Abstract
Introduction
Cardiorespiratory fitness (CRF) may mitigate Alzheimer's disease (AD) progression. This study examined the longitudinal associations of CRF with brain atrophy and cognitive decline in a late‐middle‐aged cohort of adults at risk for AD.
Methods
One hundred ten cognitively unimpaired adults (66% female, mean age at baseline 64.2 ± 5.7 years) completed a baseline graded treadmill exercise test, two brain magnetic resonance imaging scans (over 4.67 ± 1.17 years), and two to three cognitive assessments (over 3.26 ± 1.02 years). Linear mixed effects models examined the longitudinal associations adjusted for covariates.
Results
Participants with higher baseline CRF had slower annual decline in total gray matter volume (P = .013) and cognitive function (P = .048), but not hippocampal volume (P = .426). Exploratory analyses suggested these effects may be stronger among apolipoprotein E ε4 carriers.
Discussion
CRF is a modifiable physiological attribute that may be targeted during the preclinical phase of AD in effort to delay disease progression, perhaps most effectively among those with genetic risk.
Keywords: aerobic fitness, apolipoprotein E ε4, executive function, exercise, lifestyle factors, magnetic resonance imaging, memory, neuroimaging, physical activity, risk factor
1. INTRODUCTION
Currently, there are more than 55 million Americans 65 years and older. 1 Of these older adults, an estimated 5.8 million are living with Alzheimer's disease (AD). 2 The United States is at the precipice of a major aging demographic shift; Census Bureau data estimate that by the year 2050, there will be more than 85 million Americans over the age of 65. 1 Because age is the greatest risk factor for AD, 2 the forthcoming expansion of the older adult population presents a significant, urgent challenge to our society.
The pathophysiological processes of AD begin during the preclinical phase of the disease, decades prior to clinical diagnosis. 3 , 4 Brain atrophy, measured by serial magnetic resonance imaging (MRI), is a marker of neurodegeneration that has been shown to track with cognitive decline and disease progression. 5 , 6 The available evidence demonstrates that brain volume and cognition decline in mid‐late life, preceding the clinical diagnosis of AD. 3 , 4 , 5 , 6 , 7 , 8 , 9 As a result, research aimed at curbing disease progression during the preclinical phase has become a public health imperative.
There is increasing recognition that cardiorespiratory fitness (CRF), the integrated ability to deliver oxygen to the musculoskeletal system during exercise, may be an important mitigating factor for developing dementia. 10 Studies routinely report that higher levels of self‐reported exercise and physical activity are positively associated with cognition and lower rates of cognitive decline and AD in older adulthood. 11 , 12 , 13 , 14 In cognitively unimpaired adults, positive associations between objectively measured CRF, brain volume, and cognitive function have been reported by our group 15 and others (see review in Erickson et al. 16 ). However, these studies have been cross‐sectional and did not account for physical activity, thereby limiting the interpretation and impact of the findings. The purpose of this study was to determine the longitudinal associations of CRF with brain atrophy and cognitive function in a late‐middle‐aged cohort of adults at risk for AD, and ascertain whether any observed effects are modified by physical activity. We hypothesized that greater baseline CRF would be associated with a reduced rate of brain atrophy and cognitive decline. Lastly, based on data describing the presence of the apolipoprotein E ε4 allele (APOE ε4) influencing CRF 17 and future risk of AD, 18 we explored the potential effect of APOE ε4 carrier status within our sample.
2. METHODS
2.1. Participants
The Wisconsin Registry for Alzheimer's Prevention (WRAP) is a longitudinal registry of late‐middle‐aged adults that began in 2001. All WRAP participants are community‐dwelling adults who were dementia‐free at the time of enrollment. Once enrolled, participants undergo biennial neuroimaging and cognitive assessments. Additional study enrollment and design details have been previously described. 19 For the current study, potential participants were determined to be eligible for inclusion if they were cognitively unimpaired and free of confounding medical conditions (e.g., neurological disease). Exclusion criteria were as follows: documented vascular disease (i.e., coronary artery disease, cerebrovascular disease, peripheral arterial disease, or congestive heart failure), uncontrolled type I or II diabetes mellitus, severe untreated hypertension (> 200/100 mmHg), and the inability to safely walk on a treadmill. Enrolled participants underwent baseline maximal exercise testing and physical activity monitoring, and completed magnetic resonance imaging (MRI) scans, and cognitive assessments as part of their involvement in WRAP or one of its substudies. Trained staff administer all assessments following standardized protocols. The University of Wisconsin Institutional Review Board approved all study procedures, and all participants signed written informed consent.
