Abstract
Background
Subjective cognitive decline (SCD) is considered a risk factor for Alzheimer's disease (AD), highlighting the need for identifying and ranking effective interventions. This was addressed in a systematic review and network meta‐analysis (NMA) of pharmacological and non‐pharmacological interventions for SCD.
Methods
MEDLINE, Web of Science Core Collection, CENTRAL, and PsycINFO were searched for randomized controlled trials (RCTs) investigating effects on memory, global cognition, and quality of life. Random‐effect model NMAs were conducted. The Cochrane Risk‐of‐Bias‐2 tool assessed methodological quality. Prospero‐Registration: CRD42020180457.
Results
The systematic review included 56 RCTs. Education programs were most effective for improving memory, second most effective for improving global cognition. Quality of life and adverse events could not be included due to insufficient data. Overall methodological quality of studies was low.
Conclusion
Education programs were most effective for improving memory and cognition, warranting further research into effective elements of this intervention. There is urgent need to address identified methodological shortcomings in SCD intervention research.
Keywords: network meta‐analysis, non‐pharmacological interventions, pharmacological interventions, subjective cognitive decline, systematic review
1. INTRODUCTION
Subjective cognitive decline (SCD) is defined as perceived cognitive decline in the absence of objective cognitive impairment. 1 Recently, substantial interest in SCD has emerged, reflecting its recognition as a potential early manifestation of Alzheimer's disease (AD). 2 SCD is associated with a 4.5‐fold risk increase for subsequent diagnosis of mild cognitive impairment (MCI) due to AD, and a 6.5‐fold increased risk for AD. 3 Individuals with SCD are also more likely to present with AD biomarkers (i.e., increased amyloid burden, neurodegeneration). Thus, identifying effective interventions that allow counteracting or slowing of disease progression at an early stage is of utmost importance.
Several pharmacological and non‐pharmacological interventions are currently under investigation that aim to improve cognitive functioning and psychological well‐being in people with SCD. 4 A recent systematic review and meta‐analysis that investigated the effectiveness of psychological, cognitive, lifestyle, or pharmacological interventions for SCD concluded that psychological group interventions can improve psychological well‐being and that cognitive training interventions resulted in small, but statistically significant, improvement of cognitive performance. 5
However, while conventional meta‐analytical approaches can provide valuable information about the overall effectiveness of a particular treatment across included studies, comparisons of more than two interventions are not possible. This can be achieved by a network meta‐analysis (NMA), which allows direct comparisons between all different interventions in the same model by considering direct (within studies) and indirect (between studies sharing a comparable intervention) evidence simultaneously. 6 It also allows establishing efficacy rankings of different interventions for specific outcomes, which is highly relevant for clinical decisions. However, this approach has not yet been used to characterize and rank the effectiveness of pharmacological and non‐pharmacological treatments for SCD.
The aim of the present study was to (1) identify and describe all investigated interventions for individuals with SCD in a systematic review; (2) rate the overall research quality of these studies with a risk of bias judgment; (3) evaluate and compare the effectiveness of all investigated interventions on memory, global cognition, quality of life, and adverse events using network meta‐analyses; and (4) generate clinically meaningful recommendation rankings for treatment decisions for SCD.
2. METHODS
The present systematic review and NMA was pre‐registered and the review protocol can be accessed at www.crd.york.ac.uk/PROSPERO/ (ID: CRD42020180457). Reporting follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline. 7 The “PRISMA for Abstracts Checklist” and the “PRISMA checklist for systematic reviews” are depicted in Tables S1 and S2 in supporting information. Confirming consent of subjects was not necessary.
RESEARCH IN CONTEXT
Systematic Review: MEDLINE, Web of Science Core Collection, CENTRAL, and PsycINFO were searched for randomized controlled trials (RCTs) investigating effects of pharmacological and non‐pharmacological interventions in subjective cognitive decline (SCD). Our search yielded 9298 search results and identified n = 56 eligible studies.
Interpretation: Our results confirm that interventions that improved cognition and memory in other populations, like physical activity interventions and cognitive training, were also effective in SCD. Surprisingly, the overall most effective intervention type was education programs. We also identified a lack on studies that investigated quality of life and adverse events, even though such participant‐related outcomes are of utmost importance.
Future directions: SCD may provide a unique window for early interventions aimed at preventing cognitive decline before pathological impairment may manifest. Based on our results, future research on education programs as part of preventive care in SCD should be conducted, investigating participant‐related outcomes with the use of proper statistical and reporting methods.
Highlights
We conducted the first network meta‐analysis investigating effectiveness of interventions for subjective cognitive decline (SCD).
Overall, education programs were identified as most effective for improving memory and global cognition.
Several methodological shortcomings in current SCD intervention research were identified that need to be addressed in future research.
2.1. Systematic review
Systematic reviews of randomized controlled trials (RCTs) are generally considered the highest level of evidence for the relative effectiveness of interventions. 8 The following paragraphs detail the methods of the systematic review.
2.1.1. Search and study selection
We conducted a systematic search in MEDLINE Ovid, Web of Science Core Collection, CENTRAL, and PsycINFO up to April 15, 2020. Reference lists of relevant reviews were searched for additional publications. Full‐text publications were requested from the authors within a 2‐week time frame, if not otherwise accessible. Tables S3‐S6 in the supporting information provide additional information on the systematic review and search strings.
Titles and abstracts were screened according to predefined eligibility criteria by three individual review authors (MR, XH, SR) using the Covidence software (Veritas Health Innovation). Subsequently, full‐text articles of studies meeting inclusion criteria were reviewed for inclusion in the systematic review. If no consensus could be reached between reviewers, cases were discussed until consensus was reached.
2.1.2. Eligibility criteria
Studies were considered eligible if they had analyzed effects of interventions for SCD in RCTs in both female and male individuals of all ages. Both pharmacological and non‐pharmacological interventions for SCD were included. We did not limit or pre‐specify requirements and/or parameters of intervention types. SCD was defined as (1) self‐perceived persistent decline in cognitive capacity relative to previous cognitive status, unrelated to an acute event, and (2) normal performance on standardized cognitive tests used to classify MCI adjusted for age, sex, and education. 9 During the initial search, we also included studies in which SCD was not clearly defined, for example, only labeled as “self‐reported memory problems” without further specifications. Subsequently, SCD definitions were inspected and only those that had a current SCD definition were included in our main analysis. To confirm our results, a sensitivity analysis was conducted that also included studies without clear SCD definition (see section 3.7). Studies that had only included patients with MCI or dementia or patients with diagnosis of major psychiatric or medical diseases were excluded. We included studies published in English or German; only n = 3 studies in other languages were identified (see Figure 1).
FIGURE 1.
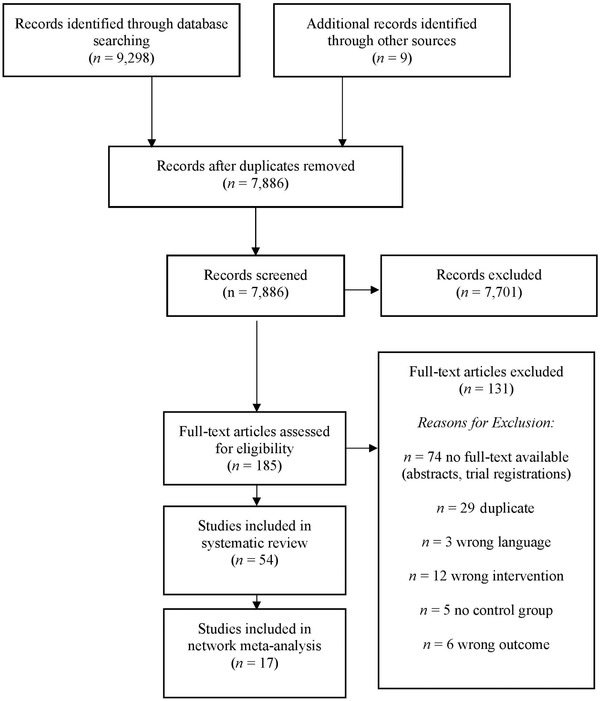
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram of the study selection process
Memory was defined as primary outcome, because it is one of the most vulnerable domains in aging, 10 one of the first domains subjectively affected in people with SCD, 1 and the core deficit in MCI and AD. Secondary outcomes were global cognition, quality of life, and adverse events. Only direct pre–post intervention outcome data were considered because only few studies reported long‐term follow‐up assessments.
