Abstract
Apoptosis‐associated speck‐like protein containing a caspase recruit domain (ASC), encoded by PYCARD gene, is a 22 kDa small molecule, which aggregates into ASC specks during inflammasome activation. ASC protein is an adaptor protein present in several inflammasome complexes that performs several intra‐ and extracellular functions, in monomeric form or as ASC specks, during physiological and pathological processes related to inflammation and adaptive immunity. Extracellular ASC specks (eASC specks) released during cell death by pyroptosis can contribute as a danger signal to the propagation of inflammation via phagocytosis and activation of surrounding cells. ASC specks are found in the circulation of patients with chronic inflammatory diseases and have been considered as relevant blood biomarkers of inflammation. eASC amplifies the inflammatory signal, may induce the production of autoantibodies, transports molecules that bind to this complex, contributing to the generation of antibodies, and can induce the maturation of cytokines promoting the modelling of the adaptive immunity. Although several advances have been registered in the last 21 years, there are numerous unknown or enigmatic gaps in the understanding of the role of eASC specks in the organism. Here, we provide an overview about the ASC protein focusing on the probable roles of eASC specks in several diseases, up to the most recent studies concerning COVID‐19.
Keywords: extracellular ASC, inflammasome, inflammasome in COVID‐19, innate immunity
We provide an overview about the ASC protein focusing on the probable roles of extracellular ASC specks in several diseases, up to the most recent studies concerning COVID‐19.

Abbreviations
- AD
Alzheimer's disease
- AIM2
absent in melanoma 2
- ASC
apoptosis‐associated speck‐like protein containing a caspase recruit domain
- Aβ
amyloid beta peptides
- CARD
caspase recruitment domain
- DAMP
damage‐associated molecular pattern
- eASC specks
extracellular ASC specks
- GSDMD
gasdermin D
- HIN
haematopoietic interferon
- LRR
leucine‐rich repeats
- NLR
nucleotide‐binding and oligomerization domain receptors
- NLRC
NLR family containing CARD domain
- NLRP
NLR family containing pyrin domain
- PYD
N‐terminal pyrin
- RIG‐I
retinoic acid‐inducible gene‐I‐like
- ROS
reactive oxygen species
- TLR
Toll‐like receptor
INTRODUCTION
Masumoto and colleagues described ASC in 1999 as a 22 kDa protein containing a caspase recruitment domain (CARD) at C‐terminal, able to form an aggregate during apoptosis cell death in the human promyelocytic leukaemia HL‐60 cell line. Considering this first report, the protein was named as apoptosis‐associated speck‐like protein containing a caspase recruit domain [1]. In 2001, the same group demonstrated that ASC has two domains, one N‐terminal Pyrin or PYD and one C‐terminal CARD, and is expressed by endothelial and leucocyte cells, mainly by CD14+ monocytes in peripheral blood [2].
Between 2002 and 2005, few studies were carried out describing the activation role of NF‐kB pathway in the ASC speck formation in response to pathogens [3, 4, 5]. At this time, the concept of inflammasome emerged as a mechanism of inflammatory activation of innate immune cells. Currently, ASC speck is considered a hallmark of inflammasome activation. ASC plays a role as a central adapter protein that bridges cytosolic sensor proteins and zymogens to form inflammasome supramolecular complexes. In 2006, Yan Qu and colleagues demonstrated that inflammasome activation involving P2X7 receptor could release IL‐1β and also exosomes composed of ASC aggregates are associated with membrane fragments containing MHC‐II receptor, which we can currently infer as a possible precursor of eASC speck studies [6]. From the end of 2005 onwards, new publications began to appear exponentially in the literature related to inflammasomes and specifically to ASC. This protein started to emerge as a player in the pathogenesis of diseases involving inflammatory processes, especially those associated with metabolic syndromes, and autoimmune and autoinflammatory diseases. In addition, intra‐ and eASC specks have been associated with physiological processes, modulating innate and adaptive immunity responses. Thus, many studies have proposed ASC as a therapeutic target contributing to the emergence of inhibitors of different targets of inflammasome complex activation [7, 8, 9].
Regarding eASC specks, some papers were published in 2006, 2008 and 2009, but only after 2014, studies began to shed light at the activities of the extracellular form of the complex [10, 11]. Figure 1 addresses the main advances related to the ASC protein studies, since the first characterization up to the recent findings involving eASC specks. The multifaceted activities of eASC remain not fully explained. In this review, we present several physiological or pathological situations in which eASC specks are found and results that underscore their probable functions in each of these conditions.
FIGURE 1.
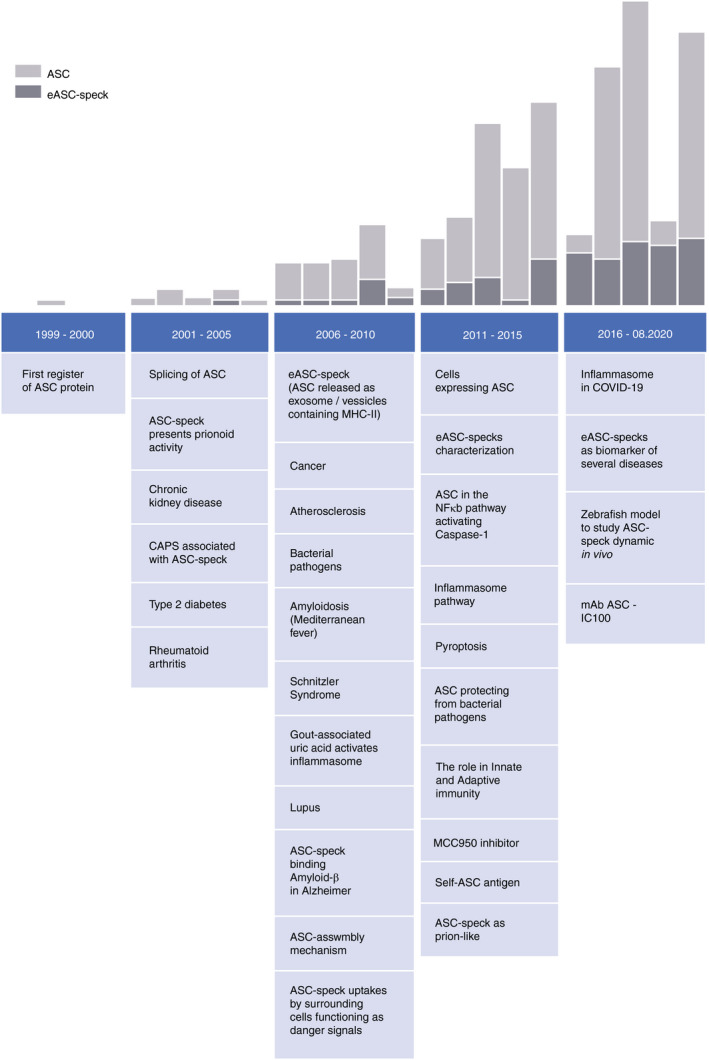
Timeline of ASC‐related publications. The data were obtained on Pubed database (https://pubmed.ncbi.nlm.nih.gov/), following the strategy search: “apoptosis‐associated speck‐like protein containing CARD” [abstract] for ASC and “Extracellular ASC, Inflammasome, ASC release” for eASC speck
THE ROLE OF ASC IN INFLAMMASOME ACTIVATION
The PYD and CARD domains of ASC molecules are death‐fold domains, which are involved in protein–protein interactions for the formation of functional complexes. These structural motifs are commonly found in proteins involved in innate immunity. ASC functions as a central adapter protein for inflammasome formation, being recruited during the activation of several cytosolic sensors from the NLR (NLRP1, NLRP3, NLRP6, NLRP7, NLRC4, NLRC5, NAIP2, NAIP5 and NAIP6) family, the haematopoietic interferon (HIN) and absent in melanoma 2 (AIM2) [12]. NLRs are composed of a conserved nucleotide‐binding and oligomerization domain (NACHT), a c‐terminal region composed of leucine‐rich repeats (LRRs), involved in ligand binding or activator sensing, and a pyrin effector domain, required for protein–protein interactions. The multiprotein inflammasome oligomeric complexes are formed through homodimeric interactions between NLR's N‐terminal pyrin and the ASC's N‐terminal pyrin and the ASC's C‐terminal CARD with the N‐terminal CARD of pro‐caspase‐1. This facilitates ASC polymerization to form long helical filaments that are condensed into an intracellular macromolecular aggregate, known as ASC speck [12]. This complex induces the activation by proximity of pro‐caspase‐1 zymogen into active caspase that cleaves the inactive pro‐IL‐1β and pro‐IL‐18 forms into bioactive cytokines that activate downstream inflammatory pathways [13], which is reviewed in detail by several groups [14, 15, 16, 17].