RESEARCH IN CONTEXT
Systematic review: The authors performed a traditional literature review related to cardiorespiratory fitness (CRF), brain volume, and cognitive function. There is evidence that CRF is cross‐sectionally associated with brain volume and cognitive function in older adulthood. However, whether CRF is associated with future brain atrophy and cognitive decline in mid‐ to late‐aged adults at risk for Alzheimer's disease (AD) is currently unknown.
Interpretation: Within a late‐middle‐aged cohort of adults at risk for AD, higher baseline CRF was associated with slower annual decline in total gray matter volume and cognitive function over 3 to 5 years of follow‐up. These findings suggest CRF may be an important physiological attribute to target during the preclinical stage of AD.
Future directions: Longitudinal studies that assess changes in CRF are needed to better elucidate causality and directionality of the observed associations.
2.2. Cardiorespiratory fitness assessment
Under the supervision of a physician, exercise tests were conducted by a certified exercise physiologist along with a trained exercise specialist after a resting 12‐lead electrocardiogram (ECG) assessment. The exercise test was completed on a motor‐driven treadmill using a modified Balke protocol. 20 Comfortable walking speeds were determined during a warm‐up period and kept constant for the duration of testing. The majority of participants walked at 3.5 miles per hour; however, if the participant indicated that this speed was uncomfortable, a slower walking speed was chosen. The exercise test began with a 2‐minute warm‐up at 0% grade that was increased by 2.5% every 2 minutes until the participant reached volitional exhaustion or indicated they could no longer continue.
During the exercise test, oxygen consumption (V̇O2) , carbon dioxide production (V̇CO2)
, carbon dioxide production (V̇CO2) , and minute ventilation (V̇E)
, and minute ventilation (V̇E) were measured using a metabolic cart (TrueOne 2400 Parvo Medics) and a two‐way non‐rebreathing mask. Heart rate (HR) was continuously measured through a 12‐channel ECG device (Schiller CS‐200 Exercise Stress System). Rating of perceived exertion (RPE)
21
and blood pressure (Welch Allyn DS‐6601‐300) were recorded at 2‐minute intervals throughout the exercise test. The flowmeter of the metabolic system was calibrated prior to each exercise test by making multiple comparisons to a 3‐L piston syringe. Oxygen and carbon dioxide sensors were calibrated by the presentation of known gas concentrations and corrected for barometric pressure. All calibration procedures were conducted within 4 hours of the exercise test to ensure accuracy.
were measured using a metabolic cart (TrueOne 2400 Parvo Medics) and a two‐way non‐rebreathing mask. Heart rate (HR) was continuously measured through a 12‐channel ECG device (Schiller CS‐200 Exercise Stress System). Rating of perceived exertion (RPE)
21
and blood pressure (Welch Allyn DS‐6601‐300) were recorded at 2‐minute intervals throughout the exercise test. The flowmeter of the metabolic system was calibrated prior to each exercise test by making multiple comparisons to a 3‐L piston syringe. Oxygen and carbon dioxide sensors were calibrated by the presentation of known gas concentrations and corrected for barometric pressure. All calibration procedures were conducted within 4 hours of the exercise test to ensure accuracy.
We have previously shown that on average, older adults struggle to provide a maximal effort during exercise testing, which results in an underestimation of their recordedV̇O2 peak. 22 Therefore, we chose the oxygen uptake efficiency slope (OUES), a measure of exercise efficiency, as the primary CRF variable for the current study, which has shown to be a valid, reliable measure that does not require maximal effort. 22 , 23 The OUES was determined for each participant by calculating the regression slope from the linear relationship of absolute V̇O2 (L/min) plotted as a function of log10 V̇E (L/min) (V̇O2 = log10 V̇E + b) providing a measure of cardiopulmonary performance that integrates cardiovascular, musculoskeletal, and respiratory function. 23 The OUES values were standardized to body surface area (BSA) to account for individual differences. The OUES calculation only included metabolic data collected during the graded exercise test and excluded warm‐up and recovery stages due to irregular ventilation often observed during those stages.
2.3. Physical activity assessment
All participants wore a triaxial GT3X+ accelerometer (ActiGraph) on their hip for 7 consecutive days after the graded exercise test. Participants were instructed to wear the device during all waking hours, except for when showering, swimming, or bathing. Standard accelerometry inclusion criteria consisted of at least 10 hours of valid wear time per day for a minimum of three weekdays and one weekend day. 24 Accelerometer data (in 1‐second epochs) were processed using the validated Sojourn‐3 Axis method, which uses an artificial neural net to identify boundaries between activities of different intensities and to estimate metabolic equivalents (METs) for each bout. 25 To calculate minutes spent in different intensity categories of physical activity, estimated METs were determined for each bout interval and then separated into physical activity categories accordingly: < 1.5 METs = sedentary, 1.5 to 2.99 METs = light, 3 to 6 METs = moderate, and > 6 METs = vigorous. Based on current physical activity recommendations, 26 our chosen physical activity measure was time spent engaged in moderate‐to‐vigorous physical activity, expressed as a percentage of total accelerometer wear time.