2.1.3. Data extraction
Three review authors (MR, XH, SR) extracted data using a standardized extraction form. If the authors were unable to reach a consensus, a fourth review author (MM) was contacted for final decision. If required, the authors of specific studies were contacted for additional information. 11
2.1.4. Quality assessment
For each included study, risk of bias was assessed using the revised Cochrane risk‐of‐bias tool for randomized trials (RoB2 tool). 12 The tool implements signaling questions for five domains leading to low/high/medium concern for risk of bias. Two review authors (MR, SR) independently assessed risk of bias for each study. If they were unable to reach a consensus, a third review author (MM) was consulted for a final decision.
2.2. Network meta‐analyses
Network meta‐analyses extend the principles of pairwise comparisons of meta‐analyses to the evaluation of multiple treatments in a single analysis. This is achieved by combining direct and indirect evidence. Direct evidence refers to evidence obtained from RCTs in a trial comparing interventions A and B, indirect evidence refers to the evidence obtained through one or more common comparators (e.g., two studies sharing a comparable control condition 6 ). Network meta‐analyses rely on the same assumptions underlying pairwise meta‐analysis, that is, the included studies are sufficiently homogenous in terms of the condition being studied, the included participants, and the definition of active and control interventions. 13 Additionally, an important precondition for the NMA is that all investigated interventions are linked via at least one direct comparison to the overall network.
2.2.1. Main analyses
We performed a NMA using a random‐effects model. To evaluate the extent to which treatments were connected, a network plot is provided for primary and secondary outcomes. For each comparison, the estimated treatment effect along with its 95% confidence interval (CI) is provided. We graphically present the results using forest plots, with either control group or placebo group as reference treatment. For studies with multiple treatment groups, we combined arms as long as they could be regarded as subtypes of the same intervention. 11 We used the R package netmeta 1.0‐1 14 for statistical analyses. To evaluate the presence of statistical heterogeneity and inconsistency within the resulting networks, we used the generalized heterogeneity Q total and the generalized I² statistic. 15 We interpreted I² values as follows: 16 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, 75% to 100% represents considerable heterogeneity. Appendix A in the supporting information provides additional details of the statistical methods used, including assessment of heterogeneity.
2.2.2. Sensitivity analysis
Sensitivity analyses were conducted to test the robustness of our results by analyzing studies with low and medium risk of bias only (primary analyses; i.e., that only included studies with proper SCD definition). A sensitivity analysis is a repeat of the primary analysis, substituting alternative decisions or ranges of values for decisions that were arbitrary or unclear. 17 We judged studies as high risk if two risk of bias domains are judged as high risk. Additional sensitivity analyses were conducted including all studies, regardless of their SCD definition. Results of these analyses are reported in supporting information.
3. RESULTS
3.1. Results of the search
Our initial search yielded n = 9298 studies. N = 9 studies were identified through other sources. After removal of duplicates, n = 7889 were screened. After abstract review, n = 185 full‐text articles were assessed for eligibility; n = 54 studies on different interventions with participants with SCD were included. N = 17 studies could be included in the network meta‐analyses. For an overview of the study selection process, see the PRISMA flow diagram in Figure 1. References of the included study are listed in supporting information.
3.2. Systematic review: characteristics of the included studies
A total of 4692 participants with SCD from n = 54 different studies were included in the systematic review, investigating all possible SCD interventions. Studies included interventions using educational programs, memory techniques, cognitive training, meditation, physical exercise, nutritional supplements, pharmaceutical interventions, and non‐invasive brain stimulation. An in‐depth overview can be found in Table 1 and Appendix B in the supporting information.
TABLE 1.
Study and sample characteristics
| Study | Participants | Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | Age (M, SD) | Sex female (in %) | Education (in years) | SCD definition | I 1 | 12 | 13 | I4 | Global cognition | Memory | QoL | Adverse events | Others | FU | |
| Andrewes et al., 1996 | 40; 20; 20 | n.a.; age range for recruitment 60–70 | 50.00 | n.a. | Subjective memory complaints; MDRS >123 | Techniques for face‐name and prospective memory | First letter mnemonic, method of loci, narrative story method | x | 4m | ||||||
| Ban et al., 2018 | 75; 30; 30; 15 | 53.66 (5.44); 54.5 (4.9); 52.3 (5.6); 54.7 (5.9) | 86.70; 86.70; 86.70; 86.70 | 14.62; 14,3; 15.2; 14.2 | Subjective cognitive complaints with no clinical impairments; MMSE >24 or CDR <0.5 | Nutritional supplement Tremella fuciformis 600mg/d, 8 weeks | Nutritional supplement Tremella fuciformis 1200 mg/d for 8 weeks | Placebo capsule | x | X | |||||
| Barnes et al., 2013 | 126; 32; 31; 31; 32; Total dropout: 26 | 73,42 (6.00); 74.8 (6.3); 71.1 (5.5); 73.8 (5.7); 73.9 (6.1) | 62.70; 62.50; 67.70; 58.10; 62.50 | 16.35; 16.7; 15.6; 16.8; 16.3 | Subjective cognitive complaint; exclusion: dementia (self‐report, physician diagnosis or TICS‐M <17) | mental activity, 60 min/d, 3 days/wk for 12 weeks (6 weeks visual tasks, 6 weeks auditory tasks) + exercise, 60 min/d, 3 days/wk for 12 weeks (10 min warm‐up, 30 min aerobic, 5 min cool‐down, 10 min strength training, 5 minutes stretching) | mental activity control (educational lectures + MC questions) + exercise, 60 min/d, 3 days/wk for 12 weeks (10 warm‐up, 30 aerobic, 5 cool‐down, 10 strength training, 5 minutes stretching) | mental activity, 60 min/d, 3 days/week for 12 weeks (6 weeks visual tasks, 6 weeks auditory tasks) + exercise control (10 min warm‐up, 30 min stretching and toning, 10 minutes strength, 10 minutes relaxation) | mental activity control (educational lectures + MC questions) + exercise control (10 min warm‐up, 30 min stretching and toning, 10 min strength, 10 min relaxation) | X | x | X | X | ||
