal: protocol for an interrupted time-series study. BMJ Open 8: e021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray-Barruel G, Cooke M, Chopra V, Mitchell M, Rickard C. (2020) The I-DECIDED clinical decision-making tool for peripheral intravenous catheter assessment and safe removal: a clinimetric evaluation. BMJ Open 10: e035239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Nursing. (2016) Standards for Infusion Therapy. 4th edn. London: RCN. [Google Scholar]
- Sharp R, Grech C, Fielder A, Mikocka-Walus A, Esterman A. (2016) Vein diameter for peripherally inserted catheter insertion: a scoping review. Journal of the Association for Vascular Access 21: 166–175. [Google Scholar]
- Simonov M, Pittiruti M, Rickard C, Chopra V. (2015) Navigating Venous Access: A Guide for Hospitalists. Journal of Hospital Medicine 10: 471–478. [DOI] [PubMed] [Google Scholar]
- Smith-Ehrhardt B, Givens K, Lee R. (2018) Making It Stick: Developing and Testing the Difficult Intravenous Access (DIVA) Tool. Advanced Journal of Nursing 118(7): 56–62. [DOI] [PubMed] [Google Scholar]
- Steele L, Ficara C, Davies M, Moureau N. (2019) Reaching One Peripheral Intravenous Catheter (PIVC) Per Patient Visit With Lean Multimodal Strategy: the PIV5Rights™ Bundle. Journal of the Association for Vascular Access 24(3): 31–43. [Google Scholar]
- Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, Berg S. (2019) Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. British Journal of Anaesthesia 122(6): 734–741. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. (2014) Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Quality & Safety 23(4): 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon F, Puijin L, Houterman S, Bouwman A. (2016) Development of the A-DIVA Scale. Medicine 96(16): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon F, van Hooff L, de Boer H, Koopman S, Buise M, Korsten H, Dierick-van Daele A, Bouwman A. (2019) The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. Journal of Clinical Medicine 8: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston V, Nightingale A, Loughlin CO, Ventura R. (2017) The implementation of the Vessel Health and Preservation framework. British Journal of Nursing 26(8): S18–S22. [DOI] [PubMed] [Google Scholar]
- Woller S, Stevens S, Evans S. (2015) The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Initiative: A Summary and Review of Peripherally Inserted Central Catheter and Venous Catheter Appropriate Use. Journal of Hospital Medicine 11: 306–310. [DOI] [PubMed] [Google Scholar]