Abstract
Aims
the objectives of this study are to reveal the potential side effects after taking the covid19 vaccines, associated risk factors with severe side effects, and to compare the three COVID-19 vaccines available in Iraq (Sinopharm, AstraZeneca-Oxford and Pfizer- BioNTech).
Methods
a randomized cross-sectional study was conducted in April 2021. A standardized questionnaire platform was utilized to collect information about the Iraqi population.
Results
1012 were enrolled in the study, 60.2% were male and 39.8% were female. 84% were symptomatic post vaccination. Young aged participants, females, participants with history of COVID19 infection, those with comorbid diseases and AstraZeneca vaccine receivers were statistically significant risk factors for having adverse reactions post vaccination, P value (0.03, 0.028, 0.007, 0.019 and 0.0001) respectively. Regarding severity of symptoms, most symptoms were mild and moderate. Residency in Kurdistan Region of Iraq and AstraZeneca vaccine were the statistically significant risk factors for getting severe symptoms P value < 0.0001 of both. Females were an associated risk factor for D-dimer elevation P value = 0.05.
Conclusion
fatigue, injection site reactions, fever, myalgia, headache and chills were the most reported side effects. Most symptoms were mild to moderate in term of severity.
Keywords: Adverse effects, COVID19 vaccine, Sinopharm, AstraZeneca-Oxford, Pfizer- BioNTech
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has spread viciously across the world with millions of infected people and deaths [1]. Hence, there was an international urgent demand to develop vaccines by the scientific community [2].
The World Health Organization (WHO) had declared the release of covid 19 vaccines in September 2020 [2]. On December 31, 2020, mRNA vaccine “Pfizer BioNTech”, and on February 15, 2021, the adenoviral vector vaccines ChAdOx1 nCoV-19 (AstraZeneca-Oxford) were granted emergency use [3].
Researchers found that efficacy of AstraZeneca and Pfizer vaccine is 70% and 95% respectively [4,5]. While the inactivated SARS-CoV-2 vaccine, BBIBP-CorV, developed by Sinopharm (Beijing, China) is safe and effective with a seroconversion of 92%–100%, its T cell responses after vaccination is low compared to the other two leading to its low effectiveness [6]. Despite that, Sinopharm was the 1st vaccine that had been administered to Iraqi population and acceptable as WHO's target was an efficacy of more than 50% [7].
Vaccine hesitancy (VH) refers to a delay of vaccine acceptance in spite of its existence, researchers consider it as a public health challenge nourished by misleading and inaccurate information of vaccines in terms of safety and efficacy [8,9].
Since the beginning of the pandemic until May 22, 2021, Iraq had reported a total of 1 164 149 confirmed cases and 16 158 deaths of SARS-CoV2 infection. It had passed through 2 prominent waves of transmission, the last of which had been initiated in the second half of January 2021. However, a study performed in Kurdistan region of Iraq, reported a decline in the mortality rate from November 2020 to February 2021, which was assumed to be due to natural immunity to the virus to an extent [10]. Regarding vaccine status in Iraq, a total of 441 121 people had received the vaccine by 10th of May, with almost 15 000 vaccinations every day [11], i.e. only 2% of population had been vaccinated.
Several studies in UK and Poland had supported the reality that fearing the vaccine symptoms is the most outstanding and frequent reason of vaccination aversion [12,13]. In Iraq, there are some myths regarding COVID19 vaccines, some believe that these vaccines are a part of conspiracy operations. Potential side effects and lack of trust towards manufacturer companies are other factors of low vaccine compliance rate [8]. These concerns had raised the need to do a study about the post-COVID19 vaccination side effects of different vaccine companies that are available in Iraq. Therefore, the objectives of this study are to reveal the potential side effects after taking the covid19 vaccines, associated risk factors with severe side effects, and to compare the three COVID-19 vaccines available in Iraq (Sinopharm, AstraZeneca-Oxford and Pfizer- BioNTech).