2.4. Neuroimaging protocol
Baseline and follow‐up MRI scans were acquired on a 3T GE X750 Discovery scanner with an 8‐ or 32‐channel phased array head coil. Three participants had follow‐up MRI scans acquired on a 3T GE SIGNA scanner. Three‐dimensional (3‐D) T1‐weighted inversion recovery‐prepared spoiled gradient echo scans were collected using the following parameters: inversion time (TI)/echo time (TE)/repetition time (TR) = 450 ms/3.2 ms/8.2 ms, flip angle = 12°, slice thickness = 1 mm no gap, field of view (FOV) = 256, matrix size = 256×256 yielding a voxel resolution of 1 mm×1 mm×1 mm. Volumetric estimates were derived from the T1‐weighted images. Tissue segmentation into gray matter, white matter, and cerebral spinal fluid (CSF) was performed with SPM version 12 (www.fil.ion.ucl.ac.uk/spm) with all images bias corrected and spatially normalized to Montreal Neurological Institute space. Intracranial volume was the sum of SPM estimated gray matter, white matter, and CSF volumes. Hippocampal volume was estimated from T1‐weighted images using the automated FSL FIRST version 6 software (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST). FSL FIRST is a segmentation tool that identifies subcortical brain regions given the observed intensity gradients (e.g., gray/white matter, CSF borders) on the T1‐weighted image. 27 The FSL FIRST segmentation methods are widely used and have been validated against other automated segmentation tools and gold‐standard manual segmentation methods. 27 , 28 All images were visually inspected to ensure they were accurately reconstructed and without topological defects. For our analyses, a priori brain outcomes of interest included total gray matter and hippocampal volumes, expressed as a percentage of intracranial volume to account for individual variability in head size. The mean ± standard deviation (SD) time between the CRF/physical activity assessment and baseline MRI was 0.74 ± 0.51 years.
2.5. Cognitive testing
Participants in WRAP undergo comprehensive cognitive testing at each study visit. 19 For the current study, the preclinical Alzheimer's Cognitive Composite (PACC), which combines scores from the Auditory Verbal Learning Test (AVLT), the Wechsler Adult Intelligence Scale (WAIS), and the Wechsler Memory Scale (WMS) was the cognitive measure of interest. 29 , 30 Specifically, the AVLT total recall score (trials I‐V), WAIS digit symbol total score, and the WMS logical memory II total score were standardized using the means and SDs among those who were cognitively unimpaired at their baseline assessment per test, and were then averaged to create the PACC score. 29 , 30 Additional PACC details have been previously described. 31 The mean ± SD time between the CRF/physical activity assessment and baseline cognitive testing was 0.78 ± 0.48 years.
2.6. Covariate measures
All participants completed a variety of health‐related questionnaires and measurements at each study visit. Variables investigated as potential confounders included age, sex, years of education, APOE ε4 carrier status, and number of previous cognitive testing visits. Age, sex, and years of education were measured by self‐report. APOE ε4 carrier status was determined through polymerase chain reaction‐based genotyping and classified as carriage of at least one APOE ε4 allele. 19 The number of previous cognitive testing visits were included to control for prior practice with the cognitive battery.
2.7. Statistical analyses
Participant characteristics were summarized with means, standard deviations, and frequencies. Linear mixed effects models, with age as the time metric, were used for the primary analyses to test longitudinal associations of (1) baseline CRF with brain atrophy (total gray matter and hippocampal volumes) while controlling for sex, years of education, and APOE ε4 carrier status and (2) baseline CRF with cognitive trajectories while controlling for sex, years of education, APOE ε4 carrier status, and number of previous cognitive testing visits. The CRF × age (i.e., time) interaction was the term of interest, as it would indicate whether longitudinal decline in brain volumes or cognition differed as a function of CRF. The variables in each interaction were mean‐centered. Sensitivity analyses were performed with additional adjustment for physical activity. Further, we conducted exploratory analyses to examine the differential effect of APOE ε4 carrier status though stratification analyses and three‐way interactions. Statistical significance was set at an alpha level of 0.05. All analyses were conducted in SPSS v 26.