MECHANISM OF ASC SPECK RELEASE
Inflammasome activation leads to cell death by pyroptosis depending on caspase‐1 cleavage of pore‐forming gasdermin D (GSDMD) in the plasmatic membrane. Changes in membrane permeability allow the release of monomeric ASC, and during the cell rupture, all internal cell content is released to the surrounding environment, including the ASC speck [18, 19]. Although this mechanism remains poorly explored, it is known that gasdermins can also be activated directly in target cells by perforin‐induced granzymes released from antigen‐specific TCD8 or NK cells. While perforin induces pore formation leading to calcium influx and potassium efflux, which activate NLRP3 inflammasome, granzymes are able to directly activate gasdermin, promoting pyroptosis and ASC speck release [20, 21]. eASC specks can act at the released site or move into the circulation and act as a danger signal [11]. The proinflammatory nature of pyroptosis could ensure a feedback amplification loop between the innate and the adaptive response.
All stages of formation and release of ASC specks can be investigated and/or visualized by classical techniques of Western blot and confocal and electron microscopy and more recently by high‐throughput methods of image flow cytometry [22].
ASC SPECK FUNCTIONS
In basal conditions, ASC is a monomer diffused in the cytosol; activated NLRP3 triggers the assembly of ASC molecules, which in turn recruit and activate caspases forming an extremely large and efficient signalling complex composed of thousands of those proteins. ASC shows a seeding behaviour or the propensity to polymerize in a prion‐like manner [11]. The recent description of prion‐like polymerization signalling, not only in diseases but also in non‐pathogenic conditions, as an activation mechanism in innate immunity, has opened a new field of research, in which ASC specks play a central role [23]. The formation of functional platforms by prion‐like propagation generates faster and stronger responses to danger, which represents an essential attribute of innate defence mechanisms; however, this response needs to be tightly regulated [13]. The PYD and CARD ASC domains are separated by a long and flexible linker that seems to play a role in autoinhibition of ASC to form spontaneous polymers in basal conditions [24]. Because of the unstructured linker, the two domains rearrange upon polymerization in multiple disordered and unstable conformations, blocking binding interfaces in PYD domains. In addition, PYCARD gene can synthetize different isoforms of ASC with different aggregation abilities due to splicing variants. Secreted monomeric forms of ASC, differently from specks, are thought to have a physiological role in inhibiting caspase activation [25].
On the other hand, upon inflammasome activation, ASC specks are extremely stable, are resistant to proteolytic degradation and are liberated by dying cells to exert important functions in innate and adaptive immunity and in the progression of several pathologies. Two independent groups of scientists in 2014 described that NLRP3 inflammasome has the ability to amplify inflammation through extracellular oligomeric complexes containing NLRP3 and ASC [10, 11]. These specks remain active, promote the extracellular activation of caspase‐1, can be engulfed by surrounding cells and act as danger signals activating and triggering an inflammatory cascade in these cells [10, 11]. Thus, eASC specks can directly activate caspase‐1 in the extracellular milieu, as well as inside the cell after being internalized by phagocytes. This process worked in vitro and in vivo. In this regard, ASC speck injection in the peritoneal cavity or in the skin of mice induced the release of IL‐1β and the local migration of leucocytes [11]. These experiments are strong indications that eASC specks may play a role in diseases where inflammasome is activated.
PHYSIOLOGICAL ROLES OF eASC
ASC specks in adaptive immune response
Although ASC represents a powerful amplification mechanism in innate immunity, the interaction between eASC speck and adaptive immunity remains poorly explored [11]. ASC speck formation is found not only in macrophages, neutrophils, natural killers, dendritic cells and T and B lymphocytes but also in non‐immune cells that play important roles in immune surveillance such as fibroblasts, and epithelial and endothelial cells [2]. A possible link between innate and adaptive immune systems can be found in the role of ASC in antigen presentation. Cytosolic proteins tend to aggregate on the ASC speck, apparently based on non‐specific hydrophobic interactions [26]. During intracellular infection, antigenic proteins could co‐aggregate with ASC specks, and the release of this complex in the extracellular space would favour antigen presentation [26]. Similar to particulate antigen delivery vehicles, which increase the duration of antigen presentation, phagocytized ASC specks are degraded slowly in phagolysosomes. Furthermore, in in vivo study using ASC‐citrine reporter mouse model, ASC specks were observed close to the ERTR7+ microtubular conduit system generated by fibroblastic reticular cells (the lymph node conduit system for draining lymph fluid) [27]. These cells form a network supporting tightly packed lymphocytes and antigen‐presenting cells in the subcapsular lymphoid compartment [28]. In vivo inflammasome activation via NLRC4 by Pseudomonas aeruginosa caused the production and accumulation of eASC specks in subcapsular macrophage area of lymph nodes as well [11]. The strategic position of ASC specks nearby subcapsular macrophages in lymph–tissue interface in the lymph node should provide conditions for enhancement of innate and adaptive immune cell activation.
