Abstract
Introduction
Mendelian randomisation (MR) has been suggested to be able to overcome biases of observational studies, but no meta-analysis is available on MR studies on abdominal aortic aneurysm (AAA). This systematic review and Meta-analysis examined the evidence of causal risk factors for AAA identified in MR studies.
Methods
Publicly available databases were systematically searched for MR studies that reported any causal risk factors for AAA diagnosis. Meta-analyses were performed using random effect models and reported as odds ratio (OR) and 95% confidence intervals (CI). Study quality was assessed using a modified version of Strengthening the Reporting of Mendelian Randomisation Studies (STROBE-MR) guidelines.
Results
Sixteen MR studies involving 34,050 patients with AAA and 2,205,894 controls were included. Meta-analyses suggested that one standard deviation increase in high density lipoprotein (HDL) significantly reduced (OR: 0.66, 95% CI: 0.61, 0.72) and one standard deviation increase in low density lipoprotein (LDL) significantly increased the risk (OR: 1.68, 95%, CI: 1.55, 1.82) of AAA. One standard deviation increase in triglycerides did not significantly increase the risk of AAA (OR: 1.21, 95% CI: 0.86, 1.71). Quality assessment suggested that ten and five studies were of low and moderate risk of bias respectively, with one study considered as high risk of bias.
Conclusion
This meta-analysis suggests LDL and HDL are positive and negative casual risk factors for AAA.
Keywords: Mendelian randomization, Abdominal aortic aneurysm, Peripheral artery disease
1. Introduction
Abdominal aortic aneurysm (AAA) rupture is responsible for approximately 200,000 deaths annually worldwide [1]. Since most AAAs are asymptomatic, they are usually diagnosed by incidental imaging or screening programs [2] The only established treatment to prevent AAA rupture is surgical repair [2], [3]. Randomised controlled trials have shown that elective surgical repair of small asymptomatic AAAs (<55 mm) [4] or large asymptomatic AAAs in people that are unfit does not reduce mortality [5]. Clinical guidelines therefore recommend surgical repair is reserved for people with symptomatic or ruptured AAAs or large asymptomatic AAAs who have an extended life expectancy [2], [3]. Most non-surgically managed AAAs continue to grow in size thereby increasing the risk of rupture [6]. Thus, there is a need to identify drug therapies able to limit AAA growth.
Thirteen previous randomised controlled trials have reported that a range of repurposed medications, such as antibiotics, beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers and mast cell inhibitors, do not slow AAA growth [7], [8], [9]. Therefore, there is a need to identify more specific drug targets for AAA. So far most AAA pathogenesis research has focused on animal models and examination of human AAA samples [10]. The heritability of AAA has been estimated to be about 70% from twins studies [11], [12] and as such there is growing interest in using genetic markers to identify drug targets [7], [10].
Mendelian randomisation uses genetic alleles as an inherited marker of a risk factor of interest, such as inherited level of low density lipoprotein-cholesterol (LDL-C) over a life-time, in order to examine if the risk factor is truly causative in the disease being investigated [13]. This approach is thought to overcome some of the biases of standard risk factor observational studies [13]. Mendelian randomisation studies have reported findings that replicate the results of large randomised controlled trials to predict the benefits of lipid modifying medications in prevention of cardiovascular events [14]. As such, mendelian randomisation may provide a way to identify drug targets. This review aimed to systematically summarise published research using Mendelian randomisation to assess causal risk factors for AAA.
2. Methods
2.1. Literature search
This systematic review was performed according to the 2015 Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement [15]. The study protocol was registered with PROSPERO (Registration number – CRD42020203479). This systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [16]. Medline (via OvidSP, 1966), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Complete (Via EBSCOhost) and Scopus databases to identify articles that used Mendelian randomisation to examine potential causal risk factors for AAA. Medical subject headings and keywords related to ‘Mendelian’ and ‘Aortic aneurysm’ were used in all databases to identify relevant studies. For Ovid, following search was conducted: (Mendelian [Title/Abstract] OR Mendelian randomisation analysis [MeSH]) AND AAA [Title/Abstract]. For Scopus, the following search was conducted: (aneurysm [Title/Abstract] OR AAA [Title/Abstract]) AND Mendelian [Title/Abstract]. For CINAHL database, the following search was conducted: (EMTREE aortic aneurysm [MeSH] OR AAA [Title/Abstract] OR aneurysm [Title/Abstract]) AND Mendelian [Title/Abstract]. The search was initially conducted on 17/08/2020 and updated on 24/01/2021 without any study design or language restrictions by one author (MI). Titles and abstracts were screened to identify relevant studies. If the suitability of a publication was unclear, the full-text article was reviewed. Reference lists of studies identified in the primary search were screened to identify additional relevant studies. To be included in this systematic review, studies needed to have conducted a Mendelian randomisation in patients with AAA. These studies must have clearly identified the potential causal factors investigated and the instrumental variables that had been used to test the causal relationship. Studies were excluded on the basis of not conducting a Mendelian randomisation, using thoracic or ascending aortic aneurysm cases only or if studies did not investigate causal factors for AAA specifically. Studies were evaluated against the inclusion criteria independently by three authors (MI, TS and ST) and any differences were resolved via discussion with a third author (JG).