3. RESULTS
One hundred ten cognitively unimpaired (Mini‐Mental State Examination [MMSE] = 24–30) participants (mean age = 64.2±5.7 years, 66% female) completed baseline CRF testing and physical activity monitoring. The supervising exercise physiologist stopped three tests due to safety concerns (arrhythmia, dyspnea, hypertension), one participant discontinued participation due to mask discomfort, and two participants recorded insufficient accelerometer wear time. Further, 12 individuals lacked repeat MRI data and four individuals did not have follow‐up cognitive data, resulting in an analytic sample of 92 participants for the brain volume analyses and 100 participants for the cognitive function analysis.
Participants had two neuroimaging MRI scans over 4.67±1.17 years and two to three cognitive tests (two, n = 64; three, n = 36) over 3.26±1.02 years of follow‐up. Removing three participants who had follow‐up MRI acquired on a different scanner did not influence any of the reported findings (P > .05). On average, participants displayed a baseline OUES value of 1.16±0.28 and engaged in 1.42±0.62 hours/day of moderate‐to‐vigorous physical activity. The OUES was weakly associated with physical activity (r = .21; P = .029). Participants had an average brain volume of 655.38±57.59 mL for total gray matter, 7746.68±876.28 mm3 for hippocampal, and 1422.06±135.47 mL for intracranial volume. At the group level, total gray matter and hippocampal volume declined at a rate of −0.73±0.90% and −0.16±1.30% per year, and cognitive trajectories declined −0.047 points (standardized score) per year, respectively. Additional participant characteristic data are detailed in Table 1.
TABLE 1.
Baseline participant demographics stratified by analysis
| Variable | Total sample | CRF‐brain | CRF‐cognition |
|---|---|---|---|
| Sample size | 110 | 92 | 100 |
| Female, % | 65.5 | 63.0 | 65.0 |
| Age, years | 64.2 (5.7) | 64.1 (5.8) | 64.1 (5.7) |
| Weight, lbs | 174.4 (36.1) | 175.8 (37.4) | 176.0 (36.0) |
| Height, in | 66.2 (3.7) | 66.4 (3.8) | 66.3 (3.7) |
| BMI, kg/m2 | 27.9 (5.5) | 28.0 (5.7) | 28.1 (5.4) |
| Education, years | 16.4 (2.3) | 16.4 (2.3) | 16.5 (2.2) |
| APOE ε4 +, % | 41.8 | 44.6 | 43.0 |
| FH +, % | 70.9 | 70.7 | 72.0 |
| MMSE, score | 29.3 (1.1) | 29.3 (1.1) | 29.3 (1.1) |
Note: Values indicate mean score and standard deviation.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CRF, cardiorespiratory fitness; FH, family history of Alzheimer's disease; MMSE, Mini‐Mental State Examination.
Results from the linear mixed effects models are presented in Tables 2 and 3. The CRF × age interaction was significant and demonstrated that participants with higher baseline CRF had slower annual decline in total gray matter volume (β = .25 standard error [SE] .10; p = .013; Figure 1) and PACC score (β = .07 SE .03; P = .048; Figure 2), but not hippocampal volume (β = .001 SE .002; P = .426). After additional adjustment for physical activity, the CRF × age interaction persisted for total gray matter volume (β = .23 SE .10; P = .023) but not PACC score (β = .05 SE .03; P = .11). When the analyses were stratified by APOE ε4 status, and adjusting for physical activity, the CRF × age interaction remained significant for carriers (gray matter: β = .34 SE .15, P = .028; PACC score: β = .11 SE .06, P = .049), but not noncarriers (gray matter: β = .14 SE .14, P = .33; PACC score: β = .01 SE .04, P = .90). Adding a CRF × age × APOE ε4 status interaction term to formally test the observed genotype differences did not result in a significant three‐way interaction for total gray matter volume (P = .32) or PACC score (P = .10).
TABLE 2.
Model predicting total gray matter volume (n = 92)
| Parameter | 95% Confidence interval | |||||
|---|---|---|---|---|---|---|
| Estimate (β) | SE | t‐value | P | Lower bound | Upper bound | |
| Intercept | 46.9149 | 1.9379 | 24.21 | .000 | 43.0646 | 50.7653 |
| Age | −.2993 | .0303 | −9.89 | .000 | −.3591 | −.2396 |
| CRF | .6502 | .9981 | .65 | .516 | −1.3314 | 2.6318 |
| Sex | −2.6020 | .6199 | −4.20 | .000 | −3.8331 | −1.3708 |
| Education | −.0318 | .1182 | −.27 | .788 | −.2667 | .2031 |
| APOE ε4 status | .0782 | .5145 | .15 | .880 | −.9436 | 1.1000 |
| CRF × age | .2496 | .0988 | 2.53 | .013 | .0544 | .4447 |
Notes: β = Unstandardized beta coefficient; SE = standard error; Sex coded 0 = female, 1 = male. APOE ε4 status coded 0 = non‐carrier, 1 = carrier; †Estimates reflect total gray matter expressed as a percentage of intracranial volume (sum of total gray matter, white matter, and CSF volumes).
