Abstract
Ultrasonography and magnetic resonance imaging have become important imaging modalities in rheumatological disorders next to standard radiography. Due to their ability to assess both morphological and functional changes they play a significant role in early diagnosis and treatment monitoring. This review presents the latest advancements in imaging of inflammatory arthritis with a focus on two main groups of rheumatic diseases: connective tissue diseases and spondyloarthritis. New developments related to peripheral and sacroiliac joints imaging are discussed, including Superb Micro Flow Imaging and Shear Wave Elastography in ultrasonography, as well as Whole Body MRI, quantitative MRI, and the recent advances in cartilage imaging in MRI, including T2-and T1p-mapping, and dGEMRIC. The role of emerging imaging techniques in the early diagnosis of inflammatory arthritis is discussed, including DECT, VIBE, BoneMRI, and pQCT.
Keywords: Inflammatory rheumatic diseases, Ultrasonography, Magnetic resonance imaging
1. Introduction
There are dozens of different types of arthritis. Increasing emphasis has been placed on the early diagnosis in patients with inflammatory arthropathies, which are the most common type of arthritis.1 They belong to two groups of diseases: connective tissue diseases and spondyloarthritis (SpA). The former group includes primarily autoimmune systemic diseases, such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic lupus erythematosus (SLE), systemic scleroderma (SSc), dermato- and polymyositis (DM, PM), fibromyalgia, eosinophilic fasciitis, Still's disease, and several others. The second group comprises diseases of both autoimmune and autoinflammatory background, including ankylosing spondylitis, psoriatic arthritis, reactive arthritis, enteropathic arthritis, and undifferentiated forms of arthritis.
Over the past decades, the imaging of inflammatory arthritis has advanced spectacularly, particularly due to constant technological improvements of cross-sectional modalities.1 Although in the very early stages of these diseases a specific diagnosis remains challenging in many cases, this review will focus on the imaging publications on inflammatory arthritis spanning the last few years to highlight advances in that field. Other arthritis types, such as metabolic, endocrine-related, or osteoarthritis, are not covered in this paper.
2. General overview of imaging features and modalities
Contemporary imaging of inflammatory arthropathies no longer relies on conventional radiography (CR) alone, but also includes ultrasound (US), magnetic resonance imaging (MRI), and in certain situations computed tomography (CT).2 Most inflammatory rheumatic diseases are characterized primarily by systemic, synovial and bone inflammation (synovitis and osteitis) leading to joint destruction (cysts, erosions, cartilage damage). Among other key inflammatory features are: tenosynovitis, bursitis, enthesitis, and panniculitis, and in specific diseases the inflammation of muscles, fasciae, skin, subdermis, and nails. Imaging is important to identify these major pathologies in order to aid the diagnosis and management.2 The role of imaging methods differs depending on their sensitivity for specific tissues, ease of quantification, and feasibility of use.2
The main tools to detect synovitis, tenosynovitis and bursitis, with sensitivity superior to the clinical examination, are US and MRI.2 The high resolution of US provides optimal multiplanar, static and dynamic evaluation of MSK soft tissue pathologies in peripheral joints. MRI shows advantages in complex, three-dimensional evaluation of all joints of the peripheral and axial skeleton, and all tissues which may be affected by arthritis. This includes the detection of bone marrow edema (BME) and inflammatory osteitis, which cannot be visualized with CR and US, but have been postulated as a precursor of bone erosions.2
Bone erosions are among the cardinal features of RA, and their central role in the pathogenesis, diagnosis and prognosis of patients with the disease is widely recognized.3 They have traditionally been considered as late-stage lesions, however, as several studies have shown that bone erosions might occur very early in the course of RA.3 Erosions and cysts, representing damage, are best imaged by CT, CR, and MRI, and only some may be diagnosed by US due to limited access to many bone surfaces. Ziegeler et al.4 found that the application of the noise estimation based low-dose technology resulted in radiographs of comparable quality to standard-dose images, allowing for a reduction of radiation exposure by up to 50%.4 High-resolution peripheral quantitative CT (HR-pQCT) remains a research tool, but its ability to provide information about the bone microstructure provided the most accurate detection of bone lesions.2 It offers high-resolution 3D imaging (typically 82 μm isotropic voxel size) of bone structure with a low radiation dose. The high-resolution and quantitative nature of this technique enables assessment of periarticular bone marrow density and cortical/trabecular microarchitecture for detecting early erosive damage, although it is limited to the imaging of peripheral joints such as the wrist/hand and ankle/feet.5
Despite high sensitivity in the diagnosis of early inflammatory lesions, the specificity of imaging findings remains challenging, since synovitis and osteitis are present in most arthropathies. This observation is reflected in the new OMERACT (The Outcome Measures in Rheumatology) definition for adults with rheumatic conditions, which lacks the elementary lesion “synovial effusion”, since it did not prove reliable, and was often detected also in healthy subjects.6 Subclinical synovitis as detected by US is also frequent in healthy individuals. Although less common and less intense, and seen in fewer joints, even power Doppler (PD) signal suggestive of subclinical synovitis is present in up to 55% of healthy subjects.7 In the study by Ji et al.,8 there was no difference between RA and non-RA groups in terms of BME, either. The location and extent of BME may help in the differential diagnosis. In early RA involving the hands and wrists, BME is usually located in the marginal area of the bone, whereas in carpal bones it may involve a larger proportion of bone away from the subchondral bone.9 In patients with psoriatic arthritis (PsA), a predominance of BME in relation to synovitis, and the presence of osteitis involving a whole bone despite minor joint involvement, are particularly suggestive of PsA.9 Erosions are common in connective tissue diseases, mainly RA and Still's disease, but they may also be seen in endocrine and metabolic related arthropathies, and are an unusual feature in SpA.10 Cartilage loss is a typical feature of arthritis, both inflammatory and noninflammatory. For the differential diagnosis, the pattern of cartilage loss is relevant, which is uniform in inflammatory connective tissues diseases, across all joint compartments, unlike, for example, in osteoarthritis (OA) or gout.