| Beck et al., 2016 | 61; 31; 30 | 77.3 (4.47); 57.5 (4.6); 57.1 (4.4) | 48.39; 60.00 | n.a. | Normal laboratory parameters, PRMQ (one item answered with “rather often”, “very often”, or five with “sometimes”) | 240 mg EGB 761 ® (Ginkgo) daily in the morning, 56±4 days | Placebo | x | |||||||
| Ben‐Itzhak et al., 2008 | 26 | 73.8 (1.2) | 65.38 | 13.2 | Subjective memory complaint; MMSE > 23; exclusion: dementia diagnosis | Single dose 20 mg methylphenidate | Cross‐over placebo | x | X | ||||||
| Boa Sorte Silva, Gill, Owen et al., 2018 7 | 127; 63; 64 | 67.45 (7.32); 67.6 (7.5); 67.4 (7.2) | 70.86; 69.80; 71.90 | 13.55; 13.3; 13.8 | Subjective cognitive complaint; IADL 8/8; MMSE > 24; exclusion: dementia, MDD | Multiple modality & mind‐motor training (M4) exercise 60 min/d, 3 d a week for 24 weeks | Multiple modality training (M2) exercise 60 min/d, 3 d a week for 24 weeks | x | x | X | 7m | ||||
| Boa Sorte Silva, Gill, Gregory et al., 2018 7 | 109;57;52 | 67.4 (7.2); 67.6 (7.5) | 71.9; 69.8 | 13.8 (3); 13.3 (2.7) | Subjective cognitive complaint; IADL 8/8; MMSE > 24; exclusion: dementia, MDD | Multiple modality & mind‐motor training (M4) exercise 60 min/d, 3 d a week for 24 weeks | Multiple modality training (M2) exercise 60 min/d, 3 d a week for 24 weeks | x | X | ||||||
| Brautigam et al., 1998 | 241; 77; 82; 82 Drop‐out: 44; 15; 14; 15 | 68.95 (7.77); 69.45 (7.18); 68.60 (8.28); 68.83 (7.86) | 59.34; 59.74; 60.98; 57.32 | n.a. | Subjective cognitive complaints; MMSE > 19; BDI < 20; Exclusion: memory loss of known origin | Ginkgo extract, 1.9 ml 3 times a day for 24 weeks | Ginkgo extract, 1.9 ml (1:1 diluted with placebo) 3 times a day for 24 weeks | Placebo | x | x | X | ||||
| Chan et al., 2017 | 48; 26; 22 | 68.96 (5.86); 69.50 (6.89); 68.34 (4.42) | 75.00; 80.77; 68.18 | 9.21; 8.69; 9.82 | CMSS > 2; CGDS‐SF < 8; BAI < 16; CDRS > 111 | Memory Intervention (Troyer et al., 2008), 90 min/session, once a week for 10 weeks | Dejian Mind‐Body Intervention, 90 min/session once a week for 10 weeks | x | X | 18m (Chan et al., 2018) | |||||
| Chan et al., 2018 [follow up to Chan et al. (2017)] | 29; 18; 11 | 68.79 (6.15); 68.6 (6.8); 69.1 (5.2) | 75.86: 77.78; 72.73 | 9.08; 8.70; 9.70 | Chinese memory symptoms scale > 2; CGDS‐SF < 8; BAI < 16; CDRS > 111 | Memory Intervention (Troyer et al., 2008), 90 min/session, once a week for 10 weeks | Dejian Mind‐Body Intervention, 90 min/session once a week for 10 weeks | x | |||||||
| Cheng et al., 2018 | 93; 47; 46 | 73.90 (7.40) | 81,00% | 6.20 (4.70) | Memory Inventory for the Chinese > 2 | Integrated Attention Training Program (IATP) | Health‐related education program | x | x | X | 6m | ||||
| Çinar & Şahiner, 2020 | 120; 30; 30; 30; 30 | 69.99 (9.48); 68.3 (10.94); 66.5 (9.79); 70.85 (7.78); 74.3 (7.56) | 55.83; 66.67; 56.67; 46.67; 53.33 | 11.08; 11.57; 13.27; 11.63; 7.83 | SCI: subjective cognitive decline without objective manifestation; AD: definded by NINCDS‐ADRDA criteria | SCI control group | SCI/BEYNEX: 15‐20 min physical and cognitive exercise daily. Used for at least 1200 min. | AD control group: Rivastigmin patch | AD/BEYNEX: 15‐20 minute physical and cognitive exercise daily. Used for at least 1200 min.+ Rivastigmine patch | x | x | X | |||
| Cohen‐Mansfield et al., 2015 | 44; 15; 15; 14 | 73.49 (5.18); 72.80 (3.78); 74.44 (5.78); 73.21 (5.97) | 72.7; 60.0; 86.7; 71.4 | 14.82; 14.25; 14.50; 16.00; | Subjective memory decline; MMSE >23 | ACTIVE (cognitive training) for 10 weeks | Health promotion for 10 weeks | Participant‐centered intervention for 10 weeks | x | x | |||||
| Epperson et al., 2011 | 16 baseline; 12 completed both arms | 54.0 (2.8) | 100 | 16.4 | Subjective cognitive decline; MMSE > 26 | Atomoxetin 80 mg/d, 6 weeks | Placebo controlled cross‐over design | x | X | ||||||
| Frankenmolen et al., 2018 | 60; 31; 29 | 67.07 (7.54); 66.2 (7.3); 68.0 (7.8) | 48.43; 32.00; 66.00 | ISCED: 4.6; 4.5; 4.7 | Subjective cognitive decline without objective manifestation; IADL | Adapted version of the memory strategy training (MST) protocol of Koning‐Haanstra et al. (1990): seven group sessions a 90 minutes, homework during the week between sessions | COGPACK attention and memory tasks. | x | x | X | 6m | ||||
| Fukuda et al., 2020 | 57; 27; 30 | 55.0 (5.3); 54.6 (5.4); 55.4 (5.3) | 52.6; 51.9; 53.3 | 14.6; 14.5; 14.7 | EMC: 3 revised questions | Matured hop bitter acids (12 weeks, 35 mg/d) | Placebo | x | |||||||
| Heath et al., 2017 | 63; 32; 31 | 67.0 (7.5); 65.7 (6.6); 68.3 (8.1) | 74; 74; 75 | 14.2 (2.8); 14.0 (2.7); 14.2 (2.8) | MoCA; IADL >6; MMSE >24 | Multiple modality & mind‐motor training (M4) exercise 60 min/d, 3 d a week for 24 weeks | Multiple modality training (M2) exercise 60 min/d, 3 d a week for 24 weeks | x | |||||||
| Hong et al., 2020 | 56; 23; 15; 18 | 65.88 (5.15); 66.22 (5.73); 65.40 (4.82); 65.83 (4.89) | 76.78; 73.9; 93,3; 66,7 | 11.37 (3.66); 10.43 (3.72); 11.20 (3.97); 12.72 (3.05) | Guideline by Jessen et al. (2014) | Multi‐domain cognitive training in small groups, twice a week with 90 minutes per session | Education Program, weekly phone calls as a reminder | x | |||||||
| Hoogenhout et al., 2012 | 50; 24; 26 | 66.05 (4.32); 66.00 (4.23); 66.10 (4.48) | 100; 100; 100 | Eight‐point scale: 4.07 (1.94); 4.14 (2.03); 4.00 (1.90) | Self‐reported subjective cognitive decline; MMSE >24 | Educational group intervention including eight 1.5‐hour sessions over 4 weeks and homework | Waiting list | x | x | 1 w | |||||
| Hooper et al., 2017 | 183; 98; 85 | 75.94 (4.55); 75.9 (4.7); 76.0 (4.4) | 65.59; 69.4; 61.2 | No. of persons reaching university level: 38; 24; 14 | No dementia: limitation in one or more IADL, or gait speed slower 0.8 m/s | n‐3‐PUFA (800 mg DHA, 225 mg EPA) supplementation, daily for three years | Placebo | x | |||||||
| Hsieh et al., 2019 | 24; 7; 17 | 68.3 (6.4); 66.0 (4.23); 67.5 (7.3) | 79.2; 71.2; 82.4 | 11.2; 10.6; 11.6 | CDR = 0; AD8 < 2; 4 CERAD questions | Physical fitness training, hand‐eye coordination, meditation, 1 hour for each modality, twice a week for 16 weeks | Same intervention, different population (non‐SMC) | x | x | ||||||
| Innes et al., 2018 2 , 3 | 53; 25; 28 | 60.47 (1.17); 60.71 (1.38); 60.2 (1.63) | 86.79; 92.00; 96.43 | ≥ 12 years: 81.13%; 88.00%; 75.00% | Guideline by Abdulrab and Heun (2008), Jessen et al. (2010), Jessen et al. (2014), & Reisberg et al. (2008) | Kirtan Kriya Meditation, 12 min/d, 12 weeks | Music listening | x | 3 m | ||||||
