Abstract
In this narrative review we summarize the literature and provide updates on recent studies of air pollution exposures and child lung function and lung function growth. We include exposures to outdoor air pollutants that are monitored and regulated through air quality standards, and air pollutants that are not routinely monitored or directly regulated, including wildfires, indoor biomass and coal burning, gas and wood stove use, and volatile organic compounds. Included is a more systematic review of the recent literature on long-term air pollution and child lung function since this is an indicator of future adult respiratory health and exposure assessment tools have improved dramatically in recent years. We present “summary observations” and “knowledge gaps.” We end by discussing what is known about what can be done at the individual/household, local/regional, and national level to overcome structural impediments, reduce air pollution exposures and improve child lung function. We found a large literature on adverse air pollution effects on children’ lung function level and growth; however, many questions remain. Important areas needing further research include: whether early life effects are fixed or reversible; and what are windows of increased susceptibility, long-term effects of repeated wildfire events, and effects of air quality interventions.
Keywords: Children, lung function, FEV1, FVC, air pollution, indoor pollution, wildfire, gas stoves, biomass
INTRODUCTION
The adverse acute effects of ambient air pollution from traffic, fossil fuel combustion, industrial facilities, and other sources on child respiratory health have been well-documented in decades of research. This research has directly informed national ambient air quality standards for ground-level ozone (O3), fine particulate matter (PM2.5) and other criteria pollutants regulated by the United States Environmental Protection Agency (US EPA)1 per the Clean Air Act, which mandates that the standard be designed to protect sensitive subpopulations including children. In the 1980s and 90s child lung function was assessed as a subclinical marker of acute pollution health effects in panel studies of children with and without asthma, and short-term decrements in lung function were observed with short-term increases in ambient air pollution.2–4 These early panel studies contributed biologic plausibility to the time-series studies of clinically-sensitive children that demonstrated short-term increases in air pollution to related with increases in adverse clinical events (e.g., urgent care visits and hospitalization for respiratory illnesses).5, 6 Air pollution and lung function studies also have direct clinical import for stepwise management of childhood asthma, since level of forced expiratory volume in one second (FEV1) and FEV1/ forced vital capacity (FVC) percent predicted are used in assessment of level of asthma severity.7
In addition to studies showing acute effects of air pollution on lung function, a growing number of longitudinal studies have suggested adverse effects of early-life or life-time long-term air pollution exposures on lung function growth during childhood. In contrast to lung function level, which is assessed at a single point in time, lung function growth is assessed based on repeated measurements with the difference between first and last lung function measure (size of increase) often used as a proxy for lung function development. As a body of evidence, these studies suggest that long-term childhood air pollution exposures may shift the entire population distribution of childhood lung function and lung function growth downward. This is portrayed in the hypothetical child lung function growth trajectories (Figure 1),8–10 where air pollution exposures in the prenatal period or during early childhood could result in reduced lung function growth (Figure 1: scenarios b or d). Persistent or additional exposures could compound the injury by increasing the risk of early, and potentially steeper lung function decline after the plateau in early adulthood (Figure 1: scenarios c or e). The consequence of either scenario could be lung function that is below the threshold for chronic obstructive pulmonary disease (COPD; post-bronchodilator FEV1/FVC below 0.70) in later adulthood.
FIGURE 1.
