Abstract
Objective
To compare the clinical effects of cervical decompression first, lumbar decompression first, or simultaneous decompression of both lesions in the treatment of tandem spinal stenosis (TSS).
Methods
This is a retrospective analysis. From January 2013 to December 2018, 51 TSS patients underwent our surgery and postoperative investigation. Among the 51 subjects, 27 females and 24 males, aged 49–77 years with an average age of 66.3 ± 6.8, were selected. According to the different operation sequences, all patients were divided into three groups. In simultaneous operation group, five patients underwent cervical and lumbar vertebrae surgery at the same time. In first cervical surgery group, 28 patients underwent cervical vertebra surgery first, followed by lumbar spine surgery after a period of recovery. And in first lumbar surgery group, 18 patients underwent lumbar vertebrae surgery first. The choice for neck surgery is posterior cervical single‐door vertebroplasty, the surgery of lumber is plate excision and decompression needle‐rod system internal fixation. The outcome measures are visual analogue scale (VAS), Japanese Orthopaedic Association cervical (JOA‐C) and lumbar (JOA‐L) scores, which were assessed at 3 months and 1 year after the operation by telephone interview. In addition, operative time, estimated blood loss, and hospital stay were also recorded.
Results
All the patients in the study had surgery performed successfully by the same group of orthopaedic surgeons. The preoperative VAS scores of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 8.00 ± 1.00, 8.36 ± 0.68, and 8.17 ± 0.71 (P > 0.05). The preoperative JOA‐C scores were 7.00 ± 2.35, 6.54 ± 1.53, and 7.83 ± 1.04 (P < 0.05). And the preoperative JOA‐L scores were 7.20 ± 2.17, 4.64 ± 2.36, and 5.78 ± 1.22 respectively (P < 0.05). During the final 1‐year follow‐up, the JOA‐C improvement rates of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 85.68% ± 5.44%, 84.27% ± 5.02%, and 83.34% ± 10.25%, respectively (P > 0.05), and the JOA‐L improvement rates were 80.04% ± 3.35%, 81.65% ± 3.74%, and 80.21% ± 4.76% (P > 0.05). The difference among them was not statistically significant. In addition, operation time (OP), blood loss (BL), and hospital stay (HS) in the simultaneous operation group were 245.00 ± 5.00 min, 480.00 ± 27.39 mL, and 16.60 ± 0.55 days, respectively. While those parameters in the first cervical surgery group were 342.50 ± 18.18 min, 528.21 ± 43.97 mL, and 22.75 ± 2.15 days, and in the first lumbar surgery group they were 346.11 ± 24.77 min, 519.44 ± 43.99 mL, and 22.89 ± 1.64 days. The average blood loss in simultaneous operation group was less (P > 0.05); meanwhile, the operation time and hospital stay time were significantly shorter in the simultaneous operation group than in the first cervical surgery group and first lumbar surgery group (P < 0.05). Only one case of fat liquefaction occurred in first cervical surgery group, which healed spontaneously after a regular change of dressing for 1 month.
Conclusions
Under the condition of ensuring the surgical effect, the choice of staged surgery or concurrent surgery according to the patients' own symptoms of cervical and lumbar symptoms could both obtain satisfactory results, and the damage of simultaneous surgery was less than that of staged surgery.
Keywords: Surgical option, Tandem spinal stenosis, TSS
The term “tandem spinal stenosis” (TSS) refers to spinal canal diameter narrowing in at least two distinct regions of the spine, often in the cervical and lumbar spine. The purpose of surgical treatment is to relieve the pain of patients and maintain stability. Under the condition of ensuring the quality of surgery, both the one‐stage operation and the staged operation according to the patients' own conditions can finally achieve good results.
Introduction
Spinal stenosis is a narrowing of the foramina and neural canal, which frequently affects more than one segment of the spine and causes the compression of the neurologic structures with corresponding symptoms 1 , 2 . The term “tandem spinal stenosis” (TSS) refers to spinal canal diameter narrowing in at least two distinct regions of the spine 3 .
TSS is considered a degenerative spinal condition, which is related to an aging‐related disease and may increase as the population ages 4 . Degenerative spondylosis and stenosis are common diseases of the elderly, usually manifested as the degenerative changes related to aging and stress, leading to progressive encroachment on the spinal canal 5 . Although TSS can occur in any segments of the spine, it most commonly occurs in the more active cervical and lumbar spine 6 . Spinal stenosis is defined as the critical narrowing of the sagittal diameter of the spinal canal (cervical spinal stenosis <10 mm, lumbar spinal stenosis <11 mm) 7 . Its clinical symptoms are the direct result of a severe reduction in sagittal diameter, which is sufficient to produce symptoms in the central spinal canal or lateral nerve foramen and lateral fossa and patient performance depends on this progressive narrowing 6 . Narrowing of the spinal canal can cause spinal cord compression leading to myelopathy.
Stenosis involving the cervical spine has symptoms such as hand clumsiness, gait spasm, sensory impairment, loss of balance, muscular atrophy, and bowel or bladder dysfunction, while lumbar stenosis is associated with neurogenic claudication and radiculopathy 5 , 8 . All of the above symptoms may occur in TSS patients due to involvement of both cervical and lumbar spine.