| Innes et al., 2016 2 , 3 | 60 (drop‐out: 7); 30; 30 | 60.58 (1.01); 60.93 (1.56); 60.23 (1.32) | 86.79; 90.00; 96.67 | 15.43; 16.17; 14.7 | Guideline by Abdulrab and Heun (2008), Jessen et al. (2010), Jessen et al. (2014), & Reisberg et al. (2008) | Kirtan Kriya Meditation, 12 min/d, 12 weeks | Music listening | x | 3 m | ||||||
| Jeon et al., 2016 | 75; 30; 30; 15 | 53.76 (5.72); 53.4 (6.4); 54.2 (5.4); 53.6 (5.2) | 76.02; 76.7; 76.7; 73.3 | 14.7; 14.5; 15.0; 14.7 | GDS ≥2; exclusion: MCI or AD | Ganglioside 660 μg/d for 8 weeks | Ganglioside 330μg/d for 8 weeks | Placebo | x | ||||||
| Kita et al., 2018 | 98; 48; 50 | 52.04 (4.76); 52.3 (4.3); 51.8 (5.2) | 85.16; 87.4; 83.0; | 14.3; 14.5; 14.1 | HDS‐R ≤20 | Whey peptide 1 g/d for 12 weeks | Placebo | x | x | ||||||
| Kwok et al., 2013 | 223; 111; 112 | 75.40 (5.81); 75.42 (5.82); 75.38 (5.83) | 85.2; 87.4; 83.0 | Secondary education; 21.1%; 18.9%; 23,2% | CMSS ≥3; Chinese MMSE ≥ 20 | 1.5 h cognitive training once a week for 12 weeks | Health‐related educational lectures | x | 9 m | ||||||
| Kwon et al., 2015 | 75; 30; 30; 15 | 40.16 (11.76); 42.5 (11.2); 37.6 (11.7); 40.6 (12.7) | 52.5; 56.7; 60.0; 26.7 | 100% high school or higher | GDS = 2; ≥1 symptoms of subjective memory impairment; CDR < 0,5; MMSE ≥25 | Herbal mixture 1200 mg/d for 8 weeks | Herbal mixture 600 mg/d for 8 weeks | Placebo | x | x | |||||
| Latorre Postigo et al., 2010 | 45; 15; 15; 15 | 66.9 (3.14); 67.8 (2.85); 65.73 (3.36); 67.4 (2.99) | 64.44; 73.3; 66.7; 53.3 | Percentage of persons with secondary education: 8.9; 13.3; 6.7; 6.7 | MEC >27; GDS <19; “yes” to ≥2 questions by Montejo et al. (1999) | Group memory training 10 times twice a week for 90 min | Wait list (control) | Health education (placebo) | x | 6 m | |||||
| Lautenschlager et al., 2008 | 170; 85; 85 | 68.65 (8.58); 68.6 (8.7); 68.7 (8.5) | 50.6; 49.4; 51.8 | 12.35; 12.1; 12.6 | MMSE ≥24; CDR < 1; able to walk for 6 minutes; no dementia diagnosis; "yes" to "Do you feel like your memory is getting worse?” | Physical activity + behavioural intervention | Education and usual care | x | x | x | x | x | 6, 12 & 18 m | ||
| Macpherson et al., 2012 | 56; 28; 28 | 71.1 (4.59); 71.9 (4.81); 70.3 (4.3) | 100; 100; 100 | 12.0; 11.9; 12.0 | MMSE ≥ 24; screening by Jorm et al. (1997); “yes” to “Do you feel like your memory is getting worse?” | Multivitamin supplementation (Swisse Women's Ultivite 50+ TM) once daily for 16 weeks | Placebo | x | |||||||
| Manenti et al., 2017 | 22; 11; 11 | 74.5 (5.9); 75.9 (7.1); 73.1 (4.7) | 63.64; 63.64; 63.64 | 9.9; 9.6; 10.3 | MMSE ≥ 27; normal 4 objective memory and cognitive performance; EMQ score > 1 SD above mean score of health older adults | Anodal transcranial current stimulation left lateral prefrontal cortex; 15 minutes; 1.5 mA | Sham stimulation | x | 30 d | ||||||
| McEwen et al., 2018 | 55; 26; 29 | 66.16 (4.2); 67.0 (5.1); 65.4 (3.0) | 65.5; 73.1 | n.a. | MoCA ≥ 23; MEM‐Q‐24 | Memory training + exercise (simultaneously) | Memory training + excercise (sequentially) | x | x | ||||||
| McNamara et al., 2018 | 76; 17; 19; 20; 20 | 67.96 (4.65); 69.0 (5.2); 68.0 (3.9); 68 (4.7); 67 (4.9) | 53.9; 41.2; 57.9; 65.0; 50.0 | 15.3; 15.6; 15.0; 16.1; 14.7 | CDR = 0; MoCA > 25 or normative CVLT | Fish oil (1.6 mg EPA, 0.8 mg DHA) daily for 24 weeks | 25 g blue berry powder daily for 24 weeks | Fish oil + blue berry powder daily for 24 weeks | Placebo | x | x | 6 m | |||
| Middleton et al., 2018 | 126; 32; 31; 31; 32 | 73.4 (6.0); 74.8 (6.1); 73.8 (5.7); 71.1 (5.5); 73.9 (6.3) | 65.0; 62.5; 58.1; 67.7; 62.5 | 16.4 (2.4); 16.7 (2.2); 16.8 (2.3); 15.6 (2.8); 16.3 (2.1) | Self‐reported subjective cognitive decline; | Aerobic exercise 60 min a day, 3 days a week for 12 weeks + computer‐based cognitive training 30 min a day, 3 times a week for 12 weeks | Computer‐based cognitive training 30 min a day, 3 times a week for 12 weeks + stretching/toning | Aerobic exercise 60 min a day, 3 days a week for 12 weeks + educational DVDs | Stretching/toning + educational DVDs | x | |||||
| Oh et al., 2018 | 53; 18; 19; 16 | 59.3 (5.0); 59.28 (5.1); 58.78 (5.0); 59.94 (5.2) | 52.8; 50.0; 52.6; 56.3 | 13.94; 14.22; 14.16; 13.38 | Subjective cognitive decline measured by the subjective memory complaints questionnaire | Smartphone‐based brain Anti‐aging and memory Reinforcement Training (SMART), 15‐20 min/d, 5 d/week for 8 weeks | Fit Brains ® (other cognitive training app), 15‐20 min/d, 5 d/week for 8 weeks | Wait‐list | x | ||||||
| Pereira‐Morales et al., 2018 | 40; 17; 12; 11 | 64.5 (4.8); 69.3 (4.8); 65.6 (7.2) | 90.00; 13.30; 09.09; 10.00 | 10.5 (4.1); 13.2 (3.1); 13.3 (3.2) | Subjective cognitive decline measured by the subjective memory complaints questionnaire | Integrated Psychostimulation Program, 8w, 90 minutes/day, 4 days/week | Computerized Cognitive Training, w, 90 minutes/day, 4 days/week | Control Group | x | x | x | ||||
| Pike et al., 2018 | 150; SCD: 53 | 73.8 (8.3) | 56.00 | 14.5 (4.2) | Level of SMD was determined using the self‐report Memory Assessment Clinics Questionnaire. | Semantic Association | Spaced Retrival | Control group | x | x | x | ||||
| Schwarz et al., 2018 5 | 28; 14; 14 | 69.0 (6.0); 70.0 (5.0) | 64.28; 64.28; 64.28; | 15.0 (2.0); 16.0 (4.0) | Subjective cognitive decline | Placebo Group | Spermidine Group | x | x | ||||||
| Scogin et al., 1985 | 47; 20; 27 | n.a. | n.a. | n.a. | Memory complaints were assessed by the Metamemory Questionnaire | Memory Training | Control Group | x | x | ||||||
| Small et al., 2006 | 17; 8; 9 | 54.0 (12.0); 53.0 (10.0) | 63.0; 67.0 | 18.0 (3.0); 17.0 (4.0) | All subjects had mild age‐related memory complaints measured by Memory Functioning Questionnaire (MFQ) | Health Lifestyle Program | Control Group | x | |||||||
| Smart et al., 2016 | 38; SCD: 15 | 69.60 (3.58) | 72.72 | 16.40 (2.69) | “Are you concerned or worried that you are experiencing significant decline in your thinking abilities, more than just normal aging?” In response to the question, “Have you ever been diagnosed with a psychological condition, such as depression or anxiety?” (yes/no), | Mindfulness Training | Psychoeducation | x | x | ||||||
| Solé‐Padullés et al., 2006 | 39; 20; 19 | 66.95 (9.43); 68.68 (7.78) | 66.66; 53.84 | n.a. | Memory complaints | rTMS | Control Group | x | |||||||
| Stoynova et al., 2019 | 26 | 68.96 (6.02) | 53.84 | n.a. | Memory complaints |
Transcranial direct current stimulation; pre‐session, 12 training sessions (three per week for 4 weeks), a post‐session 4 days |
Control Group | x | 3m | ||||||