3. Data extraction and quality assessment
Data from included studies were extracted into predefined tables by one author (MI) and independently reviewed by two more authors (TS, ST). The following data were collected from included studies: country/ethnicity, study design, criteria used to select cases and controls imaging modality, study sample size, datasets used in study, mean age, percentage of male participants, risk factors (alcohol, smoking, hypertension, diabetes, body mass index (BMI) & hyperlipidemia), aortic imaging method, as well as the number of single nucleotide polymorphism (SNPs) used for analysis of a specific causal factor. Information regarding proposed and established causal risk factors of AAA was also collected. For each reported causal risk factor of AAA, the following data were collected: Odds ratio, sample size, p value, unit of risk measurement, randomisation analysis method (such as Egger regression or inverse-variance weighted estimate) and sensitivity analyses were also collected. A quality assessment tool was adapted from the Strengthening the Reporting of Mendelian Randomisation Studies (STROBE-MR) Guidelines [17]. These guidelines were modified using previously published articles regarding Mendelian randomization reporting quality approaches [13], [18], [19]. The quality assessment tool is shown in the supplementary table 2. The quality assessment score was converted to a percentage and scores of < 80, 80–90 and > 90% were considered to represent high, medium and low risk of bias, respectively.
Table 2.
Quality assessment of all included studies.
| STUDIES → | (23) | (24) | (20) | (25) | (27) | (28) | (29) | (30) | (31) | (32) | (33) | (34) | (35) | (36) | (26) | (37) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title and Abstract | ||||||||||||||||
| Title and abstract | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Rationale and objectives | ||||||||||||||||
| Background | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Objectives | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Study Design | ||||||||||||||||
| Study design and Data sources | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Statistical methods for main analysis | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Software and pre – registration | (.) | (.) | (+) | (.) | (.) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (.) | (.) | (.) |
| Reporting | ||||||||||||||||
| Descriptive Data | (.) | (+) | (.) | (+) | (.) | (+) | (+) | (+) | (.) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Main Results | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (.) | (+) | (+) | (+) |
| Sensitivity and additional analysis | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (-) |
| Analysis | ||||||||||||||||
| Key results | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Limitations | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (.) | (+) | (.) | (+) | (+) | (+) | (.) | (.) |
| Interpretation | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (.) |
| Generalisability | (.) | (+) | (.) | (-) | (.) | (-) | (-) | (.) | (-) | (.) | (-) | (.) | (.) | (.) | (.) | (-) |
| Assessment of Assumptions | ||||||||||||||||
| Mendelian Randomisation core assumptions | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (-) | (.) | (+) | (+) | (+) | (-) |
| Total score (out of 14) | 12.5 | 13.5 | 13 | 12.5 | 12.5 | 13 | 13 | 13.5 | 12 | 13.5 | 11.5 | 13 | 13 | 13.5 | 12.5 | 9.5 |
| % | 89.3 | 96.4 | 92.9 | 89.3 | 89.3 | 92.9 | 92.9 | 96.4 | 85.7 | 96.4 | 82.1 | 92.9 | 92.9 | 96.4 | 89.3 | 67.9 |
3.1. Statistical analysis
Data were pooled in a meta-analysis when at least 2 studies were identified assessing the association of common genetic instruments with AAA diagnosis or growth. Efforts were made to minimise the impact of duplicate data by including the largest dataset, where publications using clearly overlapping data were identified. In studies where potential data overlap was considered possible but unavoidable, sensitivity analyses were performed by excluding relevant studies one at a time to assess the impact on the overall findings. Primary outcomes were defined as the association of genetic risk with AAA diagnosis, and were reported as odds ratios (OR) [95% confidence interval (CI)]. A 2-sided p-value of <0.05 was considered statistically significant. Meta-analysis was performed using the ‘meta’ package from the R program (Version 3.4.4). Detailed methodology of the meta-analysis are available elsewhere [20]. In brief, due to the small number of eligible studies, meta-analyses were performed using the inverse variance method with random effects models and applied Sidik-Jonkman method with Hartung‐Knapp modification to estimate the between-study variance (tau2) [21]. This estimator uses the Q-profile method to provide a conservative and broader CI to minimize the risk of false positive results. A minimum of ten studies were deemed as eligible to develop funnel plots to analyse publication bias [22].
4. Results
4.1. Study identification
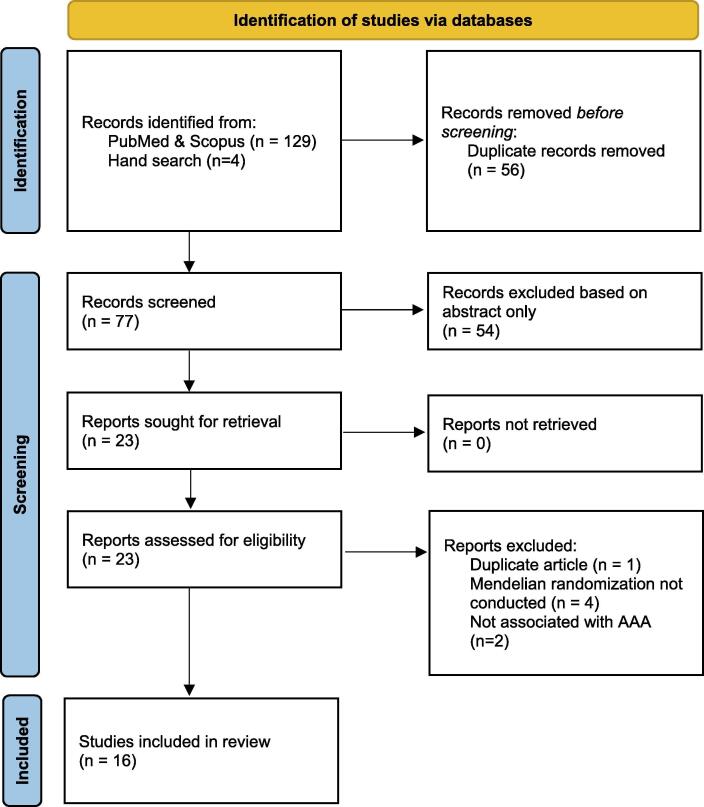
Initial database searches yielded a total of 129 results, of which 56 duplicates were removed, resulting in 73 unique records. After applying the inclusion and exclusion criteria, a total of 12 studies and 4 hand – searched studies were deemed eligible for our final qualitative analysis (Fig. 1).