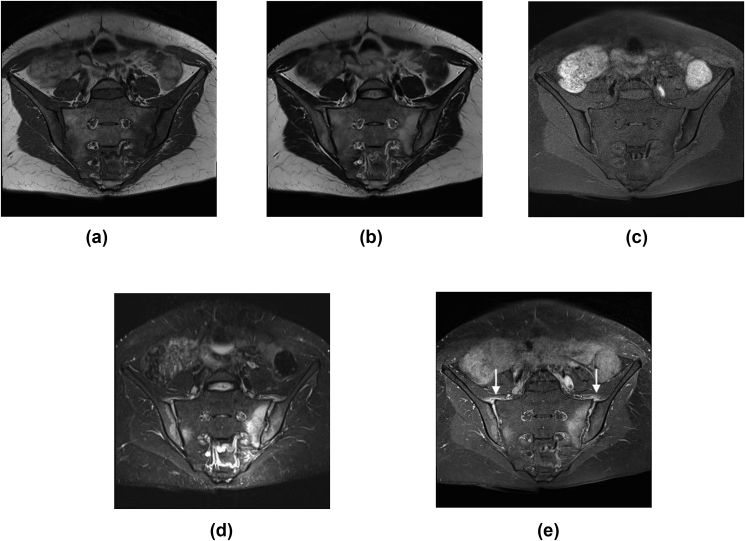
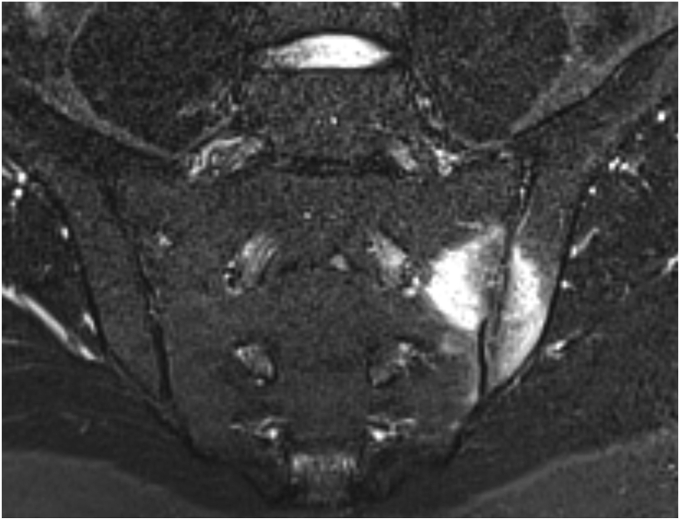
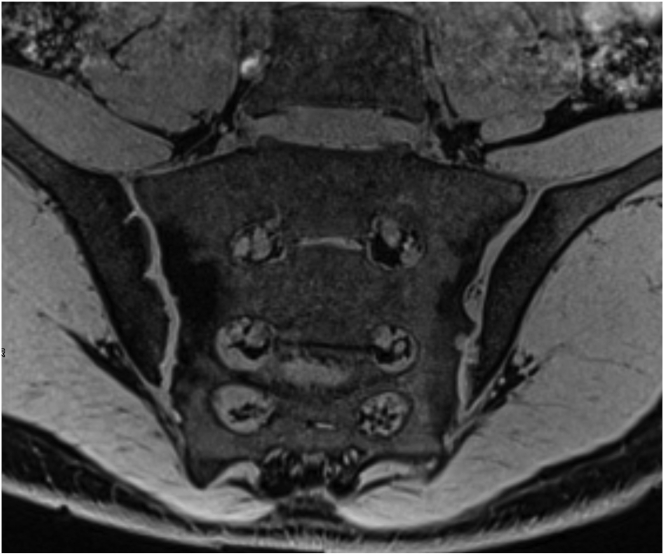
In cases of axial spondyloarthritis, the gold standard for diagnosing sacroiliitis in spondyloarthritis is MRI of the sacroiliac joints (SIJs). In 2019, the ASAS (The Assessment of SpondyloArthritis international Society) MRI working group updated the definitions for MRI lesions in the SI joint of patients with SpA.11 The definitions for capsulitis, enthesitis, fat metaplasia, erosion and ankylosis were revised. The site of capsulitis was clarified as the perimeter of the joint. For enthesitis, an increased STIR or T1FS post Gd signal in the interosseous ligaments was excluded from the new definition. For fat metaplasia, morphological characteristics were defined. For erosion, signal changes of adjacent bone marrow were added to the definition. Ankylosis was defined to be present when a bright signal of bone marrow is seen across the joint space. New definitions were provided, including joint space enhancement, joint space fluid, inflammation at the site of erosion, bone bud (new bone formation in the joint space that is not bridging the joint), and fat metaplasia in an erosion cavity (backfill).11 Synovitis was removed from the original ASAS definitions,11 since according to histological studies synovium is only present at the perimeter of the lower third of the cartilaginous portion of the SIJ12 (Fig. 1).
Fig. 1.
MRI of the sacroiliac joints in a 35-year old female with sacroiliitis: a) T1 weighted image, b) T2 weighted, c) T1 fat saturation, d) T2 TIRM, e) T1 FS contrast enhanced show bilateral osteitis, joints space enhancement, capsulitis (arrows), and erosions.
In contrast to well developed and validated SIJ MRI scoring systems in adults, no specific scoring systems have been developed for children. According to the Spondyloarthritis Research Consortium of Canada, inflammation and structural scores are practical and reliable to use for pediatric imaging, but they do not account for age-related maturational changes.13,14 The MRI in JIA working group of Outcome Measures in Rheumatology and Clinical Trials (OMERACT) seeks to develop an MRI-based scoring system for the assessment of SIJ in JIA.15 Different MRI features of SIJ inflammation defined in the revised preliminary JAMRIS-SIJ scoring system include BME, osteitis, joint space fluid, joint space enhancement, inflammation in an erosion cavity, capsulitis, and enthesitis. The MRI features of SIJ structural damage are sclerosis, erosion, fat lesion (also known as fat metaplasia), backfill, and ankylosis. A statement of ‘overarching consideration’ was included for all definitions ‘in comparison to changes normally seen in MRI of age and sex matched children’.15 This shows that diagnosing sacroiliitis in the pediatric population is particularly challenging because of normal developmental changes that are seen in the immature skeleton.16, 17, 18 Understanding the normal MRI appearance of the developing SIJ is important for distinguishing normal developmental variation from disease.
3. Update in ultrasound
US is a commonly used modality in inflammatory arthropathies. The importance of ultrasound examinations has also been highlighted by its inclusion in the two EULAR/American College of Rheumatology (ACR) classification criteria for polymyalgia rheumatica (PMR) (in 2012) and gout (in 2015).19 Advances made over the past years include, among others, quantitative ultrasound, high-frequency transducers up to 70 MHz (ultra-high frequency ultrasonography; UHFUS), and superb microvascular imaging (SMI).
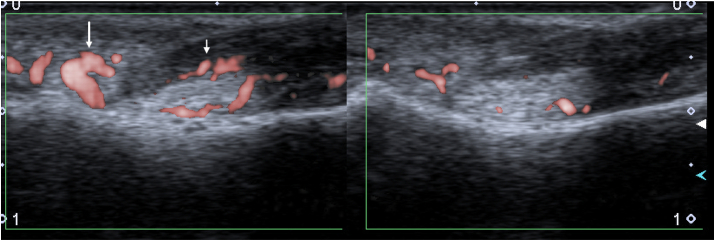
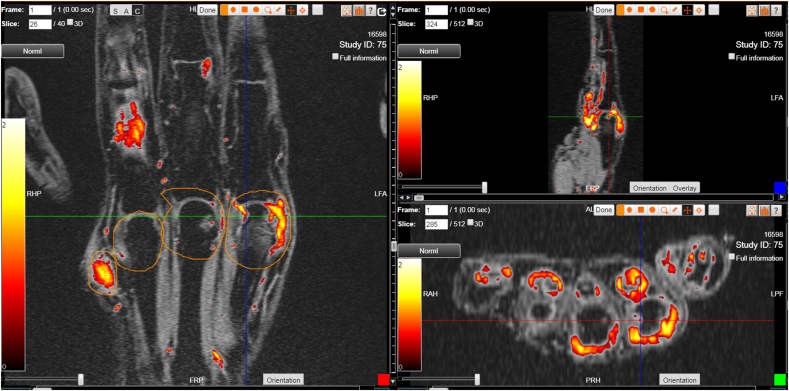
Krajewska-Włodarczyk et al.20 using 24 MHz transducer found an association between inflammation in the nail bed and PsA development. US can provide a precise assessment of the vascularity of the nail root, nail bed and matrix, and may be useful for determining the intensity of local inflammation as a prognostic factor for PsA development. SMI is an innovative Doppler technology which can visualize the smallest vascular structures that were previously not visible (Fig. 2). Additional studies with long-term follow-up are needed to validate the role of SMI in improving the diagnostic accuracy of this technology and for predicting radiographic progression.21
Fig. 2.
A 54 y.o. female with PsA and painful nail of the 2nd finger. a) Increased vascularity of the nail root (long arrow) and nail bed (short arrow) of the painful nail in color Superb Micro-Vascular Imaging (cSMI) (left part of the image) compared to standard PD of that nail (right); b) increased flow seen in monochromatic SMI (mSMI) in the nail bad of the painful nail and c) trace of vascularization in the nail bad on the contralateral asymptomatic nail.
A number of studies have focused on establishing US-based criteria for enthesitis, which is especially frequent in adults with peripheral SpA, and in children with JIA. Despite some research indicating PsA-specific lesions, further studies are needed to identify more specific features providing differentiation between inflammation-related enthesitis from metabolic, age- and overload-driven enthesopathy.7,22 A further preliminary enthesitis score developed in a recent GRAPPA study has reported the ability to differentiate between PsA and healthy controls.23
Among rheumatic diseases, RA and PsA may be especially difficult to differentiate. Paratenonitis, digital enthesitis (of tendons, plantar plate, collateral ligaments, pulleys), and flexor tenosynovitis with significantly greater tendon sheath synovial hypertrophy and tendon sheath vascularization may help differentiate PsA from RA.7 Zabotti et al.24 have developed a US-based score for dactylitis, the DACTylitis glObal Sonographic score (DACTOS). Another recent systematic review reported variable diagnostic accuracy for US in PsA.7 Therefore, in light of numerous contradictory data, further research is needed to gain insights into the problem.