The diagnosis of TSS may be proven difficult due to its varied presentation. Clinically, its most common feature is a triad of symptoms: lower extremity intermittent neurogenic claudication, gait disturbance, and combined upper and lower motor neuron syndrome. In 1964, Teng and Papatheodorou discussed 12 cases of cervical spondylosis and lumbar spondylosis, the first reported simultaneous occurrence of cervical and lumbar spinal stenosis 9 . In 1984, Epstein's team stated that in a cohort of hospitalized patients with spinal stenosis, 5% had symptoms of coexisting cervical and lumbar stenosis 10 . In 1987, Dagi et al. first used the term “tandem spinal stenosis(TSS)” to describe multiple spinal stenosis, and first reported a one‐staged combined surgical treatment of TSS 11 .The study revealed that the prevalence of cervical stenosis is approximately 5% to 20% 12 , and the rate of lumbar spinal stenosis is estimated at 8% to 11% 13 .As a proportion of patients with tandem stenosis have no clinical symptoms, there is no definitive epidemiological data on the prevalence of TSS, and available statistical data vary widely. It has been said that the prevalence of TSS is estimated to be between 5% and 25%, which is the most cited incidence reported 11 . In a cadaver study of the general population, Lee et al. found that the prevalence of tandem stenosis ranged from 0.9% to 5.4%. The study also got a statistically significant correlation between cervical and lumbar spinal stenosis: stenosis in one segment of the spine positively predicted stenosis in other parts of the spine 15.3% to 32.4% of the time 14 . In patients undergoing spinal stenosis surgery, the proportion of clinically diagnosed symptomatic tandem stenosis was 3.4% 7 . Other studies have reported moderate or more asymptomatic cervical compression in 24% of patients with lumbar spinal stenosis 15 .
The mechanism of TSS is hypothesized to be referral pain due to cervical spinal cord compression resulting in stimulation of the spinothalamic tract and/or disruption of descending raphe nuclei projections, which modulate the ascending nociceptive pathway. Upper motor neurons are situated from the cerebral cortex to the relevant segment of nerve roots, so compression of cervical spinal stenosis may result in a mixed result of upper and lower motor neurons 5 . In patients with existing cervical spinal stenosis, the presence of additional lumbar spinal stenosis leads to the presence of both central and peripheral motion disorders, making it difficult to identify the most responsible segments. It is suggested that the symptoms and signs of cervical spondylosis (e.g. gait disorders and difficulty standing) make a preoperative diagnosis of symptoms associated with lumbar spinal stenosis difficult.
The treatment of TSS is controversial. In the treatment of TSS, patients with more symptomatic cervical spinal stenosis may find that surgical treatment of the neck lesions first results in untreated symptoms of lumbar spinal stenosis over time and further progression of lower limb symptoms. Conversely, if lumbar spinal stenosis is treated first, symptoms associated with cervical spinal stenosis rapidly deteriorate, especially in patients with precursors of cervical spinal stenosis.
There are three operative strategies: cervical decompression first 16 , lumbar decompression first 7 , or one‐staged decompression of both lesions 17 . In the current published literature, there is no consensus as to who is better or worse in one‐stage of decompression compared with simultaneous multistage decompression.
Since it is difficult to ascertain the most symptomatic stenosis and the likelihood of postoperative progression, it is necessary to further study the optimal treatment of TSS. In this study, 51 patients with TSS were retrospectively analyzed to explore the choice of simultaneous or staged decompression surgery. In addition, one case of TSS was reported in detail.
Methods
With the approval of the institutional review committee of our hospital, the medical records of the patients diagnosed as tandem spinal stenosis according to the imaging manifestations and clinical symptoms from 1 January 2013 to 31 December 2018 were analyzed retrospectively. Fifty‐one patients with TSS, 27 females and 24 males, with an average age of 66.3 ± 6.8 years, were selected.
Inclusion Criteria
According to the PICOS principles, the inclusion criteria are as follows:
P (participants): (i) Patients with severe clinical symptoms (pain, numbness, limb weakness, limited lifting, radiation pain of extremity, intermittent claudication, poor precision, etc.), which have an obvious impact on the quality of daily life, and poor conservative treatment effect; (ii) Patients with cervical stenosis and lumbar stenosis at the same time (cervical spinal stenosis with a sagittal diameter of less than 10 mm and lumbar spinal stenosis with a sagittal diameter of less than 12 mm).
I (intervention): Patients with well‐defined TSS who did cervical and lumbar vertebrae surgery at the same time.
C (comparison): Well‐defined TSS patients with staged operation.
O (outcome): The data of visual analogue scale (VAS), Japanese Orthopaedic Association of cervical (JOA‐C) and lumbar (JOA‐L) scores are complete.
Exclusion Criteria
The exclusion criteria are as follows: (i) acute spinal cord injury caused by trauma; (ii) tuberculosis and tumor of spine; (iii) congenital deformity or curvature of spine; (iv) piriformis syndrome, peripheral vascular disease, and peripheral neuropathy; (v) without complete preoperative and postoperative follow‐up data.
Operation Selection
The choice for neck surgery is posterior cervical single‐door vertebroplasty. After satisfactory anesthesia, the patient was placed in the prone position, the skull was fixed on the head frame, routinely disinfected and covered with a disposable surgical drape. A 12 cm‐long incision was made in the center of the responsible segment spinous process from the middle of the neck. The skin, subcutaneous tissue, nuchal ligament, and supraspinous ligament were cut successively along the spinous process and lamina, and the erector spinae on both sides were successively stripped to the outer edge of zygapophysial joint. Under the protection of nerve stripper, the responsible segment of cervical vertebra was opened with an abrasion drill. The opening angle of one side is about 60°, and the other side is the door shaft. Screw fixation with plate is screwed in at the single open door of the responsible segment lamina. The decompression effect of nerve dissector was satisfactory (spine dura mater is expansion and has integrity), and it was washed repeatedly with normal saline. It was put in a drainage tube to connect with a negative pressure drainage ball, sutured layer by layer, and the incision was bandaged. External fixation of neck bracket is used, and patient is returned to the ward after waking from anesthesia.
