Abstract
Purpose
Numerous studies have applied a variety of methods to assess paraspinal muscle degeneration. However, the methodological differences in imaging evaluation may lead to imprecise or inconsistent results. This article aimed to provide a pragmatic summary review of the current imaging modalities, measurement protocols, and imaging parameters in the evaluation of paraspinal muscle fat infiltration (FI) in MRI studies.
Methods
Web of Science, EMBASE, and PubMed were searched from January 2005 to March 2020 to identify studies that examined the FI of paraspinal muscles on MRI among patients with lumbar degenerative diseases.
Results
Intramyocellular lipids measured by magnetic resonance spectroscopy and FI measured by chemical‐shift MRI were both correlated to low back pain and several degenerative lumbar diseases, whereas results on the relationship between FI and degenerative lumbar pathologies using conventional MRI were conflicting. Multi‐segment measurement of FI at the lesion segment and adjacent segments could be a prognostic indicator for lumbar surgery. Most studies adopted the center of the intervertebral disc or endplate as the level of slice to evaluate the FI. Compared with visual semiquantitative assessment, quantitative parameters appeared to be precise for eliminating individual or modality differences. It has been demonstrated that fat CSA/total CSA (based on area) and muscle–fat index (based on signal intensity) as quantitative FI parameters are associated with multiple lumbar diseases and clinical outcomes after surgery.
Conclusion
Having a good command of the methodology of paraspinal muscle FI on MRI was effective for diagnosis and prognosis in clinical practice.
Keywords: Degeneration, Fat infiltration, Imaging evaluation, Magnetic resonance imaging, Methodology, Introduction
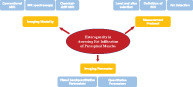
Heterogeneity in assessing the paraspinal muscle fat infiltration exists in imaging modalities, measurement protocols (including measured level and slices, region of interest definition, and fat detection methods), and imaging parameters (semiquantitative and quantitative parameters).
Introduction
Fat infiltration (FI), a crucial indicator of composition change of paraspinal muscle degeneration, may contribute to the loss of muscle strength and endurance 1 . There has been increasing interest in imaging evaluation of FI as a potential diagnostic and prognostic tool in lumbar spine health in recent decades 2 .
Systematic reviews have pointed out that the impact of FI on diseases has conflicting results 3 , 4 , 5 . These discrepancies might be due to methodological differences, such as imaging modality, measurement protocols, and parameter selection 1 , 4 , 6 . Several advanced MRI approaches have been proposed since 2006, including magnetic resonance spectroscopy (MRS) and chemical‐shift MRI 7 , 8 . Heterogeneity of measurement protocols involves the level and slice selection (e.g. slice positioning) and the definition of the region of interest (ROI) 1 . Furthermore, numerous imaging parameters hitherto have been used to describe the degree of FI, including semiquantitative and quantitative parameters 1 .
To our knowledge, no prior systematic reviews have examined these imaging methods. This review aimed to summarize the existing MRI methods of FI assessment from the methodological perspective of imaging modalities, measurement protocols, and parameter selection, and to discuss the diagnostic benefits for lumbar degenerative diseases of using various methods for measuring FI.
Literature Review
Three electronic databases (Web of Science, Embase, and PubMed) were searched from January 2005 to March 2020 to identify studies that examined the FI of the paraspinal muscles (psoas, multifidus, and erector spinae). All fields were searched for these terms: “paraspinal muscles,” “multifidus,” “transversospinales,” “erector spinae,” or “psoas major”; and “spinal degeneration” or “low back pain”. Two independent reviewers determined whether studies were included based on the following inclusion criteria: (i) recruited participants who have reported lumbar degenerative diseases (i.e. radiculopathy, disk herniation, sciatica, spinal stenosis, spondylolysis, spondylolisthesis, osteoarthritis, or facet joint osteoarthritis) or nonspecific low back pain (LBP); and (ii) employed MRI (conventional MRI, MRS, and chemical‐shift MRI) to measure the FI of paraspinal muscles. Exclusion criteria were: (i) patients without lumbar degenerative diseases or who were younger than 18 years of age to exclude some idiopathic spinal diseases; (iii) patients not involved in any FI assessments; (iii) patients evaluated only by kinematic MRI; (iv) case reports, editorials/letters, literature reviews, guidelines, and abstract‐only publications; and (v) non‐English literature.
The literature review identified 4500 articles, of which 136 full‐text articles were retrieved for full review. After the screening of titles and abstracts, the full text was retrieved and a total of 78 studies were deemed to meet the inclusion criteria. A search flow diagram is presented in Fig. 1.
Fig 1.