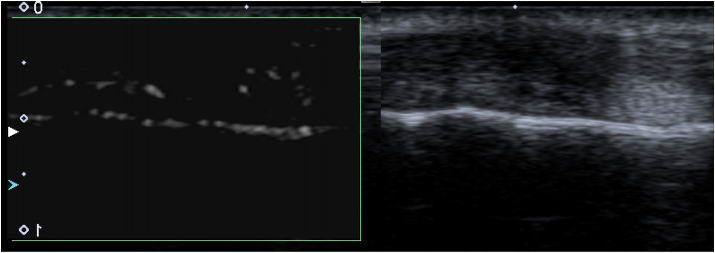
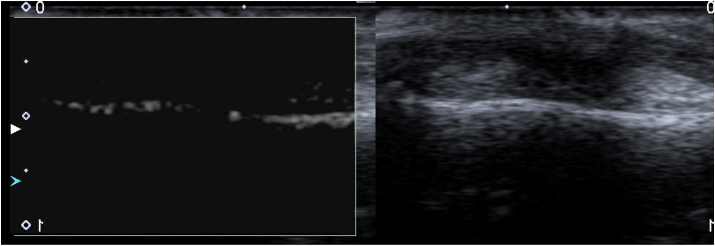
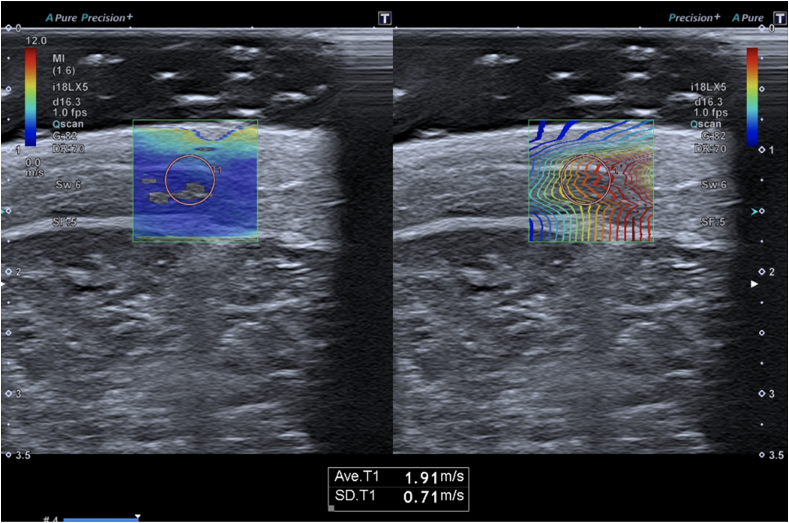
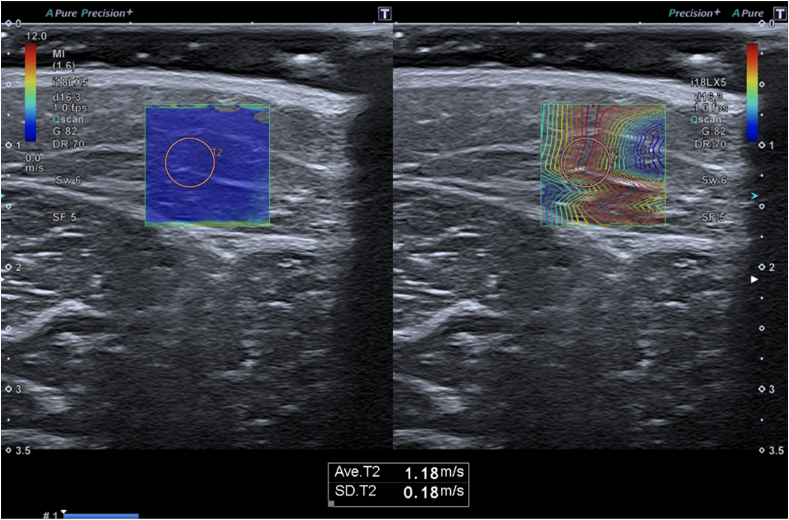
Bachasson et al. applied shear wave elastography (SWE) to the biceps brachii of 34 patients with inflammatory myopathies, finding lower muscle stiffness in patients with more severe muscle weakness.25 This may suggest the potential of SWE to differentiate between disease activity. SWE may be perfectly suited for the diagnostic work-up and follow-up of lesions involving the skin, subdermis, fascia and muscles in the course of scleroderma22,26 (Fig. 3).
Fig. 3.
A 13 y.o. boy with juvenile localized scleroderma. Shear wave (SW) elastography on 18 MHz transducer with propagation map taken from the affected area in the subdermal tissue of the thigh shows an average speed in this area of 1.91 m/s, with standard deviation (SD) of 0.71 m/s; SWE on the contralateral healthy side is 1.18 m/s and SD 0.18 m/s.
At present, ultrasound computer-aided diagnosis (USCAD) systems have been developed for multiple clinical applications.27 The four most studied rheumatic diseases where artificial intelligence, such as machine learning or artificial neural network, has been applied are: RA, OA, systemic lupus erythematosus, and idiopathic inflammatory myopathies.27 In 2019, Anderson et al.28 showed that neural network technology could be used in the scoring of disease activity on Doppler US images.
Ultrasound has been used for the assessment of bone demineralization, which is a prevalent finding in inflammatory rheumatic diseases. Bone evaluation to quantify bone mineral density (BMD) can be performed by various methods, including dual-emission X-ray absorptiometry (DEXA), quantitative ultrasound (QUS), and quantitative computed tomography (QCT).29 Although QUS may have some complementary benefits in fracture risk prediction models, the current literature does not support the substitution of QUS for DEXA in the diagnosis and monitoring of osteoporosis in rheumatic diseases.29
4. Update in magnetic resonance imaging
In recent years, substantial technical advances have been made in MRI software and hardware, leading to a reduction in scan times and improved qualitative and quantitative MR imaging.30,31
Quantitative MRI modalities include: dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), diffusion weighted imaging (DWI), and chemical shift encoded MRI (CSE-MRI).
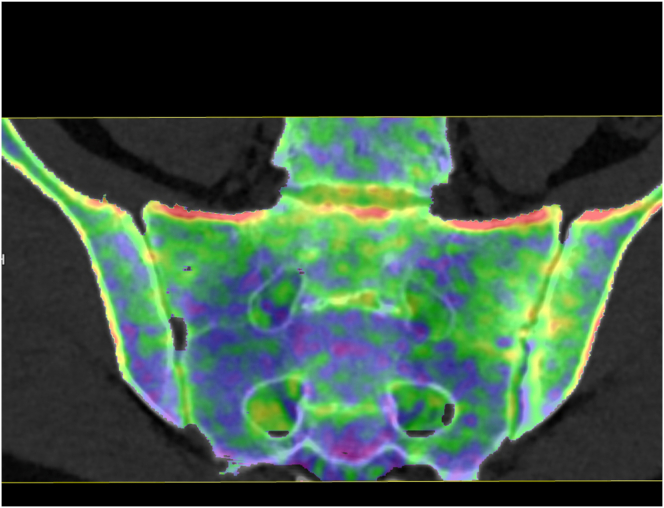
DCE-MRI is based on the T1 relaxation characteristics of gadolinium agents. It investigates the distribution of the contrast medium in the vascular and interstitial space, expressed as changes in signal intensity over the scan time. With this technique, different quantitative parameters can be extracted according to the applied approach which were successfully used to distinguish synovitis between RA and PSA or patients with early disease from healthy controls, and to assess the early response to treatment32,33 (Fig. 4, Fig. 5). Quantitative MRI measures in RA, such as Ktrans of synovitis, and semi-quantitative MRI measures, such as RAMRIS of synovitis, osteitis and bone erosions, and CARLOS system to evaluate the degree of cartilage loss, have been shown to be sensitive and reproducible measures of inflammation and structural damage.9 In children with JIA, DCE-MRI was found to be a valuable tool for detecting synovitis, including in the temporomandibular joint.34,35
Fig. 4.
MRI of sacroiliac joints in a 47-year old man with axial spondyloarthritis. Maximum Enhanced Map (DYNAMIKA) super-imposed on the original TIRM T2-weighted MR image. The colours in the map indicate presence of the inflammatory activity bilaterally: redder colour shows lower degree joints space inflammation on the right, and yellow colours indicate higher degree of joint space inflammation and osteitis on the left side.
Fig. 5.
