Summary
Ageing dramatically affects number and function of both innate and adaptive arms of immune system, particularly T cell subsets, contributing to reduced vaccination efficacy, decreased resistance to infections and increased prevalence of cancer in older people. In the present paper, we analysed the age‐related changes in the absolute number of lymphocytes in 214 Sicilian subjects, and in the percentages of T and natural killer (NK) cells in a subcohort of donors. We compared these results with the immunophenotype of the oldest living Italian supercentenarian (aged 111 years). The results were also sorted by gender. The correlation between number/percentage of cells and age in all individuals. and separately in males and females, was examined using a simple linear regression analysis. We did not record the increase in the rate of inversion of the CD4/CD8 ratio, frequently reported as being associated with ageing in literature. Our observation was the direct consequence of a flat average trend of CD4+ and CD8+ T cell percentages in ageing donors, even when gender differences were included. Our results also suggest that CD4+ and CD8+ subsets are not affected equally by age comparing females with males, and we speculated that gender may affect the response to cytomegalovirus (CMV) infection. The supercentenarian showed a unique immunophenotypic signature regarding the relative percentages of her T cell subsets, with CD4+ and CD8+ T cell percentages and CD4+ naive T cell values in line with those recorded for the octogenarian subjects. This suggests that the supercentenarian has a naive ‘younger’ T cell profile comparable to that of a >80‐year‐old female.
Keywords: CMV, gender, supercentenarian, T lymphocyte subsets
We investigated age‐related changes in the absolute number of lymphocytes among 216 Sicilian subjects (age range 22‐111) and in the percentages of circulating lymphocyte subsets in a smaller group of donors of different ages. The data were also analyzed by gender. The supercentenarian has a naïve ‘younger’ T cell profile comparable to that of an older female.

INTRODUCTION
The immune system undergoes a complex and progressive functional remodelling during the ageing process. Age‐related alterations have been documented in both innate and acquired arms of the immune system, where some immune responses are diminished, leaving others unchanged or increased (1, 2). One of the most commonly mentioned hallmarks of an aged immune system is a substantial decrease in naive CD4+ and CD8+ T cells and their differentiation into memory lymphocyte subsets (2). This phenomenon is the result of events (thymic involution and life‐long antigenic stimulation) that can occur independently, but also converge in the achievement of an ‘experienced’ T cell profile, that is, low proliferating cells that are highly active in effector cytokine production upon antigenic stimulation (3). The involution of the thymus, the central organ of T cell generation, starts at the time of puberty and is the main responsible for naive T cell decline in the periphery and lymphoid organs during ageing (2, 4). While CD4+‐ and CD8+‐naive T cell percentages generally decline with age, gender‐related differences were demonstrated. In particular, females have shown higher frequencies in both CD4+‐ and CD8+‐naive T cells, probably due to a more elevated thymic function compared to males (5).
Chronic stimulation by persistent viral infections, such as human cytomegalovirus (CMV) and, to some extent Epstein–Barr virus (EBV), could play a role in shaping age‐related immune function, driving clonal expansion of specific T cells through repetitive stimulation. CMV is responsible for a persistent and latent viral infection that rarely causes obvious disease (6). However, CMV seropositivity in older people has been included into an ‘immunological risk profile’ predicting mortality (7) (see also below). Phenotypically, CMV is thought to be responsible for increasing the presence of effector memory (EM) and terminally differentiated effector (TE) T cells with phenotypical and functional features of replicative senescence, especially in the cytotoxic compartment (8, 9). Indeed, as CMV infection is mainly controlled by cytotoxic T cells, an accumulation of high numbers of CMV‐specific senescent CD8+ T cells was observed with each cycle of viral reactivation (4). A similar but slighter effect on clonal expansions of CD8+ T cells has been observed in response to EBV infection in the Swedish population (7), although it does not seem to be confirmed in older people from West Sicily (10). In these subjects, the percentage of EBV‐specific CD8+ cells was significantly lower than in young people, showing decreased levels of CD27 and CD28 but no increase in CD45RA (10).
Overall, the expansion of late‐differentiated CD8+ T cells with ageing has been associated with an age‐related increase in the rate of inversion of the CD4/CD8 ratio (i.e. <1 versus an expected ratio of between 1–5 and 2.5 or more) in older people (11). This altered ratio is accordingly considered as a marker of frailty risk (7, 12, 13).
It becomes evident that the depicted alterations represent the direct consequence of lifelong antigen exposure or the reflection of the progressive adaptation and compensation of the individual to environmental stimuli. Overall, they are implicated in the increased frequency and severity of infections, cancer and lowered responses to vaccination in older people; that is, the phenomenon called immunosenescence (1, 2, 7).
Innate immunity is also affected by age‐related changes, as demonstrated by the increase in the overall absolute number and percentage of natural killer (NK) cells in the periphery in healthy older people. This reflects a decrease in cytokine‐producing CD56 high (CD56high) cells and the increased presence of cytotoxic CD56 low (CD56low) cells (14, 15, 16).
Centenarians, individuals who have reached 100 years of age, are considered the best model of successful ageing as they avoid, delay or overcome age‐related diseases such as cancer, neurodegenerative and cardiovascular diseases (17). Their relatively good state of health implies a surprisingly active immune system, which has been therefore extensively analysed worldwide (18, 19, 20). Becoming a centenarian was a rare phenomenon only a few decades ago. However, improvements in medical care and quality of life have led to a reduction in morbidity and mortality, with a consequent increase in life expectancy (17).
According to the database from the Italian National Institute of Statistics, as of 1 January 2019, in Italy the number of centenarians (aged 100+ years) was 14–456 (84% females), semi‐supercentenarians (aged 105+ years) was 1112 (87% females) and supercentenarians (aged 110+ years) was 21, representing a doubling in these numbers compared to 2009 (21). At the time of revising this paper, according to the Italian supercentenarians database, there are 12 validated supercentenarians and the oldest living person in Italy is a woman aged 111 years, born and living in Sicily (22).
These demographic data show a significant gender difference in life expectancy, underlining the importance of a global analysis of an ageing–environment/gender interaction. Despite their biomedical relevance, gender differences seem to be still poorly considered and inadequately investigated in ageing studies (23).
In this study, we investigated age‐related alterations in the absolute numbers of lymphocytes among 214 Sicilian subjects (age range = 22–111) and in the percentages of circulating lymphocyte subsets of 41 Sicilian donors, aged between 25 and 111 years, focusing on T and NK cells. Blood cells from a subgroup of 27 healthy donors, including the oldest living Italian supercentenarian, were used for a more complete dissection of T cell subsets. Data were also analysed according to gender. To investigate whether the differences observed in T cell subpopulations are attributable to previous CMV and EBV infections, the same cohort was screened for CMV and EBV seropositivity.