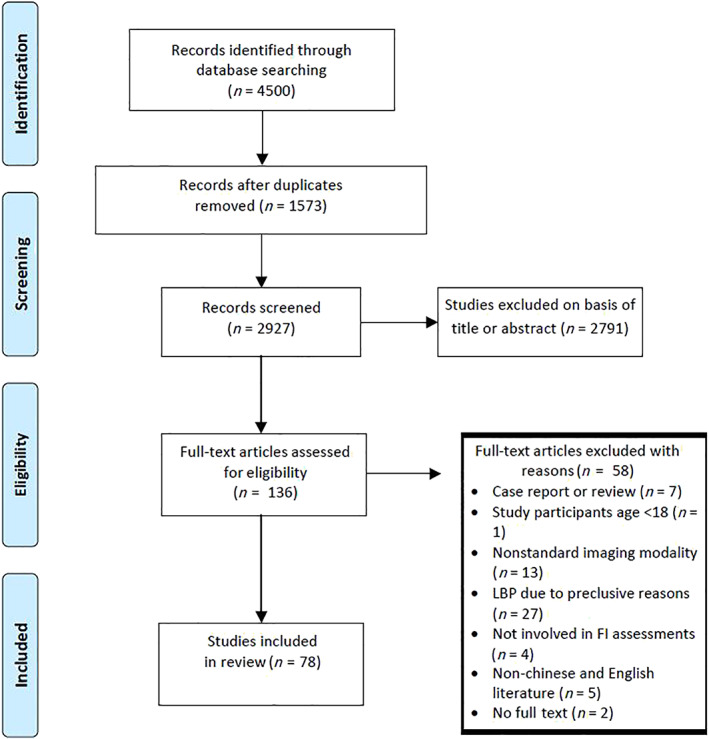
The selection flow for studies included in this review.
Imaging Modality
Conventional MRI
Most studies 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 used conventional MRI for measuring FI. In the field of MRI sequence, our results showed that T2‐weighted images 10 , 12 , 13 , 14 , 17 , 18 , 20 , 24 , 25 , 27 , 29 , 30 , 32 , 33 , 34 , 35 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 58 , 59 , 60 , 61 , 62 , 63 , 65 , 66 , 69 , 70 were used in quantitative assessments more often. It is expedient for orthopaedists to evaluate FI on frequently‐used T2‐weighted images. Suh et al. found that the intrarater and interrater reliability of parameters were generally excellent for both T1‐weighted and T2‐weighted images 53 . For predicting LBP using conventional MRI, cross‐sectional studies found that greater FI was associated with LBP 50 , 68 . However, two longitudinal studies reported no association between FI and LBP 22 , 62 .
Novel MRI Modalities
Because of the relatively limited accuracy of conventional MRI, several novel MRI modalities have emerged. We found some studies that applied MRS 7 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , chemical‐shift MRI 51 , 69 , 82 , 83 , 84 , 85 and multi‐echo MRI 75 . A comparison of different MRI modalities is included in Table 1.
TABLE 1.
Comparison of different MRI modalities
| MRI modality | Characteristic | Application |
|---|---|---|
| Conventional MRI T2‐weighted | Convenient in clinical practice; the accuracy is relatively low | The most commonly used tool in quantitative assessment |
| MRS | Can record the concentration of both IMCL and EMCL | IMCLs were correlated to several degenerative lumbar pathologies |
| Chemical‐shift MRI | Overmatches MRS in terms of EMCL; the contemporary standard for measuring EMCL | PDFF were correlated to several degenerative lumbar pathologies; and predicted the paraspinal muscle strength better than CSA |
| Multi‐echo MRI | Produces a concurring result compared with MRS | Have been performed in LBP patients |
CSA, cross‐sectional area; EMCL, extramyocellular lipids; IMCL, intramyocellular lipids; LBP, low back pain; MRS, magnetic resonance spectroscopy; PDFF, proton density fat fractions.
MRI has facilitated detailed analyses of muscular fat masses by separating and recording the concentration of extramyocellular lipids (EMCL) and intramyocellular lipids (IMCL), which is not achievable with other technology (Fig. 2) 78 . EMCL and IMCL may play different roles in degenerative pathology. Studies have demonstrated that IMCL are significantly higher in those with chronic LBP and that there is a positive correlation between IMCL and VAS 78 , 79 , 80 . IMCL are also significantly correlated with sagittal alignment and anterior annulus fibrosus degeneration 76 , 77 . In contrast, EMCL have not been found to be significantly different between chronic LBP and normal groups 76 . Thus, IMCL of paraspinal muscles might be a useful indicator for diagnosis and rehabilitation strategies.
Fig 2.
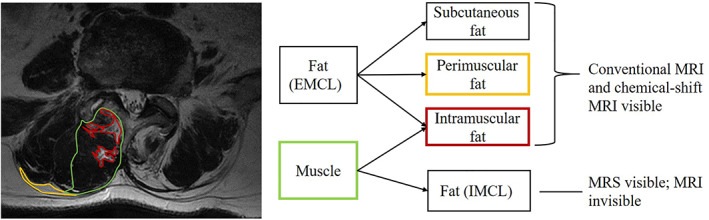
Distribution diagram of paraspinal muscle adipose tissue. Taking the T2‐weighted image of the right multifidus muscle of the L4–5 segment as an example, the green contour represents the muscle mass, the yellow contour represents perimuscular fat, and the red contour represents intramuscular fat. The perimuscular fat is stored between muscle groups and intramuscular fat is inside muscles. The perimuscular fat and intramuscular fat pertaining to extramyocellular lipids (EMCL) can be visible on conventional MRI and chemical‐shift MRI, while intramyocellular lipids (IMCL) stored in myocyte are shown only on magnetic resonance spectroscopy.