Screenshot of DYNAMIKA platform (IAG, Image Analysis Group) showing the hand of a patient with synovitis and tenosynovitis in 3 orientations. The patient scans were processed with DYNAMIKA to identify inflammation in joints and tendons sheaths in corresponding colours: the redder indicate lower inflammatory activity and yellow colours – the higher, as indicated by the colour bar on the left of each image.
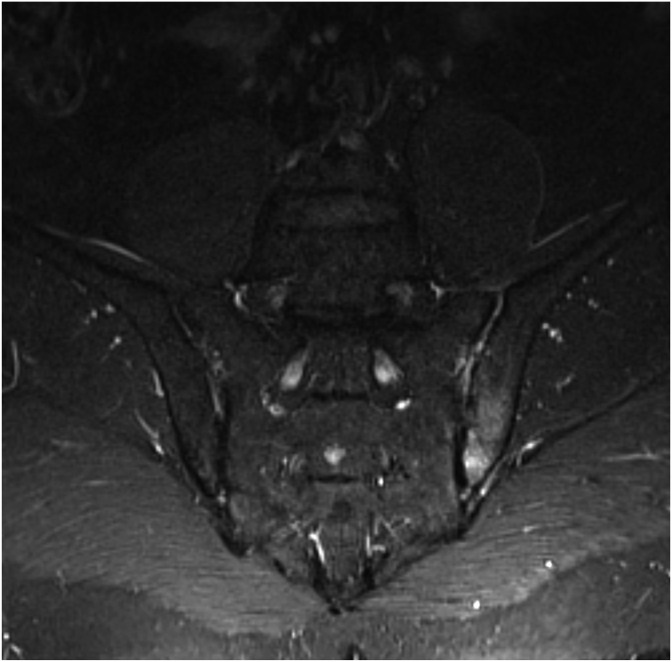
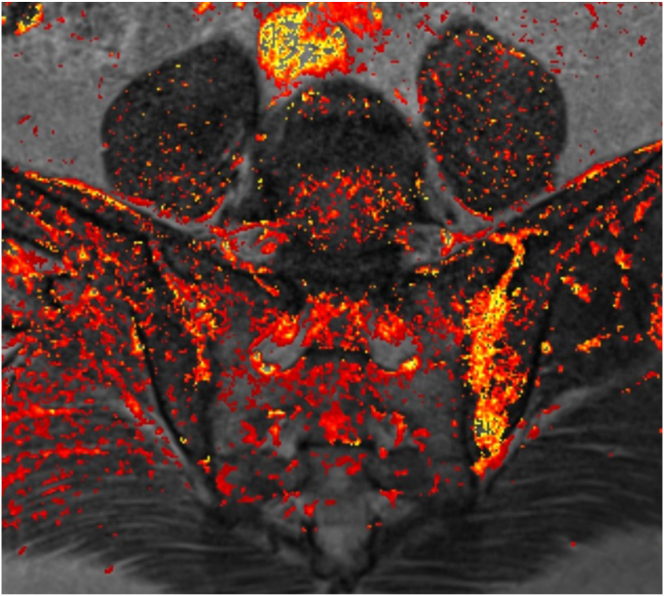
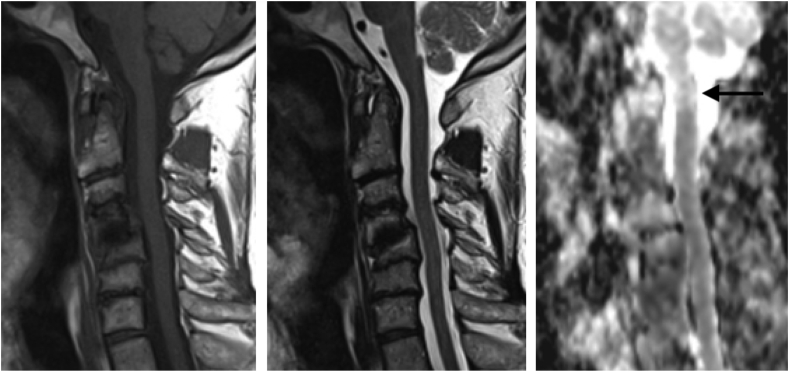
Diffusion Weighted Imaging (DWI) has been successfully applied in inflammatory rheumatic diseases.36 It has been shown that apparent diffusion coefficient (ADC) discriminates between active and inactive JIA, and identifies patients with AS, non-radiographic AS, and lower back pain not due to AS at baseline.37,38 DWI may be also useful in detecting early spinal cord damage. New research found39 that ADC value at the level of the 1st vertebra was significantly higher in rheumatoid patients with atlanto-axial instability than in the group without instability, despite the lack of signal changes in standard MR images (Fig. 6). DWI carries the intrinsic advantage of not requiring contrast medium injection but is highly sensitive to field inhomogeneities, which could adversely affect its applications.
Fig. 6.
A 67-year old female with rheumatoid arthritis and anterior subluxation at C1/C2 joint diagnosed on radiographs: a) T1-weighted and b) T2-weighted images do not show any abnormality in the spinal cord; c) ADC map shows high signal in the spinal cord at the level of the odontoid process (arrow).
Chemical shift encoded MRI (CSE-MRI) utilizes the small difference between the precession frequencies of water and fat protons when exposed to a strong external magnetic field (i.e. inside an MRI scanner).5 This phenomenon allows quantitative measurement of water and fat composition, and by the same token bone marrow edema and fat metaplasia, which reflect active inflammation and structural damage, respectively, in inflammatory arthritis.5 The technique has been successfully applied in a cohort of adolescent patients with sacroiliitis to quantify bone inflammation and healing.5
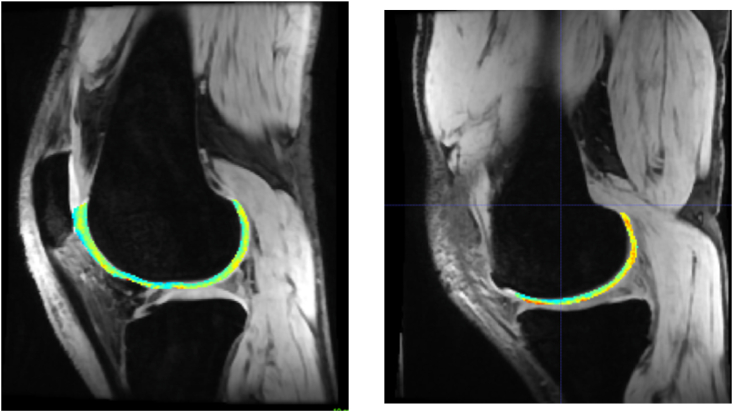
Quantitative cartilage imaging include T2-and T1p-mapping, dGEMRIC, and more recently gagCEST, and sodium (Na) imaging (Fig. 7).40
Fig. 7.
T2-mapping based on dual echo steady state (DESS) MRI of the articular cartilage of the knee in patient with early unicompartmental osteoarthritis in the medial tibiofemoral compartment. In the lateral compartment (a), a homogenous map of mostly normal (blue and green) values of T2 relaxation time is shown, whereas in the medial compartment (b) elevated (orange and red) values of T2 relaxation time are found in the weight-bearing and posterior aspect of the femoral condyle. This indicates increased water content which is associated with early alterations in the collagen content and network integrity. No gross morphological cartilage damage was visible in this patient.
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), which is based on a negatively charged gadolinium-based contrast agent, allows the evaluation of cartilage glycosaminoglycan (GAG) content. Although the use of dGEMRIC is hindered by long acquisition protocols, it provides an insight into early biochemical disturbances affecting cartilage. A recent study examining the metacarpophalangeal and proximal interphalangeal joints in non-early PSA and early RA patients showed a similar molecular cartilage composition in the two groups.41 Consequently, in the future, dGEMRIC could be applied to monitor treatment response in such patients. Regarding PSA, Abrar et al. using dGEMRIC and DCE-MRI, demonstrated a correlation between early lesions affecting the cartilage and synovial inflammation in small finger joints.42
T1ρ (i.e., spin-lattice or longitudinal relaxation time) and T2 (i.e., spin-spin or transverse relaxation time) mapping alternative quantitative MR techniques aimed at assessing early biochemical changes in cartilage, without the need of a contrast agent.,30 However, controversial results have been obtained when assessing response to treatment. One study found that T1ρ values correlated with disease activity after therapy in patients with RA, while from another study it emerged that the technique did not play any significant role in assessing response to treatment.43,44 Further research in this field is needed to fully explore the role of these quantitative analyses in the rheumatic area. From the technical point of view, for T2 mapping strong gradients, a small field of view and short interecho spacing are recommended, while for T1p, the occurrence of high specific absorption rate levels has to be taken into account. GagCEST, and sodium (Na) imaging have shown promise in assessing GAG content of cartilage, but widespread application is hindered due to the need of ultra-high field strength and specific coils.