MATERIALS AND METHODS
Study cohort
Participants were recruited between 2017 and 2020 within the project ‘Discovery of molecular and genetic/epigenetic signatures underlying resistance to age‐related diseases and comorbidities (DESIGN)’, funded by the Italian Ministry of Education, University and Research. The Ethics Committee of Palermo University Hospital (Sicily, Italy) approved the study protocol (Nutrition and Longevity, no. 032017). The study was conducted in accordance with the Declaration of Helsinki and its amendments. All study participants (or their caregivers) gave their written informed consent prior to enrolment. A total of 214 healthy donors (females = 121; males = 93) were recruited. All study participants were Sicilians, selected on the basis of their health status and aged between 22 and 111 years. Exclusion criteria were immunodepression, stroke, cancer or the use of immunomodulatory drugs within the previous 6 months. In addition, on 13 July 2020 Mrs Maria Oliva, the oldest living person in Italy, according to the Italian supercentenarians database (22), was recruited. To respect privacy, all other donors were identified with an alphanumeric code. A database was created to deal with the collected information. The participants underwent venipuncture in the morning after a fasting period of 12 h. The blood was collected in specific tubes containing ethylene diamine tetraacetic acid (EDTA) or no additives. Serum was separated by blood centrifugation of dry tubes and stored at −80°C before use. For more information about recruitment criteria, please see Aiello et al. (24).
Haematological parameter analysis
Whole blood was used for automated absolute leucocyte counts of 214 Sicilian subjects (age range = 22–111), 121 females [mean age = 68.82 years ± 26.26 standard deviation (s.d.)] and 93 males (mean age = 63.62 years ± 22.67 s.d.). Leucocyte, lymphocytes, neutrophil and monocyte absolute numbers were counted at the Unit of Transfusion Medicine of University Hospital ‘Paolo Giaccone’, Palermo, Italy.
Flow cytometry analysis
Flow cytometry analysis was performed in a subgroup of 41 subjects (23 females, 18 males) aged 25–111 years. Peripheral blood was processed freshly to determine complete blood counts and to isolate peripheral blood mononuclear cells (PBMCs). PBMCs were isolated at the Laboratory of Immunopathology and Immunosenescence of the Department of Biomedicine, Neurosciences and Advanced Diagnostics (BiND) of University of Palermo from whole blood using Ficoll‐Paque (GE Healthcare, South Plainfield, NJ, USA) density gradient centrifugation, according to the manufacturer’s instructions, within 6 h. PBMCs were frozen in 90% fetal bovine serum and 10% dimethylsulphoxide (DMSO) and stored at −80°C. Part of the samples were sent on dry ice to The Rayne Institute, King’s College London, School of Cancer and Pharmaceutical Sciences, where they were cryopreserved in liquid nitrogen tanks (−180°C) up to the day of analysis. The remaining samples were stored at −70°C in the Laboratory of Immunopathology and Immunosenescence for a few days until their characterization at the Specialistic Oncology Laboratory Unit of ARNAS Civico Hospital of Palermo.
For cell thawing, the cryopreserved vials were first transferred to a 37°C water bath and washed with X‐Vivo 15 (BioWhittaker, Walkersville, MD, USA). After centrifugation, the supernatant was discarded and PBMCs were resuspended in X‐Vivo 15 for counting. Counts and viability were determined with a haemocytometer and trypan blue dye exclusion technique. With this method, dead cells appear blue and are distinguishable from viable cells. An average of >90% live cells were obtained for each count round.
For the analysis of T and NK cell subsets, PBMCs were thawed, washed and counted. PBMCs, 1 × 106, were first stained with Fixable Viability Dye eFluor™ 780 (eBioscience, San Diego, CA, USA), following the manufacturer’s instructions, and then incubated with various combinations of monoclonal antibodies (Supporting information, Table S1). As a negative control, human unstained cells were used. Single‐stain controls were used for the automatic calculation of the compensation matrix. A minimum of 500 000 cells per sample were analysed in LSR Fortessa (BD Biosciences, San Jose, CA, USA) for the samples processed at King’s College London and in Navios EX (Beckman Coulter, Brea, CA, USA) for the samples processed in Palermo.
Table 1.
CMV and EBV serological status of the subcohort
| Clinical features | Young (n = 12) | Older (n =9 ) | LLI (n = 6) |
|---|---|---|---|
| Age (years) | |||
| Mean ± s.d. | 28.69 ± 3.47 | 82.2 ± 2.60 | 99.55 ± 5.27 |
| Range | 25‐34 | 79‐88 | 93‐111 |
| Gender | |||
| Female | 8 (66.6%) | 4 (44.4%) | 3 (50%) |
| Male | 4 (33.3%) | 5 (55.5%) | 3 (50%) |
| CMV serological status | |||
| CMV+ (n, %) | 2 (16.6%; 1 F, 1 M) | 9 (100%; 4 F, 5 M) | 6 (100%, 3 F, 3 M) |
| Anti‐CMV IgG titre (U/ml) | 103–160 | 44–177 | 160–>180 |
| EBV serological status | |||
| EBV+ (n, %) | 12 (100%; 8 F, 4 M) | 9 (100%; 4 F, 5 M) | 6 (100%, 3 F, 3 M) |
| Anti‐EBV IgG titre (U/ml) | |||
| Range | 25.4–>600 | 17.8–>600 | 20.48–>600 |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein–Barr virus; F, females; LLIs, long‐lived individuals; M, males; n, total number; s.d., standard deviation; U/mL, units per millilitre.
Lymphocyte subsets were identified through forward‐ (FSC) and side‐scatter (SSC), and further checked in the SSC/CD45 dot‐plot. An exemplificative schematic representation of the applied gating strategy for T and NK cell analysis is displayed in Figure 1. After setting the first gate in the FSC/SSC dot‐plot in the lymphocyte region events were gated in the CD3/SSC dot‐plot, as recommended by Rühle et al. (25). T cells were identified as CD3+ events. After exclusion of NK T (CD3+CD56+) cells, T cells were gated in the CD4/CD8 dot‐plot to define helper and cytotoxic subsets (25). CD4+ (helper) and CD8+ (cytotoxic) T cells were finally explored for CD197 and CD45RA expression in order to describe the fraction of naive (CD197+CD45RA+), CM (CD197+CD45RA−), EM (CD197‐CD45RA−) and TE cells (CD197−CD45RA+) (26). To complete T cell analysis, γδT cells were identified as CD3+γδ+, without distinction between the various subtypes. NK cells were identified in the ‘non‐T’ pool of lymphocytes (CD3−, including NK and B cells) according to their positivity for CD56 (25). After gating in the CD56/CD16 dot‐plot, NK cells were classified in NK1 (CD3−CD56lowCD16+) and NK2 (CD3−CD56highCD16−), as described elsewhere (25, 27).
FIGURE 1.