The surgery of lumber spine involves plate excision and decompression needle‐rod system internal fixation. After satisfactory anesthesia, the patient was placed in the prone position, routinely disinfected and covered in a disposable surgical drape. According to the line drawn before operation, the skin was cut to the subcutaneous and lumbar fascia on the responsible segment spinous process in the middle of the lumbar dorsum. The posterior cranial fossa hook was placed and bleeding was stopped layer by layer. Separation of paravertebral muscles was achieved by electrocoagulation from caudal side to cephalic side along spinous process to the zygapophysial joints. Gauze was used to stop bleeding, the other side was seperated, and the lamina retractor was placed after the exposure was satisfactory. Using the highest point of iliac crest to locate L4‐5 intervertebral space, then the L2‐3, L3‐4, and L4‐5 facet joints were determined and the insertion point (intersection of midline of lumbar transverse process and outer edge of superior articular process) was established. Guided by the pedicle cone, we determined that the bone was all around and positioned Kirschner wire. Under fluoroscopy, Kirschner wires were all located in the pedicle, and then pedicle screws were screwed in: L3 abduction 20°, head tilt 5°(65 mm * 45 mm), L4 abduction 15° head tilt 0° (65 mm * 45 mm), L5 abduction 30° head tilt 20° (65 mm * 45 mm). If the length of screw position was satisfactory through fluoroscopy, the responsible segment lamina was removed, bilateral nerve roots were completely loosened, and the posterior edge of vertebral body was separated carefully. Bipolar electrocoagulation was used to stop bleeding. After exposing lesion segment intervertebral space, the upper and lower endplates of the intervertebral discs were removed and scraped off, and then the cage model (12 mm * 26 mm) was placed. Under fluoroscopy, make sure that the cage position and size are appropriate, and then place the pre‐modified needle bar to distract the intervertebral space. Cage was inserted into the space after bone grafting in vertebral space and cage. After the fluoroscopy showed that the position was satisfactory, compression fixation was performed, transverse connection was placed, and bone graft was placed beside the transverse process. Bleeding was stopped adequately, wound was washed with plenty of normal saline, negative pressure drainage was placed on the wound, and the wound was closed layer by layer. Return patient to the ward after waking from anesthesia.
All operations were performed by the same group of surgeons. The catheter was pulled out within 1–2 days after operation, and the patients were treated with antibiotics, mannitol, and hormones. The patients were encouraged to get out of bed and move 1 week after operation.
Observation Index
The outcome measures are visual analogue scale (VAS) and Japanese Orthopaedic Association cervical (JOA‐C) and lumbar (JOA‐L) scores. At two time points, 3 months and 1 year after the operation, a student who was not involved in the operation contacted the patients by telephone to collect the JOA score data. In addition, operative time, estimated blood loss, and hospital stay were also recorded.
Visual Analogue Scale
Visual analogue scale (VAS) is used in the social and behavioral sciences to measure a patient's pain degree. Patients were asked to rate their pain on a scale of 0 to 10. A higher score represented greater pain intensity. Zero means painless, and 10 means the worst pain ever.
Japanese Orthopaedic Association
Japanese Orthopaedic Association cervical (JOA‐C) and lumbar (JOA‐L) scores were used to assess the severity of clinical symptoms pre‐ and postoperatively. JOA‐C is comprised of six domain scores: motor dysfunction in the upper and lower extremities; sensory function in the trunk, upper extremities, and lower extremities; and bladder function, scaling from 0 to 4, 4, 2, 2, 2, and 3, respectively. JOA‐C improvement rate = [(follow‐up JOA‐C score − preoperative JOA‐C score)/(17 − preoperative JOA‐C score)] × 100%. JOA‐L consists of four subsections: subjective symptoms, objective observations, restriction of activities of daily living, and urinary bladder function, scaling from 0 to 9, 6, 14 and −6, respectively. JOA‐L improvement rate = [(follow‐up JOA‐L score − preoperative JOA‐L score)/(29 − preoperative JOA‐L score)] × 100%. The higher the score, the better the patient's neurological function.
In addition, patient demography, blood loss, operation time, hospital stay, and complications were assessed and compared carefully.
Statistical Methods
SPSS25.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The measurement data was expressed by Mean ± SD, and the VAS and JOA scores were statistically analyzed by one‐way ANOVA analyses. P < 0.05 was statistically significant.
Results
After a series of screenings, 51 patients finally underwent surgery. Among the 51 cases, 24 were male and 27 were female; the maximum age was 77 years old and the minimum age was 49 years old, with an average age of 66.3 years. There were five patients who underwent cervical and lumbar vertebrae surgery at the same time (simultaneous operation group), 28 patients who underwent cervical vertebra surgery first (first cervical surgery group), and 18 patients who did lumbar vertebrae first (first lumbar surgery group). The average age of simultaneous operation group, first cervical surgery group, and first lumbar surgery group was 64.60 ± 7.64, 67.04 ± 6.53 and 65.72 ± 7.22, respectively. (Fig. S1 and Table S1).
Signs and Symptoms
The statistics of symptoms and physical signs of patients is shown in Table 1. There were 45 cases of upper extremity pain and radiation pain; 37 cases of upper extremity hypoesthesia and numbness; 23 cases of upper limb weakness, limited lifting; 14 cases of headache or dizziness; 36 cases of lumbago and lower limb radiation pain; 35 cases of lower limb hypoesthesia and numbness; 17 cases of intermittent claudication; three cases of poor urination. There were 26 cases of Hoffmann sign positive; 33 cases of Spurling sign positive; 36 cases of Babinski sign positive; 46 cases of straight leg elevation test positive; 17 cases of ankle clonus positive (Table 1).
Table 1.