As a whole, the ability of ASC specks to aggregate cytosolic proteins, to be released into the extracellular space, to localize in subcapsular niche of lymph nodes and to have controlled degradation when phagocytized gives specks the potential to contribute to antigen presentation during an infection or even in vaccination. Moreover, alum adjuvants used in human vaccines have their activity linked to the activation of ASC specks producing NLRP3 inflammasome [29, 30, 31].
The release of ASC and intracellular contents induced by inflammasome activation can also shape the adaptive immune response by activating extracellular pro‐caspase‐1. Active caspase‐1, in turn, will activate IL‐1β and IL‐18 cytokines, which promote the local recruitment of leucocytes. IL‐1β and IL‐18 play a known role in the polarization of Th17 and Th1 adaptive immune responses, respectively [32].
Thus, ASC specks present important roles in adaptive immunity independently of inflammasome activation, related to antigen uptake and presentation and polarization of immune response [31, 33].
ASC specks in labour
Inflammatory reactions are required for implantation, pregnancy maintenance and parturition, and accordingly, inflammasome components are expressed by gestational tissues in both maternal and fetal compartments. Studies have demonstrated that inflammasomes in the amniotic cavity and surrounding tissues participate in the inflammatory mechanisms of spontaneous labour at term [34, 35]. Extracellular ASC was found in amniotic fluid of women who delivered at term, and the mean concentration was higher in women who underwent spontaneous labour. The authors discuss that extracellular ASC specks in amniotic cavity act as a danger signal to amplify the inflammatory reaction to produce bioactive IL‐1β during the physiological process of spontaneous labour. This cytokine exerts important biological functions on chorioamniotic membranes, decidual and myometrial cells such as the stimulation of prostaglandin biosynthesis, cyclooxygenase 2 expression, induction of pro‐inflammatory cytokines and upregulation of matrix metalloproteinases by cervical smooth muscle cells. All these features could contribute to normal delivery, thus suggesting that ASC specks might play a beneficial role in labour; however, more studies are needed to clarify this point [34, 35]. On the other hand, NLRP3 inflammasome activation is implicated in pathological pregnancy syndromes such as preterm labour and acute histological chorioamnionitis, a placental lesion derived from infectious or sterile intra‐amniotic inflammation. Amniotic fluid and chorioamniotic membrane concentrations of ASC and gasdermin D have been found to be increased in these patients, presumably exacerbating inflammation with harmful effects [34, 35].
eASC specks in diseases
eASC specks in inflammatory and autoimmune diseases
ASC specks can be found in the circulation in many chronic inflammatory diseases and have been considered as a relevant blood biomarker of inflammation [36]. eASC specks are in high amounts in the serum of patients diagnosed with cryopyrin‐associated periodic syndrome (CAPS) during disease flare‐ups compared with periods of remission or with healthy controls. CAPS is a rare and heterogeneous disease characterized by upregulation of IL‐1β that mediates clinical symptoms of systemic inflammation such as fever, skin rash and musculoskeletal involvement. Gain‐of‐function mutant forms of NLRP3 linked to CAPS caused spontaneous aggregation of inflammasomes and ASC speck release, which are engulfed by macrophages that are in turn activated, amplifying inflammation [10, 37].
Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), gout and psoriasis share common inflammatory reactions, such as articular damage and cardiovascular disease [38, 39, 40, 41, 42]. Tissue damage occurring in these autoimmune diseases is promoted by the infiltration of activated mononuclear and polymorphonuclear cells and release of high levels of inflammatory mediators such as tumour necrosis factor (TNF), IL‐1β, IL‐6, IL‐18, IL‐17 and chemokines, besides an increased expression of inflammasome components [38, 41, 43]. The implication of ASC specks in a possible feedforward loop with NETs in synovial membrane degradation and organ failure has been described [43, 44].
Although ASC specks have been detected in the bloodstream of patients with RA, SLE, gout and psoriasis and it has been suggested as a potential biomarker for these autoimmune diseases, the exact role of ASC specks and inflammasome in their physiopathology remains unclear. It is not known whether some proteins of the ASC speck complex could contribute to the development of autoimmunity [38], but is known that autoimmune patients can produce antibodies against ASC specks, which might act as opsonin for the uptake of ASC by phagocytes, boosting inflammasome activation [11].
Several in vivo models, which partially reproduce the human clinical condition, have been used to better understand the mechanism and progression of RA. The antigen‐induced arthritis (AIA) model in mice uses methylated BSA (mBSA), injected into the knee joints, and ASC specks were detected in the knee joints in the chronic phase. On the other hand, ASC knockout mice subjected to the same AIA model, presented impaired Th1 and Th17 responses and attenuated AIA [45]. In agreement with animal models, synovial membranes from RA and osteoarthritis patients presented an abundant expression of NLRP3 and ASC in tissue cells, such as macrophages, neutrophils and B cells [17, 46, 47]. eASC specks function in these diseases’ worth further investigation.
ASC specks in myelodysplastic syndrome
Aberrant inflammasome activation and inflammasome‐directed pyroptosis in haematopoietic stem cells underlie many of the hallmark features of myelodysplastic syndromes (MDS) [48, 49]. ASC specks of 1 to 3 µm in diameter are present in bone marrow specimens and in blood from patients with MDS. Significantly lower eASC levels are found in normal age‐matched controls and in samples from patients with other lymphoid or myeloid blood malignancies [48]. The amount of ASC specks correlated with plasma concentrations of the cation binding alarmins S100A8 and S100A9 that stimulate inflammasomes through upregulated TLR4 receptors in MDS haematopoietic stem cells [49]. These findings highlight the eASC speck pathobiological relevance and its usefulness as a specific biomarker of the index of medullary pyroptotic cell death [48]. Clonal haematopoiesis of indeterminate potential (CHIP) characterized by the silent clonal acquisition of somatic mutations observed in normal individuals and with rising frequency with advancing age predisposes to MDS development. Heterogeneous somatic genetic mutations found in CHIP overlap with mutations found in MDS, involving genes of varied functional classes of regulation such as methylation, signalling, transcription or splicing [50]. These mutations induce enhanced cation flux, caspase‐1 activation, IL‐1β synthesis and ROS production that apparently modulate NLRP3 inflammasome complex activation. CHIP could therefore represent a causal link to the aberrant stimulation of innate immunity in MDS [50]. A link between somatic mutations in epigenetic modulator genes with inflammatory pathogenesis has been also considered in atherosclerotic cardiovascular diseases and type 2 diabetes in individuals with age‐related CHIP. A likely role of eASC specks in these pathologies deserves to be investigated [51].