Chemical‐shift MRI can produce water‐only and fat‐only images from dual‐echo and/or multi‐echo acquisitions that overmatch MRS when observing EMCL; thus, this is considered the contemporary standard for measuring FI. Excellent accuracy has been demonstrated for manual segmentation based on these imaging techniques compared to spectroscopy 75 and histology 81 . It is capable of quantifying proton density fat fractions (PDFF) 8 , 86 , which has revealed favorable intra‐reader and inter‐reader reproducibility. Studies have demonstrated that FI based on chemical‐shift MRI is associated with LBP 51 , 82 , 85 and herniated nucleus pulposus 69 . Moreover, using PDFF measurements could improve the prediction of paraspinal muscle strength beyond cross‐sectional areas (CSA) 86 . Multi‐echo MRI, another new approach based on exploiting chemical shift differences of water and fat resonances, produced a concurring result with the fat values derived by MRS 75 . Fischer et al. performed a quantification of fat content through multi‐echo MRI in LBP patients 75 .
Measurement Protocol
Single‐Segment or Multi‐Segment Measurement
Recent research has suggested that using a single‐segment measurement to evaluate FI is insufficient for representing the whole lumbar area 32 . Surgeons should perform a multi‐segment assessment instead of a single‐segment assessment to evaluate the overall situation of paraspinal muscle degeneration. Considering that the MR slice has a certain thickness, volumetric measures based on a three‐dimensional volume across L1–S1 (or the levels of interest) are more appropriate and realistic in representing the entire muscle volume variability 11 .
Studies that investigated nonspecific LBP were inclined to assess multiple segments in the lower lumbar region 21 , 23 , 24 , 26 , 28 , 34 , 35 , 36 , 48 , 49 , 52 , 54 , 55 , 57 , 60 , 61 , 62 , 63 , 64 , 65 , 83 , especially focusing on L4. According to Crawford et al. 87 , the fat content at L4 best represented that of the entire lumbar region in healthy participants. Hebert et al. 22 also reported that pathological change appeared most often at L4. Storheim et al. revealed that higher FI at lower lumbar segments was associated with higher Oswestry disability index (ODI) scores and greater pain intensity in chronic LBP patients 49 . This indicated that the evaluation of FI in lower lumbar segments was useful and could reflect the morbid state of patients.
For specific lumbar degenerative diseases, studies have tended to evaluate the lesion segment and adjacent segments 9 , 11 , 12 , 13 , 14 , 15 , 20 , 27 , 30 , 39 , 47 , 51 , 53 , 63 , 70 . Several studies have demonstrated that FI could be a risk factor for lumbar degenerative diseases 13 , 14 , 15 , 47 . We also found that FI of paraspinal muscles was associated with clinical outcomes. Higher FI of multifidus was correlated with lower functional status and less improvement in ODI in patients with LSS after surgery 63 , 70 . Thus, evaluating the FI at the lesion segment and adjacent segments could be a viable method for predicting the clinical outcomes of lumbar surgery.
Slice Selection
Most studies used the center of the intervertebral disc 10 , 21 , 27 , 28 , 29 , 30 , 39 , 43 , 45 , 50 , 52 , 53 , 56 , 59 , 60 , 61 , 62 , 65 , 71 , 74 , 79 or the superior/inferior endplate 9 , 15 , 19 , 20 , 26 , 31 , 34 , 35 , 41 , 44 , 47 , 48 , 49 , 57 , 63 , 64 , 66 , 68 , 69 , 70 , 82 , 83 , 85 as the level of slice. These slices are common and available in clinical practice. However, there is no research demonstrating the impact of different slice positioning on the FI results.
Defining the Novel Region of Interest
For manually defining the ROI, minute changes in muscle composition may not be clearly visible 88 . Semi‐automatic technologies emerging to define the border of paraspinal muscles have the potential to assist with this problem. Antony et al. implemented an interactive segmentation of the erector spinae and the multifidus muscles using the livewire technique (Fig. 3A,B) 24 .
Fig 3.
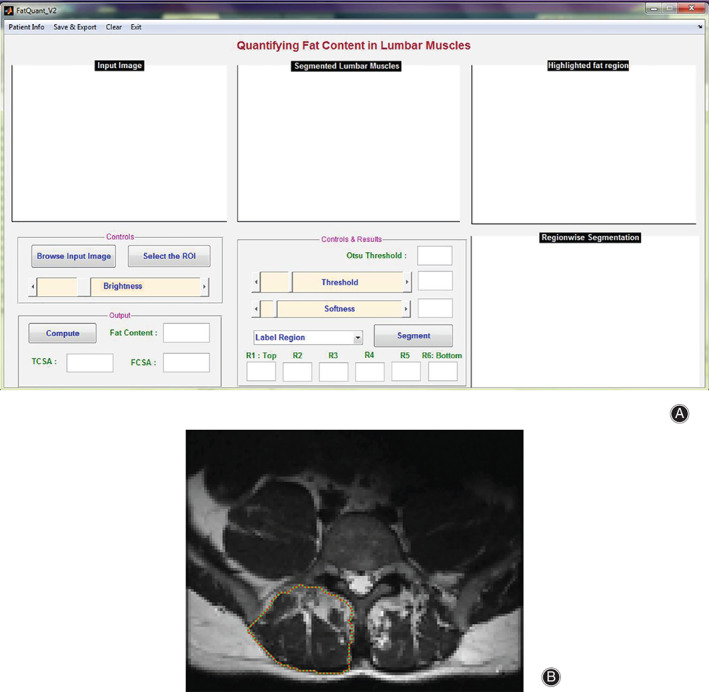
(A) A graphical user interface was developed based on interactive controls for selecting region of interest from the input image, threshold adjustment, and softness level adjustment. (B) MRI input image of the right erector spinae and the multifidus muscles, following a path that is as close as possible to image features detected as edges using Dijkstra's lowest cost path algorithm. However, the input image has to be down‐sampled in the low‐resolution image to ensure an effective running speed.