Fig. 1.
Preferred Reporting Items of Systematic Review and Meta-analyses (PRISMA) flow diagram. A total of 129 studies were screened and 12 studies were included. An additional 4 studies were added through searching references and relevant journals. AAA – Abdominal aortic aneurysm.
4.2. Study characteristics
A total of 35,144 patients with AAA and 2,572,386 controls were investigated independently in 16 studies [20], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Many studies had overlapping cohorts by using the same datasets to conduct analyses on their respective causal risk factors, with the most common overlaps occurring with the UK biobank (9 studies) [23], [27], [28], [29], [31], [34], [36], [37], [38] and the Utrecht Netherlands dataset (4 studies) [20], [25], [32], [33]. Further details regarding datasets are provided in supplement table 3.
All studies explored the association between causal factors and the risk of developing AAA, with the exception of one study that explored AAA mortality [34]. The number of cases and controls across studies ranged between 155 and 7642 and 155 to 366,549 respectively. The studies included participants recruited from multiple datasets in countries including Australia [20], [24], [25], [32], UK [20], [23], [24], [25], [27], [28], [29], [31], [32], [34], [36], [37], New Zealand [20], [25], [32], Iceland [20], [32], The Netherlands [20], [25], [32], [33], Scotland [25], China [30] and the USA [20], [32], [35]. Further details are presented in supplementary table 1. Only 8 of 16 studies summarized patient characteristics [23], [26], [27], [29], [30], [33], [34], [35], of which only 1 reported all of our predefined characteristics [30]. Studies assessed a variety of causal risk factors and thus used varying numbers of SNPs to form their analyses, ranging from using a single SNP to 396. Further detail regarding SNP number is presented in supplementary table 2. The study design, inclusion and exclusion criteria for participants varied across the datasets used within Mendelian analyses. There were 6 case – controlled datasets, 4 prospective dataset and 1 observational cohort dataset. Datasets that had reported aortic diameter as a diagnostic criterion used ≥ 30 mm as the cut-off. Four datasets (Million Veteran Program [26], The Netherlands AAA [25], [32], deCODE genetics [20], Geisinger medical study [32] did not specify either the diameter cut-off or imaging modality. Other datasets used varied criteria such as specialty encounter and emergency repair to define cases (refer to supplementary table 3). For instance, in the Aneurysm Consortium, infra-renal aortic diameter ≥ 30 mm measured via ultrasound (US) or computed tomography (CT) were considered as AAA cases, but controls were not screened with imaging to exclude AAA [20]. In contrast, the Geisinger Medical Centre study used International Classification of Diseases (ICD-9) codes, age restrictions, rupture and genetic conditions to exclude AAA in control groups [32]. The Aneurysm Consortium used controls from the Wellcome Trust Case Control Consortium [20]. Further details of all datasets including country, study design and criteria for case and control selection are provided in supplementary table 3.
Table 1.
Study characteristics of all included studies.
| Dataset | Study design | Country | Groups | Inclusion criteria | AAA diameter cut-off | AAA measurement | Imaging modality |
|---|---|---|---|---|---|---|---|
| UK Biobank [23], [27], [28], [29], [31], [34], [36], [37], [63] | Prospective | United Kingdom | AAA | NR | NR | NR | NR |
| Non-AAA controls | |||||||
| The Aneurysm Consortium[20], [32] | Case - Control | United Kingdom, Australia | AAA | Positive imaging or presentation with acute rupture | >30 mm | IRA diameter | US, CT |
| Unscreened non - AAA controls [From Wellcome Trust Case Control Consortium 2] | |||||||
| Vascular Research Consortium of New Zealand [25] | Case - Control | New Zealand | AAA | Positive imaging | ≥30 mm | IRA diameter | US, CT |
| Non-AAA controls | |||||||
| Geisinger Medical Centre [20], [32] | Case - Control | United States of America | AAA | Positive imaging, repair, rupture or 2 specialist visits of unruptured aneurysm | ≥30 mm | IRA diameter | NR |
| Non-AAA controls | |||||||
| deCODE Genetics[20], [32] | Case - Control | Reykjavik, Iceland | AAA | Positive imaging | ≥30 mm | IRA diameter | NR |
| Non-AAA controls | |||||||
| The Netherlands AAA [20], [25], [32], [33] | Case - Control | Utrecht, The Netherlands | AAA | Positive imaging, emergency AAA repair | ≥30 mm | IRA diameter | NR |
| Non-AAA controls [Nijmegen Biomedical Study and the Nijmegen Bladder Cancer Study] | |||||||
| SMART Study [20], [25] | Prospective cohort study | Utrecht, The Netherlands | AAA | Positive imaging | ≥30 mm | IRA diameter | US |
| Non-AAA controls | |||||||
| The Edinburgh Artery Study [25] | Prospective Population Based Cohort | United Kingdom | AAA | positive imaging | NR | IRA diameter | US |
| Non-AAA controls | |||||||
| Chinese PLA General Hospital [30] | Case - Control | China | AAA | positive imaging | NR | NR | US, CT |
| Age and gender matched non-AAA control group 1 | |||||||
| Age and gender matched non-AAA control group 2 | |||||||
| ARIC Study [35] | Prospective cohort study | United States of America | AAA | Positive imagery, hospital discharge with AAA, ICD9 coding, cause of death | ≥30 mm | IRA diameter | |
| Million Veteran program [26] | Observational Cohort | United States of America | AAA | NR | NR | NR | NR |
| Non-AAA controls |
AAA: Abdominal aortic aneurysm; ARIC: Atherosclerosis Risk in Communities; CT: computed tomography; IRA: Infra-renal aorta; ICD9: International Classification of Diseases; NR: Not reported; PLA: People’s Liberation Army; SMART: Secondary Manifestations of ARTerial disease; US: ultrasound; USA: United States of America