| Tabue‐Teguo et al., 2018 | 1464, non‐frail: 799 | 74.41 (4.00) | 63.70 | n.a. | Reporting subjective memory complaints, but free from clinical dementia. | Cognitive Training with polyunsaturated fatty acids | Polyunsaturated fatty acids | Cognitive Training | Placebo | x | x | ||||
| Tsai et al., 2008 | 25; 14; 11 | 69.44; 68.71; 70.36 | n.a. | n.a. | Self‐reported memory complaints | Cognitive Training | Cognitive Stimulation | x | x | ||||||
| Valentijn et al., 2005 | 149; 39; 40; 38 | 68.56 (7.43); 69.32 (7.77); 68.07 (6.58); 68.30 (8.03) | 70.0; 63.0; 63.0 | 3.81 (2.00); 3.83 (1.96); 3.74 (1.84); 3.86 (2.24)6 | Self‐reported subjective memory complaints | Memory Training (individual) | Memory Training (collective) | Control Group | x | x | 4m | ||||
| van Hooren et al., 2007 | 69; 37; 30 | 62.76 (5.62); 62.35 (5.39); 63.27 (5.95) | 82.00; 83.00 | 3.66 (1.86); 3.57 (1.76); 3.77 (2.01)6 | Self‐reported memory complaints | Goal Management Training, 6w | Control Group | x | x | ||||||
| Wahjoepramono et al., 2016 | 44; 22; cross over | n.a. | Only male participants | n.a. | Self‐reported memory complaints | Testosterone Treatment, 24 weeks | Placebo Group | x | x | x | |||||
| Watson et al., 2019 | 41; 25; 16 | 60.88 (5.79); 59.56 (5.69) | 56.00; 69.00 | n.a. | Self‐reported memory complaints | Liraglutide treatment, 12 weeks | Placebo Group | x | x | ||||||
| Wirth et al., 2018 5 | 28; 14; 14 | 69.90 (5.33); 70.4 (5.2); 69.4 (5.6) | 64.28; 64.28; 64.28; | 15.65 (2.85); 16.0 (3.70); 15.3 (1.70) | Presence of subjective cognitive complaints for at least 6 months and self‐reported associated concerns (worries) | Spermidine Treatment | Placebo Group | x | |||||||
| Youn et al., 2019 | 201; 112; 89 | 69.57 (4.89); 69.93 (5.10); 69.11 (4.60) | 57.14; 67.42 | 10.10 (3.72); 10.01 (3.89); 10.09 (3.52) | The diagnosis of SMC took place through a questionnaire validated for the Korean population consisting of 14 items with dichotomous “yes” or “no” answers and a cut‐off value of >5 | Multi‐strategic memory training of 10 weekly 90‐minutes sessions, | Control Group | x | x | x | |||||
| Youn et al., 2011 | 32; 16; 16 | 68.88 (4.00); 68.75 (4.60); 69.00 (3.45) | 62.50; 56.25 | 9.88 (2.82); 10.19 (3.19); 09.56 (2.45) | Subjective memory complaints | Multi‐strategic memory training of 10 weekly 90‐min sessions | Control Group | x | x |
x |
|||||
| Zhu et al., 2016 | 98; 47; 51 | 66.57 (10.51); 69.68 (9.52); 64.03 (10.73) | 68.40; 70.20; 66.70 | n.a. | Subjective hypomnesis/forgetfulness/memory loss (SML) and subjective attention/concentration deficits (SAD), were screened using a self‐administered 5‐point scale (1 = no symptoms or occasional slight symptoms complaints; 2 = slight/mild symptom complaints; 3 = moderate severe symptom complaints; 4 = severe symptom complaints; 5 = very severe symptom complaints) | BrainPower Advanced Capsule Treatment | Placebo Group | x | x | ||||||
| Zuniga et al., 2016 | 179 | n.a. | n.a. | n.a. | Self‐perceived memory complaints | Walking Group, 12m | Flexiblity, Toning, and Balance | x | x | x | |||||
Abbreviations: AD, Alzheimer's disease; AD8, Ascertainment of Dementia 8; ADRDA, Alzheimer's Disease and Related Disorders Association; BAI, Beck's Anxiety Inventory; BDI, Beck's Depression Inventory; CDR, Clinical Dementia Rating; CDRS, Chinese Dementia Rating Scale; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CGDS‐SF, short form Chinese Geriatric Depression Scale; CMSS, Chinese Memory Symptoms Scale; CVLT, California Verbal Learning Test; d, days; DHA, docosahexaenoic acid; EMC, Everyday Memory Checklist; EMQ, Everyday Memory Questionnaire; EPA, eicosapentaenoic acid; GDS, Global Deterioration Scale; HDS‐R, Hierarchic Dementia Scale Revised; I, intervention; IADL, Instrumental Activities of Daily Living; m, month; MDD, major depressive disorder; MDRS, Mattis Dementia Rating Scale; MCI, mild cognitive impairment; MEC, Mini‐Examen Cognoscitivo (Spanish version of the Mini‐Mental State Examination); MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; PRMQ, prospective and retrospective memory questionnaire; QoL, quality of life; rTMS, repetitive transcranial magnetic stimulation; SCD, subjective cognitive decline; SMD, standardized mean difference; TICS‐M, modified Telephone Interview for Cognitive Status; w, week
n total in study, n for I1, n for I2 and so on.
Inclusion criteria contained MCI, but the results only report subjects with SCD.
Probably the same sample.
Based on the information provided it remained unclear which test was used (Memory Function Questionnaire or Subjective Memory Questionnaire)
Probably the same sample as Schwarz et al.
Education was measured with a scale rather than in years of education.
Probably the same sample
3.3. NMA: primary outcome: memory
A total of N = 21 studies provided data on memory outcomes; however, only n = 11 studies used a sufficient SCD definition and were therefore included in the analyses, 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 leading to 17 pairwise comparisons and 6 different treatments in the NMA (see Figure 2 for the network graph on memory). Several interventions could not be included (e.g., all pharmacological studies, repetitive transcranial brain stimulation, and stretching) because they did not use and/or report current criteria for SCD. Two studies that assessed effects of matured hop bitter acids (MHBA) and whey peptide treatment provided sufficient data, but could not be integrated in the network as they were not linked to the network. 29 , 30 As the NMA in Figure 3 shows, education programs (standardized mean difference [SMD]: 2.64, 95% CI: 1.17 to 4.10), physical training (SMD: 2.71, 95% CI: 0.03 to 5.38), and cognitive training (SMD: 1.24, 95% CI: 0.27 to 2.21) were significantly superior to the control group. P‐score ranking displays education programs as the optimal treatment regarding improvement of memory, followed by physical training and cognitive training. The P‐scores measure the degree of certainty that one treatment is better than another treatment, averaged over all competing treatments. 31 , 32
FIGURE 2.
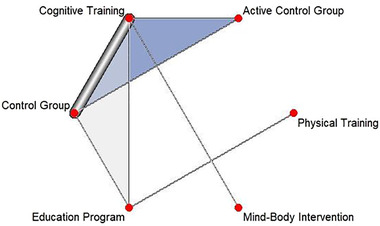
Network of analyzed comparisons for the outcome memory. Each circle corresponds to a regimen included in the analysis. Each line represents direct comparisons between these regimens, with the thickness of the line corresponding to the number of available direct within‐trial comparisons. Regimens are described in the supporting information. Blue shaded regions correspond to studies with multiple comparisons
FIGURE 3.