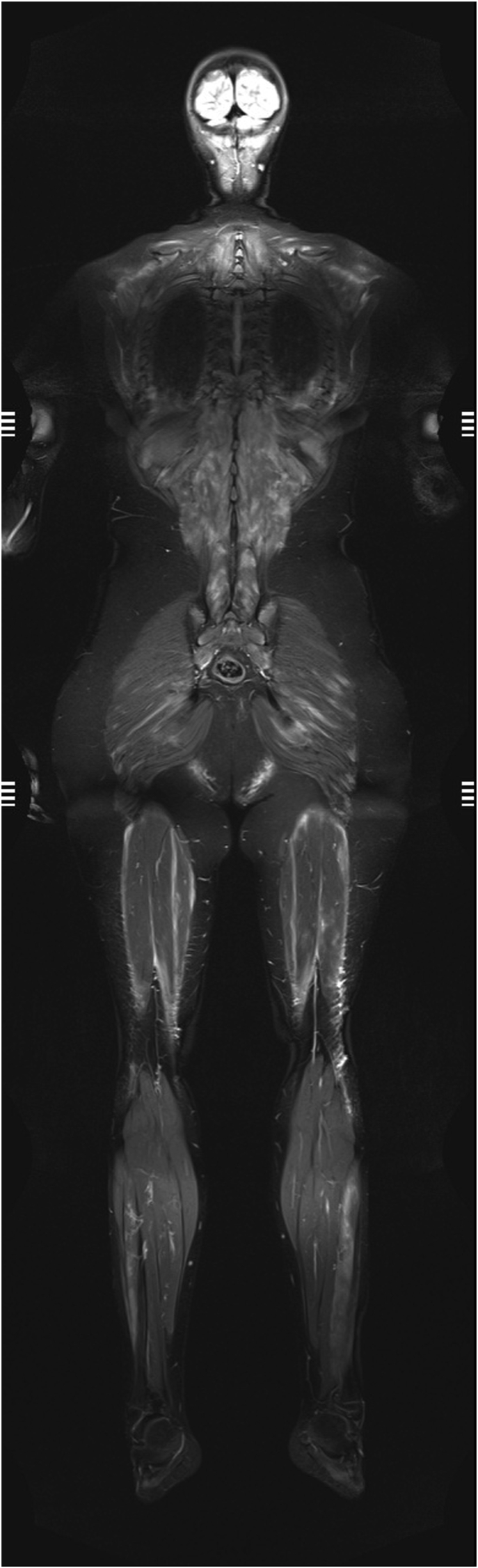
Whole body MRI (WB-MRI) is especially useful for diseases characterized by multifocal and systemic involvement, allowing an estimation of disease extension and activity. As summarized in a recent survey of the European Society of Musculoskeletal Radiology, myositis still represents one of the main applications of this technique.45 WB-MRI is the method of choice for the early diagnosis of muscle inflammation, and may also be useful for selecting a location for muscle biopsy and determining the efficacy of treatment46 (Fig. 8). Inflammatory idiopathic myositis was among the first indications for WB-MRI, also because the protocol is usually very short, without the need for additional sequences and planes.47 WB-MRI demonstrated to be especially useful in the pediatric field, in particular for chronic recurrent multifocal osteomyelitis (CRMO). By performing a whole-body examination, not only the primary site of pain can be characterized, but also additional clinically silent skeletal lesions can be easily detected with a single scan.48 Recently, interest has grown in standardized reporting and in promoting the development and use of dedicated scores, such as the SAPHO, CRMO and OMERACT scoring systems.49,50 Preliminary results show that WB-MRI scores decrease after biologic treatment.51
Fig. 8.
Whole body-MRI in a 13-year old male with juvenile DM. Increased signal in a number of muscles of the spine, upper and lower limbs, and the neck.
Dual-energy CT (DECT) provides an innovative alternative for BME detection in SpA. DECT involves the acquisition of images at two different energy levels and, therefore, is capable of differentiating between elements with a high atomic number such as calcium, and most of human body tissues with low atomic numbers including carbon, oxygen, hydrogen, and nitrogen. As such, calcium can be subtracted from the bone, and bone marrow can be visualized and displayed on a color-coded map (Fig. 9). DECT is suitable for detecting inflammatory BME in sacroiliitis, as shown by Wu et al. in a group of 47 patients with SpA, and Chen et al. in a group of 40 patients with suspected sacroiliitis.52,53 Compared with MRI, DECT demonstrated moderate to good sensitivity (81%–93%) and good specificity (91%–94%) for BME detection in SIJs.
Fig. 9.
MRI and dual-energy CT in a 28-year-old male with sacroiliitis. a) periarticular high signal on the MR STIR image in the left sacroiliac joint and b) corresponding bone marrow edema of the left sacroiliac joint seen as bright green areas with yellow and red spots on the dual-energy CT image.
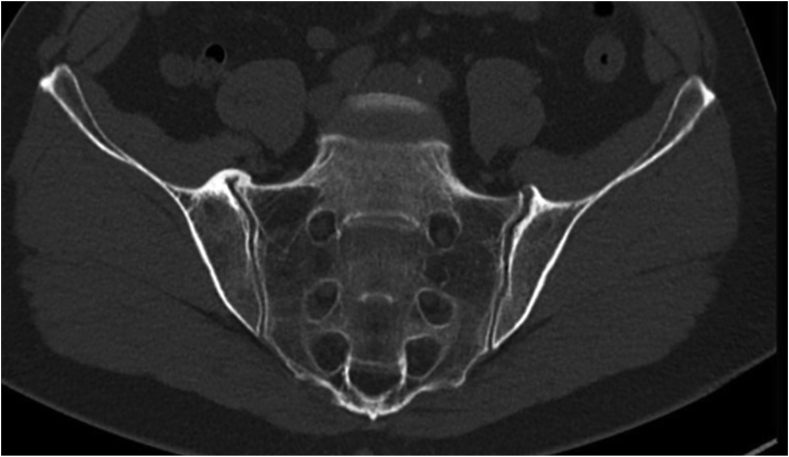
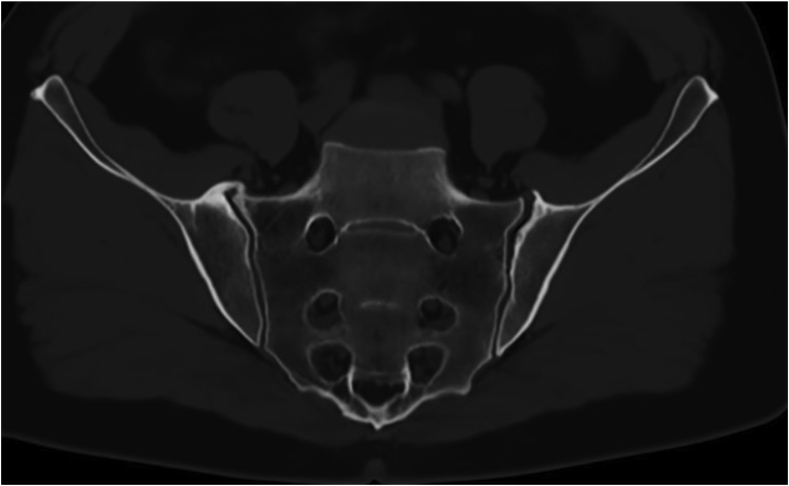
3D MRI sequences have the advantages of higher spatial resolution, lower partial volume effects, and multiplanar reconstruction. 3D volume-interpolated breath-hold examination (VIBE) sequence is of most interest for the detection of erosions54, 55, 56 (Fig. 10, Fig. 11). This is a gradient echo (GRE) sequence intrinsically, so it can potentially better depict erosions of joints by virtue of their high contrast between joint cavity, cartilage and cortical bone. In addition, it allows generation of T1-weighted images with fat suppression. The 3D VIBE sequence has displayed a higher sensitivity and better inter-reader reliability for detecting erosions compared to T1WI MRI images in patients with SpA, with reference to CT.
