Abstract
Background and Aims
A reliable predictive score system to identify the risk of symptomatic intracranial hemorrhage (sICH) after intravenous thrombolysis (IVT) in acute ischemic stroke patients is of great essence. We aimed to develop a nomogram for predicting the risk of sICH after IVT in Chinese patients.
Methods
We recruited acute ischemic stroke patients who were treated with IVT from five advanced stroke centers in China from April 2014 to November 2020. sICH was diagnosed according to the European Cooperative Acute Stroke Study II (ECASS-II) definition. Multivariable logistic regression was performed to construct the best-fit nomogram. The discrimination and calibration of the nomogram were evaluated by the area under the receiver operating characteristic curve (AUC-ROC) and calibration plot.
Results
A total of 1200 patients were enrolled, of whom 66 (5.5%) developed sICH. In the multivariate logistic regression model, atrial fibrillation (odds ratio [OR] 3.25; 95% confidence interval [CI], 1.89−5.60; P < 0.001), baseline glucose level (OR, 1.13; 95% CI, 1.07−1.20; P < 0.001), neutrophil to lymphocyte ratio (OR, 1.05; 95% CI, 1.01−1.09; P = 0.024) and baseline National Institute of Health Stroke Scale (NIHSS) (OR, 1.07; 95% CI, 1.04−1.10; P < 0.001) were independent predictors for sICH and were used to generate the nomogram. The nomogram demonstrated good discrimination as the AUC-ROC value was 0.788 (95% CI, 0.737–0.840). The calibration plot revealed good calibration.
Conclusion
The nomogram consisted of atrial fibrillation, baseline glucose level, neutrophil to lymphocyte ratio, and NIHSS score may predict the risk of sICH in Chinese acute ischemic stroke patients treated with IVT.
Keywords: stroke, intravenous thrombolysis, symptomatic intracranial hemorrhage, nomogram, predict
Introduction
Although endovascular treatment has been a promising treatment for acute ischemic stroke in recent years, intravenous thrombolysis (IVT) now still be a preferred and effective treatment for patients within the time window of 4.5 hours.1 Symptomatic intracranial hemorrhage (sICH) is a rare but dangerous complication for IVT and limits its widespread use.2 Due to different study populations and sICH definitions, the sICH rate ranges from 2.0% to 7.0%.3 Compared with other race/ethnic groups, the Asian population might take a higher risk for sICH.4
Since sICH is significantly associated with poor outcomes, a reliable prognostic model to identify patients with increased risk of sICH after IVT is essential. There have been several prognostic score systems to predict the risk of sICH after IVT.5–11 However, these score systems usually convert continuous variables such as age, blood glucose, NIHSS score to categorical variables, which might lead to loss of information because these factors are strong predictors of sICH after IVT.
Nomogram is a graphical statistical tool that uses a continuous score to calculate the probability of a clinical event for an individual patient and has been widely used in modern individualized medical decision-making.12–15 The STARTING-SICH nomogram was designed for prediction of sICH in stroke patients treated with IVT.16 However, this nomogram was derived from European-American ancestry. Therefore, it is necessary to develop a novel nomogram in the Asian population. Based on a multicenter database derived from 5 stroke centers, the present study aimed to develop a nomogram to predict the probability of sICH after IVT in Chinese stroke patients.
Methods
Study Population
The study was a retrospective cohort analysis based on prospectively collected data from five advanced stroke centers in China between April 2014 and November 2020 (including Jinling Hospital, Changsha Central Hospital, Affiliated Hospital of Yangzhou University, Wuxi People’s Hospital, and Nanjing Brain Hospital). Inclusion criteria: 1) age ≥18 years; 2) diagnosed with acute ischemic stroke; 3) treated with IVT. Patients treated with endovascular treatment after IVT, patients with severe inflammatory diseases, and those with incomplete clinical data were excluded from the analysis. This study was approved by the ethics committee of Jinling Hospital and each participating center. Due to its retrospective nature; patient consent was waived. All procedures performed in studies were under the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient data was confidentiality maintained in Jingling Hospital and each participating center.
Baseline Data Collection
Demographic characteristics, medical history, clinical data were obtained at admission. Stroke severity was evaluated by the National Institutes of Health Stroke Scale (NIHSS) score. Laboratory data including baseline blood glucose, neutrophil-to-lymphocyte ratio (NLR), platelet, total cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein were also recorded. NLR value was computed as neutrophil counts/lymphocyte counts.