2. Materials and methods
2.1. Study design
This randomized cross-sectional retrospective study was conducted in April 2021. A standardized questionnaire platform was utilized to collect information about the Iraqi population. The questionnaire included 3 major parts, in sequence: demographic data including (age, gender, residency and occupation); clinical profile (comorbid conditions, drug history and previous COVID19 infection); vaccine data (type of vaccine, side effects in term of duration and severity). An optional question regarding pre and post vaccine D-dimer level was included.
2.2. Participants
Participants were Iraqi citizens from the North (Kurdistan Region of Iraq), as well as middle and south provinces of Iraq (Baghdad, Mosul, Karbala, Najaf, Basra and Kirkuk). Individuals aged ≥18 who received Pfizer, AstraZeneca, and Sinopharm COVID19 vaccines before at least 1 week were included. Exclusion criteria were people who had been vaccinated from manufacturer companies other than the 3 included companies and Iraqi people who are living abroad. Regarding occupation, both healthcare workers (HCWs) and non-healthcare workers were included. HCWs were physicians, dentists, pharmacists and nurses. Others were students, engineers, general institutional workers, house wives, etc.
2.3. Ethical consideration
The study was reviewed and approved by the Scientific and the Ethics Committee of the College of Pharmacy at University of Duhok on March 17, 2021 (Reference No. 124). Participants had submitted their informed consents prior to participation.
2.4. Statistical analysis
The results obtained were analyzed by entering the data in Statistical Package for the Social Sciences (SPSS) software version 22 (Chicago, IL, USA). Descriptive statistics were carried out for demographic variables and medical anamnesis. Fisher's exact and Chi-square tests were used to find the associated risk factors for developing symptoms after vaccination. Odd Ratio of 1 and P value of ≤0.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
A total of 1029 participants fulfilled the questionnaire platform, however, 17 had been excluded in the study as they hadn't met the study inclusion criteria. The remaining 1012 were enrolled for the final analysis. 609 (60.2%) were male and 403 (39.8%) were female. The age of most of them was of a range (18–49) which was about 83.4%. 51.4% of participants were from Kurdistan region of Iraq and 48.6% of them were from Middle and South provinces of Iraq. 60.1% of enrolled participants had received AstraZeneca-Oxford vaccine, 29.2% had received Pfizer-BioNTech and only 10.7% had received Sinopharm. 36.6% of them had history with Covid19 virus infection. 41.7% of participants were healthcare workers.
Regarding medical anamnesis, 151 (14.9%) participants had at least one comorbid disease. Among them, 89 (58.9%) had hypertension, 48 (31.7%) had diabetes, 12 (7.9%) had thyrotoxicosis, 11 (1.1%) had asthma, the remaining ill participants were having different non communicable diseases like hyperlipidemia, arthritis, heart failure, cancer, renal failure and stroke. A total of 850 (84%) out of 1012 one had obvious adverse effects, ranging from mild to severe in term of severity, while 162 (16%) were asymptomatic, Additional demographic characteristics are shown in Table 1 .
Table 1.
Demographic characteristics of study population N = 1012.
| Variables | Frequency | Percentage |
|---|---|---|
| Sex | ||
| Male | 609 | 60.2 |
| Female | 403 | 39.8 |
| Age | ||