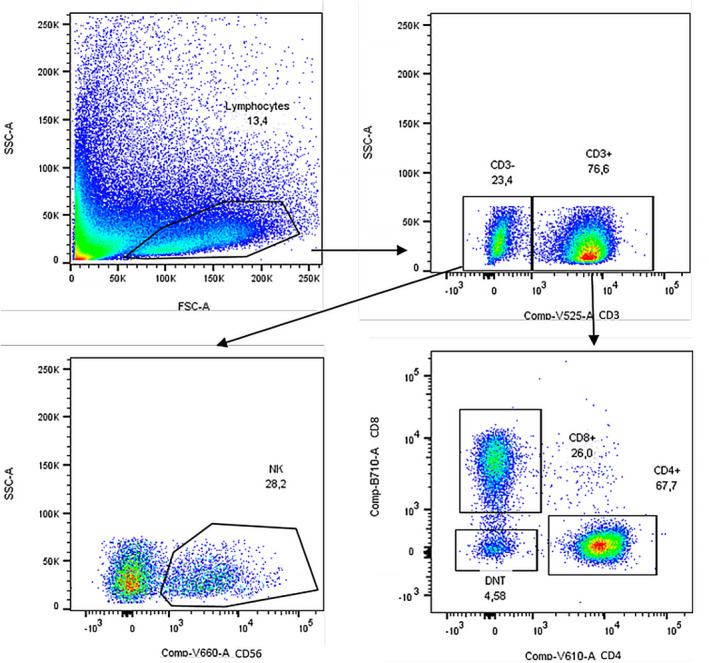
Natural killer (NK) and T cell gating strategy. Gating strategies for the analysis of NK, CD4+ and CD8+ T cell subsets. The doublet exclusion on forward‐scatter height (FCS‐H) versus forward‐scatter area (FCS)‐A followed by side‐scatter height (SSC‐H) versus side‐scatter area (SSC‐A) is not shown. A representative donor is presented
The analysed lymphocyte subsets are expressed as a fraction of the parental gated population, and reported as percentages in the graphics.
EBV and CMV serology
Specific immunoglobulin (Ig)G antibodies to EBV nuclear antigen (EBNA) and CMV in serum of 41 subjects (23 females and 18 males) aged 25–111 years were quantified by chemiluminescence immunoassay using the LIAISON® EBNA IgG kit (DiaSorin, Saluggia, Italy) and the LIAISON® CMV IgG II kit (DiaSorin), respectively, as recommended by the manufacturer. Measurements were performed by LIAISON XL (DiaSorin). The range upper limit was sect at 180 U/ml for anti‐CMV IgG and at 600 U/ml for anti‐EBV IgG.
Statistical analysis
To analyse the percentages of T, NK and NK T cells, flow cytometry data were analysed using FlowJo version 10.5.3 (Tree Star, Inc., Ashland, OR, USA) and statistical analysis was performed with GraphPad Prism version 8.1.2 (GraphPad Software, San Diego, CA, USA).
Correlation between te number/percentage of cells and age in all individuals and in males and females were examined using a simple linear regression analysis. Figures were plotted as scatter‐plots with a linear regression line and 95% confidence bands. For each statistical analysis, only P‐values < 0.05 were considered significant.
RESULTS
Analysis of haematological parameters
The number of leucocytes in peripheral blood of 214 subjects was analysed according to age and gender. Correlation analysis showed that neither age nor gender affected leucocyte, neutrophil and monocyte counts (data not shown).
In contrast, there was a significant decline in lymphocyte counts (Figure 2) for males (R 2 = 0.082, P = 0.005) but not for both genders [R 2 = 0.016, P = not significant (n.s.)] or female subjects alone (R 2 = 0.0005, P = n.s.).
FIGURE 2.
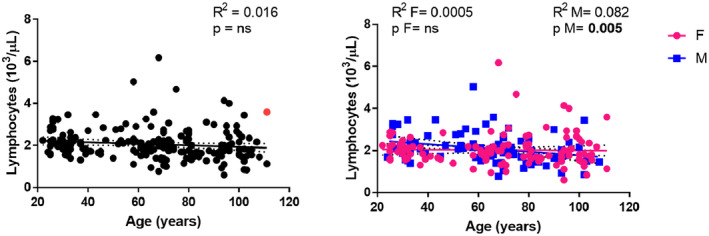
Correlations between lymphocyte counts and age. Linear regression analysis showing the relationship between lymphocyte count (103/µl) and age in all individuals (n = 214) (black line), males (n = 93) (blue line) and females (n = 121) (pink line). Each point represents data from an individual healthy donor. The supercentenarian is shown in red in the graph on the left. The coefficient of determination and P‐values are shown on the graphs. R 2 = R squared; n.s. = not significant; F = female; M = male
Analysis of T cell subsets
For the analysis of total CD4+ and CD8+ T cells, PBMCs were isolated from the blood of 41 healthy donors, 23 females (aged 25–111 years) and 18 males (aged 26–102 years) for the analysis of the percentages of T cells. PBMCs from 27 healthy donors (aged 25–111 years) divided by age and gender, as represented in Table 1, were used for a more complete analysis of T cell subsets.
To investigate the effects of age and gender on human lymphocyte subsets, the percentages of CD4+ and CD8+ T cells in the PBMC pool were determined by flow cytometric analysis using the gating strategy described in Figure 1. The percentage of CD4+ (Figure 3a) and CD8+ (Figure 3b) T cells remained almost constant with ageing, even when gender differences were included in the analysis. In contrast to the reported age‐related increase in the rate of inversion of the CD4/CD8 ratio (11), a flat average trend of change in the CD4/CD8 ratio with age was recorded in all individuals merged together (Figure 4, R 2 = 0.010, P = n.s.) and analyzed according to gender (Figure 4, females: R 2 = 0.007, P = n.s.; males: R 2 = 0.0001, P = n.s.).
FIGURE 3.
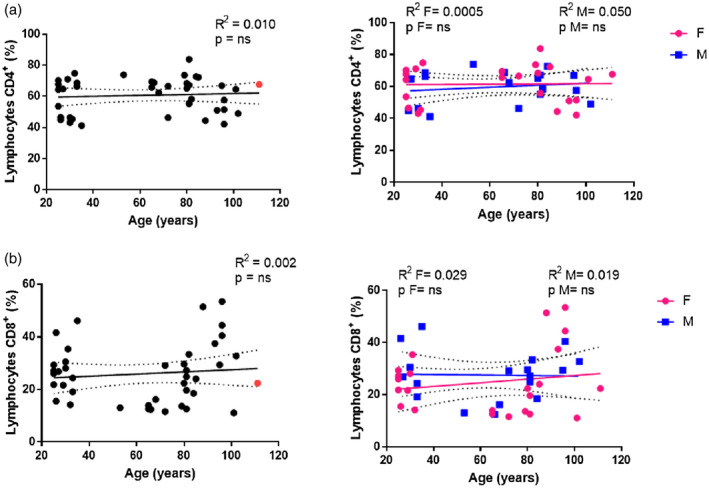
Analysis of CD4 and CD8 T cells. Linear regression analysis showing the relationship between lymphocytes CD4+ % (a), CD8+ % (b) and age in all individuals (n = 41) (black line), males (n = 18) (blue line) and females (n = 23) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. The supercentenarian is shown in red in the graphs on the left. R 2 = R squared; n.s. = not significant; F = female; M = male
FIGURE 4.