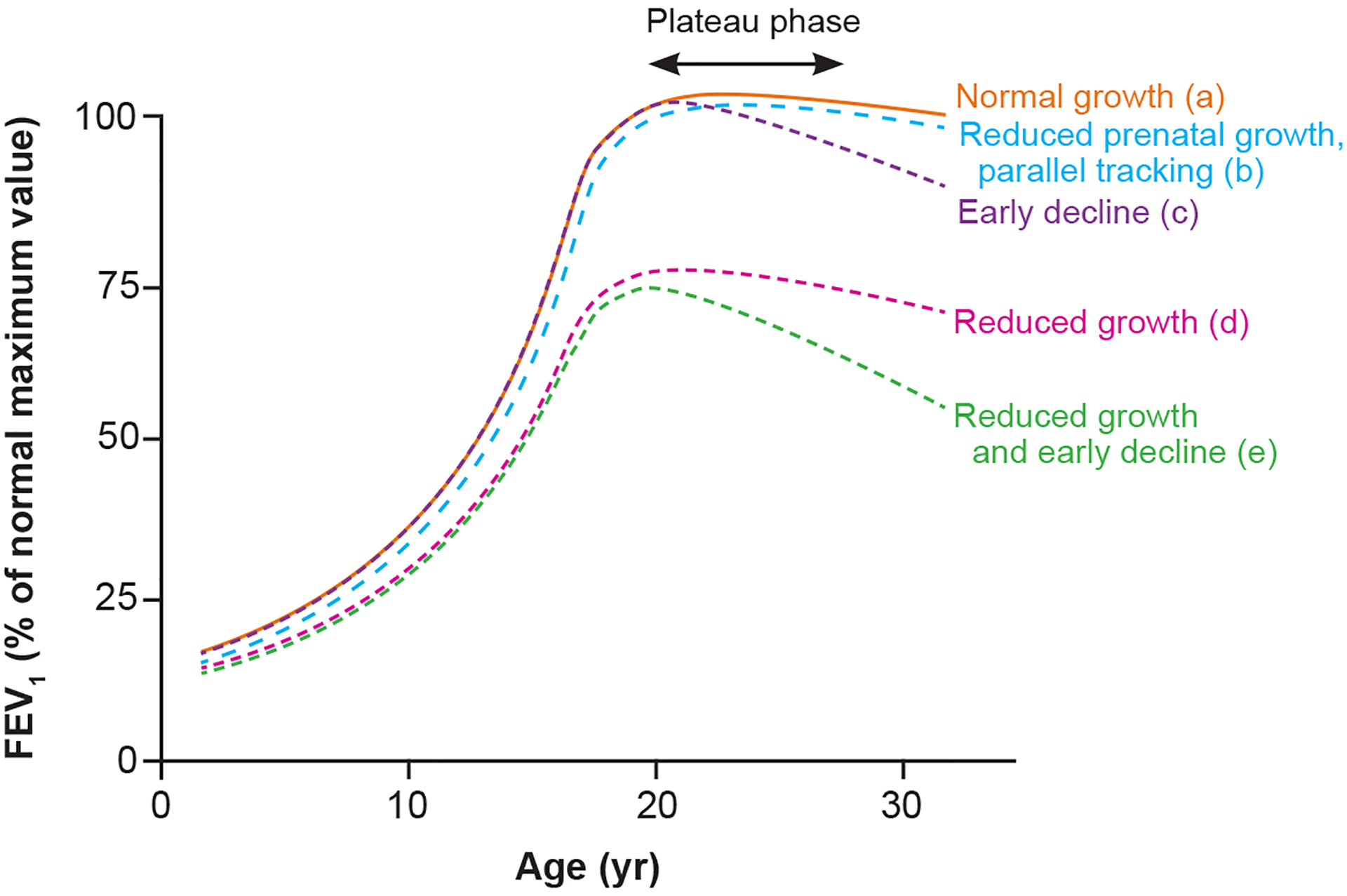
Hypothetical lung function trajectories depicting normal and reduced growth and early decline (figure adapted from McGeachie et al9).8, 10 (a) Normal pattern of lung function growth. (b) Reduced prenatal growth with postnatal growth tracking at a fixed percent predicted lower than normal. (c) Normal growth until late teen years, when peak lung function growth is reduced, and lung function begins to decline early. (d) Reduced growth and (e) reduced growth with early decline. Both (d) and (e) can result in levels of lung function consistent with diagnosis of chronic obstructive lung disease.
We provide a narrative review of air pollution exposures and their associations with child lung function and lung function growth in general pediatric populations. We primarily focus on ambient air pollutants that are primary and secondary products of fossil fuel combustion and regulated by the US EPA (e.g., particulate matter <2.5 μm [PM2.5] and <10 μm [PM10], nitrogen dioxide [NO2], ozone [O3]), reporting results separately for short- and long-term exposures. We include a more systematic review of the recent literature on long-term exposure to ambient air pollution and child lung function. We also include separate summaries of the literature on air pollution that is not currently regulated or covered by the Clean Air Act but may have adverse child lung function effects. Specifically, we focus on air pollution from wildfires, indoor biomass and coal burning, gas and wood stove use, and volatile organic compounds (VOCs) from household products. Exposure data and research on child lung function outcomes are much sparser for these pollutants compared with the large body of evidence on the outdoor criteria pollutants. For each section we provide boxed statements of “summary observations” and “knowledge gaps”.
While some families may have a few options to improve their children’s health by reducing air pollution exposures at home and during the school day, for consequential exposure reduction across the population structural changes and regulations at national, regional, and local levels are required. As neighbors and citizens, individual families can influence structural change and regulation, for example by advocating for electric buses, traffic-free zones, wildfire mitigation strategies, or public housing upgrades (e.g., ventilation, new stoves)—we acknowledge, however, that not all are empowered to do so. In the final segment of this review, we share summary observations (and knowledge gaps) about what can be done at the individual/household, local/regional, and national level to reduce air pollution exposures and improve child lung function, thereby improving child and subsequent adult respiratory health. Additionally, suggestions for future research are made.