ASC specks in chronic airway diseases
There has been growing evidence that a common factor occurring in diverse inflammatory airway diseases is an excessive inflammasome activation followed by the accumulation of ASC specks. NLRP3 inflammasome is overactivated in nasal mucosa of patients suffering from allergic rhinitis (AR) or in OVA‐induced mouse models of AR compared with normal individuals. Nasal mucosa injury and progression of AR were partially due to ASC speck accumulation and epithelial pyroptosis [52].
Pertinent to lung diseases, the presence of increased active eASC specks would promote local reaction, activating infiltrating cells and propagating systemic inflammation through an activated inflammatory cascade. Patients with chronic obstructive pulmonary disease (COPD) and mice submitted to cigarette smoke inhalation (murine model of COPD) show enrichment of eASC specks in bronchoalveolar lavage (BAL) in significantly higher amounts than in controls [53]. Excessive inflammasome activation with release of active IL‐1β and ASC specks also contributes to the pathogenesis of low Th2 asthma, a Th1‐ and Th17‐mediated non‐eosinophilic subtype of airway inflammation [53]. In addition, inflammasome activation was revealed in the sera of cystic fibrosis (CF) patients by the elevated levels of pro‐inflammatory cytokines and ASC specks comparable to the levels found in the sera of patients diagnosed with systemic autoinflammatory disease [54].
Although the link between the presence of eASC specks in BAL and serum with lung inflammation has been well established, a recent report brings doubts about the presence of extracellular oligomeric forms of ASC in BAL [25]. In fact, the study shows that extracellular ASC exists mostly, if not totally, in the monomeric form and is in high concentrations in cell‐free lung lining fluid of patients with lung inflammation. Interestingly, high levels of monomeric extracellular ASC can be found in healthy lungs. These levels are higher than the levels found in normal plasma, suggesting a physiological role for the released monomeric ASC in lung fluid [25]. ASC could act as an inhibitor since ASC monomers do not provide caspase‐1 dimerization and activation. Nevertheless, in pathological conditions extracellular monomeric ASC increases dramatically, reflecting the severity of inflammatory lung diseases and correlating with loss of lung diffusion capacity. The discrepancies observed between this study and the seminal studies of Franklin 2014 and Baroja‐Mazo 2014, and several other reports that describe the multimeric forms of eASC specks are presumably ascribed to the different methodologies used for ASC speck analysis. Further discussion is necessary to clarify these differences.
ASC specks in amyloid diseases
Diseases that result from misfolded protein aggregates called amyloids are common in humans, and about 50 amyloidogenic peptides are related to hereditary or acquired diseases. Amyloids are formed by the conversion of soluble monomeric proteins into insoluble fibrils by a nucleation‐dependent polymerization process [55]. Infections or inflammatory stimuli are supposed to drive amyloidosis through products of cell activation such as ASC specks and NETs [56]. As mentioned above, ASC shows the propensity to polymerize in a prion‐like manner [11]. Studies have shown that the aggregation of ASC into prion‐like ASC specks has the potential to aggravate amyloid pathology by cross‐seeding with several amyloidogenic peptides in different tissues. On the other side, human amyloidogenic peptides, such as β‐amyloid, α‐synuclein, serum amyloid A (SAA) and human islet amyloid polypeptide (hIAPP), have the potential to trigger NLRP3 inflammasome activation via TLR2 and to cross‐seed heterologous proteins [55, 57].
Familial Mediterranean fever (FMF) is an autoinflammatory disease characterized by frequent fever outbreaks, serositis and in some cases a consequent amyloidosis that causes kidney damage. Mediterranean fever gene (MEFV) codes for pyrin, which interacts with ASC. More than 10 years ago, a study by Balci‐Peirnircioglu (2008) suggested a role for ASC specks in AA type amyloid deposition in kidneys [58]. ASC was expressed in renal glomeruli of FMF patients but not in healthy controls. At the time, the authors suggested that higher ASC expression might result in specks that persisted in the extracellular space where they have the potential to nucleate amyloid. In a more recent study, no increase in ASC oligomers was found in the sera of FMF patients in comparison with healthy controls; however, the local formation of amyloids was not investigated [10].
Innate immune mechanisms play a role in the progression and aggravation of neurodegenerative disorders. NLRP3 inflammasome is present in microglia and astrocytes, NLRC4 inflammasome in astrocytes and the AIM2 inflammasome in neurons [59, 60].
The aggregation of amyloid beta peptides (Aβ) is a key pathology of Alzheimer's disease (AD). NLRP3 inflammasome activation has been reported in the brains of patients with AD and in APP/PS1 mice (AD mouse model, transgenic mice that develop chronic deposition of amyloid‐β), and experimental exposure of microglia to Aβ oligomers caused the formation and release of ASC specks. These aggregates, in a prion‐like mode, cross‐seed Aβ peptides through the PYD domain, increasing and accelerating the formation of the pathological Aβ‐ASC composites that are extremely toxic to microglia, leading cells to pyroptotic cell death [61]. Furthermore, lipid carrier protein ApoE (major protein regulating lipid homeostasis and a disease risk gene for AD) exacerbates Aβ‐ASC speck seeding and may serve as an opsonin for the phagocytosis of aggregates by binding to triggering receptor expressed on myeloid cells 2 (TREM2), the APoE receptor expressed by microglia in the CNS. This results in recruitment of more activated microglia and release of ASC specks contributing to the spreading of brain inflammation and activation of surrounding immune response [62, 63]. In a mouse model of Alzheimer's disease, the intrahippocampal injection of ASC specks resulted in spreading of amyloid β pathology, whereas treatment with anti‐ASC antibodies blocked the increase in this pathology [61]. Moreover, ASC deficiency by heterozygous gene expression caused a decrease in amyloid load and long‐term spatial memory recovery in 5XFAD mice, a murine model of familial Alzheimer's disease [64]. In vivo and in vitro results thus point to the contribution of eASC in AD.