Interestingly, two studies have proposed a method allowing for quantification of the spatial distribution of FI in each quartile of ROI (medial to lateral) and describing whether there is a geographical propensity for fat to accumulate (Fig. 4) 31 , 57 . They found that fat content increased per quartile from medial to lateral in males, whereas the increase of FI depended more on sagittal than transverse distribution 57 . Antony et al. proposed a new method to quantify the fat content in six regions with reference to the center of the spinal column, which represented the axis of spinal rotation 24 . These studies demonstrated that orthopaedists can keep a watchful eye on different muscle regions that might have various effects on pain levels.
Fig 4.
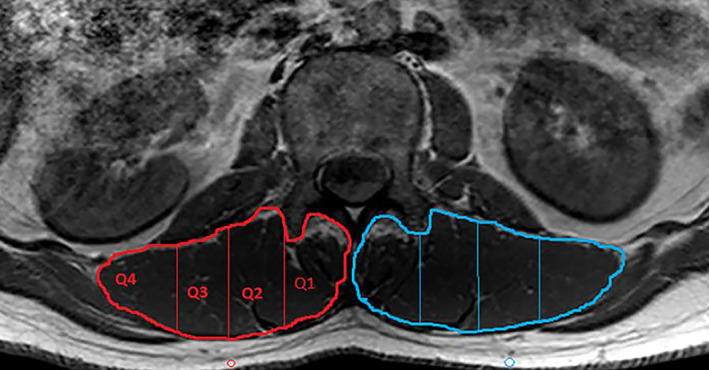
Paraspinal muscles were quartiled from medial (Q1) to lateral (Q4) with equal‐area division (demonstrated on the right side in red).
Imaging Parameters
Visual Semiquantitative Parameters
Our review showed that several early studies used semiquantitative visual grading with distinct cut‐off points (2‐point scale 19 , 67 , 3‐point scale 9 , 16 , 49 , 64 , 68 , 78 , 4‐point scale 7 , 15 , 18 , 29 , 34 , 37 , 48 , 50 , 59 , 80 , and 5‐point scale 17 , 28 , 35 , 51 ). When muscles were graded, the interobserver and intraobserver agreements were both acceptable with or without cut‐off points 7 , 34 , 51 .
Semiquantitative evaluation is convenient and intuitive in clinical practice. Studies have reported that higher FI of paraspinal muscles based on semiquantitative evaluation was correlated to functional disability, pain level, and decreased range of motion of lumbar flexion in LBP patients 9 , 49 , 64 , 68 . Teichtahl et al. also reported that paraspinal FI, but not muscle area, was associated with high‐intensity pain, disability, and structural abnormalities in community‐based adults 50 .
Quantitative Parameters
Quantitative evaluation is more accurate than semiquantitative evaluation. Numerous quantitative parameters based on area or signal intensity were applied to define FI (Table 2). In terms of area‐based indicators, fat CSA/total CSA or fat signal fractions is a universal indicator separating fat area through signal intensity difference with a threshold technology 13 , 24 , 25 , 28 , 32 , 33 , 40 , 46 . Studies have showed that LBP patients or those presenting with lumbar degenerative diseases have greater fat CSA/total CSA 13 , 25 , 40 , 46 . When multiplanar reconstruction was used, the ratio of fat volume to muscle volume outperformed fat CSA/total CSA, which was dependent upon specific slices. An MRI three‐dimensional reconstruction study found that FI increased from L1–L2 to L5–S1 level in patients with lumbar spinal stenosis 11 .
TABLE 2.
Comparison of different imaging parameters of FI
| Indicator | Suggested application | Disadvantage |
|---|---|---|
| Semiquantitative grading | Intuitive and suitable for clinical evaluation among elderly patients; correlated with functional status and clinical outcomes | The cut‐off value is uncertain and the measurement error of people with slight FI is relatively large |
| Fat CSA/total CSA | Quantitative parameters based on area; correlated with multiple lumbar degenerative diseases | Requires threshold method |
| Fat volume/muscle volume | Quantitative parameters based on volume; reflects the overall situation of muscles | Requires three‐dimensional reconstruction |
| MFI | Quantitative parameters based on signal intensity; correlated with clinical outcomes | The reference of fatty signal intensity in MFI was diverse |
| Mean MRI signal intensity | Can be influenced by individuals and measurement tools |
CSA, cross‐sectional area; FI, fat infiltration; MFI, muscle–fat index.