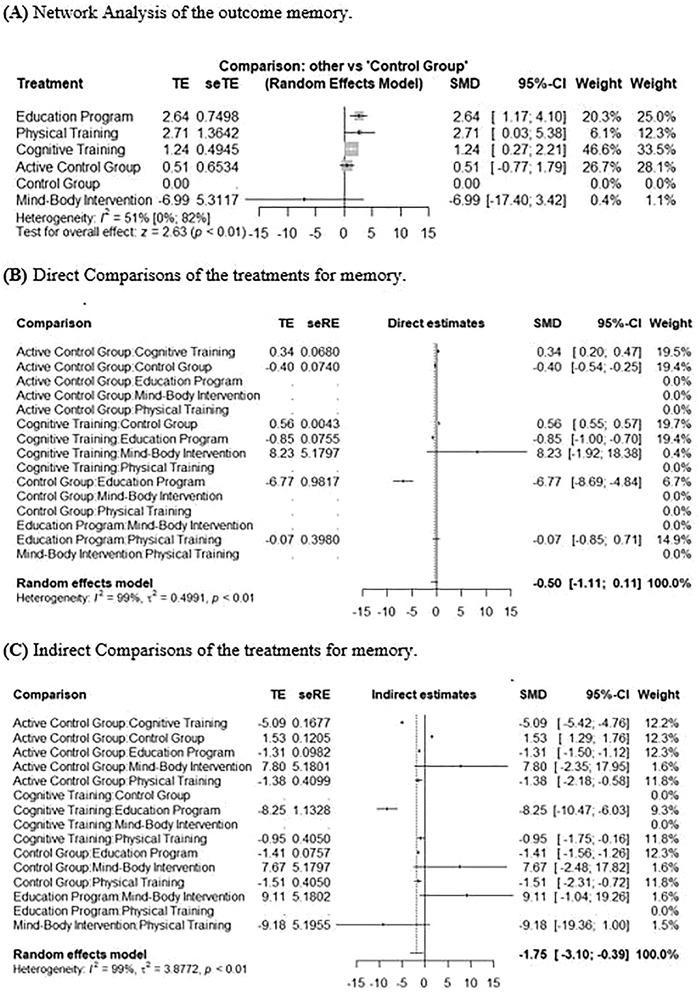
Network meta‐analysis for the outcome memory. A, Network analysis of the pooled data of five treatments compared to each other and the control group reporting memory. Treatments were ranked through P‐scores. The education program (standardized mean difference [SMD]: 2.64, 95% confidence interval [CI]: 1.17 to 4.10), physical training (SMD: 2.71, 95% CI : 0.03 to 5.38), and cognitive training (SMD: 1.24, 95% CI : 0.27 to 2.21) were significantly superior to the control group. B, Meta‐analysis of direct comparisons for memory. C, Meta‐analysis of indirect comparisons for memory. seRE, standard error of regression estimate; seTE, standard error of treatment estimate; TE, estimate of treatment effect
Regarding heterogeneity, I² was 51% in the analysis, with 95% CI ranging from 0% to 82%, indicating a moderate heterogeneity, 16 Tau² was 0.70, 95% CI from 0.0 to 37.56. Figure S1 in the supporting information shows the assessment of inconsistencies between direct and indirect comparisons.
To assess publication bias, a comparison‐adjusted funnel plot was conducted, 33 demonstrating no asymmetry in the present data (Egger's test P = .31; see also funnel plot, Figure 4).
FIGURE 4.
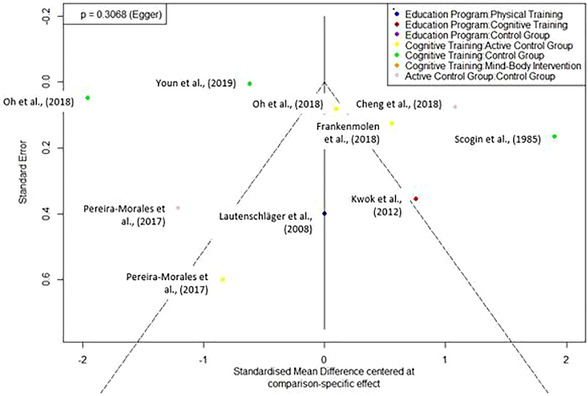
Funnel plot for studies comparing interventions on memory. A comparison‐adjusted funnel plot for the different treatment comparisons for the outcome memory. Egger's regression test for funnel plot asymmetry was not significant (P = 0.31)
3.4. Secondary outcome: global cognition
We included n = 5 studies in the analysis, 19 , 21 , 23 , 28 , 34 with seven pairwise comparisons of n = 4 treatments (cognitive training, education program, physical training, control group). The network graph is depicted in Figure S2 in the supporting information. As the NMA in Figure 5 shows, cognitive training (SMD: 1.32, 95% CI: –4.11 to 6.76) was superior to the control group, even though not significantly. P‐score ranking displays cognitive training as the optimal treatment regarding improvement in global cognition, followed by the control group. Education programs (SMD: –10.85, 95% CI: –18.42 to –3.28) and physical trainings (SMD: –12.14, 95% CI: –23.79 to –0.49) perform worse than the control group.
FIGURE 5.
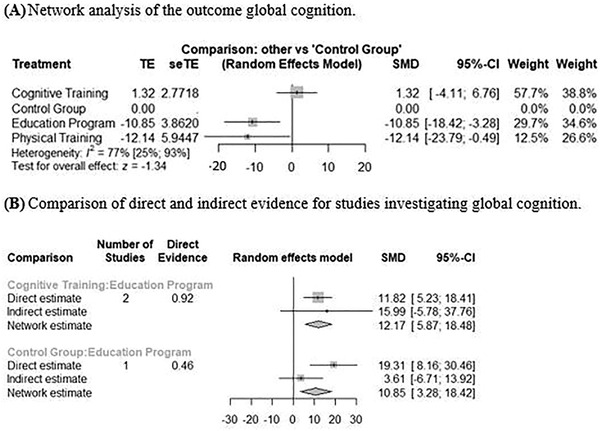
Network analyses of the secondary outcome global cognition. A, Network analysis of the pooled data of four treatments compared to each other and the control group reporting global cognition. Treatments were ranked through P‐scores. B, Meta‐analysis of direct and indirect comparisons for global cognition. CI, confidence interval; seTE, standard error of treatment assessment; SMD, standardized mean difference; TE, estimate of treatment effect
Heterogeneity was considerable with I² = 77% (95% CI: 25% to 93%) and Tau² = 52.87 (95% CI: 0.00 to 455.39). Figure S3 in the supporting information shows the assessment of inconsistencies between direct and indirect comparisons.
3.5. Secondary outcomes: quality of life and adverse events
No overall statement was possible for both outcomes due to the fact that the data were too heterogeneous and measurements took place at different assessment time points.
3.6. Risk of bias in included studies
Risk of bias assessment of the included studies is illustrated in Table 2. The overall risk of bias in the included studies is rated as low risk in n = 2 studies and high risk in n = 12 studies, leaving n = 42 studies with a medium overall risk of bias. Most studies showed a medium or high risk of bias in the domain “Risk of bias in selection of the reported results,” as they did not include a preregistration or clinical trial registration number.
TABLE 2.
Risk of bias assessment for included studies
| Domain 1: RoB arising from the randomization process | Domain 2: RoB due to deviations from the intended interventions | Domain 3: Missing outcome data | Domain 4: RoB in measurement of the outcome | Domain 5: RoB in selection of the reported results | Overall RoB | |
|---|---|---|---|---|---|---|
| Andrewes et al. (1996) | ||||||
| Ban et al. (2018) | ||||||
| Barnes et al. (2013) | ||||||
| Beck et al., (2016) | ||||||
| Ben‐Itzah et al., (2008) | ||||||
| Boa Sorte Silva, Gill, Owen et al., (2018) | ||||||
| Boa Sorte Silva, Gill, Gregory et al., (2018) | ||||||
| Brautigam et al. (1998) | ||||||
| Chan et al., (2017) | ||||||
| Cheng et al. (2018) | ||||||
| Cinar & Sahiner, (2020) | ||||||
| Cohen‐Mansfield et al., (2015) | ||||||
| Epperson et al., (2011) | ||||||
| Frankenmolen et al., (2018) | ||||||
| Fukoda et al., (2020) | ||||||
| Heath et al., (2016) | ||||||
| Hong et al., (2020) | ||||||
| Hoogenhout et al., (2012) | ||||||
| Hooper et al., (2017) | ||||||
| Hsieh et al., (2019) | ||||||
| Innes et al., (2018) | ||||||
| Innes et al., (2016) | ||||||
| Jeon et al., (2016) | ||||||
| Kita et al., (2018) | ||||||
| Kwok et al., (2012) | ||||||
| Kwon et al., (2015) | ||||||
| Latorre Postigo et al., (2013) | ||||||
| Lautenschlager et al., (2008) | ||||||
| Macpherson et al., (2012) | ||||||
| Manenti et al., (2017) | ||||||
| McEwen et al., (2018) | ||||||
| McNamara et al., (2018) | ||||||
| Middleton et al., (2018) | ||||||
| Oh et al., (2018) | ||||||
| Pereira‐Morales et al., (2018) | ||||||
| Pike et al., (2018) | ||||||
| Schwarz et al., (2018) | ||||||
| Scogin et al., (1985) | ||||||
| Small et al., (2006) | ||||||
| Smart et al., (2016) | ||||||
| Solé‐Padullés et al., (2006) | ||||||
| Stoynova et al., (2019) | ||||||
| Tabue‐Teguo et al., (2018) | ||||||
| Tsai et al., (2008) | ||||||
| Valentijn et al., (2005) | ||||||
| Van Hooren et al., (2007) | ||||||
| Wahjoepramono et al., (2016) | ||||||
| Watson et al., (2019) | ||||||
| Wirth et al., (2018) | ||||||
| Youn et al., (2011) | ||||||
| Youn et al., (2019) | ||||||
| Zhu et al., (2016) | ||||||
| Zuniga et al., (2016) |
Note. Red color indicates a high risk of bias, yellow color indicates a medium risk of bias, green color indicates a low risk of bias, assessed with the Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2).