Demographics
| Simultaneous operation | First cervical surgery | First lumbar surgery | ||
|---|---|---|---|---|
| Case | 5 | 28 | 18 | |
| Age (mean ± SD) | 64.60 ± 7.64 | 67.04 ± 6.53 | 65.72 ± 7.22 | |
| Sex, male | 2 | 14 | 8 | |
| Symptoms | Upper limb pain, radiation pain | 4 | 27 | 14 |
| Upper limb hypoesthesia, numbness | 3 | 25 | 9 | |
| Upper limb weakness, limited lifting | 2 | 13 | 8 | |
| Headache, dizziness | 1 | 9 | 4 | |
| Lumbago, lower limb radiation pain | 3 | 19 | 14 | |
| Lower limb hypoesthesia, numbness | 3 | 20 | 12 | |
| Intermittent claudication | 2 | 9 | 6 | |
| Poor urination | 1 | 1 | 1 | |
| Signs | Hoffmann sign positive | 3 | 14 | 9 |
| Spurling sign positive | 4 | 18 | 11 | |
| Babinski sign positive | 2 | 21 | 13 | |
| Straight leg elevation test positive | 4 | 26 | 16 | |
| Ankle clonus positive | 2 | 8 | 7 | |
| Imaging | Cervical physiological curvature | 5 | 28 | 17 |
| Cervical intervertebral space stenosis | 5 | 25 | 14 | |
| Cervical osteophyte formation | 4 | 20 | 10 | |
| Lumbar instability | 4 | 22 | 16 | |
| Lumbar degeneration and hyperplasia | 3 | 18 | 14 | |
| Spinal canal stenosis with intervertebral disc herniation | 5 | 25 | 16 | |
| Ossification of posterior longitudinal ligament | 4 | 24 | 13 | |
| Intervertebral disc herniation | 5 | 26 | 17 |
Imaging Features
All the 51 cases were examined by X‐ray, computed tomography (CT), and magnetic resonance imaging (MRI) of cervical and lumbar vertebrae. The X‐ray films of all subjects showed different degrees of degeneration of cervical and lumbar vertebrae. X‐ray showed the change of cervical physiological curvature in 50 cases, cervical intervertebral space stenosis in 44 cases, and cervical osteophyte formation in 34 cases. There were 42 cases of lumbar instability and 35 cases of lumbar degeneration and hyperplasia. MRI of all subjects showed different degrees of stenosis in the cervical and lumbar spinal canal. Cervical MRI showed spinal canal stenosis with intervertebral disc herniation in 46 cases. Cervical CT plain scan showed ossification of posterior longitudinal ligament in 41 cases. Lumbar CT showed intervertebral disc herniation in 48 cases (Table 1).
Intraoperative Parameters
As shown in Table 2, operation time (OP), blood loss (BL), and hospital stay (HS) in simultaneous operation group were 245.00 ± 5.00 min, 480.00 ± 27.39 mL, and 16.60 ± 0.55 days. While those parameters in the first cervical surgery group were 342.50 ± 18.18 min, 528.21 ± 43.97 mL, and 22.75 ± 2.15 days; and in first lumbar surgery group were 346.11 ± 24.77 min, 519.44 ± 43.99 mL, and 22.89 ± 1.64 days, respectively. The average blood loss in simultaneous operation group was less (P > 0.05), and operation time and hospital stay time were significantly shorter in the simultaneous operation group than in the first cervical surgery group and first lumbar surgery group (P < 0.05). (Table 2).
Table 2.
Evaluation index
| Simultaneous operation | First cervical surgery | First lumbar surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P value | ||
| Duration of surgery (min) | 245.00 | 5.00 | 342.50 | 18.18 | 346.11 | 24.77 | <0.0001 | |
| Blood loss (mL) | 480.00 | 27.39 | 528.21 | 43.97 | 519.44 | 43.99 | 0.08 | |
| Hospital stay (day) | 16.60 | 0.55 | 22.75 | 2.15 | 22.89 | 1.64 | <0.0001 | |
| VAS score | 8.00 | 1.00 | 8.36 | 0.68 | 8.17 | 0.71 | 0.49 | |
| JOA‐C | Before operation | 7.00 | 2.35 | 6.54 | 1.53 | 7.83 | 1.04 | 0.02 |
| 3 months after operation | 15.40 | 0.89 | 14.61 | 0.99 | 14.72 | 0.96 | 0.25 | |
| One year after operation | 15.60 | 0.55 | 15.32 | 0.67 | 15.44 | 0.98 | 0.72 | |
| Postoperative improvement rate of JOA‐C | 3 months after operation | 84.02% | 7.30% | 77.66% | 7.23% | 75.63% | 8.80% | 0.11 |
| One year after operation | 85.68% | 5.44% | 84.27% | 5.02% | 83.34% | 10.25% | 0.80 | |
| JOA‐L | Before operation | 7.20 | 2.17 | 4.64 | 2.36 | 5.78 | 1.22 | 0.02 |
| 3 months after operation | 23.00 | 3.16 | 23.11 | 1.66 | 23.11 | 1.57 | 0.99 | |
| One year after operation | 24.60 | 1.14 | 24.50 | 1.14 | 24.39 | 1.20 | 0.91 | |
| Postoperative improvement rate of JOA‐L | 3 months after operation | 73.35% | 11.22% | 76.04% | 5.39% | 74.78% | 5.95% | 0.61 |
| One year after operation | 80.04% | 3.35% | 81.65% | 3.74% | 80.21% | 4.76% | 0.45 | |
Evaluation Result
Patients’ Preoperative Scores
The preoperative VAS scores of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 8.00 ± 1.00, 8.36 ± 0.68, and 8.17 ± 0.71 (P > 0.05). JOA‐C scores were 7.00 ± 2.35, 6.54 ± 1.53, and 7.83 ± 1.04 (P < 0.05). And JOA‐L scores were 7.20 ± 2.17, 4.64 ± 2.36, and 5.78 ± 1.22 respectively (P < 0.05).
Postoperative Scores of Simultaneous Operation Group
For the patients who underwent cervical and lumbar surgery at the same time (simultaneous operation group), JOA‐C and JOA‐L scores were 15.40 ± 0.89 and 23.00 ± 3.16, respectively, 3 months after operation, and postoperative improvement rates of JOA‐C and JOA‐L were 84.02 ± 7.30% and 73.35 ± 11.22%. After 1 year of follow‐up, JOA‐C and JOA‐L scores were 15.60 ± 0.55 and 24.60 ± 1.14, and postoperative improvement rates were 85.68% ± 5.44% and 80.04% ± 3.35% (Table 2).