Abbreviations APOE, apolipoprotein E; CRF, cardiorespiratory fitness; CSF, cerebrospinal fluid.
TABLE 3.
Model predicting PACC score (n = 100)
| Parameter | 95% Confidence interval | |||||
|---|---|---|---|---|---|---|
| Estimate (β) | SE | t‐value | P | Lower bound | Upper bound | |
| Intercept | −1.3653 | .4765 | −2.87 | .005 | −2.3096 | −.4210 |
| Age | −.0429 | .0113 | −3.79 | .000 | −.0654 | −.0204 |
| CRF | .1742 | .2750 | .63 | .528 | −.3711 | .7194 |
| Sex | −.5386 | .1494 | −3.61 | .000 | −.8350 | −.2423 |
| Education | .0889 | .0284 | 3.13 | .002 | .0325 | .1453 |
| APOE ε4 status | −.0096 | .1225 | −.08 | .938 | −.2525 | .2333 |
| PACC exposure | .0741 | .0379 | 1.96 | .052 | −.0006 | .1488 |
| CRF × age | .0655 | .0329 | 1.99 | .048 | .0006 | .1303 |
Notes: β = Unstandardized beta coefficient; SE = standard error; Sex coded 0 = female, 1 = male. APOE ε4 status coded 0 = non‐carrier, 1 = carrier. Abbreviations APOE, apolipoprotein E; CRF, cardiorespiratory fitness; PACC, Preclinical Alzheimer's Cognitive Composite.
FIGURE 1.
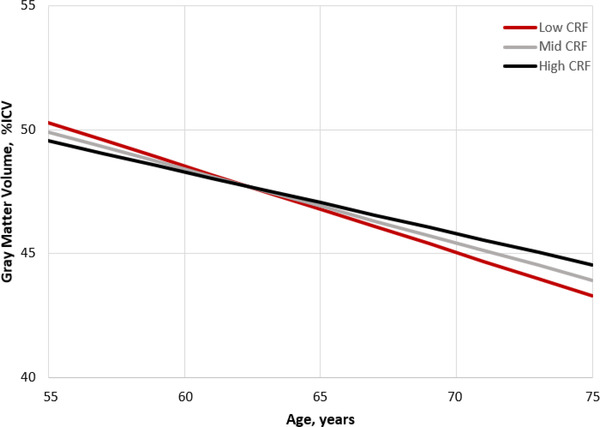
Figure displays gray matter volume change over time adjusted for covariates. The plot was extrapolated based on calculation of simple intercepts and slopes using the regression coefficients from the estimated fixed effects from the linear mixed effects model. Although CRF was included in the analysis as a continuous variable, for the purpose of graphing the findings we created groups that correspond to the average CRF level, and 1 SD below and above the mean to represent Low, Mid, and High CRF. CRF, cardiorespiratory fitness; ICV, intracranial volume; SD, standard deviation
FIGURE 2.
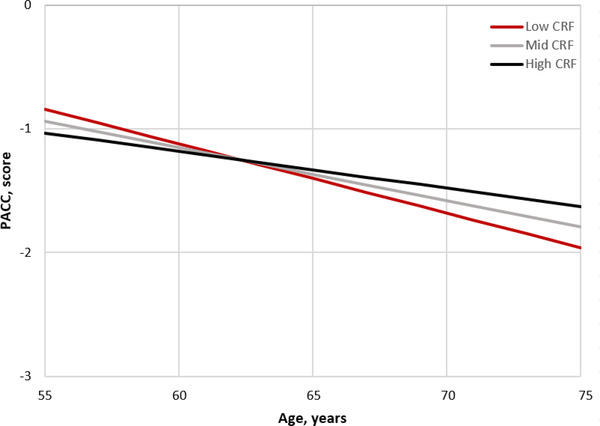
Figure displays cognitive change over time adjusted for covariates. The plot was extrapolated based on calculation of simple intercepts and slopes using the regression coefficients from the estimated fixed effects from the linear mixed effects model. Although CRF was included in the analysis as a continuous variable, for the purpose of graphing the findings we created groups that correspond to the average CRF level, and 1 SD below and above the mean to represent Low, Mid, and High CRF. CRF, cardiorespiratory fitness; PACC,= Preclinical Alzheimer's Cognitive Composite; SD, standard deviation
4. DISCUSSION
We examined the longitudinal associations of CRF with brain atrophy and cognitive decline in a sample of cognitively unimpaired late‐middle‐aged adults. Our findings suggest that higher CRF is associated with a slower rate of gray matter atrophy and cognitive decline over 3 to 5 years of follow‐up. After accounting for physical activity in our sample, CRF remained associated with longitudinal changes in gray matter volume, but not cognitive function. Notably, these results appear to be, in part, dependent on genetic risk. Our preliminary data suggest that CRF is significantly associated with longitudinal changes in total gray matter volume and cognitive function in APOE ε4 carriers, but not within non‐carriers. However, we did not observe significant three‐way interactions; therefore, the genetic specificity of our findings are uncertain.