Definition of sICH
All patients enrolled in the study underwent a CT scan within 24 hours after IVT and performed another CT whenever neurological deterioration appears. The sICH was diagnosed based on the European Cooperative Acute Stroke Study II (ECASS-II) definition: any type of intracerebral hemorrhage on post-thrombolysis imaging and increase of ≥4 NIHSS points from baseline, or the lowest score within 7 days, or leading to death.17 All image data were independently reviewed by two neurologists to assess the presence of sICH. In case of disagreement, final results were sought by consensus discussed with a third neurologist.
Statistical Analysis
Descriptive analysis was conducted as follows: continuous variables were presented as medians with interquartile ranges (IQRs) or mean ± standard deviation (SD); categorical variables were described as numbers with percentages. Differences between the groups with and without sICH were explored using the Mann–Whitney U-test or t-student test for continuous variables as appropriate. Differences between the two groups for categorical variables were analyzed by Fisher exact test or χ2 test, when appropriate. To construct the nomogram, we implemented a multivariate logistic regression analysis using the forward stepwise method to determine independent factors of sICH, all variables with P value < 0.1 in the univariate analysis were included. Variables with P value < 0.05 in the multivariate logistic regression were entered to generate the predictive model. Regression coefficient and odds ratios (OR) with 95% confidence intervals (CI) for each variable included in the model were calculated. The regression coefficient of each variable in the model was used to compute corresponding points in the scale and, eventually, obtaining the scoring system. The discriminative ability of the novel nomogram was assessed by calculating the area under the receiver-operating characteristic curve (AUC-ROC). Calibration of the predictive model that described the concordance between the observed and predicted probability based on the nomogram was tested using a calibration plot with bootstraps of 1000 resamples. All statistical analyses were performed using statistical software SPSS version 22.0 (IBM, New York, NY) and R version 3.0 (R Foundation, Vienna, Austria).
Results
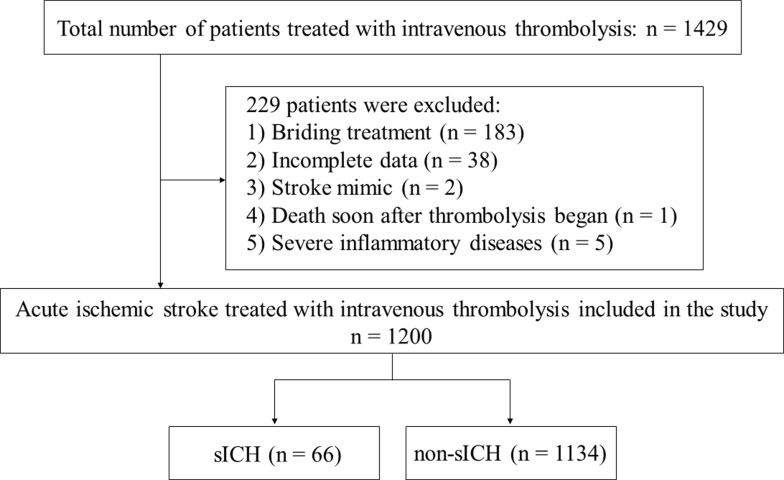
The flow chart of patient inclusion and exclusion is presented in Figure 1. There were 1200 acute ischemic stroke patients treated with IVT finally included in the present study to construct the nomogram. The clinical characteristics of patients are presented in Table 1. The mean age of the total group was 66.9 years, and 63.0% were male. According to the ECASS II definition, 66 patients (5.5%) were classified as sICH after IVT. Compared with patients without sICH, sICH patients were older (mean, 72.2 versus 66.6 years; P < 0.001), more prone to have a history of atrial fibrillation (47.0% versus 16.6%; P < 0.001), and had higher baseline blood glucose (mean, 9.8 versus 7.9 mmol/L, P < 0.001), NLR (median, 3.50 versus 2.74; P = 0.002), and baseline NIHSS (median, 15 versus 7; P < 0.001), and had lower platelet (mean, 178.0 versus 198.4 109/L; P = 0.007) (Table 1).
Figure 1.
Flow chart of patients included in the study.
Abbreviation: sICH, symptomatic intracranial hemorrhage.
Table 1.