Signal intensity‐based indicators include the muscle–fat index (MFI) 20 , 21 , 26 , 38 , 47 , 53 , 54 , 57 and mean MRI signal intensity 14 , 25 , 27 , 31 . MFI was calculated by dividing the mean signal intensity of the total muscle by the intramuscular fat to reduce individual differences, and it has been proven to be highly reliable 65 , 72 . Greater MFI was correlated to poor physical function 38 and high incidence of proximal junctional kyphosis 20 . However, the reference of fatty signal intensity was used in a variety of ways. Some studies used a homogenous region of perimuscular fat 21 , 38 , 54 or subcutaneous fat 20 , 47 , 57 as fat region when identifying intramuscular fat was impracticable. Applying cerebrospinal fluid from the same axial level of each MRI scan as the reference of signal intensity could also lessen variations in MRI background intensity 66 , 71 , 72 . Like CT attenuation, the mean MRI signal intensity was also considered to reflect FI in some studies but might be affected by individual differences and MRI operation differences 14 , 25 , 27 , 31 . We recommend combining parameters based on area and signal intensity for use as an indicator of FI.
Conclusion
Novel technologies like MRS and chemical‐shift MRI have emerged to provide details on intramyocellular or extracellular fatty concentration and could be used in distinguishing degeneration of the lumbar spine. Studies might perform a multi‐segment assessment including at least L4 instead of a single‐segment assessment. Adopting the center of the intervertebral disc or endplate as the level of slice is expedient. The spatial distribution of FI might have a particularity in degenerative lumbar spines. Numerous quantitative parameters based on area or signal intensity were applied to define the FI, among which fat CSA/total CSA and MFI seem to be the better choices in diagnosing and predicting clinical outcomes.
Grant Sources: The National Natural Science Foundation of China (No. 51804012).
Disclosure: The authors declare that they have no conflict of interest.
References
- 1. Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int, 2017, 2017: 2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging‐state of the art. J Orthop Transl, 2018, 15: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J, 2017, 17: 1729–1748. [DOI] [PubMed] [Google Scholar]
- 4. Vagaska E, Andrasina T, Vohanka S, Adamova B. Changes of paraspinal muscle morpholocy in patients with chronic non‐specific low back pain. Ceska a Slovenska Neurologie a Neurochirurgie, 2019, 82: 505–512. [Google Scholar]
- 5. Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non‐specific low back pain: a systematic review. Pain Physician, 2016, 19: 985–1000. [PubMed] [Google Scholar]
- 6. Cooley JR, Walker BF, Ardakani ME, Kjaer P, Jensen TS, Hebert JJ. Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta‐analysis. BMC Musculoskeletal Disord, 2018, 19: 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mengiardi B, Schmid MR, Boos N, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology, 2006, 240: 786–792. [DOI] [PubMed] [Google Scholar]
- 8. Burian E, Syvaeri J, Holzapfel C, et al. Gender‐ and age‐related changes in trunk muscle composition using chemical shift encoding‐based water‐fat MRI. Nutrients, 2018, 10: 1972–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salah El‐din Mahmoud W, Yousef A, Manssor E, Ahmed S. The relationship between pain and functional disability with morphological changes of psoas major in discogenic low back pain patients. Int J Ther Rehabil, 2016, 23: 363–370. [Google Scholar]
- 10. Lee JC, Cha J‐G, Kim Y, Kim Y‐I, Shin B‐J. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis ‐ comparison with the normal controls. Spine, 2008, 33: 318–325. [DOI] [PubMed] [Google Scholar]
- 11. Boissiere L, Moal B, Gille O, et al. Lumbar spinal muscles and spinal canal study by MRI three‐dimensional reconstruction in adult lumbar spinal stenosis. Orthop Traumatol Surg Res, 2017, 103: 279–283. [DOI] [PubMed] [Google Scholar]
- 12. Kong BJ, Lim JS, Kim K. A study on dispersion and rate of fat infiltration in the lumbar spine of patients with herniated nucleus polpusus. J Phys Ther Sci, 2014, 26: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang J, Wang H, Wang L, et al. Multifidus degeneration, a new risk factor for lumbar spinal stenosis: a case–control study. World Neurosurg, 2017, 99: 226–231. [DOI] [PubMed] [Google Scholar]
- 14. Fortin M, Lazary A, Varga PP, McCall I, Battie MC. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J, 2016, 25: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 15. Yanyu C, Jwoluen P, Chenkun L, Weili H, Rongsen Y. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J, 2014, 23: 999–1006. [DOI] [PubMed] [Google Scholar]
- 16. Kang CH, Shin MJ, Kim SM, Lee SH, Lee CS. MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol, 2007, 62: 479–486. [DOI] [PubMed] [Google Scholar]
- 17. Betz M, Burgstaller JM, Held U, et al. Influence of paravertebral muscle quality on treatment efficacy of epidural steroid infiltration or surgical decompression in lumbar spinal stenosis‐analysis of the lumbar spinal outcome study (LSOS) data: a swiss prospective multicenter cohort study. Spine (Phila Pa 1976), 2017, 42: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 18. Teichtahl AJ, Urquhart DM, Wang Y, et al. Lumbar disc degeneration is associated with modic change and high paraspinal fat content ‐ a 3.0T magnetic resonance imaging study. BMC Musculoskelet Disord, 2016, 17: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kong MH, Hymanson HJ, Song KY, et al. Kinetic magnetic resonance imaging analysis of abnormal segmental motion of the functional spine unit clinical article. J Neurosurg Spine, 2009, 10: 357–365. [DOI] [PubMed] [Google Scholar]