Parkinson disease (PD) is characterized by the death of dopaminergic neurons and the presence of Lewy bodies in the brain. These inclusions are rich in misfolded neurotoxic α‐synuclein with a characteristic β‐sheet structure that is capable of triggering robust activation of microglial NLRP3 inflammasome [65]. ASC specks were found to be increased in the substantia nigra of post‐mortem brains of patients with PD compared with age‐matched controls. ASC specks were found inside and also outside of microglial cells, similar to what occurs in patients with AD, probably contributing to chronic brain inflammation [66].
Inflammatory proteins, particularly ASC, have been considered biomarkers of stroke. Components of atherosclerotic plaques such as crystalline cholesterol and oxidized low‐density lipoprotein (oxLDL) activate NLRP3 inflammasomes establishing long‐term inflammation after stroke [67, 68]. Accordingly, ASC specks were detected in cerebrospinal fluid following brain injury. In experimental recurrent stroke in mice, ASC specks were found surrounding the infarcted area of the brain following ischaemia and the amount of ASC specks was positively correlated with the damage. Furthermore, ASC was able to induce microglial polarization from type 2 (M2) CD206‐positive to type 1 (M1) CD16 cells, acting as a bridge between innate and adaptive immune responses [68].
Serum levels of ASC are increased in patients with the autoimmune demyelinating disease multiple sclerosis (MS), in which there is disruption of communication between the central nervous system and the peripheral system, causing serious physical and mental problems [69]. In experimental autoimmune encephalomyelitis (mouse model that mimics MS), caspase‐1, IL‐18 and IL‐1β, and NLRP3 inflammasome products were implicated in different stages of EAE development [70]. Furthermore, it has been shown that anti‐ASC antibody treatment improves functional activities and ASC−/− mice are protected from disease progression [71].
The total set of evidence therefore suggests that eASC specks function as biomarkers and could participate in the pathogenesis of amyloid disorders and in diseases with neuroinflammatory components. Despite the well‐founded experimental and clinical findings, the actual role of eASC specks still needs to be clarified.
ASC specks in cancer
The role of inflammasome activation in the formation, progression and invasion of different types of tumours remains enigmatic. Conflicting results arise from studies in different tumour types and organs. Few studies report the presence of eASC specks in tumours, and ASC specks have been found in pancreatic adenocarcinoma surgical samples. Indeed, Brunetto et al [72], reported that pancreatic cancer cell lines release ASC specks. Inside the tumour, eASC specks acting as alarmins could be phagocytized by tumour‐associated macrophages (TAM) activating them to liberate IL‐1β and IL‐1α. These cytokines in turn activate cancer‐associated fibroblasts (CAFs) to produce thymic stromal lymphopoietin (TSLP). This cytokine is required for Th2 immunity development, through the conditioning of dendritic cells endowed with Th2‐polarizing capability, which express TSLP receptors. Th2‐type response is correlated with reduced patient's survival. Thus, it is possible that ASC specks could indirectly favour Th2 type of immune response; their relevance in the disease is suggested by the inverse correlation observed between tumoral ASC expression and survival rate in pancreatic cancer patients [72, 73].
ASC specks in viral infections
Inflammasome activation to fighting viral pathogens in macrophages and other cells starts by sensing viral DNA or RNA by retinoic acid‐inducible gene‐I‐like (RIG‐I) and TOLL‐like receptors (TLR)3, TLR7, TLR8 and TLR9 that activate the NF‐κB pathway. Alarmins such as cytokines, chemokines, LDH, ATP, ROS and viral particles are released through pyroptosis initiating an activation cascade in surrounding cells [74]. During HIV‐1 retroviral infection, inflammasome is activated not only in abortively infected CD4+ T cells in lymphatic tissues but also in peripheral blood CD14+ CD16+ monocytes that undergo pyroptosis, releasing ASC specks in the bloodstream. The amount of ASC speck‐positive cells correlated with lower CD4+ T‐cell numbers and higher viral load, suggesting that ASC specks contribute to the pathogenicity of HIV‐1. The observation indicates a possible mechanism to explain the increased inflammation and aberrant immune response that occurs in HIV‐1 infection [75].
Chronic inflammation induced by hepatitis B virus (HBV) is implicated in an increased risk of liver diseases such as hepatitis, cirrhosis and hepatocellular carcinoma [76, 77]. A recent study showed that HBV X protein activates NLRP3 inflammasome in liver cells under stress condition in vitro, inducing pyroptosis and release of ASC [78]. Furthermore, the serum eASC content levels of HBV‐positive patients were higher than in the HBV‐negative patients. ASC specks released from infected liver cells could promote the spread of liver inflammation during HBV infection predisposing to cancer initiation and development [78].
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the aetiological agent of coronavirus disease 2019 (COVID‐19), initiated in December 2019 in China and considered a pandemic by the World Health Organization (WHO) in March 2020. SARS‐CoV‐2 can infect epithelial cells of the lung and of other organs, as well as neurons and immune cells [79, 80, 81]. Decrease in T‐lymphocyte counts has been reported in severely affected patients. The active replication of the virus causes pyroptosis of host cells with the release of virus particles, DAMPs such as ATP and nucleic acids. The so‐called cytokine storm or the increase of systemic pro inflammatory cytokines, in particular interleukin‐1β and interleukin‐18 among others, and of lactate dehydrogenase (LDH) levels, a cytosolic enzyme used to monitor pyroptotic cell death, indicates that SARS‐CoV‐2 infection induces pathogenic NLRP3 inflammasome activation [82, 83, 84]. Accordingly, Sars‐CoV‐2 has been shown to induce NLRP3 inflammasome activity in several host cells, similar to other coronavirus causing ARDS such as SARS‐CoV, although there are differences in the sequences of proteins that activate inflammasome such as envelope (E), ORF3a and ORF8b between these virus [82, 83, 84]. NLRP3 Inflammasome activation has been implicated in the aggravation of neurodegenerative processes that occur in Sars‐CoV‐2 infection, similar to what occurs in AD, PD or stroke described before in which ASC specks could play a significant role. Furthermore, patients with severe compared to patients with mild disease show significantly higher frequency of circulating CD14+ CD16+ inflammatory monocytes, which might release ASC specks in the bloodstream [85] In fact, eASC specks were detected in peripheral blood from patients with COVID‐19 and in lung tissues obtained from autopsies of deceased patients. The number of ASC inflammasome specks was significantly higher in the lungs of patients with fatal COVID‐19 as compared to the lungs of control subjects [82, 86].