4.3. Quality assessment
The results from the quality assessment are reported in Table 2. Studies were assigned a percentage score based on the sum of 14 criteria. Ten studies were deemed to have low risk of bias [20], [24], [27], [28], [29], [30], [32], [34], [35], [36], five studies were deemed to have medium risk of bias [23], [25], [26], [31], [33] and one study was deemed to have a high risk of bias [37]. All studies clearly identified their analysis method as Mendelian randomisation within their title or abstract and provided a rationale for specifically conducting a Mendelian randomisation. All included studies provided a description of the study design and underlying populations. All studies reported the methods for acquiring SNPs for analysis. With regards to the main analysis, 12 studies used the inverse – variance weighted (IVW) method to report their core results [20], [24], [26], [27], [28], [29], [31], [33], [34], [35], [36], [37]. One study did not specify the main MR estimator [30]. Seven studies did not fully detail the software versions and packages used to perform the analysis [23], [24], [25], [26], [27], [36], [37]. Eleven studies provided complete descriptive data and summary statistics of their analysed populations [24], [25], [26], [28], [29], [30], [32], [33], [34], [35], [36]. All included studies provided a clear and complete discussion regarding the main results, such as the associations between genetic variants, exposures and outcomes, as well as causal effect estimates. Sensitivity or additional analyses were performed by all included studies to assess the robustness of their results, primarily using methods such as MR – Egger [20], [23], [26], [27], [28], [29], [31], [32], [33], [34], [35], weighted Median [20], [23], [26], [27], [28], [29], [31], [32], [33], [34], [35], MR – PRESSO [26], [27], [28] and leave one out analysis [25], [26], [28], [31]. Further methods used included: Inverse variance weighted (IVW) analysis [23], multivariable analysis [20], [28], [29], alternate genetic risk scores [37] and adjustment for specific variables [23], [28], [29], [30], [34]. Further details are provided in supplementary table 4. Four studies did not provide a complete analysis of the limitations or bias present within their analysis [26], [31], [33], [37]. Overall, the generalisability of studies was poor, with studies neglecting to comment on the application of their results to other populations, exposure periods or levels of exposure. Thirteen studies assessed the core Mendelian assumptions sufficiently, either via validation or by reporting from previous studies [20], [23], [24], [25], [26], [27], [28], [29], [30], [31], [34], [35], [36].
4.4. Association of causal risk factors with AAA
A variety of causal risk factors were explored by the included studies. This included lipid fractions [20], [23], [35], [37], lipid drug targets [20], [23], cytokines [24], [25], [31], body mass index (BMI) [27], [33], [34], alcohol [28], smoking [26], [29], C-reactive protein (CRP) [30], telomere length [32], waist-hip ratio adjusted BMI (WHRadjBMI) [33], type 2 diabetes mellitus (T2DM) [33], fatty acid plasma levels [36] and blood pressure [26]. These results were provided in supplementary table 4.
Two studies reported that BMI in a total of 1576 cases and 369,949 control participants was not significantly associated with an increased lifetime risk of AAA (OR: 1.06, 95% CI: 0.96, 1.16 [27] and OR: 1.63, 95% CI: 0.99, 2.61 [33]. WHRadjBMI had no significant association with AAA risk (OR: 1.84, 95% CI 0.92, 3.57) [33], however fat free mass index was demonstrated to have a significant protective effect from risk of AAA (OR: 0.64, 95% CI 0.42, 0.95) [27]. One study investigated the role of BMI in AAA associated mortality and found no significant association (OR: 0.80, 95% CI: 0.56, 1.15) [34].
Two studies investigated the causal role of interleukin 6 (IL-6) in AAA risk using 1579 cases and 368,267 controls [25], [31]. One study reported that a one allele deviation in IL-6 receptor decreased the risk of AAA significantly (OR: 0.84, 95% CI: 0.80, 0.89) [25]. Another study reported that genetically predicted soluble form of IL-6R (sIL-6R) also significantly decreased the risk of AAA (OR: 0.84, 95% CI: 0.80, 0.89) [31]. A study consisting of 4682 cases and 38,739 controls looking at the causal role of IL-1Ra in the risk of AAA development found significant per allele associations between IL and 1Ra and AAA risk (OR: 1.08, 95% CI: 1.04, 1.12) [24].
Two studies investigated the causal role of smoking in AAA risk using 8736 cases and 538,721 controls. One study showed that smoking initiation (OR: 2.71, 95% CI: 1.83, 4.01) and heaviness (in cigarettes per day) (OR: 2.53, 95% CI: 1.78, 3.61) were significantly associated with AAA risk, and that smoking cessation carried a significant protective effect (OR: 0.21, 95% CI: 0.05, 0.89) [26]. This was supported by another study that demonstrated significant associations between smoking initiation (OR: 1.74, 95% CI: 1.33, 2.26), lifetime smoking (OR: 5.51, 95% CI: 3.14, 9.68) and the risk of AAA [29].
One study investigated the causal role of alcohol in AAA with 1094 cases and 366,942 controls and demonstrated a significant association between alcohol consumption and the risk of AAA (OR: 2.60, 95% CI: 1.15, 5.89). However, this finding was non-significant when a multivariable approach was used to adjust for smoking (which is genetically correlated with alcohol consumption) (OR: 1.10, 95% CI: 0.50, 2.45) [28].
One study identified a causal role of Lipoprotein A (Lp(a)) in raising AAA risk (OR: 1.42, 95% CI: 1.29, 1.59). This finding was robust when compared to an alternate genetic instrument comprised of 2 SNPs.