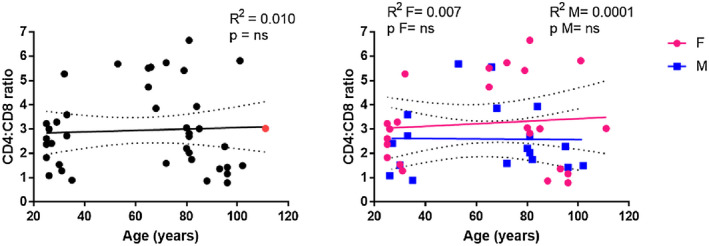
Correlations between the CD4/CD8 ratio and age. CD4/CD8 ratio of 41 donors (M = 18; F = 23) was calculated by dividing the CD4 T cell percentage by CD8 T cell percentage. The supercentenarian is shown in red in the graph on the left. R 2 = R squared; n.s. = not significant; F = female; M = male
In order to investigate the impact of ageing upon T cell subsets, we examined age‐related changes in the markers of differentiation within the CD4+ and CD8+ T cell subsets in PBMCs from a subcohort of 27 healthy donors divided by age and gender, as described in Table 1. Gating was performed on the CD4/CD8 dot‐plot, followed by CD4+ or CD8+ specific subgating according to CD45RA and CD197 (CCR7) expression, as described above. An example of the applied gating strategy is displayed in Figure 5.
FIGURE 5.
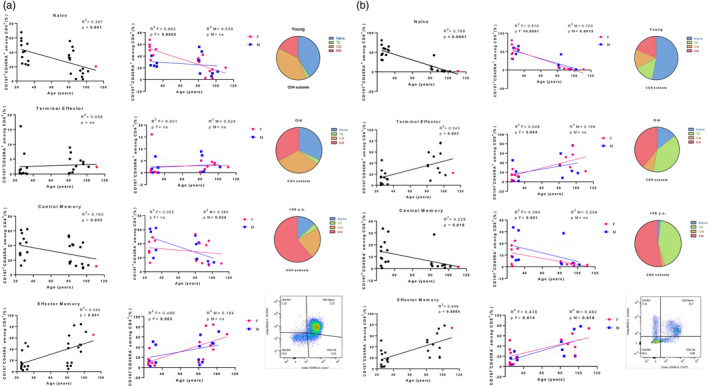
Analysis of CD4 and CD8 T cell subsets. Linear regression analysis shows the relationship between CD4+ (a), CD8+ (b) subsets and age in all individuals (n = 27) (black line), males (n = 12) (blue line) and females (F = 15) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. The supercentenarian is shown in red in the graphs (a,b) on the left. R 2 = R squared; n.s. = not significant; F = female; M = male. Right (a,b): representative fluorescence activated cell sorter (FACS) gating of (a) CD4+ and (b) CD8+ subsets. In the pie charts is depicted an overview of the differences among young donors, old people and long‐lived individuals (LLI) donors in the proportions of T cell subsets
The percentages of CD4+‐naive cells significantly declined with age in the combined group of males and females (Figure 5a, R 2 = 0.347, P = 0.001), but this was attributable to a significant decrease in CD4+CD197+CD45RA+ cells in female subjects (R 2 = 0.662, P = 0.0002), while a constant trend was observed in the older males (R 2 = 0.039, P = n.s.). These results might suggest that older males were able to better maintain a naive phenotype of CD4+ T cell subset during ageing. However, it should be taken into account that the young females in our cohort showed a higher percentage of naive CD4+ T cells (ranging from 33 to 68%, mean = 51.38 ± 11.42) than their male counterparts (ranging from 22 to 41%, mean = 27.8 ± 7.45). Similarly, the significant increase in the EM CD4+ T subset observed in ungrouped individuals (R 2 = 0.345, P = 0.001) was mainly ascribable to the female group (R 2 = 0.496, P = 0.003), while a significant decrease in CM (R 2 = 0.163, P = 0.033) CD4+ T cells was recorded in males (R 2 = 0.380, P = 0.024) but not in females. For TE CD4+ T cell subset, no significant age‐dependent effects were detected.
Consistent with other reports (6, 7, 12, 13), in our subcohort the percentages of CD8+‐naive cells significantly declined with age (R 2 = 0.769, P < 0.0001), these data being confirmed in both females (R 2 = 0.816, P < 0.0001) and males (R 2 = 0.700, P = 0.0013). The data showed a concomitant significant increase in the percentage of both TE (R 2 = 0.343, P = 0.003; females: R 2 = 0.548, P = 0.004) and EM (R 2 = 0.448, P = 0.0004; females: R 2 = 0.438, P = 0.014; males: R 2 = 0.482, P = 0.018), and a significant decrease in CM (R 2 = 0.229, P = 0.018; females: R 2 = 0.394, P = 0.021) CD8+ subsets (Figure 5b). Although naive CD8+ cells as well as the less represented CM CD8+ subset declined dramatically with age, the concomitant increase in TE and EM CD8+subsets contributed to the maintenance of an almost constant total CD8+ T cell percentage with age.
An overview of the differences among young donors, older people and long‐lived individuals (LLI; aged >90 years) in the proportions of T cell subsets is depicted in the pie charts in Figure 5a,b.
In order to evaluate the general effect of persistent viral infection on T cell subset distribution, we screened our subcohort of 27 subjects for CMV and EBV seropositivity. As shown in Table 1, all individuals were EBV‐seropositive, while 16.6% of young donors and 100% of older subjects and LLI were CMV‐seropositive. Thus, it would be plausible to hypothesize that the progressive accumulation of T cells with a TE and EM phenotype in older individuals of our cohort might be directly influenced by CMV infection, but not by EBV infection. However, a CMV‐specific T analysis should be conducted to confirm this inference.
Analysis of γδT cells
We also analysed the γδT cell percentage from 25 healthy donors, 14 females (aged 31–102 years) and 11 males (aged 35–101 years), identified by their γδ expression on CD3+ T cells. Correlation analysis in the combined group indicated that the percentage of γδT cells remained unchanged with age (Figure 6). However, when analysed separately, the male and female groups showed a reverse trend in the γδT cell percentages. Specifically, a lower trend in γδT cell percentages with age was observed in the female group, while in the males the fraction of these cells was increased.
FIGURE 6.