Thus, the participation of detrimental inflammasome overactivation in the pathogenesis of COVID‐19 has been widely discussed and NLRP3 inflammasome may be the culprit in certain manifestations of the disease. These facts raise the question of whether compounds that modulate inflammasome activity and specifically ASC speck formation could find clinical application for COVID‐19. Several patents protecting this mechanism as a target therapy have been applied to treat ARDS, SARS and MERS or as a strategy to improve antigen processing and presentation in the development of vaccines [87, 88].
CONCLUDING REMARKS
We have based our review on two special features of eASC specks, which could underlie most of their functions: the prion‐like propensity of ASC to polymerize and the fundamental discovery that eASC specks are active, spread inflammation in the adjacent environment and can reach the circulation. These abilities give these aggregates diverse functions from playing a pivotal role in defence mechanisms and homeostasis, acting as a bridge between innate and adaptive immunity, to their implication in a wide spectrum of pathologies such as certain types of cancer, cardiovascular and neurodegenerative disorders, and autoimmune and autoinflammatory diseases (as shown Table 1).
TABLE 1.
ASC speck‐related diseases
| Disease | Disturbance | Predominant site | ASC role in the disease | Reference | |
|---|---|---|---|---|---|
| Amyloidosis | Alzheimer's disease (AD) | Dementia | Microglia | Cross‐seed Aβ peptides | [62, 63, 64] |
| Parkinson's disease (PD) | Neuromuscular degeneration | Substantia nigra | Chronic brain inflammation | [66] | |
| Familial Mediterranean fever (FMF) | Kidney damage | Renal glomeruli | Deposition of AA type amyloid | [58] | |
| Autoimmune disease | Gout | Joint inflammation (deposition of uric acid crystals) | Joints and bloodstream | Unknown | [11, 40] |
| Rheumatoid arthritis (RA) | Joint inflammation | Bloodstream | Unknown | [45, 46] | |
| Systemic lupus erythematosus | Affect almost any organ or system | Bloodstream | Unknown | [43] | |
| Psoriasis | Epithelial–chronic inflammatory disease | Bloodstream | Unknown | [38] | |
| Multiple sclerosis | Demyelination | Serum | Biomarker | [68] | |
| Autoinflammatory diseases | Schnitzler syndrome | Systemic autoinflammatory response | Serum | Unknown | [36] |
| Cryopyrin‐associated periodic syndrome (CAPS) | Multisystemic autoinflammatory disease | Bloodstream | Biomarker | [37] | |
| Myelodysplastic syndromes (MDS) | Bone marrow blood cell maturation | Bone marrow/bloodstream | Biomarker | [48] | |
| Airway diseases | Allergic rhinitis (AR) | Inflammation of nasal mucosa | Nasal mucosa | Nasal epithelium pyroptosis | [52] |
| Chronic obstructive pulmonary disease (COPD) | Obstruction of respiratory airway influx | Bronchoalveolar lavage (BAL) | Airway inflammation | [53] | |
| Cystic fibrosis (CF) | Fluid accumulation into the lung and digestive tract | Epithelia and serum | Unknown | [54] | |
| Cancer | Pancreatic cancer | Organ failure | Tumour microenvironment | Favours Th2 immune response | [72] |
| Hepatocellular carcinoma | Hepatocytosis | Serum from HBV patients | Amplify inflammation in the liver | [78] | |
| Viral disease | HIV‐1 | Immunodeficiency | Bloodstream | Unknown | [75] |
| COVID‐19 | Respiratory syndrome/systemic disorders/cytokine storm | Bloodstream/lung tissues | Unknown | [82, 83, 85, 86] | |
| Others | Stroke | Obstruction of blood vessels in the brain | Bloodstream | Biomarker | [67, 68] |
These activities, however, seem to be strictly associated with the wide range of proteins that aggregate to form the complex, which play a role in inflammation, in a given pathology or in connection to the innate immunity. This association happens regardless of eASC speck itself, which would serve mainly as an aggregating and loader nucleus.
Under normal conditions, eASC specks or monomeric ASC is found in body fluids, perhaps as sentinels for protection. Under pathological conditions, concentrations increase significantly and eASC specks have been considered reliable biomarkers to aid in diagnosis and prognosis. ASC specks have been suggested as therapeutic targets; however, essential functions in protection should be maintained.
ASC specks are present in circulation and target organs in patients with severe Sars‐CoV‐2 infection. Although it is extremely difficult to demonstrate in vivo the role of eASC specks in pathogenesis of this and other diseases, ASC specks are able to propagate inflammation and might contribute to the hyperactivation of the inflammatory response to the virus, which is a hallmark in poor prognosis patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
All the authors equally contributed to this review.
ACKNOWLEDGEMENTS
We would like to thank Doctor Riffo‐Vasquez, Yanira for analysis of the manuscript and Rodrigo G. Ribeiro for his help with the illustrations.
de Souza JG, Starobinas N, Ibañez OM. Unknown/enigmatic functions of extracellular ASC. Immunology. 2021;163:377–388. 10.1111/imm.13375
Funding information
This study was supported by Grant 2017/06736‐0, 2019/20713‐8, São Paulo Research Foundation (FAPESP) and Grant 2015/50040‐4, São Paulo Research Foundation and GlaxoSmithKline.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Enigmatic inflammasomes‐Sequel (Part 2). Immunology 2021, 163: 345‐347.
Metabolic regulators of enigmatic inflammasomes in autoimmune diseases and crosstalk with innate immune receptors. Immunology 2021, 163: 348‐362.
NLRP7: From inflammasome regulation to human disease. Immunology 2021, 163: 363‐376.