A study exploring the causal role of fatty acid plasma levels in AAA development found both significantly protective and causative fatty acids with regards to AAA risk (full details listed supplement table 3) [36]. Individual studies explored the association between CRP (non-significant) [30], telomere length (non-significant) [32], T2DM (non-significant) [33], systolic blood pressure (non-significant) [26], diastolic blood pressure (significantly increased) [26] and AAA risk.
4.5. Meta-analysis of eligible studies reporting causal risk factors in AAA
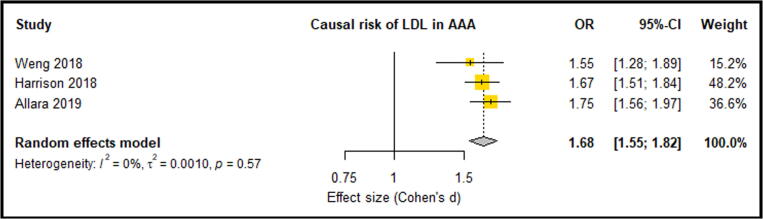
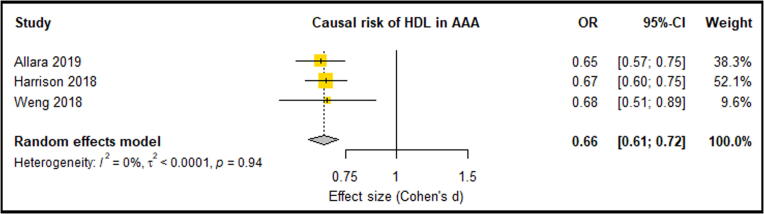
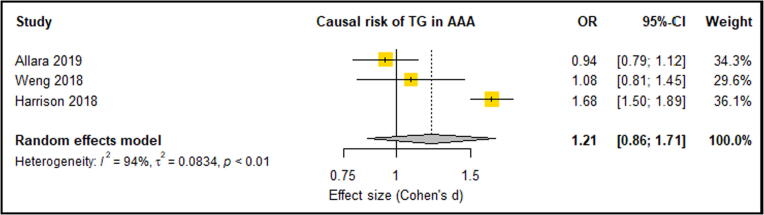
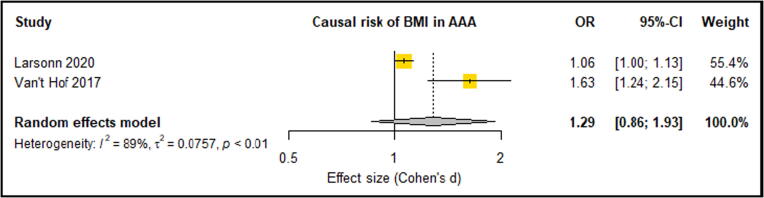
Meta-analysis of three eligible studies consisting of 6396 AAA Cases and 423,015 control participants reported LDL-C, HDL-C and TG. These studies suggested that significantly increased LDL-C was an associated causal risk factor for an increased lifetime risk of AAA [20], [23], [35] (OR: 1.68, 95% CI: 0.55, 0.82) (Fig. 2). These three studies also showed that HDL-C provided a protective effect against the risk of AAA, however this association was only significant in two of the three studies [20], [23]. Overall meta-analysis suggested that a significantly lowered HDL-C levels were a causal risk factor for AAA risk (OR: 0.66, 95% CI: 0.61, 0.72) (Fig. 3). Only one analysis demonstrated that triglycerides were significantly associated with AAA risk [20]. Meta-analysis suggested triglycerides as not being a causal risk factor associated with AAA risk (OR: 1.21, 95% CI: 0.86, 1.71) (Fig. 4). A meta-analysis of two separate studies exploring the causal risk of BMI in AAA deemed the association insignificant (OR: 1.29, 95% CI: 0.86, 1.93) (Fig. 5). Sensitivity analyses showed that the meta-analysis findings of all HDL, LDL, TG and BMI were robust (Supplementary Fig. 1, Fig. 2, Fig. 3, Fig. 4). Risk of bias assessment across studies was not possible was not possible due to limited number of eligible studies. Genetically predicted total cholesterol was also shown to significantly increase the risk of AAA, however, was not reported in at least 3 studies, so meta-analysis was not possible [35].
Fig. 2.
Forest plot suggested that increased LDL levels significantly increased the causal risk of AAA with no between-study heterogeneity (0%). AAA – Abdominal aortic aneurysm; CI –Confidence interval; LDL – Low density lipoprotein; OR – Odds ratio.
Fig. 3.
Forest plot suggested that lowered HDL levels significantly reduced the causal risk of AAA with no between-study heterogeneity (0%). AAA – Abdominal aortic aneurysm; CI –Confidence interval; HDL – High density lipoprotein; OR – Odds ratio.
Fig. 4.
Forest plot suggested unchanged TG levels had no causal risk of AAA with high between-study heterogeneity (94%). AAA – Abdominal aortic aneurysm; CI –Confidence interval; TG – Triglycerides; OR – Odds ratio.
Fig. 5.
Forest plot suggested unchanged BMI levels had no causal risk of AAA with high between-study heterogeneity (89%). AAA – Abdominal aortic aneurysm; CI –Confidence interval; BMI – Body mass index; OR – Odds ratio.
Additional findings from the meta-analysed studies included: LDL receptor (LDLR) (OR: 3.09, 95% CI: 1.81, 5.28) [23], lipoprotein lipase (LPL) (OR: 2.69, 95% CI: 1.44, 5.00) [23], 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) (OR: 0.93, 95% CI: 0.89, 0.98) (20], Cholesterol ester transfer protein (CETP) (OR: 0.89, 95% CI: 0.85, 0.94) [20] and total cholesterol (OR: 1.48, 95% CI: 1.02, 2.16) [35].