| 18–30 | 420 | 41.5 |
| 31–49 | 424 | 41.9 |
| 50–69 | 150 | 14.8 |
| 70 and more | 18 | 1.8 |
| Occupation | ||
| HCWs | 422 | 41.7 |
| Non HCWs | 590 | 58.3 |
| Residence | ||
| Kurdistan Region of Iraq | 520 | 51.4 |
| Middle and south region of Iraq | 492 | 48.6 |
| History of infection with covid | ||
| Yes | 370 | 36.6 |
| No | 642 | 63.4 |
| Smoker | ||
| Yes | 241 | 23.8 |
| No | 771 | 76.2 |
| Vaccine type | ||
| AstraZeneca | 608 | 60.1 |
| Pfizer | 296 | 29.2 |
| Sinopharm | 108 | 10.7 |
| Symptoms after vaccination | ||
| Yes | 850 | 84 |
| No | 162 | 16 |
| Severity of symptoms N = 850 | ||
| Mild | 378 | 44.5 |
| Moderate | 333 | 39.2 |
| Sever | 139 | 16.4 |
| Duration of symptoms N = 850 | ||
| < 24 h s | 329 | 38.8 |
| 1–3 days | 463 | 54.6 |
| 4days – a week | 43 | 5.1 |
| > week | 13 | 1.5 |
3.2. Prevalence of general adverse effects
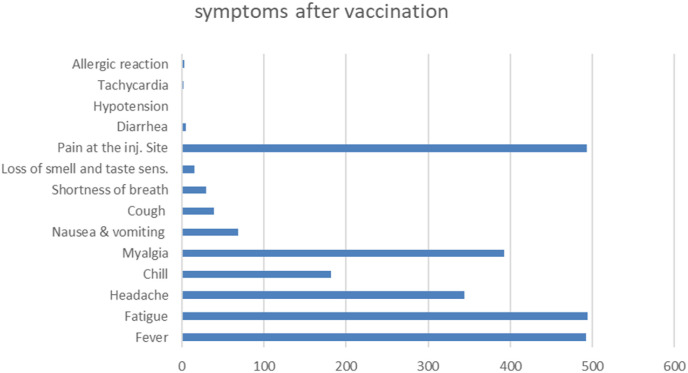
Most common post vaccination symptoms were fatigue N = 494, injection site reaction (pain, redness and swallowing) N = 493, fever N = 492, myalgia and muscle pain N = 393, headache N = 344, chills N = 182, nausea and vomiting N = 69, the less likely appeared symptoms were cough and shortness of breath N = 39, N = 30 respectively, more symptoms are shown in Fig. 1 .
Fig. 1.
Types of adverse reactions.
The frequencies of each symptom with the three taken vaccine manufacturers are demonstrated in Table 2 , 66.9% of those who took Pfizer vaccine suffered from injection site reaction, which was more common when compared with AstraZeneca and Sinopharm one 54.2%, 54.5% respectively. Fever, fatigue, headache, myalgia and chills were more common among AstraZeneca vaccine. Three participants had allergic reaction after taking the vaccine by 5–15 min. One of them was an AstraZeneca recipient and two were Pfizer recipients. More details are shown in below table.
Table 2.
Adverse Reactions to Different Vaccines among recipients of different vaccines N = 850.
| Symptoms | AstraZeneca (N = 533) | Pfizer (N = 251) | Sinopharm (N = 66) |
|---|---|---|---|
| Fever N = 492 | 365 (68.4) | 102 (40.6) | 25 (37.8) |
| Fatigue N = 494 | 346 (64.9) | 121 (48.2) | 27 (40.9) |
| Headache N = 344 | 256 (48) | 66 (26.2) | 22 (33.3) |
| Chill N = 182 | 149 (28) | 20 (7.9) | 13 (19.6) |
| Myalgia N = 393 | 289 (54.2) | 80 (31.9) | 24 (36.3) |
| Nausea & vomiting N = 69 | 55 (10.3) | 7 (2.7) | 7 (10.6) |
| Cough N = 39 | 24 (4.5) | 10 (3.9) | 5 (7.5) |
| Shortness of breath N = 30 | 22 (4.1) | 6 (2.3) | 2 (3) |
| Loss of smell and taste sens.N = 15 | 11 (2) | 2 (0.7) | 2 (3) |
| Injection site reaction N = 493 | 289 (54.2) | 168 (66.9) | 36 (54.5) |
| Diarrhea N = 5 | 3 (0.5) | 2 (0.7) | 0 (0) |
| Hypotension N = 1 | 0 (0) | 1 (0.3) | 0 (0) |
| Tachycardia N = 2 | 1 (0.1) | 1 (0.3) | 0 (0) |
| Allergic reaction N = 3 | 1 (0.1) | 2 (0.7) | 0 (0) |
3.3. Associated risk factors of having post vaccination adverse effects
Participants who aged less than 50 years old were more prone to have symptoms after vaccination; p value = 0.003. The chi-squared test revealed that females, participants with history of COVID19 infection, those with comorbid diseases as hypertension, diabetes, asthma, arthritis etc., and AstraZeneca vaccine receivers were statistically significant risk factors for having adverse reactions post vaccination, p value (0.028, 0.007 and 0.019 and 0.0001 respectively. In the other hand, residency and occupation have no correlation with the appearance of symptoms post vaccination. See Table 3 .