The impact of exercise on brain and cognitive health has received considerable attention in recent years. Regular exercise is associated with a delay in onset of AD 14 and our group's recent cross‐sectional research has demonstrated CRF is positively associated with several indices of brain and cognitive health. 15 , 32 Although exercise and CRF appear to be beneficial, randomized controlled trials have not provided strong evidence that structured exercise can substantially impact the pathophysiological processes of aging and AD (e.g., neurodegeneration). 33 An intrinsic limitation to exercise trials is the relatively short durations; the typical 6‐ to 12‐month intervention may be insufficient to significantly impact the slow progressive pathophysiology of the disease (but see also Erickson et al. 34 ). There is a need to investigate CRF in relation to brain and cognitive changes over the course of several years to further understand how this modifiable physiological attribute influences the pathophysiology of AD.
In our sample, we observed a decline in total gray matter and hippocampal volume over a 5‐year period, which is consistent with data describing brain volume declines during mid to late adulthood, prior to cognitive impairment. 35 , 36 Although several cross‐sectional studies have reported CRF is positively associated with gray matter volume in cognitively unimpaired adults (see review in Erickson et al. 16 ), few have investigated how CRF relates to future rates of brain atrophy. Vidoni et al. reported that baseline CRF was associated with hippocampal atrophy over a 2‐year period in participants with AD, but not in cognitively unimpaired participants. 37 Moreover, research from the Baltimore Longitudinal Study of Aging did not find evidence that midlife CRF prospectively predicted age‐related gray matter or hippocampal volume decline among middle‐aged adults. 38 In the present study, the absence of a longitudinal association between CRF and hippocampal volume corresponds with the existing literature; however, the finding of an association with total gray matter volume contributes to a relatively limited body of prospective research investigating objectively measured CRF and brain atrophy. Although this is a growing area of study, the varying results may be explained by sample demographics (e.g., cognitive status, genetic risk), neuroimaging methods, follow‐up period, and CRF testing methodology; as our group has previously detailed, exercise testing considerations are critical when investigating brain and cognitive health outcomes in mid‐ to late‐aged adults. 22
Similar to brain volume, cognitive function declines with age and the progression of AD. 7 , 39 Specifically, memory and executive functions decline during the preclinical phase and are the cognitive domains most related to disease progression. 31 , 40 , 41 , 42 Barnes et al. reported that low levels of baseline CRF were associated with greater decline on the MMSE 6 years later. 43 In comparable samples of cognitively unimpaired adults, CRF predicted cognitive performance over time, including tests on memory and executive functions. 44 , 45 These prior investigations suggest higher levels of CRF are related to favorable cognitive trajectories. However, it is important to note that numerous cognitive tests were not significantly related to CRF, making it uncertain which cognitive domains may be affected. 43 , 44 , 45 An important aspect of the current study was the use of the PACC score, which has been shown to be a more sensitive measure to detect subtle age‐related cognitive decline than single cognitive tests. 31 Our finding that greater mid‐ to late‐life CRF is associated with a slower decline in PACC score is intriguing because this cognitive composite captures the domains that decline during the preclinical phase of AD. 31 , 40 , 41 , 42 These data suggest CRF may be important for maintaining cognitive health throughout the early stages of the disease.