We briefly describe our review methods. We searched the database PubMed with combinations of the following search terms: “‘lung function,” “pulmonary function,” “pollution,” “pollutant,” “traffic,” “child,” “children,” “childhood,” “adolescent,” “adolescence,” “in utero,” “prenatal,” and “early life.” We also searched using the following MeSH Terms in combination: “lung function test,” “air pollutants, environmental,” and “children.” Studies not in English were excluded, but we did not restrict by country—the scope was global. We conducted our search on October 15, 2020. We also reviewed the most recent US EPA Integrated Science Assessments (ISAs) for NOx, PM, O3 for additional citations.11–13 The ISAs were also helpful in providing context for interpretation of individual citations, as they provided a committee-based comprehensive review of experimental, toxicologic, and epidemiologic data to evaluate the specificity, temporality, and plausibility of associations of specific pollutants with a large range of child and adult health outcomes. Studies of household air pollution and wildfire smoke are not consistently reported as studies of pollution, and therefore we extracted studies from the above PubMed search and performed additional targeted literature searches using the terms “wildfire,” “smoke,” “household air pollution”, “gas stove,” “wood stove,” “biomass burning,” and “volatile organic compounds” to review the sparser literature on household/indoor and wildfire pollution and child lung function. In select cases where epidemiologic evidence is scant and toxicologic data was compelling to us (e.g., O3 effects on lung development relevant to growth), we drew from the toxicology literature. We provide a complete summary of all studies found in our search that were published 2018 or later only for long-term ambient air pollution exposure and lung function level or growth because long-term exposure assessment has improved in the past several years due to satellite data availability, child lung function level and growth is a key indicator of future adult respiratory health, and these most recent studies have not previously been included in prior reviews.
SHORT-TERM AMBIENT AIR POLLUTION EXPOSURE AND CHILD LUNG FUNCTION
A large literature indicates that short-term air pollution exposures can acutely reduce lung function in children. This literature has been previously reviewed11–16 and we will briefly summarize the findings here, focusing on studies of acute effects of ambient gases and particles in general pediatric populations. It is important to recognize that in panel studies of children—as well as any observational air pollution study—, inhalation exposure is to a mixture of air pollutants, thus the responses being measured are responses to that mixture. Air pollutant mixtures, however, may be dominated by one pollutant, or another, depending on the source of the mixture and whether the pollutant mixture is dominated by local sources (e.g., local traffic or nearby industries with ground-level emissions of primary pollutants), or regional pollution, with exposure to transported primary and secondary pollutants. Nevertheless, below we report a summary of studies that focused on specific pollutants, sometimes interpretable as representing sources or mixtures, and their acute influences on children’s lung function.
Higher O3 exposure has been linked with lower levels of lung function (both FEV1 and FVC) in healthy children and those with asthma, with much evidence coming from panel studies of children attending schools or summer camps.12, 14, 17–20 Short-term PM2.5 exposure has been associated with acute decrements in lung function, including lower FEV1/FVC, in healthy pediatric populations.13, 21 Short-term PM10 exposure has also been linked with acute lung function decrements in children.22, 23
Epidemiologic studies have found associations between higher short-term ambient NO2 (often considered as an indicator of exposure to traffic-related air pollution) with acute lung function decrements in children,11, 16, 24, 25 although not all studies support this relation.26, 27 For observed NO2 associations, there is uncertainty as to whether NO2 is the causal factor, there is confounding by correlated pollutants (e.g., PM2.5), or NO2 serves as a representation of a pollution mixture (e.g., traffic pollution) that is the causal factor.11
Often at significantly higher levels of exposure than in the U.S., recent panel studies of schoolchildren from sub-Saharan Africa, Bangladesh, and China add supporting evidence for acute lung function impacts of NO228 and PM2.529–31 and PM10.29, 31 Air pollution concentrations at study locations are important to consider (i.e., whether concentrations are below or well above recommended US EPA/European standards). In settings of low air pollution levels greater sample size is needed to detect associations should they exist.