5. Discussion
The main findings of this meta-analysis were that high LDL-C and low HDL-C levels were causal risk factors for increased lifetime risk of AAA. Triglycerides however were not identified as a causal risk factor for AAA. This is in line with prior meta-analyses that demonstrated the key role of lipids in the AAA aetiology and suggested LDL-C lowering as potentially effective treatment strategy for prevention of AAA and management of existing AAA patients [20]. The current study suggests that treatments to lower LDL-C may reduce the risk of developing AAA. Whether this translates into LDL-C lowering being an effective treatment to slow AAA growth is unclear [39], [40]. A genetic proxy for HMGCR inhibitors (using rs12916 SNP) has been previously associated with lower AAA risk in a prior meta-analysis [20]. This association was shown to carry a similar direction in MR analysis, however the relationship was deemed to be insignificant [23]. This provides support for the use of statins to limit progression of AAA in keeping with a recent meta-analysis of observational studies [41], [42]. Currently however there are no randomised trials which have tested the benefit of LDL-C lowering in limiting AAA growth and thus it remains uncertain whether statins or other LDL-C lowering therapies are effective in slowing AAA growth.
Mendelian randomisation analysis on the effect of LDLR, LDL [23] and total cholesterol [35] demonstrated a causative relationship with AAA development. The LDLR SNP rs6511720 was reported to be significantly associated with AAA in a GWAS study [43]. CETP has been implicated in atherosclerosis and lipid balance. Human studies regarding use of CETP inhibitors have demonstrated a HDL-C raising and LDL-C lowering effect, countering dyslipidaemia [44]. This has also translated into cardiovascular protective effects [45] and reduced risk of major vascular events [46], [47]. Mendelian randomisation analysis affirms this notion and suggests a protective causal relationship between CETP (SNP rs3764261, CETP inhibitor proxy) and AAA [20]. While there is conflicting evidence regarding the true protective effect of CETP inhibition and risks in doing so [48], CETP inhibitors may carry potential to reduce cardiovascular and AAA risk [49].
The Physician’s Health Study showed that relative to men with BMI < 25, those with BMI in the range of 25–30 and > 30 had 30–70% higher risk of developing AAA [50], and was corroborated by prior studies from Sweden [51] and Australia [52]. Despite these large-scale studies, BMI and WHRadjBMI were shown not to be causal risk factors for AAA [27], [33]. A mouse study demonstrated IL-18 to co-localize with its receptor at regions rich in adipocytes, suggesting a role of adipocytes via IL-18 in promoting AAA development [53]. It is possible that inflammatory markers, generally increased in obesity, could increase the risk for AAA development rather than obesity per se. This could be the reason for the contradicting results of genetic studies [27], [33] and observational studies [50], [51], [52].
The hypothesis of potential role of inflammatory markers is in line with the results from genetic studies that reported a significant causal risk of IL-1Ra, IL-6R and sIL-6R with AAA development [24], [25], [31]. In contrast, CRP was reported to have no causal role in AAA in genetic studies and the association between CRP and AAA likely reflects the systematic inflammatory response in people with AAA [30]. More Mendelian randomization studies investigating the causal risk factors for AAA are required to draw reliable conclusions.
Hypertension is a well-established risk factor for AAA [54]. Observational studies suggest that high diastolic blood pressure (DBP) has been reported to have a non-linear dose dependant association with increased risk of AAA [55]. In support of this, Mendelian randomisation indicate a causative role of high DBP in AAA [26].
People with diabetes have a lower risk of AAA diagnosis in population studies [56]. This has been attributed to a number of effects of diabetes including high blood glucose promoting extra-cellular matrix glycation and modulation of aortic wall matrix remodelling and inflammation. [57]. Concurrent use of metformin [58], [59] and statins [60], [61], [62] could also be an alternative explanation for the protective effect of AAA seen in people with diabetes. Interestingly, Mendelian randomisation suggest that diabetes mellitus is not causally associated with AAA [33]. This further suggests that the observed association between protective effect of diabetes mellitus and AAA is potentially confounded possibly due to diabetes medications, rather than causatively related.
A number of limitations of this systematic review should be acknowledged. The review was limited by the small number of previous studies and therefore, lack of individual study level MR assumptions. The causative risk factors for AAA development may not be the same for AAA growth and thus it is not possible to confidently extrapolate findings from Mendelian randomisation studies focused on risk of AAA to inform treatments for established AAA. It should also be acknowledged that the analyses are limited by the number of available trials and heterogeneity of the included populations and further trials are needed for robust conclusions.
In conclusion this systematic review suggests that high LDL-C and low HDL-C are causal risk factors for AAA but high BMI and triglycerides are not. This lends strength to the utility of interventions targeting such risk factors at a preventative level, and may help identify those at higher risk of developing AAA.