Comparison of Demographics and Clinical Data in Patients with or without sICH
| Variable | with sICH n = 66 |
without sICH n = 1134 |
P value |
|---|---|---|---|
| Demographics | |||
| Age, years | 72.2 ± 11.8 | 66.6 ± 12.5 | < 0.001 |
| Male, n (%) | 37 (56.1) | 719 (63.4) | 0.23 |
| Weight, kg | 63.4 ± 13.7 | 64.5 ± 11.7 | 0.224 |
| Medical history, n (%) | |||
| Hypertension | 47 (71.2) | 796 (70.2) | 0.86 |
| Diabetes mellitus | 14 (21.2) | 275 (24.3) | 0.575 |
| Hyperlipidemia | 1 (1.5) | 57 (5.0) | 0.196 |
| Atrial fibrillation | 31 (47.0) | 188(16.6) | <0.001 |
| Smoking | 21 (31.8) | 381 (33.6) | 0.766 |
| Coronary heart disease | 16 (24.2) | 208 (18.3) | 0.232 |
| Previous stroke | 13 (19.7) | 224 (19.8) | 0.991 |
| Clinical data | |||
| Systolic blood pressure, mmHg | 161.4 ± 29.8 | 155.3 ± 26.4 | 0.063 |
| Diastolic blood pressure, mmHg | 91.4 ± 17.3 | 87.1 ± 15.3 | 0.056 |
| Time from onset to treatment, min | 174 (128, 219) | 165 (126, 209) | 0.295 |
| Baseline NIHSS, score | 15 (11, 21) | 7 (4, 13) | <0.001 |
| Laboratory data | |||
| Platelet, 109/L | 178.0 ± 51.4 | 198.4 ± 63.5 | 0.007 |
| Total cholesterol, mmol/L | 3.4 ± 1.7 | 3.5 ± 1.8 | 0.853 |
| Triglyceride, mmol/L | 1.24 (0.91, 3.44) | 1.8 (1.12, 4.04) | 0.105 |
| Low density lipoprotein, mmol/L | 1.94 (0.77, 2.73) | 2.1 (0.62, 2.84) | 0.927 |
| High-density lipoprotein, mmol/L | 1.6 ± 0.8 | 1.5 ± 0.8 | 0.153 |
| Baseline blood glucose, mmol/L | 9.8 ± 3.2 | 7.9 ± 3.4 | <0.001 |
| NLR | 3.50 (2.18, 7.43) | 2.74 (1.80, 4.46) | 0.002 |
Abbreviations: sICH, symptomatic intracranial hemorrhage; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio.
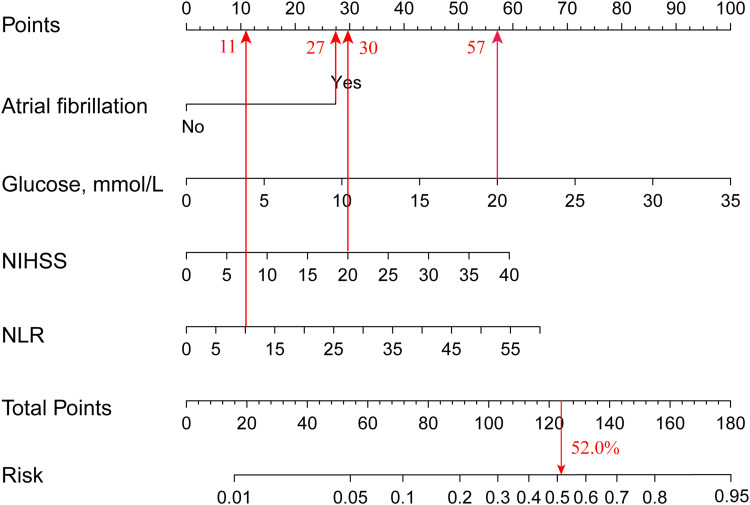
The results of the multivariate logistic regression model are shown in Table 2. Using the forward stepwise method, the following 4 variables were independent risk factors of sICH: atrial fibrillation (OR 3.25; 95% CI, 1.89−5.60; P < 0.001), baseline glucose level (OR, 1.13; 95% CI, 1.07−1.20; P < 0.001), NLR (OR, 1.05; 95% CI, 1.01−1.09; P = 0.024) and baseline NIHSS (OR, 1.07; 95% CI, 1.04−1.10; P < 0.001). Using the predictors (atrial fibrillation, baseline glucose level, NLR, baseline NIHSS) in the multivariable model, we constructed the nomogram to predict sICH risk. The nomogram was created by assigning a graphic preliminary score to each of the four predictors with a point ranging from 0−100, and then summed up all the points of each predictor to obtain the total point, eventually converted into a percentage representing an individual probability of sICH after IVT. The detailed nomogram is shown in Figure 2. For example, a patient with atrial fibrillation, blood glucose at 20 mmol/L, baseline NIHSS at 20 points, NLR at 10 would have a total of 125 points (27 points for atrial fibrillation, 57 points for blood glucose, 30 points for baseline NIHSS, and 11 points for NLR). The predicted sICH risk after IVT was approximately 52.0% for this patient.