Abbreviations: RoB, Risk of Bias.
3.7. Sensitivity analyses
Sensitivity analyses including all studies with sufficient data regardless of whether the study provided an adequate SCD definition were conducted for all outcomes (Figures S4‐S9 in the supporting information). This analysis also included pharmacological and other interventions that could not be included in the primary analysis.
Regarding memory, n = 21 studies were included with 41 pairwise comparisons of n = 15 treatments. Education programs were still rated as the most effective treatment program (SMD: 2.57, 95% CI: 1.50 to 3.65), followed by testosterone treatment (SMD: 2.45, 95% CI: –0.33 to 5.24; even though results of this study have to be interpreted carefully as only male participants were included and the study had a cross‐over design) and repetitive transcranial magnetic stimulation (SMD: 2.23, 95% CI: –0.26 to 4.72). Liraglutide treatment (a medication that was originally used to treat diabetes and obesity; SMD: –1.22, 95% CI: –4.18 to 1.75) and mind–body interventions (SMD: –6.71, 95% CI: –17.06 to 3.65) were less effective than the control group.
The ranking of treatments for global cognition was largely consistent with the main analyses. Cognitive stimulation (SMD: 2.31, 95% CI: –0.89 to 5.52), testosterone treatment (SMD: 1.30, 95% CI : –2.10 to 4.70), and cognitive training plus polyunsaturated fatty acid treatment (SMD: 1.29, 95% CI : –1.74 to 4.33) were the most effective; education programs (SMD: –3.63, 95% CI : –5.98 to –1.28) and physical training (SMD: –4.29, 95% CI: –8.3 to –1.49) were less effective than the control group.
In the network analysis investigating quality of life, n = 5 studies were included, including n = 5 treatments (cognitive training, education program, physical training, active control group, control group) in seven pairwise comparisons. The active control group was ranked as most successful in improving quality of life in participants with SCD (SMD: 3.62, 95% CI : –2.72 to 9.96), followed by cognitive training (SMD: 2.01, 95% CI : –1.93 to 5.95) and physical training (SMD: 0.76, 95% CI: –5.08 to 6.60). Education programs were ranked as less effective than the control group (SMD: 0.00, 95% CI: –3.31 to 3.32).
We performed an additional sensitivity analysis including only studies with low and medium risk of bias and an adequate SCD definition. Only n = 2 studies had to be excluded from the analyses due to low‐rated study quality; 23 , 34 results are displayed in Figure S10A‐C in the supporting information.
4. DISCUSSION
To the best of our knowledge, this is the first NMA that investigated the effectiveness of pharmacological and non‐pharmacological interventions for SCD while using the most recent reporting standards in the field. We identified 56 eligible studies that had included 4692 participants. A total of 16 different interventions were investigated in these studies. N = 17 studies that had used proper SCD criteria were included in the network meta‐analyses. Overall risk of bias in these studies was medium to high, indicating overall low quality of evidence in this field. With regard to our primary outcome measure, education programs, physical interventions and cognitive trainings were the most effective interventions for improving memory performance in SCD. Cognitive training was most effective in improving global cognition, followed by education programs. Due to a high heterogeneity in the included studies and a lack of sufficient data, effects on quality of life and adverse events could not be assessed. Results of the sensitivity analyses that included all studies regardless of their SCD definition were largely consistent with the primary analysis and revealed additional information on the potential effectiveness of pharmacological and other interventions that could not be included in the main analysis due to a lack of proper SCD definition. Specifically, education programs were still ranked as most effective for improving memory in SCD, followed by testosterone treatment and repetitive transcranial magnetic stimulation. Liraglutide treatment and mind–body interventions were less effective than passive control groups.
Regarding our primary outcome (memory), education programs were identified as the most effective intervention for people with SCD in both our main and sensitivity analyses. Importantly, these programs provided the participants with information about different healthy lifestyle strategies associated with lowering the risk of dementia (e.g., dietary modification, relaxation techniques), but also mnemonic strategies. 19 , 21 , 22 , 23 , 27 The current study was not designed to identify the specific active components underlying the superior effectiveness of these education programs. Tentatively, however, it is conceivable that making available several potential interventions to the participants and providing additional information and guidelines may have increased self‐efficacy and motivation to address the self‐perceived cognitive impairment. 27
Physical interventions were ranked as the second most effective treatment to improve memory performance in SCD. This finding is in line with evidence from epidemiological, cross‐sectional, and neuroimaging studies showing that moderate‐intensity physical exercise can be beneficial to cognitive health, including memory, even though evidence from RCTs is still mixed. 35 Exercise‐induced molecular cascades, which affect neural plasticity, may play an import role in explaining the effects of physical interventions on cognition and especially memory by promoting brain vascularization, hippocampal neurogenesis, and other neuro‐functional changes. 36 , 37 These beneficial physiological effects on brain function are likely responsible for the observed improvement of memory function in SCD, which are in line with those reported previously in other populations.
Cognitive training was ranked as the third most effective intervention for improving memory functions in SCD and also most effective for improving global cognition. The latter result is largely consistent with previous meta‐analytic studies on non‐pharmacological interventions for SCD. For example, Smart et al. 4 demonstrated that such interventions improved cognitive outcomes with a small effect size and those were more pronounced compared to other intervention types. However, because cognitive training usually targets specific cognitive domains and transfer effects are often limited to closely related tasks, 38 , 39 effects are likely stronger for measures of global cognition compared to memory.
Of all the reviewed studies that were initially considered eligible in the systematic review, the majority (37 of 54) had to be excluded from the NMA due to the lack a proper SCD definition, a problem that earlier reviews had also discussed, 4 resulting in a total of n = 17 studies that were included in the present NMA.
Definitions in these studies ranged from asking a single question (e.g., “Do you have the feeling that your memory gets worse?”) to only assuming that participants have SCD (e.g., advertising a study for adults with SCD but never asking if they feel that their memory gets worse). It is important to acknowledge that many of the included studies were published before SCD was formally defined in the literature 9 and until today, variations of these criteria have been used. 40 Clear definitions and diagnostic criteria are of utmost importance to ensure comparability among studies. In the present review and NMA, we therefore decided to only include studies that adhered to the currently most widely used definition of SCD. Nonetheless, it is important to note that the results of the sensitivity analysis that included all studies were largely consistent with those reported in the main analysis. Heterogeneity assessment for our primary outcome memory shows moderate heterogeneity, which is expected when comparing different interventions in a NMA. The outcome global cognition, however, showed substantial heterogeneity (I² = 77%). Yet, results of our sensitivity analyses that included only studies with low or medium risk of bias decreased this heterogeneity, without affecting the effectiveness rankings of the interventions. For further details on the sensitivity analyses and results, please see Appendix A and Figure S10 in the supporting information.
Unsurprisingly, interventions that have been shown to be effective for improving memory and cognition in MCI and AD (e.g., cognitive or physical training) are also effective for individuals with SCD. However, our results also demonstrate for the first time that education programs, which are often used as a control rather than experimental group, are overall most effective in individuals with SCD. Therefore, future research into these programs is warranted to identify the key elements by which these programs improve memory and cognitive functions in this particular population.
The present systematic review and NMA also identified several important shortcomings in this field: Data on patient‐related outcomes like quality of life, depression, anxiety, and adverse events were often not assessed and/or reported. This is rather surprising because these outcomes are highly relevant in a population that has no objective cognitive impairment but suffers from insecurity and the fear of cognitive decline. Future research should include these participant‐related outcomes to assess if specific interventions are suited to improve quality of life and psychological well‐being in individuals with SCD. Moreover, overall risk of bias judgment of the investigated studies was rather poor and most of the studies were rated as moderate and high risk of bias. In most instances, this was because studies were not pre‐registered and/or pre‐registration was not clearly stated in the paper. To increase transparency and to reduce the possibility of publication bias, pre‐registration of studies is strongly encouraged for future studies.