REFERENCES
- 1. Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, et al. ASC, a novel 22‐kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL‐60 cells. J Biol Chem. 1999;274:33835–8. [DOI] [PubMed] [Google Scholar]
- 2. Masumoto J, Taniguchi S, Nakayama J, Shiohara M, Hidaka E, Katsuyama T, et al. Expression of apoptosis‐associated speck‐like protein containing a caspase recruitment domain, a pyrin N‐terminal homology domain‐containing protein, in normal human tissues. J Histochem Cytochem. 2001;49:1269–75. [DOI] [PubMed] [Google Scholar]
- 3. Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. The PAAD/PYRIN‐only protein POP1/ASC2 is a modulator of ASC‐mediated nuclear‐factor‐kappa B and pro‐caspase‐1 regulation. Biochem J. 2003;373(Pt 1):101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis‐associated speck‐like protein containing a caspase recruitment domain is a regulator of procaspase‐1 activation. J Immunol. 2003;171:6154–63. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Hasegawa M, Imamura R, Kinoshita T, Kondo C, Konaka K, et al. PYNOD, a novel Apaf‐1/CED4‐like protein is an inhibitor of ASC and caspase‐1. Int Immunol. 2004;16:777–86. [DOI] [PubMed] [Google Scholar]
- 6. Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL‐1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–25. [DOI] [PubMed] [Google Scholar]
- 7. Chen YQ, Wang SN, Shi YJ, Chen J, Ding SQ, Tang J, et al. CRID3, a blocker of apoptosis associated speck like protein containing a card, ameliorates murine spinal cord injury by improving local immune microenvironment. J Neuroinflammation. 2020;17:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8:8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu S, Li X, Liu Y, Xia Y, Chang R, Zhang C. Inflammasome inhibitors: promising therapeutic approaches against cancer. J Hematol Oncol. 2019;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baroja‐Mazo A, Martin‐Sanchez F, Gomez AI, Martinez CM, Amores‐Iniesta J, Compan V, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–48. [DOI] [PubMed] [Google Scholar]
- 11. Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agrawal I, Jha S. Comprehensive review of ASC structure and function in immune homeostasis and disease. Mol Biol Rep. 2020;47:3077–96. [DOI] [PubMed] [Google Scholar]
- 15. Hoss F, Rodriguez‐Alcazar JF, Latz E. Assembly and regulation of ASC specks. Cell Mol Life Sci. 2017;74:1211–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christgen S, Place DE, Kanneganti TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franklin BS, Latz E, Schmidt FI. The intra‐ and extracellular functions of ASC specks. Immunol Rev. 2018;281:74–87. [DOI] [PubMed] [Google Scholar]
- 18. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin‐1beta secretion. Cell Res. 2015;25:1285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tapia VS, Daniels MJD, Palazon‐Riquelme P, Dewhurst M, Luheshi NM, Rivers‐Auty J, et al. The three cytokines IL‐1beta, IL‐18, and IL‐1alpha share related but distinct secretory routes. J Biol Chem. 2019;294:8325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao Y, Chen S, Cao M, Fan X, Yang T, Huang Y, et al. Antigen‐specific CD8(+) T cell feedback activates NLRP3 inflammasome in antigen‐presenting cells through perforin. Nat Commun. 2017;8:15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. [DOI] [PubMed] [Google Scholar]
- 22. Nagar A, DeMarco RA, Harton JA. Inflammasome and Caspase‐1 activity characterization and evaluation: an imaging flow cytometer‐based detection and assessment of inflammasome specks and Caspase‐1 Activation. J Immunol. 2019;202:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Carroll A, Coyle J, Gambin Y. Prions and Prion‐like assemblies in neurodegeneration and immunity: the emergence of universal mechanisms across health and disease. Semin Cell Dev Biol. 2020;99:115–30. [DOI] [PubMed] [Google Scholar]
- 24. Eldridge MJ, Shenoy AR. Antimicrobial inflammasomes: unified signalling against diverse bacterial pathogens. Curr Opin Microbiol. 2015;23:32–41. [DOI] [PubMed] [Google Scholar]
- 25. Gavrilin MA, McAndrew CC, Prather ER, Tsai M, Spitzer CR, Song MA, et al. Inflammasome adaptor ASC is highly elevated in lung over plasma and relates to inflammation and lung diffusion in the absence of speck formation. Front Immunol. 2020;11:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahillioglu AC, Ozoren N. Artificial loading of ASC specks with cytosolic antigens. PLoS One. 2015;10:e0134912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzeng TC, Schattgen S, Monks B, Wang D, Cerny A, Latz E, et al. A fluorescent reporter mouse for inflammasome assembly demonstrates an important role for cell‐bound and free ASC specks during in vivo infection. Cell Rep. 2016;16:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perez‐Shibayama C, Gil‐Cruz C, Ludewig B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol Rev. 2019;289:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ivanov K, Garanina E, Rizvanov A, Khaiboullina S. Inflammasomes as targets for adjuvants. Pathogens. 2020;9(4):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deng J, Yu XQ, Wang PH. Inflammasome activation and Th17 responses. Mol Immunol. 2019;107:142–64. [DOI] [PubMed] [Google Scholar]
- 33. Ippagunta SK, Malireddi RK, Shaw PJ, Neale GA, Vande Walle L, Green DR, et al. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2‐mediated Rac activation and actin polymerization. Nat Immunol. 2011;12:1010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gomez‐Lopez N, Motomura K, Miller D, Garcia‐Flores V, Galaz J, Romero R. Inflammasomes: their role in normal and complicated pregnancies. J Immunol. 2019;203:2757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panaitescu B, Romero R, Gomez‐Lopez N, Xu Y, Leng Y, Maymon E, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med. 2019;32:1978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rowczenio DM, Pathak S, Arostegui JI, Mensa‐Vilaro A, Omoyinmi E, Brogan P, et al. Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome. Blood. 2018;131:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu JR, Leslie KS. Cryopyrin‐associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep. 2011;11:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forouzandeh M, Besen J, Keane RW, de Rivero Vaccari JP . The inflammasome signaling proteins ASC and IL‐18 as Biomarkers of psoriasis. Front Pharmacol. 2020;11:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cui D, Liu S, Tang M, Lu Y, Zhao M, Mao R, et al. Phloretin ameliorates hyperuricemia‐induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine. 2020;66:153111. [DOI] [PubMed] [Google Scholar]
- 40. Pope RM, Tschopp J. The role of interleukin‐1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8. [DOI] [PubMed] [Google Scholar]
- 41. Ruscica M, Corsini A, Ferri N, Banach M, Sirtori CR. Clinical approach to the inflammatory etiology of cardiovascular diseases. Pharmacol Res. 2020;159:104916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sankowski AJ, Lebkowska UM, Cwikla J, Walecka I, Walecki J. Psoriatic arthritis. Pol J Radiol. 2013;78:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahlenberg JM, Carmona‐Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap‐associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chadha S, Behl T, Bungau S, Kumar A, Arora R, Gupta A, et al. Mechanistic insights into the role of pyroptosis in rheumatoid arthritis. Curr Res Transl Med. 2020;68(4):151–8. [DOI] [PubMed] [Google Scholar]