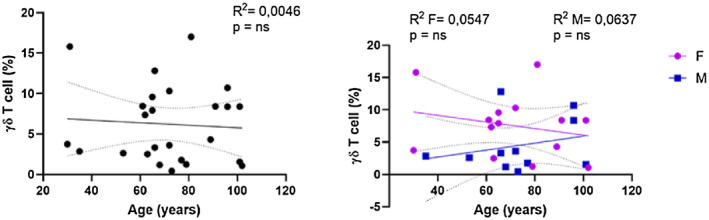
Analysis of γδT cells. Linear regression analysis shows the relationship between CD3+ γδ+ T cells and age in all individuals (n = 26) (black line), males (n = 12) (blue line) and females (n = 14) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. R 2 = R squared; n.s. = not significant; F = female; M = male
Analysis of NK and NK T cells
NK and NK T cells from 40 healthy donors, 22 females (aged 25 101 years) and 18 males (aged 26–102 years) were analysed. NK cells (defined as CD3−, CD16negative to positive and CD56+) were divided into two subsets based on their CD56 and CD16 expression (25, 27). The CD56lowCD16+ subset (NK1, Figure 8a) is mainly responsible for natural cytotoxicity by releasing cytoplasmic granules containing perforin and granzymes B. In contrast, the CD56highCD16− subset (Figure 8b) is described as secreting chemokines and cytokines.
FIGURE 8.
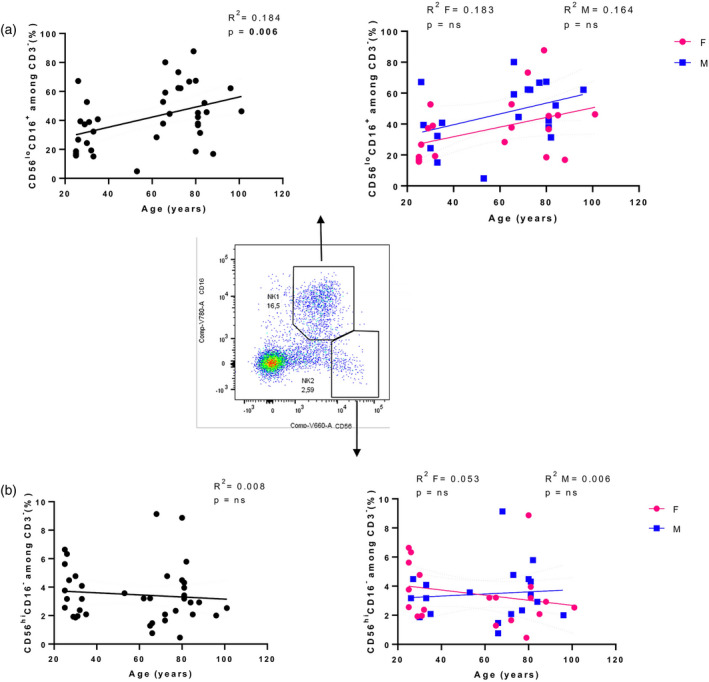
Analysis of natural killer (NK) subsets. Linear regression analysis showing the relationship between cytotoxic (a), secreting cytokines (b) NK cell subsets and age in all individuals (n = 40) (black line), males (n = 18) (blue line) and females (n = 22) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. Centre: representative FACS gating of CD56lowCD16+ and CD56highCD16‐ NK cell subsets; R 2 = R squared; n.s. = not significant; F = female; M = male
An increase in the percentage of NK cells in healthy ageing has previously been reported, in association with a reduced fraction of CD56high NK cell subset and an expansion of the CD56low NK cells (15, 16). Accordingly, NK cell percentages in the peripheral blood of the older people showed a significant increase depending on age (Figure 7, R 2 = 0.124, P = 0.025), becoming not significant when genders were analysed separately.
FIGURE 7.
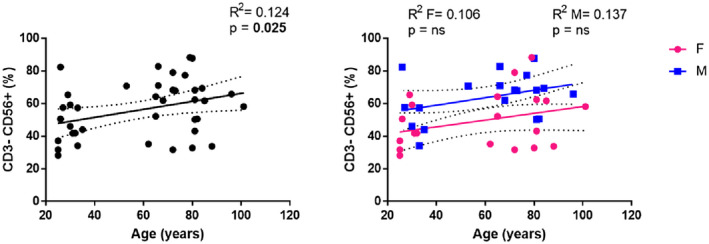
Analysis of natural killer (NK) cells. Linear regression analysis showing the relationship between CD3−CD56+ NK cells and age in all individuals (n = 40) (black line), males (n = 18) (blue line) and females (n = 22) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. R 2 = R squared; n.s. = not significant; F = female; M = male
Also, when CD3−CD56+ cells were subdivided into CD56lowCD16+ and CD56highCD16− NK cells there was a significant increase in cytotoxic NK cells in parallel with increasing age (Figure 8a, R 2 = 0.184, P = 0.006). In contrast, only a non‐significant lower trend in the frequency of cytokine‐secreting NK cells in the older population was detected (Figure 8b, R 2 = 0.008, P = n.s.).
NK T cells, a cell type sharing some functional and phenotypical characteristics with NK cells (28), were determined by their CD3 and CD56 co‐expression (Figure 9). Overall, a non‐significant age‐related increase was observed in the NK T cells (R 2 = 0.023, P = n.s.), mainly attributable to the female group.
FIGURE 9.
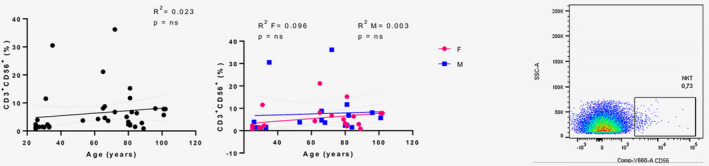
Analysis of natural killer (NK) T cells. Linear regression analysis showing the relationship between CD3+CD56+ NK T cells and age in all individuals (n = 40) (black line), males (n = 18) (blue line) and females (n = 22) (pink line). The coefficient of determination and P‐values are shown on the graphs. Each point represents data from an individual healthy donor. Right: an example of the gating strategy for NK T cells. R 2 = R squared; n.s. = not significant; F = female; M = male
Comparison with the T subset profile of a supercentenarian
Analyses of the T cell subset included a cohort of 41 subjects with the oldest living Italian supercentenarian. In the graphs displaying gender‐merged analysis, the values for the supercentenarian are reported as a red dot. It is possible to note that in the CD4+ T cell percentage graph the red dot (Figure 3, 66.6%) seems to be close to the upper limit of the 95% confidence interval, while in the CD8+ T cell percentage graph the same point (Figure 3, 22.35%) is localized at the lower limit line. The resulting CD4/CD8 ratio was 3.03 (Figure 4), just located on the regression line, in contrast to the other dots that are widely scattered around the cohort regression line. The values of both CD4+‐ and CD8+‐naive T cells for the supercentenarian were lower than those observed in the young females (Figure 5a,b). However, compared to the individuals aged 90–100 years, the CD4+‐naive T cell value for the supercentenarian (Figure 5a, 20.38%) is above the regression line, as in the case of values recorded for the octogenarian subjects. In contrast, the percentage of EM CD4+ T cells for the supercentenarian (Figure 5a, 65.44%) seems to be above the line as the LLIs, differently to the octogenarian subjects whose values are clustered below. Regarding the cytotoxic compartment, our Italian doyenne shows one of the highest values of EM CD8+ T cells (Figure 5b, 74.32%) but a percentage of CM CD8+ T cells (Figure 5b, 1.56%), comparable to their younger counterparts.