Table 2.
Multivariate Regression Analysis for sICH
| Variable | Univariate Regression Model | Multivariate Regression Model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | Regression Coefficient | OR (95% CI) | P value | |
| Age, years | 1.04 (1.02−1.06) | 0.001 | |||
| Atrial fibrillation | 4.46 (2.68−7.41) | <0.001 | 1.18 | 3.25 (1.89−5.60) | <0.001 |
| Systolic blood pressure, mmHg | 1.01 (0.99−1.02) | 0.073 | |||
| Diastolic blood pressure, mmHg | 1.02 (1.00−1.03) | 0.027 | |||
| Baseline NIHSS, score | 1.08 (1.05−1.11) | <0.001 | 0.064 | 1.07 (1.04−1.10) | <0.001 |
| Platelet, 109/L | 0.99 (0.99−1.00) | 0.01 | |||
| Baseline glucose (per 1 mmol/l increase) | 1.12 (1.06−1.19) | <0.001 | 0.123 | 1.13 (1.07−1.20) | <0.001 |
| NLR | 1.06 (1.03−1.10) | 0.001 | 0.047 | 1.05 (1.01−1.09) | 0.024 |
Abbreviations: CI, confidence interval; OR, odd ratio; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio; sICH, symptomatic intracranial hemorrhage.
Figure 2.
The nomogram to predict the probability of sICH in stroke patients treated with intravenous thrombolysis.
Abbreviations: sICH, symptomatic intracranial hemorrhage; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio.
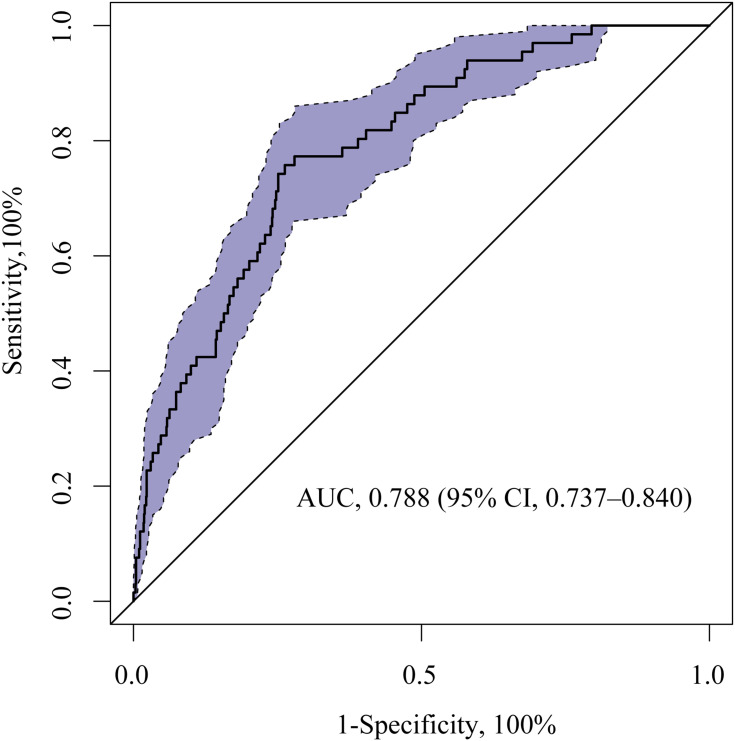
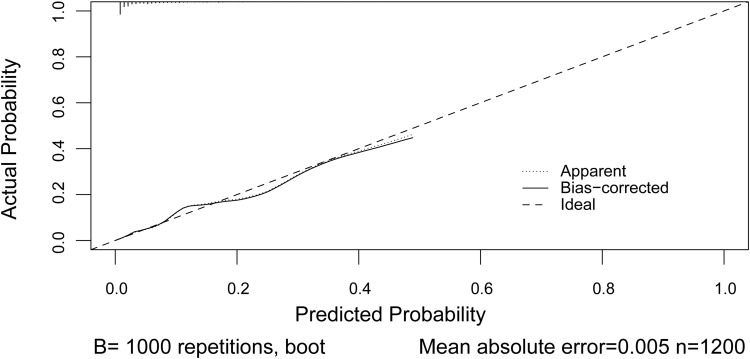
We calculated the AUC-ROC value to investigate the discriminative ability of the nomogram, which was 0.788 (95% CI, 0.737–0.840) (Figure 3). The calibration plot revealed good predictive accuracy between the actual probability and predicted probability (Figure 4).