Postoperative Scores of First Cervical Surgery Group
Three months after operation, the JOA‐C and JOA‐L scores of patients with primary cervical surgery and second lumber surgery (first cervical surgery group) were 14.61 ± 0.99 and 23.11 ± 1.66, respectively, and postoperative improvement rates of JOA‐C and JOA‐L were 77.66% ± 7.23% and 76.04% ± 5.39%. After 1 year of follow‐up, JOA‐C and JOA‐L scores were 15.32 ± 0.67 and 24.50 ± 1.14, and postoperative improvement rates were 84.2%7 ± 5.02% and 81.65% ± 3.74% (Table 2).
Postoperative Scores of First Lumbar Surgery Group
Three months after surgery JOA‐C and JOA‐L scores of patients who had lumber surgery in the first stage and cervical surgery in the second stage (first lumbar surgery group) were 14.72 ± 0.96 and 23.11 ± 1.57, respectively, and postoperative improvement rates of JOA‐C and JOA‐L were 75.63% ± 8.80% and 74.78% ± 5.95%. After 1 year of follow‐up, JOA‐C and JOA‐L scores were 15.44 ± 0.98 and 24.39 ± 1.20, and postoperative improvement rates were 83.34% ± 10.25% and 80.21% ± 4.76% (Table 2).
Comparison of Postoperative Results
JOA‐C
Three months after surgery, the JOA‐C scores of simultaneous operation group, first cervical surgery group and first lumbar surgery group were 15.40 ± 0.89, 14.61 ± 0.99, and 14.72 ± 0.96, respectively, the difference was not statistically significant (P > 0.05). While 1 year after surgery, the JOA‐C scores of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 15.60 ± 0.55, 15.32 ± 0.67 and 15.44 ± 0.98, respectively, the difference was also not statistically significant (P > 0.05). (Table 2).
Postoperative Improvement Rates of JOA‐C
Three months after surgery, the simultaneous operation group, first cervical surgery group and first lumbar surgery group postoperative improvement rates of JOA‐C scores were 84.02% ± 7.30%, 77.66% ± 7.23%, and 75.63% ± 8.80%, respectively, the difference was not statistically significant (P > 0.05). While 1 year after surgery, simultaneous operation group, first cervical surgery group, and first lumbar surgery group postoperative improvement rates of JOA‐C were 85.68% ± 5.44%, 84.27% ± 5.02%, and 83.34% ± 10.25%, respectively, the difference was also not statistically significant (P > 0.05). (Table 2).
JOA‐L
Three months after surgery, the JOA‐L scores of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 23.00 ± 3.16, 23.11 ± 1.66, and 23.11 ± 1.57, respectively, the difference was not statistically significant (P > 0.05). While 1 year after surgery, the JOA‐C scores of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 24.60 ± 1.14, 24.50 ± 1.14, and 24.39 ± 1.20, respectively, the difference was also not statistically significant (P > 0.05). (Table 2).
Postoperative Improvement Rates of JOA‐L
Three months after surgery, the simultaneous operation group, first cervical surgery group, and first lumbar surgery group postoperative improvement rates of JOA‐L scores were 73.35% ± 11.22%, 76.04% ± 5.39%, and 74.78% ± 5.95%, respectively, the difference was not statistically significant (P > 0.05). While 1 year after surgery, simultaneous operation group, first cervical surgery group, and first lumbar surgery group postoperative improvement rates of JOA‐L were 80.04% ± 3.35%, 81.65% ± 3.74%, and 80.21% ± 4.76%, respectively, the difference was also not statistically significant (P > 0.05). (Table 2).
Complications
Postoperative X‐ray examination showed that the position of internal plant was good and that no fixation loosening, fracture, or prolapse occurred in any of the patients. The physiological curvature of lumbar vertebrae and cervical vertebrae recovered. All the incisions were healed and there were no complications such as wound bleeding and infection. One case of fat liquefaction occurred in first cervical surgery group, which healed spontaneously after a regular change of dressing for 1 month. There were no serious complications such as dural tear, cerebrospinal fluid leakage, epidural hematoma, and spinal cord injury.
Case Report
A 70‐year‐old woman was hospitalized for “neck discomfort with unstable walking for more than 5 months, aggravating for 1 month, and urinary incontinence for 3 days”. Before this time (5 months previously), there was no obvious cause of neck discomfort accompanied with unstable walking, occasional radiation pain of both lower limbs, no abnormal sensation, no abnormal fine movement of limbs, no dizziness, nausea and vomiting, and no further treatment was given. One month ago, the symptoms gradually worsened, and the patient appeared to have intermittent claudication, only able to walk 100 m, accompanied by weakness and numbness of both lower limbs, which was aggravated when walking and relieved at rest. Three days before admission, there was no obvious inducement, and the symptoms of urinary incontinence appeared. The patient has a history of diabetes and hypertension, and blood pressure and blood glucose can be controlled by drugs. Babinski and Hoffman reflexes were positive on both sides, and the deep reflex was weakened. Preoperative VAS score was 8, Nurick score was 3, JOA‐C score was 7, JOA‐L score was 5. MRI results showed that the degree of cervical spinal stenosis was severe at C3‐C7 level, and lumbar spinal stenosis was severe at L2‐S1 level. Due to the severe symptoms caused by lumbar spinal stenosis, she underwent L2‐4 lumbar laminectomy, decompression, and needle rod system internal fixation in our hospital. Three months later, the symptoms of urinary incontinence and intermittent claudication improved. Postoperative VAS score was 6, JOA‐C score was 11, JOA‐L score was 12. No related complications occurred, but the symptoms of bilateral gait imbalance, weakness and numbness caused by cervical spinal stenosis gradually aggravated, which seriously reduced her quality of life. She then underwent posterior cervical single‐door vertebroplasty for C3‐C7. Postoperative VAS score was 3, JOA‐C score was 15, JOA‐L score was 24, final JOA‐C improvement rate was 80.00%, JOA‐L improvement rate was 79.17%. The symptoms of sensory disturbance were relieved, the pathological reflex turned negative, the tendon reflex returned to normal, and the postoperative quality of life was significantly improved (Figs 1 and 2).