Funding
Funding from James Cook University (Strategic Research Investment Fund) and Queensland Government supported this work. JG holds a Practitioner Fellowships from the National Health and Medical Research Council (1117061) and a Senior Clinical Research Fellowship from the Queensland Government, Australia. The funders played no role in study design, conduct, data collection, analysis and interpretation, and did not assist in preparation or review of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100836.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References:
- 1.Mortality G.B.D. Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanhainen A., Verzini F., Van Herzeele I., Allaire E., Bown M., Cohnert T. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Chaikof E.L., Dalman R.L., Eskandari M.K., Jackson B.M., Lee W.A., Mansour M.A. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77 e2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Filardo G., Powell J.T., Martinez M.A., Ballard D.J. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015;2(2):CD001835. doi: 10.1002/14651858.CD001835.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeting M.J.P.R., Powell J.T., Greenhalgh R.M. E.V.A.R. Trial Investigators Endovascular Repair of Abdominal Aortic Aneurysm in Patients Physically Ineligible for Open Repair: Very Long-term Follow-up in the EVAR-2 Randomized Controlled Trial. Ann Surg. 2017;266(5):713–719. doi: 10.1097/SLA.0000000000002392. [DOI] [PubMed] [Google Scholar]
- 6.Lederle F.A., Johnson G.R., Wilson S.E., Ballard D.J., Jordan W.D., Jr., Blebea J. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287(22):2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 7.Golledge J., Moxon J.V., Singh T.P., Bown M.J., Mani K., Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med. 2020;288(1):6–22. doi: 10.1111/joim.12958. [DOI] [PubMed] [Google Scholar]
- 8.Golledge J., Pinchbeck J., Tomee S.M., Rowbotham S.E., Singh T.P., Moxon J.V. Efficacy of Telmisartan to Slow Growth of Small Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA Cardiology. 2020;5(12):1374–1381. doi: 10.1001/jamacardio.2020.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter B.T.M.J., Curci J.A., McBride R., Larson L., Blackwelder W., Lam D., Wijesinha M., Terrin M. N-TA3CT Investigators. Effect of Doxycycline on Aneurysm Growth Among Patients With Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA. 2020;323(20):2029–2038. doi: 10.1001/jama.2020.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16(4):225–242. doi: 10.1038/s41569-018-0114-9. [DOI] [PubMed] [Google Scholar]
- 11.Wahlgren C.M., Larsson E., Magnusson P.K., Hultgren R., Swedenborg J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J Vasc Surg. 2010;51(1):3–7. doi: 10.1016/j.jvs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Joergensen T., Christensen K., Lindholt J.S., Larsen L.A., Green A., Houlind K. Editor's choice–high heritability of liability to abdominal aortic aneurysms: a population based twin study. European Journal of Vascular and Endovascular Surgery. 2016;52(1):41–46. doi: 10.1016/j.ejvs.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362 doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ference B.A., Kastelein J.J.P., Ray K.K., Ginsberg H.N., Chapman M.J., Packard C.J. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA. 2019;321(4):364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med. 2009;6(7) [PMC free article] [PubMed] [Google Scholar]
- 17.Smith G.D., Davies N.M., Dimou N., Egger M., Gallo V., Golub R. STROBE-MR: Guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ Preprints. 2019;Report No:2167–9843. [Google Scholar]
- 18.Boef A.G., Dekkers O.M., Le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. International journal of epidemiology. 2015;44(2):496–511. doi: 10.1093/ije/dyv071. [DOI] [PubMed] [Google Scholar]
- 19.Burgess S., Davey Smith G., Davies N.M., Dudbridge F., Gill D., Glymour M.M. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison S.C., Holmes M.V., Burgess S., Asselbergs F.W., Jones G.T., Baas A.F. Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA Cardiol. 2018;3(1):26–33. doi: 10.1001/jamacardio.2017.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartung J., Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 22.Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 23.Allara E., Morani G., Carter P., Gkatzionis A., Zuber V., Foley C.N. Genetic Determinants of Lipids and Cardiovascular Disease Outcomes: A Wide-Angled Mendelian Randomization Investigation. Circ Genomic Precis Med. 2019;12(12) doi: 10.1161/CIRCGEN.119.002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitag D., Butterworth A.S., Willeit P., Howson J.M.M., Burgess S., Kaptoge S. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2015;3(4):243–253. doi: 10.1016/S2213-8587(15)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison S.C., Smith A.J., Jones G.T., Swerdlow D.I., Rampuri R., Bown M.J. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34(48):3707–3716. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klarin D., Verma S.S., Judy R., Dikilitas O., Wolford B.N., Paranjpe I. Genetic Architecture of Abdominal Aortic Aneurysm in the Million Veteran Program. Circulation. 2020;142(17):1633–1646. doi: 10.1161/CIRCULATIONAHA.120.047544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson S.C., Back M., Rees J.M.B., Mason A.M., Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221–226. doi: 10.1093/eurheartj/ehz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson S.C., Burgess S., Mason A.M., Michaelsson K. Alcohol Consumption and Cardiovascular Disease: A Mendelian Randomization Study. Circ Genomic Precis Med. 2020;13(3) doi: 10.1161/CIRCGEN.119.002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson S.C., Mason A.M., Back M., Klarin D., Damrauer S.M., Million Veteran P. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J. 2020;41(35):3304–3310. doi: 10.1093/eurheartj/ehaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X.Y., Juan J., Xiang X., Wei Y.Q., Zuo S.W., Huang T. Plasma C-Reactive Protein and Abdominal Aortic Aneurysm: A Mendelian Randomization Analysis. Chin Med J. 2018;131(21):2630–2633. doi: 10.4103/0366-6999.244124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa M., Chignon A., Li Z., Boulanger M.C., Arsenault B.J., Bosse Y. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. npj Genom Med. 2019:4(1)–23. doi: 10.1038/s41525-019-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telomeres Mendelian Randomization C., Haycock P.C., Burgess S., Nounu A., Zheng J., Okoli G.N. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA. Oncol. 2017;3(5):636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van 't Hof FN, Vaucher J, Holmes MV, de Wilde A, Baas AF, Blankensteijn JD, et al. Genetic variants associated with type 2 diabetes and adiposity and risk of intracranial and abdominal aortic aneurysms. European journal of human genetics : EJHG. 2017;25(6):758-62. [DOI] [PMC free article] [PubMed]