- 20. Hyun S‐J, Kim YJ, Rhim S‐C. Patients with proximal junctional kyphosis after stopping at thoracolumbar junction have lower muscularity, fatty degeneration at the thoracolumbar area. Spine J, 2016, 16: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 21. Sions JM, Smith AC, Hicks GE, Elliott JM. Trunk muscle size and composition assessment in older adults with chronic low back pain: an intra‐examiner and inter‐examiner reliability study. Pain Med, 2016, 17: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9‐year population‐based prospective cohort study. Spine (Phila Pa 1976)., 2014, 39: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 23. Le Cara EC, Marcus RL, Dempsey AR, Hoffman MD, Hebert JJ. Morphology versus function: the relationship between lumbar multifidus intramuscular adipose tissue and muscle function among patients with low back pain. Arch Phys Med Rehabil, 2014, 95: 1846–1852. [DOI] [PubMed] [Google Scholar]
- 24. Antony J, McGuinness K, Welch N, et al. An interactive segmentation tool for quantifying fat in lumbar muscles using axial lumbar‐spine MRI. IRBM, 2016, 37: 11–22. [Google Scholar]
- 25. Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol, 2015, 88: 20140546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'hooge R, Cagnie B, Crombez G, Vanderstraeten G, Dolphens M, Danneels L. Increased intramuscular fatty infiltration without differences in lumbar muscle cross‐sectional area during remission of unilateral recurrent low back pain. Man Ther., 2012, 17: 584–588. [DOI] [PubMed] [Google Scholar]
- 27. Battie MC, Niemelainen R, Gibbons LE, Dhillon S. Is level‐ and side‐specific multifidus asymmetry a marker for lumbar disc pathology? Spine J, 2012, 12: 932–939. [DOI] [PubMed] [Google Scholar]
- 28. Battaglia PJ, Maeda Y, Welk A, Hough B, Kettner N. Reliability of the Goutallier classification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. J Manipulative Physiol Ther, 2014, 37: 190–197. [DOI] [PubMed] [Google Scholar]
- 29. Arbanas J, Pavlovic I, Marijancic V, et al. MRI features of the psoas major muscle in patients with low back pain. Eur Spine J, 2013, 22: 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takayama K, Kita T, Nakamura H, et al. New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine (Phila Pa 1976)., 2016, 41: 84–90. [DOI] [PubMed] [Google Scholar]
- 31. Mhuiris AN, Volken T, Elliott JM, Hoggarth M, Samartzis D, Crawford RJ. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1‐weighted MRI. BMC Musculoskelet Disord, 2016, 17: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Urrutia J, Besa P, Lobos D, Andia M, Arrieta C, Uribe S. Is a single‐level measurement of paraspinal muscle fat infiltration and cross‐sectional area representative of the entire lumbar spine? Skeletal Radiol, 2018, 47: 939–945. [DOI] [PubMed] [Google Scholar]
- 33. Berry DB, Padwal J, Johnson S, Parra CL, Ward SR, Shahidi B. Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord, 2018, 19: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozcan‐Eksi EE, Eksi MS, Akcal MA. Severe lumbar intervertebral disc degeneration is associated with modic changes and fatty infiltration in the paraspinal muscles at all lumbar levels, except for l1‐l2: a cross‐sectional analysis of 50 symptomatic women and 50 age‐matched symptomatic men. World Neurosurg, 2019, 122: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 35. Atci IB, Yilmaz H, Samanci MY, Atci AG, Karagoz Y. The prevalence of lumbar paraspinal muscle fatty degeneration in patients with modic type i and i/ii end plate changes. Asian Spine J, 2020, 14: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hebert JJ, Le Cara EC, Koppenhaver SL, et al. Predictors of clinical success with stabilization exercise are associated with lower levels of lumbar multifidus intramuscular adipose tissue in patients with low back pain. Disabil Rehabil, 2020, 42: 679–684. [DOI] [PubMed] [Google Scholar]
- 37. Mannil M, Burgstaller JM, Thanabalasingam A, et al. Texture analysis of paraspinal musculature in MRI of the lumbar spine: analysis of the lumbar stenosis outcome study (LSOS) data. Skeletal Radiol, 2018, 47: 947–954. [DOI] [PubMed] [Google Scholar]
- 38. Sions JM, Coyle PC, Velasco TO, Elliott JM, Hicks GE. Multifidi muscle characteristics and physical function among older adults with and without chronic low back pain. Arch Phys Med Rehabil, 2017, 98: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Truszczynska‐Baszak A, Tarnowski A. Paraspinal muscle hypotrophy and chronic discogenic low back pain. Biomed Hum Kinet, 2018, 10: 19–24. [Google Scholar]
- 40. Urrutia J, Besa P, Lobos D, Campos M, et al. Lumbar paraspinal muscle fat infiltration is independently associated with sex, age, and inter‐vertebral disc degeneration in symptomatic patients. Skeletal Radiol, 2018, 47: 955–961. [DOI] [PubMed] [Google Scholar]
- 41. Zhong Y, Liu J, Zhou W, Yu D. Relationship between straight leg‐raising test measurements and area of fat infiltration in multifidus muscles in patients with lumbar disc hernation. J Back Musculoskelet Rehabil, 2020, 33: 57–63. [DOI] [PubMed] [Google Scholar]