Table 3.
Univariate analysis of associated risk factors with symptoms after covid vaccines.
| Variables | Symptomatic N = 850 (%) |
Asymptomatic N = 162 (%) |
Total N = 1012 (%) |
Odd Ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 499 (81.9) | 110 (18.1) | 609 (60.2) | 0.672 (0.470–0.960) | 0.028 |
| Female | 351 (87.1) | 52 (12.9) | 403 (39.8) | ||
| Age | |||||
| 18–49 | 722 (85.5) | 122 (14.5) | 844 (83.4) | 1.849 (1.235–2.768) | 0.003 |
| 50 and above | 128 (76.2) | 40 (23.8) | 168 (16.6) | ||
| Occupation | |||||
| HCWs | 353 (83.6) | 69 (16.4) | 422 (41.7) | 0.957 (0.681–1.345) | 0.801 |
| Non HCWs | 497 (84.2) | 93 (15.8) | 590 (58.3) | ||
| Residence | |||||
| Kurdistan | 435 (83.7) | 85 (16.3) | 520 (51.4) | 0.949 (0.6783–1.329) | 0.763 |
| Iraq | 415 (84.3) | 77 (15.7) | 471 (46.5) | ||
| History with covid 19 infection | |||||
| Yes | 326 (88.1) | 44 (11.9) | 370 (36.6) | 1.668 (1.149–2.422) | 0.007 |
| No | 524 (81.6) | 118 (18.4) | 642 (63.4) | ||
| Smoker | |||||
| Yes | 202 (83.8) | 39 (16.2) | 241 (23.8) | 0.983 (0.663–1.456) | 0.932 |
| No | 648 (84) | 123 (16) | 771 (76.2) | ||
| Comorbid condition | |||||
| No diseases | 733 (85.1) | 128 (14.9) | 861 (85.1) | 0.6 (0.392–0.919) | 0.019 |
| Diseases | 117 (77.4) | 34 (22.6) | 151 (14.9) | ||
| Vaccine type | |||||
| AstraZeneca ∗∗ | 533 (87.7) | 75 (12.3) | 608 (60.1) | 0.0001 | |
| Pfizer | 251 (84.8) | 45 (15.2) | 296 (29.2) | ||
| Sinopharm | 66 (61.1) | 42 (38.9) | 108 (10.7) | ||
3.4. Associated risk factors for getting severe post vaccination adverse effects
Regarding severity of symptoms, Only 139 out of 850 symptomatic participants faced severe to critical side effects; prevalence (16.3%). 87.7% of severe symptoms had occurred in those whom aged (18–49 years old). Residency in Kurdistan Region of Iraq and AstraZeneca-Oxford vaccine were the statistically significant risk factors for getting sever symptoms P value < 0.0001 of both. See Table 4 .
Table 4.
Variables correlation with severity of symptoms N = 850.