DISCUSSION
In the present study, we analysed the effect of ageing on the composition of immune system cell subsets, in correlation with gender and CMV and EBV serostatus, in a cohort of Sicilian donors, ranging from young individuals to a supercentenarian. We set our focus on T and NK cells. Our approach may be considered of some interest, as it includes the immunophenotypical characterization of the supercentenarian immune system. Data concerning supercentenarian immune subsets are extremely rare in literature and, to the best of our knowledge, have only a transcriptomic focus (29).
In more than 15 years of research in the field it has been well documented that ageing is associated with a decrease in the absolute count of CD4+ T lymphocytes, with higher numbers reported for females, expansion of effector/memory CD8+ T cells and contraction of both naive CD4+ and CD8+ T cell compartments (12, 30, 31, 32, 33, 34). These processes cannot be simply considered as the direct consequence of thymic involution, as they are coupled with stimulation exerted by pathogens (especially by latent herpesviruses) during the whole life of an individual (2, 7, 8, 9, 32, 34, 35, 36, 37, 38). The most notable manifestation of the listed events is represented by the inverted CD4/CD8 ratio. The probability to detect an inverted CD4/CD8 ratio increases with age, and is more prominent in males than in females (11, 32, 33, 39). As stated in the Introduction, controversies still exist regarding this altered ratio as it is considered to be a marker of risk of frailty, although this association was not found in all populations studied (37).
The results of our study show a constant trend in the percentages of both CD4+ and CD8+ T cells with age. Consequently, we did not observe the described age‐related increase in the rate of inversion of the CD4/CD8 ratio, whereas this value tends to increase in LLIs and to decrease in the supercentenarian. As depicted by the linear regression graph, the total CD4+ and CD8+ T cell percentages in individuals aged 90–100 years appear to exhibit different trends to those recorded in octogenarian subjects. Most 90–100‐year‐old donors show lower CD4+ T cell percentages, in line with data for the supercentenarian and higher CD8+ T cells percentages than octogenarian donors. It remains to be assessed if these fluctuations in CD4+ and CD8+ T cell percentages are correlated with the probability of reaching successful ageing. Another possible explanation may be represented by other concomitant causes determining the reduced detection of the CD4/CD8 ratio inversion in our pool of donors, such as different lifestyle and environmental factors such as diet, gut microbiota, access to health care, exposure to pathogens and pollution, as well as genetic background (37).
As mentioned above, an age‐related shift from antigen‐inexperienced naive T lymphocytes to antigen‐experienced memory and effector T cells has been extensively reported. These phenomena are due to both long‐term repeated exposure to antigens together with reduced thymopoiesis (2, 5, 12, 35). Our analyses demonstrated that age influences diversely lymphocyte subpopulations in the whole sampled population. Similar to previous reports (30, 31, 32), ageing was associated with a significant reduction in both naive CD4+ and CD8+ T cells. Also, a significant reduction in CM CD4+ and CD8+ T cells was observed in our cohort, contradicting those reports recording an age‐related increase in both T cell fractions (31, 40, 41). Finally, a significant increase in EM CD4+ and CD8+ T cells and in TE CD8+ T cells was observed, once again confirming previous reports documenting that the EM population increase with age (30, 40, 42).
Age‐related differences in T cell subset distribution may partly be explained by the presence of chronic viral infections. As CMV is more frequent in older individuals of our cohort, CMV seropositivity may have influenced the T cell subset specific expansion and survival with age (30, 40, 42). In contrast, EBV infection seems to play no role, as all individuals in this study were EBV‐seropositive. Indeed, accumulation of late‐stage CD8+ T cells, some of which may indeed be senescent and contribute to age‐related diseases (43), are predominantly observed in CMV‐seropositive older people, whereas older people infected with other persistent herpesviruses, such as EBV, do not show similar effects seemingly limited to CMV (38). Moreover, it has been claimed that a small proportion of CMV‐infected individuals is able to counteract the pathological effects of the CMV‐related accumulation of these cells attaining longevity (44), as seems to be happening in our LLIs, including the supercentenarian.
Our data also demonstrated that γδT cells appeared to remain unchanged with increasing age. This is consistent with the demonstration by Argentati et al. (45), that the percentage of CD3+ γδT cells in the peripheral blood was heterogeneous in the different age groups, with mean values not significantly different among the young donors, older subjects, and centenarians.
NK cell subsets are known to be differentially affected by ageing with a gradual decrease in the more immature CD56+ NK cells, while the percentage and the absolute number of CD56+CD16+ NK cells have been variably reported as maintained, increased or decreased in the older subjects and consistently increased in the centenarians (2, 16, 19, 46, 47). Accordingly, our results showed a significant increase in cytotoxic NK cells with ageing, but no significant lower trend in the frequency of cytokine‐secreting NK cells in the older population. Finally, the number of circulating NK cells was slightly lower in females than in males.
NK T cells have been described to decrease in older people and increase in centenarians (19). In our study, a slightly age‐related increase was observed in the NK T cells, mainly attributable to the female group.
Because these non‐significant gender‐related changes of NK and NK T cells, our data also suggest that the CD4+ and CD8+ T cell subsets are not affected by age in an equal manner between females and males. Indeed, the influence of gender was significant in various lymphocyte subpopulations. First, a significant age‐related decline in the lymphocyte counts in males was identified. Males also showed significantly lower percentages of CM CD4+ T cells. The reduction in naive and the increase in EM CD8+T cells were confirmed as significant in both genders, although the significance is higher in women, especially for the naive compartment. This is in contrast with a previous report (32), where both very old and middle‐aged females showed a higher percentage of naive CD8+ T cells than males. Females also exhibited a significant decrease in naive CD4+ and CM CD8+ T cells.
Biological causes such as hormonal differences or the presence of two X chromosomes, in addition to other gender effects, could partially explain the age‐dependent differences in cell counts and percentages emerging comparing male and female donors in our study (48). We might also speculate that gender could impact the response to CMV infection. In fact, it is well known that females are more resistant to infections (1, 48).
A recent epigenetics study has confirmed that PBMCs of females and males significantly differ after 65 years of age. This analysis revealed that older females have higher genomic activity for B and T cells, suggesting that the age‐related decline in T cells is greater in men. Older males, in contrast, have higher activity for monocytes and inflammation (49).
Centenarians are considered a model of successful ageing (17, 50, 51). Therefore, supercentenarians, i.e. people who have reached 110 years of age, are a superb model of successful ageing (29). Their characteristics of delayed onset of age‐related diseases and compression of morbidity (52, 53) imply that their immune system remains functional (29). Here, we analysed the immunological profile of the oldest living woman in Italy (aged 111 years), born and bred in Sicily, and compared her immunophenotype to that of Sicilian young, older and female LLIs donors.