Wildfires
Air quality regulations, technological advances such as improved catalytic converters, and a transition away from coal burning in many regions of Europe and the US have contributed to a downward trend in outdoor PM pollution. Despite these advances, in the Northwestern US and Northern California, air quality has in fact worsened between 1988 and 2016 as a result of wildfire activity in the US and Canada.32 In the US, wildfires have become the #1 single source of PM and contribute more than a third of the total annual burden of PM2.5 in the atmosphere according to the US EPA Emissions Inventory.33
North America has experienced a sharp increase in the frequency of large, devastating wildfires as a result of longer, hotter fire seasons due to climate change, and years of fire suppression that have led to an over-accumulation of fire-prone organic matter.34 Many other parts of the world, including Australia, Brazil, Southeast Asia, and Russia, are also grappling with increased wildfire activity—a worrisome trend that will continue globally due to climate change.
The composition of wildfire smoke varies depending on the type of fuel burned (e.g., forests, grasslands, peat bogs, human-made structures). Wildland smoke includes not only high levels of particles from organic sources, but also gases such as carbon monoxide (CO), nitrogen oxides (NOx; that also lead to O3 formation), and volatile organic compounds including carcinogens such as benzene, benzo[a]pyrene, formaldehyde, and acetaldehyde.34, 35 Even though wildfire smoke events may be relatively short in duration (e.g., days to weeks), the pollution levels can be extremely high. For example, PM2.5 levels in San Francisco exceeded 200 μg/m3 during the Camp Fire, while the average PM2.5 level for the city is about 9 μg/m3. In addition, smoke travels great distances. Children living in wildfire-prone areas today are exposed to smoke pollution from multiple regional fires during each wildfire season, which now continues for weeks to months longer than the wildfire season of a generation ago.
The respiratory effects of individual wildfire smoke pollutants (e.g., PM2.5, NOx, O3) are likely to be similar to the effects of those pollutants as identified in epidemiologic and mechanistic studies without regard for emission source. However, the composition of PM2.5 emitting by burning wildlands is different from PM2.5 emitted by fossil fuel combustion, and there are likely to be unique airway toxicities of the complex wildfire-generated smoke mixture of gases and particles. Among children (and adults) with asthma, for example, PM2.5 from wildfires appears to be more likely to trigger an asthma exacerbation per unit mass than PM2.5 from other sources, suggesting a possible enhanced inflammatory effect of the wildfire smoke mixture on asthmatic airways.36, 37 There is evidence that healthy children who have smaller airways, as determined by a low ratio of the forced expiratory flow at 25–75% of the pulmonary volume (FEF25–75) to the FVC, are more likely to experience respiratory symptoms when exposed to wildfire smoke than children with normal lung function.38 These findings suggest an acute inflammatory effect of wildfire smoke on small airways.
Relatively few studies have examined the acute effects of short-term exposure to wildfire smoke on child lung function. A panel study measuring daily peak flow among 234 generally healthy schoolchildren aged 6 to 15 exposed to seasonal wildfires in the Brazilian Amazon found that higher levels of PM10 and PM2.5 in the preceding 5 days were associated with lower peak flow.39 In this study, younger children (aged 6–8) experienced greater decrements in peak expiratory flow rate (PEFR) in association with wildfire-PM than older children. A panel study of 309 schoolchildren exposed to smoke from biomass burning in the Brazilian Amazon also found associations between short-term PM2.5 exposure and daily PEFR, with greater effects for non-asthmatic children.40 In one of very few studies of chronic effects of wildfires on child lung function, infants exposed to a 6 week coal mine fire had lower lung reactance as measured by forced oscillation technique at age 3 in association with their level of PM2.5 exposure during the fire.41 A study of rhesus macaque monkeys exposed as infants to ambient smoke from a series of Northern California wildfires in 2008 while housed in an outdoor colony had lower lung function at age 3 compared to unexposed monkeys.42 These studies suggest that such extreme fire pollution events may have long term sequelae for lung function growth. However, there is a need for more studies on the chronic effects of long-term and repeated exposure to wildfire smoke on child lung function level and lung function growth.
LONG-TERM AMBIENT AIR POLLUTION EXPOSURE AND CHILD LUNG FUNCTION
Associations with subsequent level and growth of lung function
We first summarize the literature on long-term air pollution exposure and children’s lung function in general pediatric populations and then present the findings from our systematic review of studies since 2018. Studies up to through approximately 2018 have been previously reviewed or summarized.11–14, 43 A range of exposure windows, both in duration and timing, have been considered in prior studies. In studies of chronic air pollution exposure, lung function outcomes have often been assessed either (1) at a single time point (e.g., at age ~12 years; “lung function level”), or (2) at relatively few repeated time points with the difference (size of increase) between first and last lung function measure sometimes used as a proxy for lung function development (e.g., change in FVC between ages 8 and 16 years; “lung function growth”). We should note, however, that the use of only 2 to 3 repeated measures to represent lung function growth over time may lead to limitations in interpretation of the outcome (see below).