Although it is generally accepted that CRF and physical activity are important for brain and cognitive health, few studies have investigated their independent contributions. Among our sample, accounting for baseline physical activity did not modify the longitudinal association between CRF and total gray matter volume but did influence the association with cognitive function. With respect to total gray matter volume, these data agree with the few cross‐sectional studies among mid‐ to late‐aged adults 32 , 46 and suggest CRF has a significant impact on brain health that is not explained by physical activity behaviors. On the other hand, inclusion of physical activity resulted in a non‐significant association between CRF and cognitive function, suggesting that physical activity behaviors may be a contributing factor. Collectively, these findings highlight the necessity to consider both the physiological attribute of CRF and the behavior of physical activity to better understand the unique contributions each may confer. Further investigation is warranted to elucidate the physiological mechanisms underlying these associations. One hypothesized pathway is through cerebral vascular function. Adequate blood supply is essential for neuronal functioning, and vascular dysfunction is likely a contributor to neuronal cell death (e.g., atrophy). 47 Our group has previously shown CRF, but not physical activity, is associated with cerebral blood flow in brain regions vulnerable to hypoperfusion in aging and AD, 32 which, in turn, may mitigate brain atrophy and preserve cognitive functions. Other systems that CRF and exercise may act through to protect against brain atrophy and cognitive decline include insulin regulation, mitochondrial function, inflammation, and stress (e.g., hypothalamic–pituitary–adrenal axis). 48 Importantly, these pathways activated through exercise do not act in isolation and there are likely complex interactions among numerous processes that exert protective effects.
The greatest known genetic risk for AD is the presence of APOE ε4, where carriers have a three‐fold increased risk. 18 Recent research suggests that APOE ε4 carriers have lower CRF than non‐carriers 17 raising the possibility that decreased CRF may be a contributing factor to the increased prevalence of AD observed in this genotype. Exploratory analyses suggest higher CRF at baseline was associated with a slower decline in total gray matter volume and cognitive function among carriers of APOE ε4, but not within non‐carriers. However, the three‐way interactions were non‐significant; therefore, these exploratory findings should be interpreted with caution. A few studies have reported exercise and physical activity exert greater benefit for APOE ε4 carriers with respect to AD risk and brain health; however, the literature overall is inconclusive (see review in de Frutos‐Lucas et al. 49 ). To our knowledge, no study has investigated the role of APOE ε4 status with CRF, physical activity, and longitudinal changes in brain volume and cognitive function. Because adults who harbor the APOE ε4 genotype are at greatest need for treatments to slow disease progression, our preliminary finding that CRF partially mitigates brain atrophy and cognitive decline among these at‐risk adults is of heightened importance.
Future longitudinal studies that assess changes in CRF are needed to better elucidate causality and directionality of the observed associations. Further, we cannot rule out the potential fluctuations of physical activity behaviors, which would influence our accelerometer measures; however, this does not present a concern with CRF testing. Although our sample size was sufficient to test our primary aims, we may have been underpowered when examining three‐way interactions. The WRAP is a well‐characterized sample of highly educated White participants, making it unknown whether these findings generalize to more socioeconomically diverse populations. These limitations should be considered in the context of the study novelties, which include an at‐risk population, objectively measured CRF and physical activity, and examination of brain atrophy and cognitive decline over a critical period of aging.
The forthcoming expansion of the older adult population will substantially increase AD cases nationwide. There is a need to identify treatments that can be implemented during the preclinical phase to delay the progression of AD, which, in turn, will decrease disease prevalence. If discovered, a treatment postponing symptom onset by as little as 5 years is estimated to reduce the prevalence of AD by half, substantially decreasing the societal burden. 50 Our study provides evidence that within a late‐middle‐aged cohort, greater CRF was associated with a slower rate of total gray matter atrophy and cognitive decline over 3 to 5 years of follow‐up. Notably, these findings were most pronounced in adults who harbor the APOE ε4 genotype, suggesting CRF may be an important physiological attribute to target during the preclinical stage of AD.
CONFLICTS OF INTEREST
The authors have no competing interests to declare.
ACKNOWLEDGMENTS
This work was supported by National Institute on Aging grants F31 AG062009 (RJD), T32 AG027668 (RJD), K23 AG045957 (OCO), R21 AG051858 (OCO), R01 AG027161 (SCJ), and R01 AG021155 (SCJ), and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Extendicare Foundation, the Alzheimer's Association, Wisconsin Alumni Research Foundation, the Helen Bader Foundation, Northwestern Mutual Foundation, and the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin. The contents do not represent the views of the National Institutes of Health, Department of Veterans Affairs, or the United States government.