- 34.Wade K.H., Carslake D., Sattar N., Davey Smith G., Timpson N.J. BMI and Mortality in UK Biobank: Revised Estimates Using Mendelian Randomization. Obesity (Silver Spring). 2018;26(11):1796–1806. doi: 10.1002/oby.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng L.C., Roetker N.S., Lutsey P.L., Alonso A., Guan W., Pankow J.S. Evaluation of the relationship between plasma lipids and abdominal aortic aneurysm: A Mendelian randomization study. PLoS ONE. 2018;13(4) doi: 10.1371/journal.pone.0195719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan S., Back M., Bruzelius M., Mason A.M., Burgess S., Larsson S. Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients. 2019;11(12):3001-. doi: 10.3390/nu11123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson S.C., Gill D., Mason A.M., Jiang T., Back M., Butterworth A.S. Lipoprotein(a) in Alzheimer, Atherosclerotic, Cerebrovascular, Thrombotic, and Valvular Disease: Mendelian Randomization Investigation. Circulation. 2020;141(22):1826–1828. doi: 10.1161/CIRCULATIONAHA.120.045826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson C.D., Clancy P., Bourke B., Walker P.J., Dear A., Buckenham T. Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J. 2010;159(2):307–313. doi: 10.1016/j.ahj.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karrowni W., Dughman S., Hajj G.P., Miller F.J., Jr. Statin therapy reduces growth of abdominal aortic aneurysms. J Investig Med. 2011;59(8):1239–1243. doi: 10.231/JIM.0b013e31823548e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukhija R., Aronow W.S., Sandhu R., Kakar P., Babu S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. The American journal of cardiology. 2006;97(2):279–280. doi: 10.1016/j.amjcard.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Schouten O., van Laanen J.H., Boersma E., Vidakovic R., Feringa H.H., Dunkelgrun M. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg. 2006;32(1):21–26. doi: 10.1016/j.ejvs.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 43.Bradley D.T., Hughes A.E., Badger S.A., Jones G.T., Harrison S.C., Wright B.J. A variant in LDLR is associated with abdominal aortic aneurysm. Circulation: Cardiovascular. Genetics. 2013;6(5):498–504. doi: 10.1161/CIRCGENETICS.113.000165. [DOI] [PubMed] [Google Scholar]
- 44.de Grooth G.J., Klerkx A.H., Stroes E.S., Stalenhoef A.F., Kastelein J.J., Kuivenhoven J.A. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004;45(11):1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Fayad Z.A., Mani V., Woodward M., Kallend D., Abt M., Burgess T. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. The Lancet. 2011;378(9802):1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ference B.A., Kastelein J.J.P., Ginsberg H.N., Chapman M.J., Nicholls S.J., Ray K.K. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA. 2017;318(10):947–956. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sikorski J.A. Oral cholesteryl ester transfer protein (CETP) inhibitors: a potential new approach for treating coronary artery disease. J Med Chem. 2006;49(1):1–22. doi: 10.1021/jm058224l. [DOI] [PubMed] [Google Scholar]
- 48.Miller NE. CETP inhibitors and cardiovascular disease: time to think again. F1000Research. 2014;3. [DOI] [PMC free article] [PubMed]
- 49.Dullaart R.P., Dallinga-Thie G.M., Wolffenbuttel B.H., van Tol A. CETP inhibition in cardiovascular risk management: a critical appraisal. Eur J Clin Invest. 2007;37(2):90–98. doi: 10.1111/j.1365-2362.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Djousse L., Song Y., Akinkuolie A.O., Matsumoto C., Manson J.E. Associations of Diabetes and Obesity with Risk of Abdominal Aortic Aneurysm in Men. J Obes. 2017;2017:3521649. doi: 10.1155/2017/3521649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stackelberg O., Bjorck M., Sadr-Azodi O., Larsson S.C., Orsini N., Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100(3):360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 52.Golledge J., Clancy P., Jamrozik K., Norman P.E. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116(20):2275–2279. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 53.Liu C.L., Ren J., Wang Y., Zhang X., Sukhova G.K., Liao M. Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur Heart J. 2020;41(26):2456–2468. doi: 10.1093/eurheartj/ehz856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornuz J., Sidoti Pinto C., Tevaearai H., Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14(4):343–349. doi: 10.1093/eurpub/14.4.343. [DOI] [PubMed] [Google Scholar]
- 55.Kobeissi E., Hibino M., Pan H., Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2019;34(6):547–555. doi: 10.1007/s10654-019-00510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aune D., Schlesinger S., Norat T., Riboli E. Diabetes mellitus and the risk of abdominal aortic aneurysm: A systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2018;32(12):1169–1174. doi: 10.1016/j.jdiacomp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Norman P.E., Davis T.M., Le M.T., Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res. 2007;48(3):125–131. doi: 10.1080/03008200701331524. [DOI] [PubMed] [Google Scholar]
- 58.Yu X., Jiang D., Wang J., Wang R., Chen T., Wang K. Metformin prescription and aortic aneurysm: systematic review and meta-analysis. Heart. 2019;105(17):1351–1357. doi: 10.1136/heartjnl-2018-314639. [DOI] [PubMed] [Google Scholar]
- 59.Raffort J., Hassen-Khodja R., Jean-Baptiste E., Lareyre F. Relationship between metformin and abdominal aortic aneurysm. Journal of vascular surgery. 2020;71(3):1056–1062. doi: 10.1016/j.jvs.2019.08.270. [DOI] [PubMed] [Google Scholar]
- 60.Twine C.P., Williams I.M. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98(3):346–353. doi: 10.1002/bjs.7343. [DOI] [PubMed] [Google Scholar]
- 61.Salata K., Syed M., Hussain M.A., de Mestral C., Greco E., Mamdani M. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7(19) doi: 10.1161/JAHA.118.008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Q., Yang H., Lin Q., Hu M., Meng Y., Qin X. Effect of Statin Therapy on Survival After Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-analysis. World J Surg. 2018;42(10):3443–3450. doi: 10.1007/s00268-018-4586-x. [DOI] [PubMed] [Google Scholar]
- 63.Allen N., Sudlow C., Downey P., Peakman T., Danesh J., Elliott P. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1(3):123–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.