Figure 3.
ROC curve of the nomogram for predicting sICH in stroke patients treated with intravenous thrombolysis.
Abbreviations: AUC, area under the ROC curve; ROC, receiver operating characteristic; sICH, symptomatic intracranial hemorrhage.
Figure 4.
Calibration plot of the nomogram model. Dotted line is the performance of the nomogram, while the solid line corrects for any bias in the nomogram. Dashed line is the reference line where a nomogram would lie.
Discussion
In this study, we found that the presence of atrial fibrillation, baseline glucose level, NLR, and NIHSS score were independent predictors for sICH after IVT. Based on the four independent factors, we constructed a nomogram to individually predict the probability of sICH for acute ischemic stroke patients treated with IVT in the Chinese population.
We found that 66 patients (5.5%) experienced sICH after IVT in the present study. Raymond C.S. Seet reviewed the incidence of sICH following IVT for acute ischemic stroke, including randomized controlled trials, registry studies, and cohort studies, and found the mean sICH rates for these studies were 7.5%, 3.5%, and 5.9%, respectively, and the mean overall incidence of sICH was 5.6%.3 The difference in rates of sICH among studies might be owing to the different definitions of sICH and study populations. Prior studies show that the ECASS-II criteria may have the highest interrater agreement and are strongly correlated with poor outcome at 3 months.2,18 The sICH incidence in our study was a little higher than that reported in the STARTING-SICH cohort (3.6%), which was conducted in the European-American ancestry16 because the Asian population may have a higher risk of sICH than other race/ethnic groups.4
Consistent with prior studies, our study found that atrial fibrillation was associated with sICH after IVT. Hu et al carried out a meta-analysis about the safety of IVT for acute ischemic stroke with atrial fibrillation and found that the incidence of sICH in atrial fibrillation patients was higher than that in non-atrial fibrillation patients (6.4% vs 4.1%; P < 0.001).19 The following points might explain the reason that atrial fibrillation patients have a higher risk of sICH after IVT: First, atrial fibrillation patients are more likely to have old and organized thrombi, which are often insensitive to IVT, leading to the time extension of recanalization, and increase the risk of bleeding.20 Second, patients with atrial fibrillation usually have poor collateral circulation, severe intracranial hypoperfusion, and large infarct sizes, thus increase the reperfusion hemorrhage risk.21 Third, a subgroup of atrial fibrillation patients take anticoagulant drugs before IVT, and it may also increase the bleeding risk. Higher baseline glucose level is another predicted factor in our nomogram model. Hyperglycemia can result in increased matrix metalloproteinase-9 and a series of oxidative stress reactions, which may disrupt the integrity of the blood brain barrier and increase the permeability of the blood brain barrier, leading to the development of sICH.22 Baseline NIHSS score, which reflects the severity of the stroke, has been confirmed as the risk factor for sICH following IVT.9,23,24 Our nomogram model indicated that patients with higher baseline NIHSS score might have an increased risk of sICH.
Our study also revealed that NLR was an independent risk factor for sICH. The inflammatory response mediated by the migration of inflammatory cells and the activation of extracellular matrix kinase (mainly matrix metalloproteinases), which leading to the disruption of blood brain barrier integrity has been proven to play a vital role in hemorrhagic transformation.25,26 The neutrophils can increase the permeability of the blood-brain barrier by release related cytokines, chemokines, adhesion molecules, and different proteases, while the activation of lymphocytes may reduce the destruction of the blood-brain barrier. As the balance between neutrophils and lymphocytes, NLR is considered the biomarker for systemic inflammation. Maestrini et al reported that NLR was the best predictor for sICH after IVT, and an NLR ≥4.8 before thrombolysis showed a 3.7-fold increased risk for sICH.27 A clinical study from Guo et al reported that the dynamic change of NLR was associated with sICH after IVT in acute ischemic stroke patients.28 To our knowledge, our study is the first predictive score system that includes NLR as a predictor to predict the probability of sICH after IVT.