- 42. Sasaki T, Yoshimura N, Hashizume H, et al. MRI‐defined paraspinal muscle morphology in Japanese population: the Wakayama Spine Study. PLoS One, 2017, 12: e0187765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie D, Zhang J, Ding W, et al. Abnormal change of paravertebral muscle in adult degenerative scoliosis and its association with bony structural parameters. Eur Spine J, 2019, 28: 1626–1637. [DOI] [PubMed] [Google Scholar]
- 44. Xia W, Fu H, Zhu Z, et al. Association between back muscle degeneration and spinal‐pelvic parameters in patients with degenerative spinal kyphosis. BMC Musculoskelet Disord, 2019, 20: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shafaq N, Suzuki A, Matsumura A, et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine (Phila Pa 1976)., 2012, 37: 1398–1406. [DOI] [PubMed] [Google Scholar]
- 46. Shahidi B, Parra CL, Berry DB, et al. Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine, 2017, 42: 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu B, Jiang K, Li X, Zhang J, Liu Z. Correlation of the features of the lumbar multifidus muscle with facet joint osteoarthritis. Orthopedics, 2017, 40: 793–800. [DOI] [PubMed] [Google Scholar]
- 48. Prasarn ML, Kostantinos V, Coyne E, Wright J, Rechtine GR. Does lumbar paraspinal muscle fatty degeneration correlate with aerobic index and Oswestry disability index? Surg Neurol Int, 2015, 6: 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Storheim K, Berg L, Hellum C, et al. Fat in the lumbar multifidus muscles ‐ predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2‐year follow‐up of a randomized trial. BMC Musculoskelet Disord, 2017, 18: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teichtahl AJ, Urquhart DM, Wang Y, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community‐based adults. Spine J, 2015, 15: 1593–1601. [DOI] [PubMed] [Google Scholar]
- 51. Yanik B, Keyik B, Conkbayir I. Fatty degeneration of multifidus muscle in patients with chronic low back pain and in asymptomatic volunteers: quantification with chemical shift magnetic resonance imaging. Skeletal Radiol, 2013, 42: 771–778. [DOI] [PubMed] [Google Scholar]
- 52. Wesselink E, de Raaij E, Pevenage P, van der Kaay N, Pool J. Fear‐avoidance beliefs are associated with a high fat content in the erector spinae: a 1.5 tesla magnetic resonance imaging study. Chiropr Man Ther, 2019, 27: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suh DW, Kim Y, Lee M, Lee S, Park SJ, Yoon B. Reliability of histographic analysis for paraspinal muscle degeneration in patients with unilateral back pain using magnetic resonance imaging. J Back Musculoskelet Rehabil, 2017, 30: 403–412. [DOI] [PubMed] [Google Scholar]
- 54. Sions JM, Elliott JM, Pohlig RT, Hicks GE. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther, 2017, 47: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ali I, Ulbricht C, McGregor AH. Degeneration of the extensor muscle group in a surgical low back and leg pain population. J Back Musculoskelet Rehabil, 2011, 24: 23–30. [DOI] [PubMed] [Google Scholar]
- 56. Bhadresha A, Lawrence OJ, McCarthy MJ. A comparison of magnetic resonance imaging muscle fat content in the lumbar paraspinal muscles with patient‐reported outcome measures in patients with lumbar degenerative disk disease and focal disk prolapse. Global Spine J, 2016, 6: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crawford RJ, Volken T, Mhuiris AN, et al. Geography of lumbar paravertebral muscle fatty infiltration the influence of demographics, low back pain, and disability. Spine, 2019, 44: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 58. Dohzono S, Toyoda H, Takahashi S, et al. Factors associated with improvement in sagittal spinal alignment after microendoscopic laminotomy in patients with lumbar spinal canal stenosis. J Neurosurg Spine, 2016, 25: 39–45. [DOI] [PubMed] [Google Scholar]
- 59. Faur C, Patrascu JM, Haragus H, Anglitoiu B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet Disord, 2019, 20: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fortin M, Battié MC. Quantitative paraspinal muscle measurements: inter‐software reliability and agreement using OsiriX and ImageJ. Phys Ther, 2012, 92: 853–864. [DOI] [PubMed] [Google Scholar]
- 61. Fortin M, Gibbons LE, Videman T, Battié MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports, 2015, 25: 880–887. [DOI] [PubMed] [Google Scholar]
- 62. Fortin M, Omidyeganeh M, Battie MC, Ahmad O, Rivaz H. Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomed Eng Online, 2017, 16: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fortin M, Lazary A, Varga PP, Battie MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J, 2017, 26: 2543–2551. [DOI] [PubMed] [Google Scholar]
- 64. Hildebrandt M, Fankhauser G, Meichtry A, Luomajoki H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord, 2017, 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu Z‐J, He J, Zhao F‐D, Fang X‐Q, Zhou L‐N, Fan S‐W. An assessment of the intra‐ and inter‐reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine, 2011, 36: 868–874. [DOI] [PubMed] [Google Scholar]
- 66. Hyun SJ, Bae CW, Lee SH, Rhim SC. Fatty degeneration of the paraspinal muscle in patients with degenerative lumbar kyphosis: a new evaluation method of quantitative digital analysis using MRI and CT scan. Clin Spine Surg, 2016, 29: 441–447. [DOI] [PubMed] [Google Scholar]