Fig. 1.
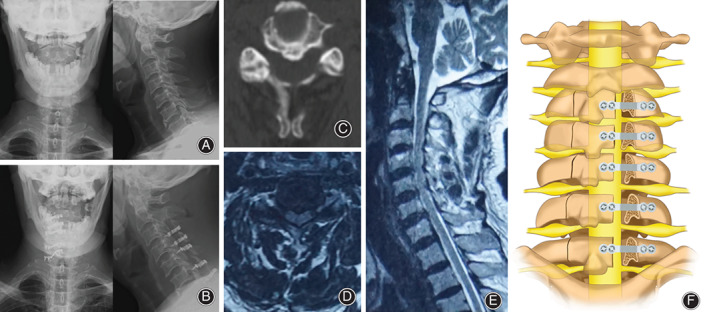
(A) Preoperative DX showed that the physiological curvature of the cervical spine became straight. (B) Postoperative DX showed that the internal fixation position was good. (C) CT showed C4‐5 level spinal stenosis. (D) MRI cross‐section showed C4‐5 level spinal stenosis. (E) MRI sagittal plane showed cervical spinal stenosis. (F) Schematic illustration.
Fig. 2.
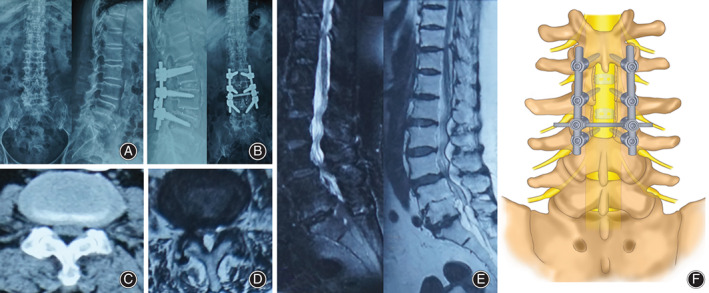
(A) Preoperative DX showed that the physiological curvature of the lumbar spine was straightened; (B) Postoperative DX showed good internal fixation position; (C) CT shows L3‐4 spinal stenosis; (D) MRI cross section shows L3‐4 spinal stenosis; (E) MRI sagittal plane shows lumbar spinal stenosis. (F) Schematic illustration.
Discussion
TSS refers to simultaneous narrowing of disjointed regions of the spinal canal, and stenosis could occur at any level throughout the spinal column, particularly the cervical and lumbar regions 18 , 19 . A recent, large‐scale Japanese study based on whole‐spine magnetic resonance imaging (MRI) found that the prevalence of CSS was 25%, lumbar spinal stenosis (LSS) was 30%, and TSS was 11% 20 . The classic symptoms in TSS includes gait disturbance, intermittent claudication, and mixed upper and lower extremity symptoms 11 , 21 . A comprehensive history and examination are essential to reduce the risk of missing TSS.
Surgical selection for TSS patients with clinical symptoms of cervical spinal stenosis and lumbar spinal stenosis is still controversial 1 , 14 . Either cervical spinal stenosis or lumbar spinal stenosis can lead to anesthesia and pain from lower limbs 22 ; if the symptoms of lumbar stenosis with cervical stenosis were managed only by lumbar decompression, the residual cervical symptoms after surgery could result in unimproved surgical outcomes 11 , 23 , 24 . Cervical decompression surgery followed by lumbar decompression in some reports has been advocated because of the decompression of the cervical spine resulting in the connection tract between the corticospinal tract and lower limbs, which might pass through the stenosis segments of cervical spine, and was also decompressed, making lumbar symptoms improve 16 , 25 . Cervical decompression surgery after lumbar decompression was promoted as lumbar decompression could correct the anteflexion of lumbar vertebra and promote greater space for the cervical spinal cord 11 . Some authors believe that the main symptoms rather than the absolute stenosis on the radiograph were the primary issue to be treated by decompression surgery 10 . Recently, some articles have suggested that electrophysiological examination (electroneuromyography [ENMG] and motor‐ and sensory‐evoked potential [MEP‐SEP]) could be carried out before operation to identify the most suitable area for operation and choose the mode of operation 3 . Simultaneous decompressions for patients with coexisting cervical and lumbar spinal stenosis were recommended recently with faster functional recovery and less hospital stays and costs 26 .
Principles of Surgical Selection
The principles of selection of cervical and lumbar surgery do not only aim to fully remove the compression factors. They also aim to minimize the damage to the stability of the spine, in line with the biomechanical characteristics of the spine, and reduce the occurrence of various complications. Generally speaking, the surgical methods can be divided into one‐stage operation and staged operation according to the time. The patients selected by our team for one‐stage operation are patients with good physical fitness, no contraindications to surgery, younger age (less than 60 years old), and severe neck and waist symptoms. Under the premise of adequate preoperative preparation, the first‐stage operation of cervical vertebrae and lumbar vertebrae was performed. Staging surgery is for patients with TSS whose symptoms are mainly in a single site. We can consider a single‐site operation first, depending on the degree of symptom relief to determine whether to perform another part of the operation. In addition, patients with poor physical condition and low surgical tolerance must be operated on in stages to reduce the incidence of intraoperative and postoperative accidents. When choosing the preferred surgical site for staging operation, the imaging manifestations, clinical symptoms, patients' opinions, and economic conditions should be considered comprehensively (Fig. 3).
Fig. 3.