Several predictive scoring systems have been published for predicting sICH after IVT in acute ischemic stroke patients5–11 (Table 3). However, these scores often converted the discrete/continuous variables into a dichotomous or multi-categorical variable, thus might lead to the loss of information and decrease the predictive accuracy. Nomogram is a graphical calculation instrument that can assign an accurate numerical probability of a clinical event for an individual patient, which bypasses the categorization of continuous variables into ≥2 different groups.12 The STARTING-SICH predicted model composed of 10 clinical variables was the first nomogram model to predict the risk probability of sICH after IVT, and it was conducted in the European-American ancestry.16 Considering the differences in sICH risk between European-American and Asian populations, it is necessary to construct a nomogram to predict the risk of sICH in Asians. As far as we know, our predictive model is the first nomogram to predict sICH risk in Asian populations. There were a good discriminative and a calibration ability for our nomogram model. With this nomogram, clinicians can easily and quickly discern patients who are at increased risk of developing sICH that require thrombolysis, take intensive monitoring for an individual patient, and also help guide the expectations of patients and their families.
Table 3.
Description of the Representative sICH Predictive Model
| Predictive Model | Variables in the Model | Definition of sICH |
|---|---|---|
| GRASPS | Age, sex, ethnicity, NIHSS, blood glucose, systolic blood pressure | NINDS |
| HAT | Diabetes mellitus /blood glucose, NIHSS, hypodensity on CT (MCA territory) | NINDS |
| SEDAN | Age, NIHSS, blood glucose, early infarct signs, hyperdense cerebral artery sign | ECASS-II |
| SITS-SICH | Age, body weight, hypertension, NIHSS, blood glucose, systolic blood pressure, onset to treatment time, aspirin alone, aspirin plus clopidogrel | SITS-MOST |
| THRIVE | Age, NIHSS, hypertension, diabetes mellitus, and atrial fibrillation | NINDS |
| SPAN-100 | Age, NIHSS | NINDS |
| STARTING-SICH | Age, aspirin alone, aspirin plus clopidogrel, oral anticoagulant with INR ≤1.7, pre-stroke modified Rankin Scale score >0, NIHSS, systolic blood pressure, blood glucose, onset to treatment time, hyperdense cerebral artery sign, current infarction sign | ECASS-II |
Abbreviations: sICH, symptomatic intracranial hemorrhage; NIHSS, National Institute of Health Stroke Scale; SITS, Safe Implementation of Thrombolysis in Stroke; SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study; GRASPS, Glucose Race Age Sex Pressure Stroke Severity; HAT, hemorrhage after thrombolysis; SEDAN, blood sugar, early infarct signs, hyperdense cerebral artery sign, age, NIH Stroke Scale; THRIVE, totaled health risks in vascular events; SPAN-100, stroke prognostication using age and NIH stroke scale-100; STARTING-SICH, systolic blood pressure, age, onset-to-treatment time for thrombolysis, NIHSS score, glucose, aspirin alone, aspirin plus clopidogrel, anticoagulant with INR ≤1.7, current infarction sign, hyperdense artery sign; NINDS, National Institute of Neurological Disorders and Stroke; ECASS-II, European Cooperative Acute Stroke Study II.
The strengths of our study are as follows: the data are derived from multiple stroke centers in China, the sample size is relatively large, and the prognostic factors included in the nomogram can be easily and quickly achieved at admission. Nevertheless, there are some limitations to our study. First, this is a retrospective analysis of multicenter data, the accuracy of the predictive model may be influenced by missing data. Second, several neuroradiologic predictors, such as early infarction signs and hyperdense middle cerebral artery signs were not available in the study. Our nomogram might be suitable for those who cannot accurately interpret the image of early infarction signs. Third, although we tried to include study parameters as many as possible, some major confounders which may influence sICH were not available, such as previous anticoagulant use, previous antiplatelet agents use, blood coagulation status on admission, blood pressure variability, and cerebral microbleeds. Finally, further external validations in other different cohorts are still warranted.