From the graphs displaying values for naive and EM CD4+ T cells, it is interesting to note that the octogenarians seem to have higher percentages of naive CD4+ T cells than nonagenarians and centenarians, and the supercentenarian seems to represent a meeting‐point between these two groups. Conversely, nonagenarians and centenarians show higher percentages of EM CD4+ T cells than octogenarians, but these values are similar to those reported for the supercentenarian. Our report is limited by the small number of analysed samples. However, it would be interesting to monitor these octogenarian subjects over time to see if with advancing age their immune profile in the context of CD4+ T cells changes like that of their older counterparts and, especially, that of the supercentenarian.
Recently, circulating immune cells of supercentenarians have been analysed at single‐cell resolution. This permitted the identification of CD4+ T cells that have cytotoxic characteristics. This feature is truly unique to supercentenarians, as CD4+ T cells generally have helper functions, but no cytotoxic activity (29). This may represent an essential adaptation to achieve exceptional longevity by sustaining immune responses to infections and diseases.
Thus, an in‐depth immunophenotypical and functional characterization of immune subsets of octogenarians followed over time could help to more clearly understand if our experimental evidence, together with previously reported data, could be helpful in predicting the entity of changes affecting the immune system during ageing, and the probability of reaching an advanced age with a functional and adequately adapted defensive repertoire.
In conclusion, ageing dramatically affects both the relative presence and function of the different T cell subsets. These changes are likely to be among factors contributing to the reduced vaccination efficacy and decreased resistance to infections, as well as increased prevalence of cancer in the older population; i.e. immunosenescence (1, 2, 7). However, our knowledge of immunosenescence is likely to be still very incomplete. More detailed data are needed, particularly on the immune phenotype of semi‐ and super‐centenarians, to identify strategies that may counteract the effects of ageing on the immune system. A principal objective of such studies will be the identification of potential interventions that could reduce the incidence of and morbidity for age‐associated diseases by better preservation and stimulation of functional immune competence in the course of chronological ageing.
Supercentenarians show a unique immunophenotypical signature regarding the relative percentages of their T cell subsets. Recent reports also confirm that their immune cells also show previously unknown functional properties (29). It would be reasonable to think that the key of successful ageing may be encountered in the supercentenarians’ uncommon immune characteristics. An internationally coordinated effort would be highly recommended in order to extend the characterization of supercentenarians’ immune system beyond the geographical borders, and to create an immunophenotypical and genomic database to share the details at a worldwide level. Such a global commitment may be beneficial in terms of sharing these rare pieces of information and of speeding up the extrapolation of a possible predictive ‘successful‐ageing‐profile’.
CONFLICT OF INTEREST
The authors have no competing interests or conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
M. E. L. and F. P. performed statistical analysis of the data and drafted the manuscript. C. C. revised the manuscript and all authors contributed with the critical revising of the manuscript. M. E. L., M. B., F. G. and N. Z. performed the cytometric analyses. F. B. and G. G. performed the serological analyses. M. E. L., C. C., G. C. and F. F. conceived the study. C. C. was responsible with G. A., A. A. and S. A. for patient recruitment and characterization. All authors checked statistical analysis of the data. All authors approved the final version of the manuscript
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of Palermo University Hospital (Sicily, Italy) approved the study protocol (Nutrition and Longevity, no. 032017), and written informed consent was obtained from all participants.
Supporting information
Table S1
ACKNOWLEDGEMENTS
The research was funded by Italian Ministry of University (PRIN: progetti di ricerca di rilevante interesse nazionale—Bando 2015 Prot 20157ATSLF Discovery of molecular and genetic/epigenetic signatures underlying resistance to age‐related diseases and comorbidities) to C. C. and G. C. Work in the Molecular Medicine Group at King’s College London was supported by CRUK, the Experimental Cancer Medicine Centre, and the NIHR Biomedical Research Centres (BRC) based at King’s Health Partners. The authors thank Doctor Sergio Rizzo, Chief of Unit of Transfusion Medicine of University Hospital ‘Paolo Giaccone’ for performing leukocyte counts. The authors also thank all the donors for their kind participation in this study.
Ligotti and Aiello contributed equally as co‐first authors.
Caruso, Farzaneh, and Candore contributed equally as senior authors.
DATA AVAILABILITY STATEMENT
The data sets generated and/or analysed during the current study are not publicly available due to privacy reasons, but are available in anonymized form from the authors on reasonable request.
REFERENCES
- 1. Caruso C, Vasto S. Immunity and aging. In: Ratcliffe MJH, editor. Encyclopedia of immunobiology, vol. 5. Oxford: Academic Press. 2016. p. 127–32. [Google Scholar]
- 2. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10:2247. 10.3389/fimmu.2019.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu W, Larbi A. Markers of T cell senescence in humans. Int J Mol Sci. 2017;18:1742. 10.3390/ijms18081742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020;134:110887. 10.1016/j.exger.2020.110887 [DOI] [PubMed] [Google Scholar]
- 5. Clave E, Araujo IL, Alanio C, et al., Milieu Intérieur Consortium . Human thymopoiesis is influenced by a common genetic variant within the TCRA‐TCRD locus. Sci Transl Med. 2018;10:eaao2966. 10.1126/scitranslmed.aao2966 [DOI] [PubMed] [Google Scholar]
- 6. Aiello A, Accardi G, Candore G, Caruso C, Colomba C, Di Bona D, et al. Role of immunogenetics in the outcome of HCMV Infection: implications for ageing. Int J Mol Sci. 2019;20:685. 10.3390/ijms20030685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pawelec G, Akbar A, Caruso C, Solana R, Grubeck‐Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. 10.1111/j.0105-2896.2005.00271.x [DOI] [PubMed] [Google Scholar]
- 8. Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–55. 10.4049/jimmunol.1301721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Benedetto S, Derhovanessian E, Steinhagen‐Thiessen E, Goldeck D, Müller L, Pawelec G. Impact of age, sex and CMV‐infection on peripheral T cell phenotypes: results from the Berlin BASE‐II Study. Biogerontology 2015;16:631–43. 10.1007/s10522-015-9563-2. [DOI] [PubMed] [Google Scholar]
- 10. Colonna‐Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, et al. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West‐Sicily. Exp Gerontol. 2007;42:995–1002. 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11. McBride JA, Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T‐cell ratio tell us about HIV and health? PLOS Pathog. 2017;13:e1006624. 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson BO, Ferguson FG. Age‐related change in peripheral blood T‐lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 13. Luz Correa B, Ornaghi AP, Cerutti Muller G, Engroff P, Pestana Lopes R, Gomes da Silva Filho I, et al. The inverted CD4:CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. NeuroImmunomodulation 2014;21:206–12. 10.1159/000356827. [DOI] [PubMed] [Google Scholar]
- 14. Hooten N, Longo DL, Evans M. Age‐ and race‐related changes in subpopulations of peripheral blood lymphocytes in humans. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook of Immunosenescence. Cham: Springer, 2018: pp. 396–424. 10.1007/978-3-319-64597-1_85-1. [DOI] [Google Scholar]
- 15. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–41. 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 16. Witkowski JM, Larbi A, Le Page A, Fülöp T. Natural killer cells, aging, and vaccination. Interdiscip Top Gerontol Geriatr. 2020;43:18–35. 10.1159/000504493. Epub 2020 Apr 9. [DOI] [PubMed] [Google Scholar]
- 17. Caruso C, ed. Centenarians. An example of positive biology. Cham: Springer, 2019. 10.1007/978-3-030-20762-5. [DOI] [Google Scholar]
- 18. Bulati M, Caruso C, Colonna‐Romano G. B cells in centenarians and their offspring. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook of immunosenescence. Cham: Springer, 2019; pp. 841–842. 10.1007/978-3-319-99375-1_88 [DOI] [Google Scholar]
- 19. Bianchini E, Pecorini S, De Biasi S, Gibellini L, Nasi M, Cossarizza A, et al. Lymphocyte subtypes and functions in centenarians as models for successful aging. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook of immunosenescence. Cham: Springer, 2018: pp. 1–37. 10.1007/978-3-319-64597-1_2-1 [DOI] [Google Scholar]
- 20. Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–6. 10.1016/0167-5699(95)80064-6 [DOI] [PubMed] [Google Scholar]
- 21. Italian National Institute of Statistics [in Italian]. Available at: https://www.istat.it/it/archivio/232302 (accessed 28 September 2020).