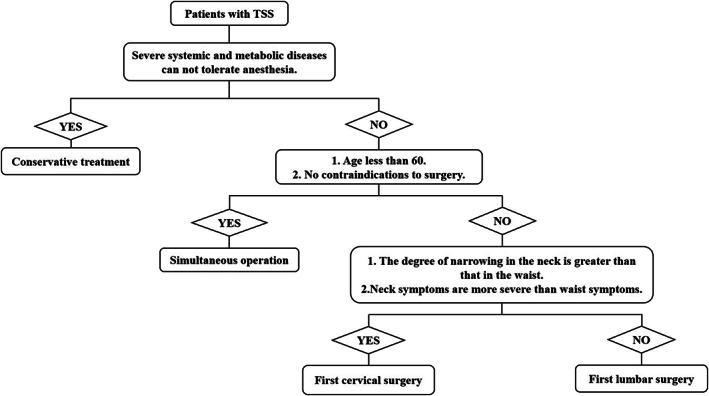
Schematic decision diagrams.
Case Report Analysis
In this paper, a case of TSS was reported in detail. As the patient was elderly and had relatively serious symptoms of lumbar spinal stenosis, this case primarily received a lumbar decompression after that the symptoms of urinary incontinence and intermittent claudication improved. Then the second‐stage cervical decompression surgery was executed, the sensory disturbance of upper and lower limbs was relieved, and the tendon reflex and Hoffmann sign returned to normal. Both results of postoperative and follow‐up were satisfactory in these three patients. The prognosis of patients with tandem spinal stenosis is not worse than that of a single patient with cervical stenosis or lumbar stenosis 27 , 28 . There was no significant correlation between the surgical outcome and the choice of surgical procedure in patients with coexisting cervical spinal stenosis and lumbar spinal stenosis 29 , 30 .
All Data Analysis
Among the 51 patients selected in this paper, 28 patients underwent cervical surgery, 18 patients underwent lumbar surgery, and five patients underwent both cervical and lumbar surgery. During the final one‐year follow‐up, the JOA‐C improvement rates of simultaneous surgery, first cervical surgery, and first lumbar surgery were 85.68% ± 5.44%, 84.27% ± 5.02%, and 83.34% ± 10.25%, respectively, and the JOA‐L improvement rates were 80.04 ± 3.35%, 81.65 ± 3.74%, and 80.21 ± 4.76%, respectively. The difference was not statistically significant, suggesting that under the condition of ensuring the surgical effect, the choice of staged surgery or concurrent surgery according to the patients' own severe symptoms of cervical and lumbar symptoms have little effect on the final results. But in our study, the total operative time of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 245.00 ± 5.00 min, 342.50 ± 18.18 min, and 346.11 ± 24.77 min; the operation time of simultaneous operation group was significantly less than that of first cervical surgery group and first lumbar surgery group (P < 0.05). Meanwhile the hospital stays of simultaneous operation group, first cervical surgery group, and first lumbar surgery group were 16.60 ± 0.55 days, 22.75 ± 2.15 days, and 22.89 ± 1.64 days; the hospitalization time of simultaneous operation group was also less than that of first cervical surgery group and first lumbar surgery group (P < 0.05). Although the difference in intraoperative blood loss was not statistically significant, the value of simultaneous operation group (480.00 ± 27.39 mL) was intuitively smaller than that of first cervical surgery group (528.21 ± 43.97 mL) and first lumbar surgery group (519.44 ± 43.99 mL). So, from the point of view of the injury to the patient, simultaneous surgery may be better than staged surgery.
Limitations
Inevitably, the study has some potential limitations. The first is its retrospective and non‐random model. Because our subjects were patients undergoing TSS surgery, the stenosis degree and nerve compression problems were different, which could not be randomized and blinded like other drug trials. Secondly, the sample size of this paper is relatively small, so the conclusions obtained in this paper may not be extended to all TSS patients. Thirdly, the JOA scores used in this paper are mainly used to assess simple cervical or lumbar diseases, so we cannot determine their reliability to TSS. Currently, the published literature does not provide a specific score to evaluate TSS patients. With the increase of TSS research, this problem may be solved in the near future.
Conclusion
The principles of cervical and lumbar surgery selection are to fully relieve the pressure factors and minimize the damage to spine stability, which is in line with the biomechanical characteristics of the spine and reduces the occurrence of various complications. The purpose of surgical treatment is to reduce pressure and sustain stability. According to our research, to ensure the best‐quality operation and achieve good results, either one‐stage operation or staged operation can be selected based on the patient's own situation. Finally, the damage of simultaneous surgery was less than that of staged surgery.
Supporting information
Appendix S1: Supplementary Information
Contributor Information
Kai Wang, Email: wangkaiy48@126.com.
Liang Zhang, Email: zhdoctor2008@me.com.