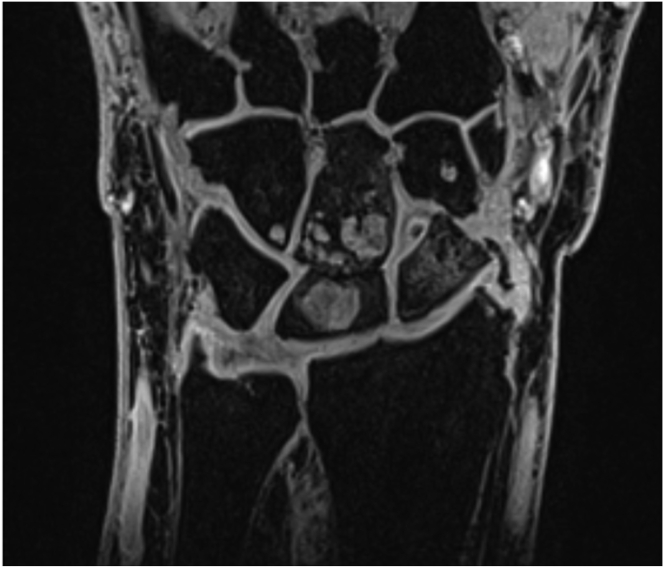
Fig. 10.
3D volume-interpolated breath-hold examination (VIBE) MR image of a 13-year old boy with sacroiliitis. The cortical outline and erosions are better depicted on VIBE image (b) than on T1-weighted MR image (a).
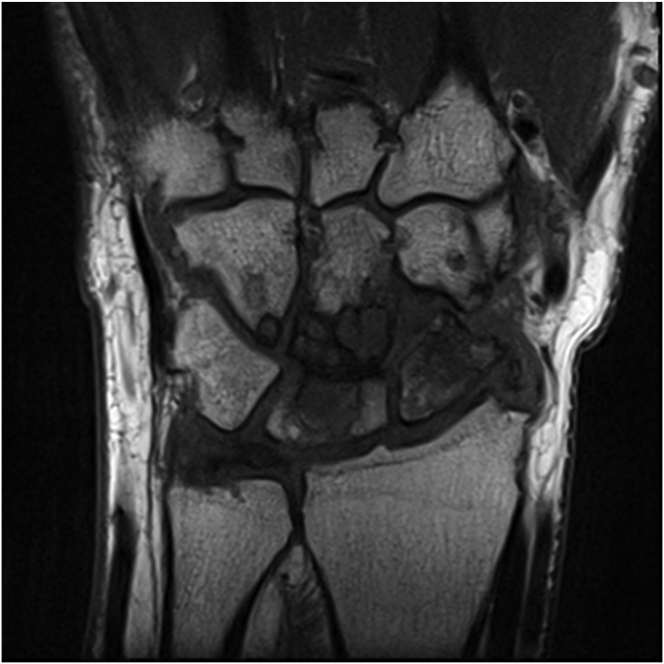
Fig. 11.
Numerous erosions in carpal joints in a 67-year old female with rheumatoid arthritis in T1-weighted image (a) and on VIBE (b) MRI.
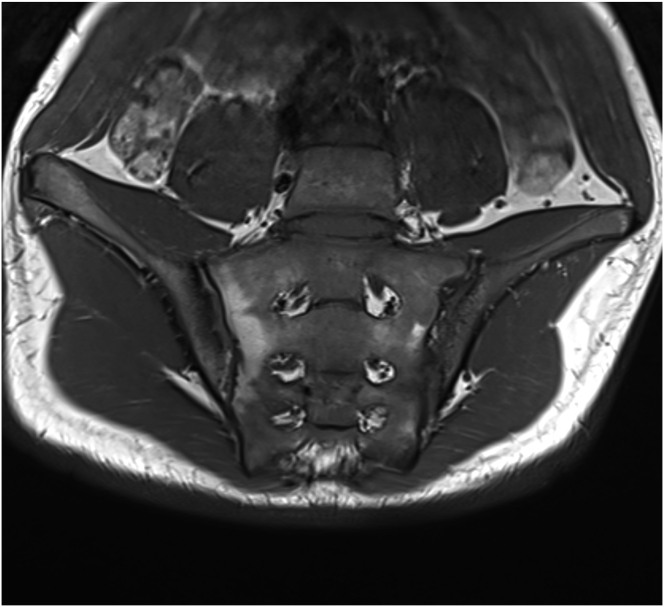
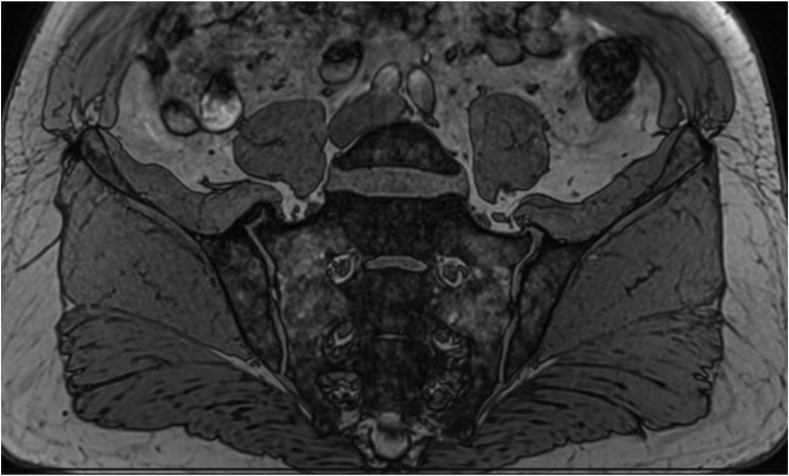
MRI-based synthetic CT (BoneMRI) is a deep learning-based technology generating CT-like images from 3-dimensional T1-weighted multiple gradient echo sequence (3DT1MGE). The technique aims at specific visualization of the osseous morphology based on Hounsfield Unit (HU) estimation. With commercially available software, BoneMRI images can be automatically reconstructed without any manual post-processing. CT-like BoneMRI images have a high similarity to CT images. In cases involving sacroiliac joints, the osseous morphology of the joints is well depicted on CT-like BoneMRI images and much easier assessed in comparison to the routine T1-weighted image. Recently, this technique was applied by Jans et al. in detecting structural lesions in the SI joint in patients with suspected sacroiliitis (57) (Fig. 12). With CT used as a reference standard, BoneMRI was shown to have a higher diagnostic accuracy and inter-reader reliability for the evaluation of erosions, sclerosis and ankylosis in comparison to the routine T1-weighted sequence. Moreover, BoneMRI increased the diagnostic confidence of radiologists.
Fig. 12.
T1-weighted MRI (a), CT scan (b) and CT-like image of the sacroiliac joints acquired using BoneMRI (c).
In addition to the imaging modalities discussed so far, which rely on the detection of anatomical and structural changes related to the disease, molecular imaging provides insight into the preceding molecular and cellular changes in the very early stages of pathogenesis, and allows visualization of the underlying biochemical processes inducing the disease. Special probes are bound to imaging tracers that target various disease processes (inflammation, T/B-cell activation, activated macrophages, activated vascular endothelium, and apoptosis) and are detected using positron-emission tomography CT (PET-CT) and single photon emission CT (SPECT).5 Molecular imaging has also shown great potential in the development of personalized therapy.5
5. Conclusion
Imaging plays a vital role in the diagnosis and management of inflammatory arthropathies. Advances in the hardware and software of US, MRI and CT increase our ability in the early diagnosis, follow-up of the disease, and assessment of treatment responses and therapeutic outcome. High-field strength MRI systems are increasingly being used in the clinical practice, providing increased spatial resolution and signal-to-noise ratio. Qualitative, semiquantitative and quantitative assessments allow more objective evaluation and standardization of imaging-based assessment. The specificity of imaging findings remains the main problem, so imaging results need to be interpreted together with the overall clinical picture and any additional available data. With increasing availability of biologic therapies that target specific disease pathogenesis, it is more important than ever for clinicians to be able to differentiate between different types of inflammatory arthritis.19
Conflict of interest
On behalf of all coauthors I declare no conflict of interest, regarding the submitted paper: IMAGING UPDATE IN INFLAMMATORY ARTHRITIS.