This is the first systematic review and NMA investigating all possible pharmacological and non‐pharmacological interventions for SCD. Identifying suitable intervention options is of utmost importance because these interventions may not only improve participants’ memory and global cognition, but also help in improving quality of life and overall well‐being. It is highlighted that more research on the effective elements of education programs, which were ranked as most effective for improving memory in our NMA, is required. Our analysis also identifies a number of limitations in current SCD intervention research that need to be addressed in the future, including use of proper SCD definitions, improvement of trial quality, and inclusion of patient‐related outcomes.
5. CONCLUSIONS
SCD may provide a unique window for early interventions aimed at preventing cognitive decline before pathological impairment may manifest or even to prevent progression to dementia. The current review and NMA shows that education programs, physical interventions, and cognitive training were most effective for improving memory in participants with SCD and that cognitive training was most effective in improving global cognition. We also identified a lack of studies that investigated quality of life and adverse events, even though such participant‐related outcomes are of utmost importance in individuals with subjectively perceived cognitive impairment. These outcomes need to receive more attention in future research. Frequently, current research did not use proper SCD definitions and several shortcomings were identified, including lack of study pre‐registration and low methodological transparency.
In sum, our findings suggest that education programs as part of preventive care in SCD have potential to empower individuals to take proactive steps in support of their own cognitive and emotional well‐being, which in turn may decrease future burdens on healthcare systems. Important shortcomings in SCD intervention research were identified that need to be addressed in future studies.
CONFLICTS OF INTEREST
Agnes Flöel is co‐author of one of the reviewed studies (Wirth et al.); however, she was not involved in data extraction and quality assessment of this particular study to avoid a potential conflict of interest. All other authors do not have any conflict of interest.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Roheger M, Hennersdorf X‐S, Riemann S, Flöel A, Meinzer M. A systematic review and network meta‐analysis of interventions for subjective cognitive decline. Alzheimer's Dement. 2021;7:e12180. 10.1002/trc2.12180
REFERENCES
- 1. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020;19:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol 2017;13:369‐396. [DOI] [PubMed] [Google Scholar]
- 3. Lin Y, Shan P‐Y, Jiang W‐J, et al. Subjective cognitive decline: preclinical manifestation of Alzheimer's disease. Neurol Sci 2019;40:41‐49. [DOI] [PubMed] [Google Scholar]
- 4. Smart CM, Karr JE, Areshenkoff CN, et al. Non‐pharmacologic interventions for older adults with subjective cognitive decline: systematic review, meta‐analysis, and preliminary recommendations. Neuropsychol Rev 2017;27:245‐257. [DOI] [PubMed] [Google Scholar]
- 5. Bhome R, Berry AJ, Huntley JD, et al. Interventions for subjective cognitive decline: systematic review and meta‐analysis. BMJ Open 2018;8:e021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dias S, Caldwell DM. Network meta‐analysis explained. Arch Dis Child Fetal Neonatal Ed 2019;104:F8‐F12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs 2003;12:77‐84. [DOI] [PubMed] [Google Scholar]
- 9. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salthouse TA. Effects of age and ability on components of cognitive change. Intelligence 2013;41:501‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thomas, J , Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane 2019; 2019. [Google Scholar]
- 12. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 13. Rouse B, Chaimani A, Li T. Network meta‐analysis: an introduction for clinicians. Intern Emerg Med 2017;12:103‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rücker G, Krahn U, König J, et al. Netmeta:Network Meta‐Analysis Using Frequentist Methods. R packageversion 1.1‐0.; 2019. [Google Scholar]
- 15. Schwarzer G, Carpenter JR, Rücker G. Meta‐Analysis with R. Cham: Springer, 2015. [Google Scholar]
- 16. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT (ed.) Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell; 2008:243‐296. [Google Scholar]
- 17. Higgins JPT (ed). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008. [Google Scholar]
- 18. Chan AS, Cheung WK, Yeung MK, et al. A Chinese chan‐based mind‐body intervention improves memory of older adults. Front Aging Neurosci 2017;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng CP‐W, Chiu‐Wa Lam L, Cheng S‐T. The effects of integrated attention training for older chinese adults with subjective cognitive complaints: a randomized controlled study. J Appl Gerontol 2018;37:1195‐1214. [DOI] [PubMed] [Google Scholar]
- 20. Frankenmolen NL, Overdorp EJ, Fasotti L, et al. Memory strategy training in older adults with subjective memory complaints: a randomized controlled trial. J Int Neuropsychol Soc 2018;24:1110‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong YJ, Lee JH, Choi EJ, et al. Efficacies of cognitive interventions in the elderly with subjective cognitive decline: a prospective, three‐arm, controlled trial. J Clin Neurol 2020;16:304‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwok TCY, Bai X, Li JCY, et al. Effectiveness of cognitive training in Chinese older people with subjective cognitive complaints: a randomized placebo‐controlled trial. Int J Geriatr Psychiatry 2013;28:208‐215. [DOI] [PubMed] [Google Scholar]
- 23. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027‐1037. [DOI] [PubMed] [Google Scholar]
- 24. Oh SJ, Seo S, Lee JH, et al. Effects of smartphone‐based memory training for older adults with subjective memory complaints: a randomized controlled trial. Aging Ment Health 2018;22:526‐534. [DOI] [PubMed] [Google Scholar]
- 25. Pereira‐Morales AJ, Cruz‐Salinas AF, Aponte J, et al. Efficacy of a computer‐based cognitive training program in older people with subjective memory complaints: a randomized study. Int J Neurosci 2018;128:1‐9. [DOI] [PubMed] [Google Scholar]
- 26. Scogin F, Storandt M, Lott L. Memory‐skills training, memory complaints, and depression in older adults. J Gerontol 1985;40:562‐568. [DOI] [PubMed] [Google Scholar]
- 27. Small GW, Silverman DHS, Siddarth P, et al. Effects of a 14‐day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry 2006;14:538‐545. [DOI] [PubMed] [Google Scholar]
- 28. Youn J‐H, Ryu S‐H, Lee J‐Y, et al. Brain structural changes after multi‐strategic metamemory training in older adults with subjective memory complaints: a randomized controlled trial. Brain Behav 2019;9:e01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukuda T, Obara K, Saito J, et al. Effects of hop bitter acids, bitter components in beer, on cognition in healthy adults: a randomized controlled trial. J Agric Food Chem 2020;68:206‐212. [DOI] [PubMed] [Google Scholar]
- 30. Kita M, Obara K, Kondo S, et al. Effect of supplementation of a whey peptide rich in tryptophan‐tyrosine‐related peptides on cognitive performance in healthy adults: a randomized, double‐blind, placebo‐controlled study. Nutrients 2018;10:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rücker G. Network meta‐analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312‐324. [DOI] [PubMed] [Google Scholar]
- 32. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta‐analysis. PLoS ONE 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Çinar N, Şahiner TAH. Effects of the online computerized cognitive training program BEYNEX on the cognitive tests of individuals with subjective cognitive impairment and Alzheimer's disease on rivastigmine therapy. Turk J Med Sci 2020;50:231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelly ME, Loughrey D, Lawlor BA, et al. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta‐analysis. Ageing Res Rev 2014;16:12‐31. [DOI] [PubMed] [Google Scholar]
- 36. Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 2013;37:2243‐2257. [DOI] [PubMed] [Google Scholar]
- 37. Voss MW, Soto C, Yoo S, et al. Exercise and hippocampal memory systems. Trends Cogn Sci 2019;23:318‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly ME, Loughrey D, Lawlor BA, et al. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta‐analysis. Ageing Res Rev 2014;15:28‐43. [DOI] [PubMed] [Google Scholar]
- 39. Karbach J, Verhaeghen P. Making working memory work: a meta‐analysis of executive‐control and working memory training in older adults. Psychol Sci 2014;25:2027‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rabin LA, Smart CM, Crane PK, et al. Subjective Cognitive decline in older adults: an overview of self‐report measures used across 19 International Research Studies. J Alzheimers Dis 2015;48(Suppl 1):S63‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