| Variables | Mild-moderate symptoms N = 177 (%) | Severe symptoms N = 139 (%) | Odd ratio 95% CI | P value |
|---|---|---|---|---|
| Sex | ||||
| Male | 422 (84.6) | 77 (15.4) | 1.17 (0.81–1.69) | 0.386 |
| Female | 289 (82.3) | 62 (17.7) | ||
| Age | ||||
| 18–49 yrs | 600 (83.1) | 122 (16.9) | 1.32 (0.76–2.29) | 0.308 |
| 50 yrs and above | 111 (86.7) | 17 (13.3) | ||
| Occupation | ||||
| HCWs | 291 (82.4) | 62 (17.6) | 1.16 (0.80–1.67) | 0.421 |
| Non-HCWs | 420 (84.5) | 77 (15.5) | ||
| Residence | ||||
| Kurdistan Region of Iraq | 333 (76.6) | 102 (23.4) | 3.12 (2.08–4.68) | < 0.0001 |
| Middle and south Iraq | 378 (91.1) | 37 (8.9) | ||
| History of infection with covid 19 | ||||
| Yes | 267 (81.9) | 59 (18.1) | 1.22 (0.84–1.77) | 0.278 |
| No | 444 (84.7) | 80 (15.3) | ||
| Smoking | ||||
| Yes | 164 (81.2) | 38 (18.8) | 1.25 (0.83–1.89) | 0.279 |
| No | 547 (84.4) | 101 (15.6) | ||
| Comorbid conditions | ||||
| Diseases | 99 (84.6) | 18 (15.4) | 0.91 (0.53–1.57) | 0.760 |
| No diseases | 612 (83.5) | 121 (16.5) | ||
| Vaccine type | ||||
| AstraZeneca∗ | 411 (77.1) | 122 (22.9) | < 0.0001 | |
| Pfizer | 236 (94) | 15 (6) | ||
| Sinopharm | 64 (97) | 2 (3) | ||
3.5. Associated variables with D-dimer elevation
220 participants underwent the D-dimer test (205 patients receiving Astra Zeneca vaccine and 15 patients receiving Sinopharm and Pfizer vaccines). The elevations were found among 6 women, all of them were AstraZeneca vaccine receivers and only one of them were aged more than 50 years old, 3 D-dimer elevated women progressed to petechiae and hematomas and one needed hospitalization to treat the thromboembolism. Hence, females were an associated risk factor for D-dimer elevation p value = 0.05. See Table 5 .
Table 5.
Variables correlation with D-dimer elevations N = 220.
| Variables | D-dimer not elevated (%) | D-dimer elevated (%) | P value |
|---|---|---|---|
| Gender | |||
| Male | 83 (100) | 0 (0) | 0.05 |
| Female | 131 (95.6) | 6 (4.4) | |
| Age | |||
| 18–49 | 177 (97.2%) | 5 (2.8%) | 0.6645 |
| ≥ 50 | 37 (97.3%) | 1 (2.7%) | |
| Vaccine type | |||
| AstraZeneca | 199 (97%) | 6 (3%) | 0.65 |
| Pfizer and Sinopharm | 15 (100%) | 0 (0%) | |
4. Discussion
Recently, COVID-19 vaccines have been introduced to control the pandemic; however, similar to other vaccines, it is not without adverse effects. In our study, out of 1012 participants, 850 (84%) were symptomatic and 162 (16%) were asymptomatic. The most common symptoms were: fatigue (58.1%), local injection site reactions, (58%), fever (57.8%), myalgia and muscle pain (46.2%), headache (40.4%), and chills (21.4%). Our finding was similar to other studies for e.g. a study from Czech republic reported fatigue, headache, muscle pain, and chills the most common side effects [14]. Similarly, a study from Wuhan showed fever, fatigue, headache, and muscle pain in vaccinated individuals by (46%), (44%), (39%), and (17%), respectively [15]. Pain at the injection site occurred in mRNA (66.9%), adenoviral vector (54.2%), and Sinopharm (54.5%) vaccine recipients. On the contrary, the Pfizer manufacturer reported a higher rate of local injection site reactions (83.1%); while, the same prevalence rate was reported in the adenoviral vector vaccine (54%) [5,15,16].
Post vaccination allergic reaction occurred in three participants; of whom 1 (0.1%) was from AstraZeneca vaccine, and 2 (0.7%) were from Pfizer vaccine. This rate was lower than other studies where they reported 1.7% allergic reactions [17]. This hypersensitivity is believed to be due to polyethylene glycol (PEG) excipient in the mRNA vaccines (Pfizer) and polysorbate 80 in the adenoviral vector vaccines ChAdOx1 nCoV-19 (AstraZeneca-Oxford) [18,19]. Those excipients are added to many pharmaceutical products in order to increase solubility, improve absorption and stability, create specific appearance or influence palatability [18].
In the current study, several associated risk factors for developing side effects after COVID19 vaccines were recognized, namely: younger people, females, history of COVID-19 infection, comorbidity, and AstraZeneca vaccine. In contrast, older people were less likely to develop these symptoms. This finding was in parallel to the report of Food and Drug Administration (FDA), stating that people of ≥55 years were less likely to suffer from adverse effects [5]. In correspondence, a study from Czech Republic also found that young adults were more likely to have side effects with Pfizer vaccine [14]. Randomized controlled trails in Brazil, South Africa, and UK on the safety and efficacy of ChAdOx1 nCoV-19 vaccine “AstraZeneca” also documented a lower number and intensity of side effects in older adults [4].