Reference
- 1. Adamova B, Bednarik J, Andrasinova T, et al. Does lumbar spinal stenosis increase the risk of spondylotic cervical spinal cord compression? Eur Spine J, 2015, 24: 2946–2953. [DOI] [PubMed] [Google Scholar]
- 2. Schaffer JC, Raudenbush BL, Molinari C, Molinari RW. Symptomatic triple‐region spinal stenosis treated with simultaneous surgery: case report and review of the literature. Global Spine J, 2015, 5: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jannelli G, Baticam NS, Tizi K, Truffert A, Lascano AM, Tessitore E. Symptomatic tandem spinal stenosis: a clinical, diagnostic, and surgical challenge. Neurosurg Rev, 2020, 43: 1289–1295. 10.1007/s10143-019-01154-9. [DOI] [PubMed] [Google Scholar]
- 4. Pennington Z, Alentado VJ, Lubelski D, et al. Quality of life changes after lumbar decompression in patients with tandem spinal stenosis. Clin Neurol Neurosurg, 2019, 184: 105455. [DOI] [PubMed] [Google Scholar]
- 5. Felbaum DR, Fayed I, Stewart JJ, Sandhu FA. Relief of lumbar symptoms after cervical decompression in patients with tandem spinal stenosis presenting with primarily lumbar pain. Cureus, 2016, 8: e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LaBan MM. Iliopsoas weakness: a clinical sign of lumbar spinal stenosis. Am J Phys Med Rehabil, 2004, 83: 224–225. [DOI] [PubMed] [Google Scholar]
- 7. Aydogan M, Ozturk C, Mirzanli C, Karatoprak O, Tezer M, Hamzaoglu A. Treatment approach in tandem (concurrent) cervical and lumbar spinal stenosis. Acta Orthop Belg, 2007, 73: 234–237. [PubMed] [Google Scholar]
- 8. Ono K, Ebara S, Fuji T, Yonenobu K, Fujiwara K, Yamashita K. Myelopathy hand. new clinical signs of cervical cord damage. Bone Joint J, 1987, 69‐B: 215–219. [DOI] [PubMed] [Google Scholar]
- 9. TENG P, PAPATHEODOROU C. Combined cervical and lumbar spondylosis. Arch Neurol, 1964, 10: 298–307. [DOI] [PubMed] [Google Scholar]
- 10. Epstein Nancy E, Epstein JA, Carras R, Murthy VS, Hyman RA. Coexisting cervical and lumbar spinal stenosis: diagnosis and management. Neurosurgery, 1984, 15: 489–496. [DOI] [PubMed] [Google Scholar]
- 11. Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg, 1987, 66: 842–849. [DOI] [PubMed] [Google Scholar]
- 12. Lee MJ, Cassinelli EH, Riew KD. Prevalence of cervical spine stenosis. Anatomic study in cadavers. J Bone Joint Surg Am, 2007, 89: 376–380. [DOI] [PubMed] [Google Scholar]
- 13. Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976), 2000, 25: 389–394. [DOI] [PubMed] [Google Scholar]
- 14. Lee MJ, Garcia R, Cassinelli EH, Furey C, Riew KD. Tandem stenosis: a cadaveric study in osseous morphology. Spine J, 2008, 8: 1003–1006. [DOI] [PubMed] [Google Scholar]
- 15. Lee SH, Kim KT, Suk KS, et al. Asymptomatic cervical cord compression in lumbar spinal stenosis patients: a whole spine magnetic resonance imaging study. Spine (Phila Pa 1976), 2010, 35: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 16. Naderi S, Mertol T. Simultaneous cervical and lumbar surgery for combined symptomatic cervical and lumbar spinal stenoses. J Spinal Disord Tech, 2002, 15: 229–231; discussion 231–222. [DOI] [PubMed] [Google Scholar]
- 17. Kikuike K, Miyamoto K, Hosoe H, Shimizu K. One‐staged combined cervical and lumbar decompression for patients with tandem spinal stenosis on cervical and lumbar spine: analyses of clinical outcomes with minimum 3 years follow‐up. J Spinal Disord Tech, 2009, 22: 593–601. [DOI] [PubMed] [Google Scholar]
- 18. Hong CC, Liu KP. A rare case of multiregional spinal stenosis: clinical description, surgical complication, and management concept review. Global Spine J, 2015, 5: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu PP, Yu M, Liu XG, Liu ZJ, Jiang L. Surgeries for patients with tandem spinal stenosis in cervical and thoracic spine: combined or staged surgeries? World Neurosurg, 2017, 107: 115–123. [DOI] [PubMed] [Google Scholar]
- 20. Nagata K, Yoshimura N, Hashizume H, et al. The prevalence of tandem spinal stenosis and its characteristics in a population‐based MRI study: the Wakayama spine study. Eur Spine J, 2017, 26: 2529–2535. [DOI] [PubMed] [Google Scholar]
- 21. Alvin MD, Alentado VJ, Lubelski D, Benzel EC, Mroz TE. Cervical spine surgery for tandem spinal stenosis: the impact on low back pain. Clin Neurol Neurosurg, 2018, 166: 50–53. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi J, Kobayashi H, Wakabayashi S, et al. The effect of a prostaglandin E1 derivative on the symptoms and quality of life of patients with lumbar spinal stenosis. J Orthop Sci, 2013, 18: 208–215. [DOI] [PubMed] [Google Scholar]
- 23. LaBan MM, Green ML. Concurrent (tandem) cervical and lumbar spinal stenosis: a 10‐yr review of 54 hospitalized patients. Am J Phys Med Rehabil, 2004, 83: 187–190. [DOI] [PubMed] [Google Scholar]
- 24. Vogt MT, Cawthon PM, Kang JD, Donaldson WF, Cauley JA, Nevitt MC. Prevalence of symptoms of cervical and lumbar stenosis among participants in the osteoporotic fractures in men study. Spine (Phila Pa 1976), 2006, 31: 1445–1451. [DOI] [PubMed] [Google Scholar]
- 25. Richter M, Kluger P, Puhl W. [Diagnosis and therapy of spinal stenosis in the elderly]. Z Orthop Ihre Grenzgeb, 1999, 137: 474–481. [DOI] [PubMed] [Google Scholar]
- 26. Uehara M, Tsutsumimoto T, Yui M, Ohta H, Ohba H, Misawa H. Single‐stage surgery for compressive thoracic myelopathy associated with compressive cervical myelopathy and/or lumbar spinal canal stenosis. Eur Spine J, 2016, 25: 1904–1911. [DOI] [PubMed] [Google Scholar]
- 27. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management?: a prospective 10‐year study. Spine (Phila Pa 1976), 2000, 25: 1424–1435; discussion 1435–1426. [DOI] [PubMed] [Google Scholar]
- 28. Seichi A, Takeshita K, Ohishi I, et al. Long‐term results of double‐door laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976), 2001, 26: 479–487. [DOI] [PubMed] [Google Scholar]
- 29. Eskander MS, Aubin ME, Drew JM, et al. Is there a difference between simultaneous or staged decompressions for combined cervical and lumbar stenosis? J Spinal Disord Tech, 2011, 24: 409–413. [DOI] [PubMed] [Google Scholar]
- 30. Swanson BT. Tandem spinal stenosis: a case of stenotic cauda equina syndrome following cervical decompression and fusion for spondylotic cervical myelopathy. J Man Manip Ther, 2012, 20: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