- 22. The Italian supercentenarians database [in Italian]. Available at: https://www.supercentenariditalia.it/persone‐viventi‐piu‐longeve‐in‐italia (accessed 29 March 2020).
- 23. Ostan R, Monti D, Gueresi P, Bussolotto M, Franceschi C, Baggio G. Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender‐specific medicine. Clin Sci. 2016;130:1711–25. 10.1042/CS20160004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiello A, Accardi G, Aprile S, Caldarella R, Carru C, Ciaccio M, et al. Age and gender‐related variations of molecular and phenotypic parameters in a cohort of Sicilian population: from young to centenarians. Aging Dis 2021;12. 10.14336/AD.2021.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rühle PF, Fietkau R, Gaipl US, Frey B. Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int J Mol Sci. 2016;17:1316. 10.3390/ijms17081316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clénet ML, Gagnon F, Moratalla AC, Viel EC, Arbour N. Peripheral human CD4+CD8+ T lymphocytes exhibit a memory phenotype and enhanced responses to IL‐2, IL‐7 and IL‐15. Sci Rep. 2017;7:11612. 10.1038/s41598-017-11926-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009;126:458–65. 10.1111/j.1365-2567.2008.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–84. 10.1111/j.1474-9728.2004.00107.x [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto K, Kouno T, Ikawa T, Hayatsu N, Miyajima Y, Yabukami H, et al. Single‐cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc Natl Acad Sci USA. 2019;116:24242–51. 10.1073/pnas.1907883116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong MS, Dan JM, Choi JY, Kang I. Age‐associated changes in the frequency of naïve, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–8. 10.1016/j.mad.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 31. Stervbo U, Bozzetti C, Baron U, Jürchott K, Meier S, Mälzer JN, et al. Effects of aging on human leukocytes (part II): immunophenotyping of adaptive immune B and T cell subsets. Age 2015;37:93. 10.1007/s11357-015-9829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wikby A, Månsson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 2008;9:299–308. 10.1007/s10522-008-9138-6 [DOI] [PubMed] [Google Scholar]
- 33. Strindhall J, Skog M, Ernerudh J, Bengner M, Löfgren S, Matussek A, et al. The inverted CD4/CD8 ratio and associated parameters in 66‐year‐old individuals: the Swedish HEXA immune study. Age 2013;35:985–91. 10.1007/s11357-012-9400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Weyand CM, Goronzy JJ. Hallmarks of the aging T cell system. FEBS J. 2021. doi: 10.1111/febs.15770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111. 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wikby A, Nilsson B‐O, Forsey R, Thompson J, Strindhall J, Löfgren S, et al. The immune risk phenotype is associated with IL‐6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 37. Pawelec G. Immune parameters associated with mortality in the elderly are context‐dependent: lessons from Sweden, Holland and Belgium. Biogerontology 2018;19:537–45. 10.1007/s10522-017-9739-z [DOI] [PubMed] [Google Scholar]
- 38. Derhovanessian E, Maier AB, Hähnel K, Beck R, de Craen AJM, Slagboom EP, et al. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late‐differentiated CD4+ and CD8+ T‐cells in humans. J Gen Virol. 2011;92(Pt 12):2746–56. 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 39. Strindhall J, Löfgren S, Främsth C et al. CD4/CD8 ratio <1 is associated with lymphocyte subsets, CMV and gender in 71‐year old individuals: 5‐year follow‐up of the Swedish HEXA Immune Longitudinal Study. Exp Gerontol. 2017;95:82–7. 10.1016/j.exger.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 40. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–81. 10.1016/j.mad.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 41. Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17:476–9. 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drijvers JM, Sharpe AH, Haigis MC. The effects of age and systemic metabolism on anti‐tumor T cell responses. Elife 2020;9:e62420. 10.7554/eLife.62420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience. 2017;39:261–71. 10.1007/s11357-017-9983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Derhovanessian E, Maier AB, Beck R, Jahn G, Hähnel K, Slagboom PE, et al. Hallmark features of immunosenescence are absent in familial longevity. J Immunol. 2010;185:4618–24. 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 45. Argentati K, Re F, Donnini A, Tucci MG, Franceschi C, Bartozzi B, et al. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J Leukoc Biol. 2002;72:65–71. [PubMed] [Google Scholar]
- 46. Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. 2014;29:56–61. 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 47. Solana C, Tarazona R, Solana R. Immunosenescence of natural killer cells, inflammation, and Alzheimer’s disease. Int J Alzheimers Dis. 2018;2018:3128758. 10.1155/2018/3128758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caruso C, Accardi G, Virruso C, Candore G. Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun Ageing. 2013;10:20. 10.1186/1742-4933-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Márquez EJ, Chung C‐H, Marches R, Rossi RJ, Nehar‐Belaid D, Eroglu A, et al. Sexual‐dimorphism in human immune system aging. Nat Commun. 2020;11:751. 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caruso C, Passarino G, Puca A, Scapagnini G. ‘Positive biology’: the centenarian lesson. Immun Ageing. 2012;9:5. 10.1186/1742-4933-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lio D, Scola L, Giarratana RM, Candore G, Colonna‐Romano G, Caruso C, et al. SARS CoV2 infection _The longevity study perspectives. Ageing Res Rev. 2021;67:101299. 10.1016/j.arr.2021.101299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Accardi G, Aiello A, Aprile S, Caldarella R, Cammarata G, Carru C, et al. The phenotypic characterization of the Cammalleri sisters, an example of exceptional longevity. Rejuvenation Res. 2020;23:476–84. 10.1089/rej.2019.2299. [DOI] [PubMed] [Google Scholar]
- 53. Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data sets generated and/or analysed during the current study are not publicly available due to privacy reasons, but are available in anonymized form from the authors on reasonable request.