Female gender was a significant risk factor for adverse effects (P value = 0.028); this was in line with other literatures [14,17]. In general, younger individuals and females tend to develop stronger immune responses than older individuals and males, respectively [4]. Hence, they are more likely to develop more frequent and more intense side effects.
The history of SARS CoV-2 infection was a significant risk factor for frequent adverse reactions among vaccinated people (p value = 0.007). In concordance, a prospective observational study conducted in the UK by Menni et al. reported more common local and systematic side effects among individuals with previous infections [17]. Although there is no clear explanation, this could be linked with the increase of immunogenicity as a result of vaccination, which in turn increases reactogenicity [17]. Coexisting diseases such as bronchial asthma, hypertension, and DM were a recognized risk factor for post vaccine side effects (P value = 0.019). This finding was in contrast to other studies [20]. The exact explanation for this finding is unclear at the moment; however, further prospective studies with a larger sample size to ascertain this association is indicated.
In our study, AstraZeneca vaccine recipients were more prone to side effects in comparison to the other two (P value = 0.0001). Likewise, other studies pointed out this finding [17]. Regarding severity of symptoms, most symptoms were mild (44.5%) and moderate (39.2%), only 139 out of 850 reported severe side effects. This finding is in concordance to a study from Wuhan where they reported that most symptoms were mild to moderate in severity [15]. Individuals aged<50 years experienced severe symptoms in 87.7%. Additionally, the side effects were more severe among AstraZeneca-Oxford vaccine recipients (P value < 0.0001). In line to the present study, a study from Jordan, a neighboring country, found that the number and severity of side effects were associated with the type of vaccine [21]. Another study by Mathioudakis et al. found that Pfizer vaccine causes milder, less frequent systemic adverse effects but more common local side effects [22].). For the most part, in order to understand the variety of diversities regarding vaccines further studies need to be executed.
Participants from Kurdistan region of Iraq reported severe symptoms after vaccination (P value < 0.0001), this finding was obscure and needs further studies.
In the current study, D-dimer elevation was seen among 6 women out of 220 people who underwent the test. The D-dimer test represents an excellent non-invasive triage test in patients with suspected venous thromboembolism [23]. Female gender was a significant risk factor for developing D-dimer elevation (P value 0.05); all were AstraZeneca vaccine recipients. This finding is satisfying the AstraZeneca vaccine scattered reports from more than 20 countries of rare blood-clotting conditions, which was found mostly among women aged 55 years or younger [24]. This rare event of AstraZeneca vaccine is named “vaccine-induced immune thrombotic thrombocytopenia” (VITT), which is pathologically resembling heparin-induced thrombocytopenia (HIT). This results from the development of immune mediated thrombocytopenia by platelet-activating antibodies against platelet factor 4 (PF4) [25,26]. Several reports documented this finding among mRNA vaccines recipients [27]; however, we did not find such a case in this study, which may be explained by the small sample size; furthermore all participants did not undergo laboratory investigations. In general, D-dimer and anti-PF4/heparin antibodies tests are recommended for people who intend to get vaccinated, especially young women, to prevent possible VITT.
5. Conclusion
In conclusion, keeping with recorded literatures, fatigue, injection site reactions, fever, myalgia, headache and chills were the most reported side effects. Covid-19 vaccine adverse reactions is a fact that occurred in more than 80% of vaccine recipients; however, most symptoms were mild to moderate in term of severity and can be tolerated. Hence, COVID-19 vaccines are safe and our community is encouraged to receive vaccination. Females, young people, patients with comorbidity, history of past COVID-19 infection, and AstraZeneca vaccine recipients were associated risk factors of developing post-vaccination side effects. Female gender was a significant risk factor for developing D-dimer elevation, which in turn may cause rare clotting disorders.
Further prospective studies with a larger sample size and long duration follow-up, including important laboratory parameters such as D-dimer are warranted to better understand side effects in COVID-19 vaccine recipients.