- 45. Kolly L, Karababa M, Joosten LA, Narayan S, Salvi R, Petrilli V, et al. Inflammatory role of ASC in antigen‐induced arthritis is independent of caspase‐1, NALP‐3, and IPAF. J Immunol. 2009;183:4003–12. [DOI] [PubMed] [Google Scholar]
- 46. Kolly L, Busso N, Palmer G, Talabot‐Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, et al. Self double‐stranded (ds)DNA induces IL‐1beta production from human monocytes by activating NLRP3 inflammasome in the presence of anti‐dsDNA antibodies. J Immunol. 2013;190:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basiorka AA, McGraw KL, Abbas‐Aghababazadeh F, McLemore AF, Vincelette ND, Ward GA, et al. Assessment of ASC specks as a putative biomarker of pyroptosis in myelodysplastic syndromes: an observational cohort study. Lancet Haematol. 2018;5:e393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang Z, Liang C, Wang T, Zou Q, Zhou M, Cheng Y, et al. NLRP3 inflammasome activation promotes the development of allergic rhinitis via epithelium pyroptosis. Biochem Biophys Res Commun. 2020;522:61–7. [DOI] [PubMed] [Google Scholar]
- 53. Kim V, Criner GJ. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med. 2015;21:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scambler T, Jarosz‐Griffiths HH, Lara‐Reyna S, Pathak S, Wong C, Holbrook J, et al. ENaC‐mediated sodium influx exacerbates NLRP3‐dependent inflammation in cystic fibrosis. Elife. 2019;8:49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68. [DOI] [PubMed] [Google Scholar]
- 56. Westwell‐Roper C, Verchere CB. Modulation of innate immunity by amyloidogenic peptides. Trends Immunol. 2019;40:762–80. [DOI] [PubMed] [Google Scholar]
- 57. Westwell‐Roper C, Denroche HC, Ehses JA, Verchere CB. Differential activation of innate immune pathways by distinct islet amyloid polypeptide (IAPP) aggregates. J Biol Chem. 2016;291:8908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balci‐Peynircioglu B, Waite AL, Schaner P, Taskiran ZE, Richards N, Orhan D, et al. Expression of ASC in renal tissues of familial mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Exp Biol Med (Maywood). 2008;233:1324–33. [DOI] [PubMed] [Google Scholar]
- 59. Adamczak SE, de Rivero Vaccari JP , Dale G, Brand FJ 3rd, Nonner D, Bullock MR, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214:1351–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, et al. Microglia‐derived ASC specks cross‐seed amyloid‐beta in Alzheimer's disease. Nature. 2017;552:355–61. [DOI] [PubMed] [Google Scholar]
- 62. Marttinen M, Takalo M, Natunen T, Wittrahm R, Gabbouj S, Kemppainen S, et al. Molecular mechanisms of synaptotoxicity and neuroinflammation in Alzheimer's Disease. Front Neurosci. 2018;12:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018;18:759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Couturier J, Stancu IC, Schakman O, Pierrot N, Huaux F, Kienlen‐Campard P, et al. Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J Neuroinflammation. 2016;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. Triggering of inflammasome by aggregated alpha‐synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8:e55375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson FL, von Herrmann KM , Andrew AS, Kuras YI, Young AL, Scherzer CR, et al. Plasma‐borne indicators of inflammasome activity in Parkinson's disease patients. NPJ Parkinsons Dis. 2021;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. He XF, Zeng YX, Li G, Feng YK, Wu C, Liang FY, et al. Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3‐dependent manner. J Cereb Blood Flow Metab. 2020;40:1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kerr N, Garcia‐Contreras M, Abbassi S, Mejias NH, Desousa BR, Ricordi C, et al. Inflammasome proteins in serum and serum‐derived extracellular vesicles as Biomarkers of Stroke. Front Mol Neurosci. 2018;11:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Keane RW, Dietrich WD, de Rivero Vaccari JP . Inflammasome proteins as biomarkers of multiple sclerosis. Front Neurol. 2018;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fusco M, Skaper SD, Coaccioli S, Varrassi G, Paladini A. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017;17:522–32. [DOI] [PubMed] [Google Scholar]
- 71. Desu HL, Plastini M, Illiano P, Bramlett HM, Dietrich WD, de Rivero Vaccari JP , et al. IC100: a novel anti‐ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J Neuroinflammation. 2020;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brunetto E, De Monte L, Balzano G, Camisa B, Laino V, Riba M, et al. The IL‐1/IL‐1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J Immunother Cancer. 2019;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Protti MP, De Monte L. Dual role of inflammasome adaptor ASC in cancer. Front Cell Dev Biol. 2020;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayward JA, Mathur A, Ngo C, Man SM. Cytosolic recognition of microbes and pathogens: inflammasomes in action. Microbiol Mol Biol Rev. 2018;82:e00015‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahmad F, Mishra N, Ahrenstorf G, Franklin BS, Latz E, Schmidt RE, et al. Evidence of inflammasome activation and formation of monocyte‐derived ASC specks in HIV‐1 positive patients. AIDS. 2018;32:299–307. [DOI] [PubMed] [Google Scholar]
- 76. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 77. Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV‐related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. [DOI] [PubMed] [Google Scholar]
- 78. Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69:683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ehre C. SARS‐CoV‐2 infection of airway cells. N Engl J Med. 2020;383:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu K, He J, Wu Y, Xie B, Liu X, Wei B, et al. Dysregulated adaptive immune response contributes to severe COVID‐19. Cell Res. 2020;30:814–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, et al. SARS‐CoV‐2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30(10):928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rodrigues TS, de Sa KSG , Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS‐CoV‐2 infection and are associated with COVID‐19 severity in patients. J Exp Med. 2021;218(3):e20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rodrigues TS, Sa KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasome activation in COVID‐19 patients. medRxiv 2020:2020.2008.2005.20168872. [Google Scholar]
- 84. Yap JKY, Moriyama M, Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID‐19. J Immunol. 2020;205:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in COVID‐19. Clin Infect Dis. 2020;71:2272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannata A, et al. Inflammasome formation in the lungs of patients with fatal COVID‐19. Inflamm Res. 2021;70:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. March KB, Natalia . Patent analysis of use of ASC and ASC‐CM to treat ARDS, SARS, and MERS. vol. US10143709. Westfield, IN, US; 2018.
- 88. Sahillioglu ACO. Method for antigen delivery. vol. 9725491, C07K 14/47 (20060101); C07K 14/50 (20060101) edn. Istanbul; 2015.