Conclusions
In conclusion, we developed a novel nomogram predictive model, which is consisted of atrial fibrillation, baseline glucose level, NIHSS score and NLR to predict the risk probability of sICH after IVT treatment in Chinese acute ischemic stroke patients. Further external validation is warranted to validate the efficacy of the nomogram in different populations.
Acknowledgments
We express our gratitude to all the researchers and patients who participated in this study.
Funding Statement
This study was supported in part by the National Natural Science Foundation of China (NO. U20A20357, 81870947), the National Key R&D project (NO. 2017YFC1307901) and Key R&D Program of Jiangsu Province (Clinical Frontier Technology Project) (NO. BE2020700).
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author (Xinfeng Liu).
Disclosure
All the authors declare that there are no conflicts of interest.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. 2011;77(4):341–348. doi: 10.1212/WNL.0b013e3182267b8c [DOI] [PubMed] [Google Scholar]
- 3.Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. 2012;34(2):106–114. doi: 10.1159/000339675 [DOI] [PubMed] [Google Scholar]
- 4.Mehta RH, Cox M, Smith EE, et al. Race/Ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke. 2014;45(8):2263–2269. doi: 10.1161/STROKEAHA.114.005019 [DOI] [PubMed] [Google Scholar]
- 5.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43(9):2293–2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 6.Cucchiara B, Tanne D, Levine SR, et al. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17(6):331–333. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 7.Lou M, Safdar A, Mehdiratta M, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71(18):1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strbian D, Engelter S, Michel P, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71(5):634–641. doi: 10.1002/ana.23546 [DOI] [PubMed] [Google Scholar]
- 9.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe implementation of treatments in stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43(6):1524–1531. doi: 10.1161/STROKEAHA.111.644815 [DOI] [PubMed] [Google Scholar]
- 10.Flint AC, Faigeles BS, Cullen SP, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke. 2013;44(12):3365–3369. doi: 10.1161/STROKEAHA.113.002794 [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Guzik AK, Reeves M, et al. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology. 2013;80(1):21–28. doi: 10.1212/WNL.0b013e31827b1ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shariat SF, Capitanio U, Jeldres C, et al. Can nomograms be superior to other prediction tools? BJU Int. 2009;103(4):492–495. doi: 10.1111/j.1464-410X.2008.08073.x [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Yuan K, Wang H, et al. Nomogram to predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J Am Heart Assoc. 2020;9(3):e014899. doi: 10.1161/JAHA.119.014899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–290. doi: 10.1016/S1474-4422(14)70325-4 [DOI] [PubMed] [Google Scholar]
- 15.Cappellari M, Mangiafico S, Saia V, et al. IER-SICH nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50(4):909–916. doi: 10.1161/STROKEAHA.118.023316 [DOI] [PubMed] [Google Scholar]
- 16.Cappellari M, Turcato G, Forlivesi S, et al. STARTING-SICH nomogram to predict symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke. Stroke. 2018;49(2):397–404. doi: 10.1161/STROKEAHA.117.018427 [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352(9136):1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.Gumbinger C, Gruschka P, Böttinger M, et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke. 2012;43(1):240–242. doi: 10.1161/STROKEAHA.111.623033 [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Ji C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta-analysis. BMC Neurol. 2021;21(1):66. doi: 10.1186/s12883-021-02095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura K, Iguchi Y, Shibazaki K, et al. IV t-PA therapy in acute stroke patients with atrial fibrillation. J Neurol Sci. 2009;276(1–2):6–8. doi: 10.1016/j.jns.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 21.Tu HT, Campbell BC, Christensen S, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10(4):534–540. doi: 10.1111/ijs.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won SJ, Tang XN, Suh SW, et al. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70(4):583–590. doi: 10.1002/ana.22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, Pan Y, Zhou L, et al. Predictors of post-thrombolysis symptomatic intracranial hemorrhage in Chinese patients with acute ischemic stroke. PLoS One. 2017;12(9):e0184646. doi: 10.1371/journal.pone.0184646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34(2):185–199. doi: 10.1038/jcbfm.2013.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. doi: 10.1161/01.CIR.0000046451.38849.90 [DOI] [PubMed] [Google Scholar]
- 27.Maestrini I, Strbian D, Gautier S, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85(16):1408–1416. doi: 10.1212/WNL.0000000000002029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Z, Yu S, Xiao L, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflammation. 2016;13(1):199. doi: 10.1186/s12974-016-0680-x [DOI] [PMC free article] [PubMed] [Google Scholar]