Author contributions
Conceptualization: H.B.A. and M.A.M.; methodology: H.B.A., S.A.M., A.M.A; formal Analysis: H.B.A.; writing—original draft preparation: H.B.A.; review and editing: M.A.M.
Funding
This research received no external funding.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgment
Greatly appreciating all the people who participated in this study, who spent much time fulfilling the questionnaire. Additionally, a huge thank goes to Dr. Rezvan Faisal Abduljabbar for helping us during the study, his effort is highly appreciated.
References
- 1.Dong E., Gardner H. DuL. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta Kaur S.P.V. COVID-19 Vaccine: a comprehensive status report. Virus Res. 2020 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2021. Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. Geneva, Switzerland. [Google Scholar]
- 4.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. 10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Local Reactions C.D.C. 2021. Systemic reactions, adverse events, and Serious adverse events: Pfizer-BioNTech COVID-19 vaccine. [cited 2021 7 March 2021] [Google Scholar]
- 6.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Helath Organization . 2020. WHO target product profiles for COVID-19 vaccines. Version 3-29. [Google Scholar]
- 8.Wu Harrison E.A.J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020;35(4):325–330. doi: 10.1007/s10654-020-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merza M.A., Abdulah D.M., MohammedA H.M., Yones M. 2021. Epidemiological Trends of coronavirus disease 2019 in Iraqi Kurdistan. Disaster medicine and public health Preparedness; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . 2021. WHO confirms the safety and efficacy of COVID-19 vaccines and urges Iraqis to register and vaccinate to help defeat the pandemic.http://www.emro.who.int/irq/iraq-news/ [cited 2021 11 April 2021]; Available from: [Google Scholar]
- 12.Luyten J., BruyneelA L., van Hoek J. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine. 2019;37(18):2494–2501. doi: 10.1016/j.vaccine.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Szmyd B., Karuga F.F., Bartoszek A., Staniecka K., Siwecka N., Bartoszek A. Attitude and behaviors towards SARS-CoV-2 vaccination among healthcare workers: a cross-sectional study from Poland. Vaccines. 2021;9(3):218. doi: 10.3390/vaccines9030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riad A., Pokorná A., Attia S., Klugarová J., Klugar M. KoščíkM. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech republic. J Clin Med. 2021;10(7):1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. 10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021:939–949. doi: 10.1016/S1473-3099(21)00224-3. https://www.sciencedirect.com/science/article/pii/S1473309921002243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice S.M., Ferree S.D., MesinkovskaA N.A., Kourosh S. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Women’s Dermatol. 2021:209–212. doi: 10.1016/j.ijwd.2021.01.007. https://www.sciencedirect.com/science/article/pii/S2352647521000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9(3):221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi W.S.H.J. Cheong. COVID-19 vaccination for people with comorbidities. Infect Chemother. 2021;53(1):155. doi: 10.3947/ic.2021.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatmal M.m.M., Al-Hatamleh M.A., Olaimat A.N., Hatmal M., Alhaj-Qasem D.M., Olaimat T.M. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):556. doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathioudakis A.G., Ghrew M., Ustianowski A., Ahmad S., Borrow R., Papavasileiou L.P. Self-reported real-world safety and reactogenicity of covid-19 vaccines: a vaccine recipient survey. Life. 2021;11(3):249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Nisio M., Squizzato A., Rutjes A.W., Büller H.R., ZwindermanP A.H., Bossuyt M. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemostasis. 2007;5(2):296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 24.Mallapaty S.E. Callaway. What scientists do and don't know about the Oxford-AstraZeneca COVID vaccine. Nature. 2021;592(7852):15–17. doi: 10.1038/d41586-021-00785-7. [DOI] [PubMed] [Google Scholar]
- 25.Nazy I., Sachs U.J., Arnold D.M., McKenzie S.E., Atlhaus P. ChoiK. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemostasis. 2021 doi: 10.1111/JTH.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Eichinger P. KyrleS. 2021. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. [Google Scholar]
- 27.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. 2021 doi: 10.1002/ajh.26132. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8014568/ [DOI] [PMC free article] [PubMed] [Google Scholar]