References
- 1.Sudoł-Szopińska I., Greenspan A. MSK imaging in Rheumatology. Semin Muscoskel Radiol. 2018;22:125–126. doi: 10.1055/s-0038-1639483. [DOI] [PubMed] [Google Scholar]
- 2.Isbel M., Paramalingam S., Conaghan P.G., Keen H.I. An update on imaging in rheumatoid arthritis. Curr Treat Options in Rheum. 2020;6:370–381. [Google Scholar]
- 3.Di Matteo A., Mankia K., Duquenne L. Ultrasound erosions in the feet best predict progression to inflammatory arthritis in anti-CCP positive at-risk individuals without clinical synovitis. Ann Rheum Dis. 2020;79:901–907. doi: 10.1136/annrheumdis-2020-217215. [DOI] [PubMed] [Google Scholar]
- 4.Ziegeler K., Siepmann S., Hermann S. Application of an advanced noise reduction algorithm for imaging of hands in rheumatic diseases: evaluation of image quality compared to standard-dose images. Rheumatol Int. 2020;40:893–899. doi: 10.1007/s00296-020-04560-1. [DOI] [PubMed] [Google Scholar]
- 5.King Kenneth Cheung K.K., Hall-Craggs M.A. Update on imaging in Rheumatology – recent advances. Medicine. 2018;46:170–174. [Google Scholar]
- 6.Bruyn G.A., Iagnocco A., Naredo E. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 Years on. J Rheumatol. 2019;46:1388–1393. doi: 10.3899/jrheum.181095. [DOI] [PubMed] [Google Scholar]
- 7.Dubash S.R., De Marco G., Wakefield R.J., Tan A.L., McGonagle D., Marzo-Ortega H. Ultrasound imaging in psoriatic arthritis: what have we learnt in the last five years? Front Med. 2020;7:487. doi: 10.3389/fmed.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L., Li G., Xu Y., Zhou W., Zhang Z. Early prediction of rheumatoid arthritis by magnetic resonance imaging in the absence of anti-cyclic citrullinated peptide antibodies and radiographic erosions in undifferentiated inflammatory arthritis patients: a prospective study. Int J Rheum Dis. 2015;18:859–865. doi: 10.1111/1756-185X.12420. [DOI] [PubMed] [Google Scholar]
- 9.Sudoł-Szopińska I., Teh J., Cotton A. Rheumatoid hand and other hand-deforming rheumatic conditions. Semin Muscoskel Radiol. 2021;25:232–245. doi: 10.1055/s-0041-1729150. [DOI] [PubMed] [Google Scholar]
- 10.Scullion S., Grainger A.J., Greenspan A. Radiologic imaging of metabolic and endocrine disorders as they affect the hand and wrist. Semin Muscoskel Radiol. 2021;25:1–14. doi: 10.1055/s-0041-1727192. [DOI] [PubMed] [Google Scholar]
- 11.Maksymowych W.P., Lambert R.G.W., Østergaard M. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. 2019;78(11):1550–1558. doi: 10.1136/annrheumdis-2019-215589. [DOI] [PubMed] [Google Scholar]
- 12.Puhakka K.B., Melsen F., Jurik A.G. MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol. 2004;33:15–28. doi: 10.1007/s00256-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 13.Weiss P.F., Maksymowych W.P., Xiao R. Spondyloarthritis research consortium of Canada sacroiliac joint inflammation and structural scores: change score reliability and recalibration utility in children. Arthritis Res Ther. 2020;22(1):58. doi: 10.1186/s13075-020-02157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss P.F., Maksymowych W.P., Lambert R.G. Feasibility and reliability of the spondyloarthritis research consortium of Canada sacroiliac joint structural score in children. J Rheumatol. 2018;45(10):1411–1417. doi: 10.3899/jrheum.171329. [DOI] [PubMed] [Google Scholar]
- 15.Otobo T.M., Conaghan P.G., Maksymowych W.P. Preliminary definitions for sacroiliac joint pathologies in the OMERACT juvenile idiopathic arthritis MRI score (OMERACT JAMRIS-SIJ) J Rheumatol. 2019;46(9):1192–1197. doi: 10.3899/jrheum.181115. [DOI] [PubMed] [Google Scholar]
- 16.Herregods N., Jans L.B.O., Chen M. Normal subchondral high T2 signal on MRI mimicking sacroiliitis in children: frequency, age distribution, and relationship to skeletal maturity. Eur Radiol. 2020;29 doi: 10.1007/s00330-020-07328-0. [DOI] [PubMed] [Google Scholar]
- 17.Chauvin N.A., Xiao R., Brandon T.G. MRI of the sacroiliac joint in healthy children. AJR Am J Roentgenol. 2019;212(6):1303–1309. doi: 10.2214/AJR.18.20708. [DOI] [PubMed] [Google Scholar]
- 18.Weiss P.F., Brandon T.G., Bohnsack J. Variability in magnetic resonance imaging interpretation of the pediatric sacroiliac joint. Arthritis Care Res. 2020;11 doi: 10.1002/acr.24206. [DOI] [PubMed] [Google Scholar]
- 19.Kaeley G.S., Catherine Bakewell C., Deodhar A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: a narrative review. Arthritis Res Ther. 2020;22:1. doi: 10.1186/s13075-019-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krajewska-Włodarczyk M., Owczarczyk-Saczonek A., Placek W., Wojtkiewicz M., Wiktorowicz A., Wojtkiewicz J. Ultrasound assessment of changes in nails in psoriasis and psoriatic arthritis. Hindawi BioMed Research Int. 2018 doi: 10.1155/2018/8251097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota K., Wada T.T., Akiyama Y., Mimura T. Detection of synovial inflammation in rheumatic diseases using superb microvascular imaging: comparison with conventional power Doppler imaging. Mod Rheumatol. 2018;28(2):327–333. doi: 10.1080/14397595.2017.1337288. [DOI] [PubMed] [Google Scholar]
- 22.Pracoń G., Aparisi P., Simoni P., Gietka P., Sudoł-Szopińska I. Conventional radiography and ultrasound imaging of rheumatic diseases affecting the paediatric population. Semin Muscoskel Radiol. 2021;25:68–81. doi: 10.1055/s-0041-1726014. [DOI] [PubMed] [Google Scholar]
- 23.Tom S. GRAPPA development of a preliminary ultrasonographic enthesitis score in psoriatic arthritis — GRAPPA ultrasound working group. J Rheumatol. 2019;46:384–390. doi: 10.3899/jrheum.171465. [DOI] [PubMed] [Google Scholar]
- 24.Zabotti A., Sakellariou G., Tinazzi I. Clinical science novel and reliable DACTylitis global sonographic (DACTOS) score in psoriatic arthritis. Ann Rheum Dis. 2020:1–7. doi: 10.1136/annrheumdis-2020-217191. [DOI] [PubMed] [Google Scholar]
- 25.Bachannsson D., Dubois G.J.R., Allenbach Y., Benveniste O., Hogrel J.Y. Muscle shear wave elastography in inclusion body myositis: feasibility, reliability and relationship with muscle impairments. Ultrasound Med Biol. 2018;44:1423–1432. doi: 10.1016/j.ultrasmedbio.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Idzior M., Sotniczuk M., Michalski E., Gietka P., Sudoł-Szopińska I., Ultrasonography MRI and classic radiography of skin and MSK involvement in juvenile scleroderma. J Ultrason. 2020;20(83):311–317. doi: 10.15557/JoU.2020.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez-Martínez J., Pineda C., Sandoval H., Bernal-González A. Computer-aided diagnosis in rheumatic diseases using ultrasound: an overview. Clin Rheumatol. 2020;39:993–1005. doi: 10.1007/s10067-019-04791-z. [DOI] [PubMed] [Google Scholar]
- 28.Andersen J.K.H., Pedersen J.S., Laursen M.S. Neural networks for automatic scoring of arthritis disease activity on ultrasound images. RMD Open. 2019;5 doi: 10.1136/rmdopen-2018-000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oo W.M., Naganathan V., Bo M.T., Hunter D.J. Linical utilities of quantitative ultrasound in osteoporosis associated with inflammatory rheumatic diseases. Quant Imag Med Surg. 2018;8(1):100–113. doi: 10.21037/qims.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juras V., Schreiner M., Laurent D. The comparison of the performance of 3 T and 7 T T2 mapping for untreated low-grade cartilage lesions. Magn Reson Imaging. 2019;55:86–92. doi: 10.1016/j.mri.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giraudo C., Kainberger F., Boesen M., Trattnig S. Quantitative imaging in inflammatory arthritis: between tradition and innovation. Semin Muscoskel Radiol. 2020;24(4):337–354. doi: 10.1055/s-0040-1708823. [DOI] [PubMed] [Google Scholar]