- 67. Keshavarz E, Dehghani Z, Mohammadi K, Haghighatkhah H, Shobeirian F. Comparison of pathologic findings of lumbosacral MRI between low back pain patients and the controls. J Res Med Den Sci, 2018, 6: 213–216. [Google Scholar]
- 68. Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf‐Yde C. Are MRI‐defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med, 2007, 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee SK, Jung JY, Kang YR, Jung JH, Yang JJ. Fat quantification of multifidus muscle using T2‐weighted Dixon: which measurement methods are best suited for revealing the relationship between fat infiltration and herniated nucleus pulposus. Skeletal Radiol, 2020, 49: 263–271. [DOI] [PubMed] [Google Scholar]
- 70. Yang L, Yuzeng L, Yong H, Tie L, Li G, Chen X. Fat infiltration in the multifidus muscle as a predictor of prognosis after decompression and fusion in patients with single‐segment degenerative lumbar spinal stenosis: an ambispective cohort study based on propensity score matching. World Neurosurg, 2019, 128: 989–1001. [DOI] [PubMed] [Google Scholar]
- 71. Lee JH, Lee SH. Does lumbar paraspinal muscles improve after corrective fusion surgery in degenerative flat black? Indian J Orthop, 2017, 51: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ropponen A, Videman T, Battie MC. The reliability of paraspinal muscles composition measurements using routine spine MRI and their association with back function. Man Ther, 2008, 13: 349–356. [DOI] [PubMed] [Google Scholar]
- 73. Fortin M, Macedo LG. Multifidus and paraspinal muscle group cross‐sectional areas of patients with low back pain and control patients: a systematic review with a focus on blinding. Phys Ther, 2013, 93: 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paalanne N, Niinimaki J, Karppinen J, et al. Assessment of association between low back pain and paraspinal muscle atrophy using opposed‐phase magnetic resonance imaging: a population‐based study among young adults. Spine (Phila Pa 1976)., 2011, 36: 1961–1968. [DOI] [PubMed] [Google Scholar]
- 75. Fischer MA, Nanz D, Shimakawa A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi‐echo MR imaging with single‐voxel MR spectroscopy. Radiology, 2013, 266: 555–563. [DOI] [PubMed] [Google Scholar]
- 76. Ogon I, Takebayashi T, Takashima H, et al. Magnetic resonance spectroscopic analysis of multifidus muscles lipid content and association with spinopelvic malalignment in chronic low back pain. Br J Radiol, 2017, 90: 20160753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ogon I, Takebayashi T, Takashima H, et al. Multifidus muscles lipid content is associated with intervertebral disc degeneration: a quantitative magnetic resonance imaging study. Asian Spine J., 2019, 13: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ogon I, Takebayashi T, Takashima H, et al. Quantitative analysis concerning atrophy and fat infiltration of the multifidus muscle with magnetic resonance spectroscopy in chronic low back pain. Spine Surg Relat Res, 2019, 3: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Takashima H, Takebayashi T, Ogon I, et al. Analysis of intra and extramyocellular lipids in the multifidus muscle in patients with chronic low back pain using MR spectroscopy. Br J Radiol, 2018, 91: 20170536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Takashima H, Takebayashi T, Ogon I, et al. Evaluation of intramyocellular and extramyocellular lipids in the paraspinal muscle in patients with chronic low back pain using MR spectroscopy: preliminary results. Br J Radiol, 2016, 89: 20160136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Smith AC, Parrish TB, Abbott R, et al. Muscle‐fat MRI: 1.5 tesla and 3.0 tesla versus histology. Muscle Nerve, 2014, 50: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Goubert D, De Pauw R, Meeus M, et al. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross‐sectional study. Spine J, 2017, 17: 1285–1296. [DOI] [PubMed] [Google Scholar]
- 83. Bailey JF, Fields AJ, Ballatori A, et al. The relationship between endplate pathology and patient‐reported symptoms for chronic low back pain depends on lumbar paraspinal muscle quality. Spine (Phila Pa 1976)., 2019, 44: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yoo YH, Kim H‐S, Lee YH, et al. Comparison of multi‐echo dixon methods with volume interpolated breath‐hold gradient echo magnetic resonance imaging in fat‐signal fraction quantification of paravertebral muscle. Korean J Radiol, 2015, 16: 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Goubert D, Meeus M, Willems T, et al. The association between back muscle characteristics and pressure pain sensitivity in low back pain patients. Scand J Pain, 2018, 18: 281–293. [DOI] [PubMed] [Google Scholar]
- 86. Schlaeger S, Inhuber S, Rohrmeier A, et al. Association of paraspinal muscle water‐fat MRI‐based measurements with isometric strength measurements. Eur Radiol, 2019, 29: 599–608. [DOI] [PubMed] [Google Scholar]
- 87. Crawford RJ, Elliott JM, Volken T. Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur Spine J, 2017, 26: 3059–3067. [DOI] [PubMed] [Google Scholar]
- 88. Crawford RJ, Cornwall J, Abbott R, Elliott JM. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross‐reference. BMC Musculoskeletal Disord, 2017, 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]