Dougherty RJ, Jonaitis EM, Gaitán JM, et al. Cardiorespiratory fitness mitigates brain atrophy and cognitive decline in adults at risk for Alzheimer's disease. Alzheimer's Dement. 2021;13:e12212. 10.1002/dad2.12212
REFERENCES
- 1. Vespa J, Armstrong DM, Medina L. Demographic turning points for the United States: Population projections for 2020 to 2060. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau. 2018. [Google Scholar]
- 2. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010‐2050) estimated using the 2010 census. Neurology. 2013;80(19):1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villemagne VL, Burnham S, Bourgeat P. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. [DOI] [PubMed] [Google Scholar]
- 4. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iturria‐Medina Y, Sotero RC, Toussaint PJ, Mateos‐Pérez JM, Evans AC. Early role of vascular dysregulation on late‐onset Alzheimer's disease based on multifactorial data‐driven analysis. Nat Commun. 2016;7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Betthauser TJ, Koscik RL, Jonaitis EM, et al. Amyloid and tau imaging biomarkers explain cognitive decline from late middle‐age. Brain. 2019;143(1):320‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allison SL, Koscik RL, Cary RP, et al. Comparison of different MRI‐based morphometric estimates for defining neurodegeneration across the Alzheimer's disease continuum. Neuroimage Clin. 2019;23:101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the. Circulation. 2016;134(24):e653‐e699. [DOI] [PubMed] [Google Scholar]
- 11. Yaffe K, Barnes D, Nevitt M, Lui Li‐Y, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703‐1708. [DOI] [PubMed] [Google Scholar]
- 12. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J Intern Med. 2011;269(1):107‐117. [DOI] [PubMed] [Google Scholar]
- 13. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 14. Larson EB, Wang Li, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73‐81. [DOI] [PubMed] [Google Scholar]
- 15. Dougherty RJ, Schultz SA, Boots EA, et al. Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer's disease. Brain Behav. 2017;7(3):e00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35 Suppl 2:S20‐S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris JK, Zhang G, Dougherty RJ, et al. Collective effects of age, sex, genotype, and cognitive status on fitness outcomes. Alzheimers Dement (Amst). 2020;12(1):e12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 19. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. BALKE B, WARE RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675‐688. [PubMed] [Google Scholar]
- 21. Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377‐381. [PubMed] [Google Scholar]
- 22. Dougherty RJ, Lindheimer JB, Stegner AJ, Van Riper S, Okonkwo OC, Cook DB. An objective method to accurately measure cardiorespiratory fitness in older adults who cannot satisfy widely used oxygen consumption criteria. J Alzheimers Dis. 2018;61(2):601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28(6):1567‐1572. [DOI] [PubMed] [Google Scholar]
- 24. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181‐188. [DOI] [PubMed] [Google Scholar]
- 25. Lyden K, Keadle SK, Staudenmayer J, Freedson PS. A method to estimate free‐living active and sedentary behavior from an accelerometer. Med Sci Sports Exerc. 2014;46(2):386‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoemaker D, Buss C, Head K, et al. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 2016;129:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid‐related decline. JAMA Neurol. 2014;71(8):961‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. 2017;13(9):1004‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonaitis EM, Koscik RL, Clark LR, et al. Measuring longitudinal cognition: individual tests versus composites. Alzheimers Dement (Amst). 2019;11:74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dougherty RJ, Boots EA, Lindheimer JB, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer's disease. Brain Imaging Behav. 2020;14(4):1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta‐analysis. Neuroimage. 2018;166:230‐238. [DOI] [PubMed] [Google Scholar]
- 34. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017‐3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676‐1689. [DOI] [PubMed] [Google Scholar]
- 36. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295‐3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer's and aging over 2 years. Neurobiol Aging. 2012;33(8):1624‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tian Qu, Studenski SA, Resnick SM, Davatzikos C, Ferrucci L. Midlife and late‐life cardiorespiratory fitness and brain volume changes in late adulthood: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71(1):124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lyketsos CG, Chen Li‐S, Anthony JC. Cognitive decline in adulthood: an 11.5‐year follow‐up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry. 1999;156(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 40. Hedden T, Oh H, Younger AP, Patel TA. Meta‐analysis of amyloid‐cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable neuropsychological differences in early preclinical Alzheimer's disease: a meta‐analysis. Neuropsychol Rev. 2017;27(4):305‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clark LR, Berman SE, Norton D, et al. Age‐accelerated cognitive decline in asymptomatic adults with CSF β‐amyloid. Neurology. 2018;90(15):e1306‐e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 44. Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014;69(4):455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pentikäinen H, Savonen K, Ngandu T, et al. Cardiorespiratory fitness and cognition: longitudinal associations in the FINGER Study. J Alzheimers Dis. 2019;68(3):961‐968. [DOI] [PubMed] [Google Scholar]
- 46. Voss MW, Weng TB, Burzynska AZ, et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage. 2016;131:113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age‐associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis. 2017;55(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 49. de Frutos‐Lucas J, Frost N, Erickson KI, et al. Does APOE genotype moderate the relationship between physical activity, brain health and dementia risk? A systematic review. Ageing Res Rev. 2020;64:101173. [DOI] [PubMed] [Google Scholar]
- 50. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]