- 32.Boesen M., Kubassova O., Sudoł-Szopińska I. MR Imaging of joint infection and inflammation with emphasis on dynamic contrast-enhanced MR imaging. Pet Clin. 2018;13(4):523–550. doi: 10.1016/j.cpet.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Axelsen M.B., Boesen M., Bliddal H., Jacobsson L., Hansen M.S., Østergaard M. Responsiveness of different dynamic contrast- enhanced magnetic resonance imaging approaches: a post-hoc analysis of a randomized controlled trial of certolizumab pegol in rheumatoid arthritis. Scand J Rheumatol. 2019;16:1–7. doi: 10.1080/03009742.2019.1639820. [DOI] [PubMed] [Google Scholar]
- 34.Nusman C., Lavini C., Hemke R. Dynamic contrast-enhanced magnetic resonance imaging of the wrist in children with juvenile idiopathic arthritis. Pediatr Radiol. 2017;47:205–213. doi: 10.1007/s00247-016-3736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starck S., Andersen E., Macicek O. Effects of motion correction, sampling rate and parametric modelling in dynamic contrast enhanced MRI of the temporomandibular joint in children affected with juvenile idiopathic arthritis. Magn Reson Imag. 2021;77:204–212. doi: 10.1016/j.mri.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 36.de Mello R., Ma Y., Ji Y., Du J., Chang E.Y. Quantitative MRI musculoskeletal techniques: an update. AJR Am J Roentgenol. 2019;213(3):524–533. doi: 10.2214/AJR.19.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barendregt A.M., van Gulik E.C., Lavini C. Diffusion-weighted imaging for assessment of synovial inflammation in juvenile idiopathic arthritis: a promising imaging biomarker as an alternative to gadolinium-based contrast agents. Eur Radiol. 2017;27(11):4889–4899. doi: 10.1007/s00330-017-4876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradbury L.A., Hollis K.A., Gautier B. Diffusion-weighted imaging is a sensitive and specific magnetic resonance sequence in the diagnosis of ankylosing spondylitis. J Rheumatol. 2018;45(6):771–778. doi: 10.3899/jrheum.170312. [DOI] [PubMed] [Google Scholar]
- 39.Mańczak M., Pracoń G., Sudoł-Szopińska I., Gasik R. Apparent diffusion coefficient as an indicator of spinal cord compression due to anterior atlanto-axial subluxation in rheumatoid arthritis patients. Eur Spine J. 2019;28(10):2352–2358. doi: 10.1007/s00586-019-06058-2. [DOI] [PubMed] [Google Scholar]
- 40.Oei E.H.G., Wick M.C., Müller-Lutz A., Schleich C., Miese F.R. Cartilage imaging: techniques and developments. Semin Muscoskel Radiol. 2018;22(2):245–260. doi: 10.1055/s-0038-1639471. [DOI] [PubMed] [Google Scholar]
- 41.Abrar D., Schleich C., Frenken M. DGEMRIC in the assessment of pre-morphological cartilage degeneration in rheumatic disease: rheumatoid arthritis vs. psoriatic arthritis. Diagnostics. 2021;11(2):147. doi: 10.3390/diagnostics11020147. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrar D., Schleich C., Muller-Lutz A. Cartilage degradation in psoriatic arthritis is associated with increased synovial perfusion as detected by magnetic resonance imaging. Front Med. 2020;25(7) doi: 10.3389/fmed.2020.539870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ku E., Pedoia V., Tanaka M. Evaluating radiocarpal cartilage matrix changes 3-months after anti-TNF treatment for rheumatoid arthritis using MR T1ρ imaging. J Magn Reson Imag. 2017;45(5):1514–1522. doi: 10.1002/jmri.25448. [DOI] [PubMed] [Google Scholar]
- 44.Hirose J., Nishioka H., Tsukano M., Matsubara S., Usuku K., Mizuta H. Matrix changes in articular cartilage in the knee of patients with rheumatoid arthritis after biological therapy: 1-year follow-up evaluation by T2 and T1ρ MRI quantification. Clin Radiol. 2018;73(11):984. doi: 10.1016/j.crad.2018.06.020. e11–984.e18. [DOI] [PubMed] [Google Scholar]
- 45.Giraudo C., Lecouvet F., Cotten A. Whole-body magnetic resonance imaging in inflammatory diseases: where are we now? Results of an international survey by the European Society of Musculoskeletal Radiology. Eur J Radiol. 2021;136:109533. doi: 10.1016/j.ejrad.2021.109533. [DOI] [PubMed] [Google Scholar]
- 46.Sudoł-Szopińska I., Jacques t, Gietka P., Cotten A. Imaging in dermatomyositis in adults and children. J Ultrason. 2020;20:e36–e42. doi: 10.15557/JoU.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellutti F.B., Bader-Meunier, Baildam E. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76:329–340. doi: 10.1136/annrheumdis-2016-209247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andronikou S., Kraft J.K., Offiah A.C. Whole-body MRI in the diagnosis of paediatric CNO/CRMO. Rheumatology. 2020;59(10):2671–2680. doi: 10.1093/rheumatology/keaa303. [DOI] [PubMed] [Google Scholar]
- 49.Arnoldi A.P., Schlett C.L., Douis H. Whole-body MRI in patients with non-bacterial osteitis: radiological findings and correlation with clinical data. Eur Radiol. 2017;27:2391–2399. doi: 10.1007/s00330-016-4586-x. [DOI] [PubMed] [Google Scholar]
- 50.Assmann G., Kueck O., Kirchhoff T. Efficacy of antibiotic therapy for SAPHO syndrome is lost after its discontinuation: an interventional study. Arthritis Res Ther. 2009;11:R140. doi: 10.1186/ar2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choida V., Madenidou A.V., Sen D., Hall-Craggs M., Ciurtin C. A systematic review of the ability of whole body MRI to assess disease activity and treatment response in inflammatory arthritis. Ann Rheum Dis. 2020;79:AB1084. doi: 10.1136/annrheumdis-2020-eular.2576. [DOI] [PubMed] [Google Scholar]
- 52.Wu H., Zhang G., Shi L. Axial Spondyloarthritis: dual-energy virtual noncalcium CT in the detection of bone marrow edema in the sacroiliac joints. Radiology. 2019;290:157–164. doi: 10.1148/radiol.2018181168. [DOI] [PubMed] [Google Scholar]
- 53.Chen M., Herregods N., Jaremko J.L. Bone marrow edema in sacroiliitis: detection with dual-energy CT. Eur Radiol. 2020;30(6):3393–3400. doi: 10.1007/s00330-020-06670-7. [DOI] [PubMed] [Google Scholar]
- 54.Baraliakos X., Hoffmann F., Deng X. Detection of erosions in the sacroiliac joints of patients with axial spondyloarthritis using the magnetic resonance imaging VIBE technique. J Rheumatol. 2019;46(11):1445–1449. doi: 10.3899/jrheum.181304. [DOI] [PubMed] [Google Scholar]
- 55.Diekhoff T., Greese J., Sieper J. Improved detection of erosions in the sacroiliac joints on MRI with volumetric interpolated breath-hold examination (VIBE): results from the SIMACT study. Ann Rheum Dis. 2018;77:1585–1589. doi: 10.1136/annrheumdis-2018-213393. [DOI] [PubMed] [Google Scholar]
- 56.Jans L.B.O., Chen M., Elewaut D. MRI-based synthetic CT in the detection of structural lesions in patients with suspected sacroiliitis: comparison with MRI. Radiology. 2021;289:343–349. doi: 10.1148/radiol.2020201537. [DOI] [PubMed] [Google Scholar]