In their recent comprehensive review that extended beyond examination of the literature on traffic exposure and lung function to include pollution exposures from other sources, Schultz et al. concluded that regardless of the timing of exposure early life and school-age exposure to traffic-related air pollution adversely affects children’s lung function.43 The authors did not find firm evidence from the studies evaluated that exposure during certain age windows were more relevant for subsequent lung function. They instead concluded that exposure over the entire childhood period is of importance but suggested that more studies may be needed to define whether children were more vulnerable at specific life-stages.43
Defining effects of specific long-term pollutant exposures by particle size fraction, particle component, or level of pollutant gases
A large number of studies provide evidence that that long-term PM2.5 is associated with lower lung function levels and slower lung function growth in children.13 Studies have been conducted in multiple cohorts from several locations, with differing exposure assessment methods and from different time periods, adding to the robustness of the evidence. The evidence for the larger size range of PM10—which includes road dust (tire and brake wear, road salt), wildfires smoke, and aeroallergens—is less robust due to lower availability of long-term PM10 models.13 Two studies that have been conducted, both in Europe, have found null associations between long-term PM10 exposure and child lung function.44, 45 Recently, there is a renewed interest in the study of the coarse particle fraction (2.5μm-10μm; PM10−2.5),46 which is primarily composed of organic debris, crustal elements, and suspended road dust metals.47 The effects of particles in this size fraction on child lung function, possibly mediated through their effects on the upper airway, including the nose, is an area of active investigation.48
Associations between long-term NO2 exposure and decrements in lung function levels in children are also well-documented in the epidemiologic literature from studies in various locations with varied follow-up time and using different methods for exposure assessment and lung function measurements.11 The evidence for adverse NO2 effects is stronger for FEV1, representing airway mechanical properties and/or airway caliber, compared with that for FVC, representing lung volume.11 Uncertainty, however, remains as to whether NO2 is the causal agent, if results are confounded by copollutant exposure, or if NO2 represents a source that is the causal factor. Results from NO2 models with adjustment for PM2.5 are mixed, and it is therefore difficult to conclude whether the NO2 associations with lung function are explained by effects of traffic particles that are imperfectly measured by NO2 and PM2.511
Based on epidemiologic studies previously reviewed, studies with evidence supporting associations of long-term O3 exposure with lower lung function levels and growth are inconsistent, suggesting perhaps that this pollutant may have its greatest chronic effects in regions of the world where exposure is more constantly elevated and where indoor penetration of outdoor air is greater.12, 49–55 Toxicologic studies involving rodents and primates, in contrast, provide support of prenatal or early life effects of O3 exposure on lung development, with suggestive evidence in Shore’s rodent models of interactions amongst O3, diet/obesity, and allergen exposures in their influences on the risk of airflow obstruction.12, 56–62
Following up on these prior thorough reviews and summaries, in Table 1 and in the text below we present a summary of recent lung function studies of general pediatric populations and long-term air pollution exposure assessment published since 2018, using the literature search methodology described in the introduction. We defined long-term air pollution exposure as exposure in some earlier time period (i.e., not concurrent, and not only the preceding 12 months). Lung function assessment must have included one of the following measures: FEV1, FVC, or FEF25–75 (the latter being the same as flow mean flow between 25% and 75% of FVC [MEF25–75] and maximum mid-expiratory flow [MMEF]). We identified eight studies fitting these criteria. Five of the studies were based in Europe,44, 48, 63–65 two in Asia,66, 67 and one in the U.S.68 Of these eight studies: four included exposure to NO2 all of which found a negative association with lung function; two included O3 of which one found a negative association with lung function; one included PM2.5 which found a negative association with lung function; and five included PM10 of which three found a negative association with lung function. While all studies examined lung function levels and only one study48 also examined lung function growth. This study, the Dutch Prevention and Incidence of Asthma and Mite Allergy cohort, assessed associations between air pollution exposure from birth-4 years of age with lung function growth between ages 8 and 16 years in 721 children. They included into their models an exposure-age interaction term—which can be interpreted as the association of pollution exposure with annual rate of change in lung function.48 They reported that PM2.5 exposure was related to reduced FEV1 growth.48
Table 1.
Summary of studies on long-term air pollution and lung function in healthy or population-based children published since 2018.
| Reference | Population (location, cohort name) | Study design | Exposure focus | Exposure Assessment | Lung function Assessment | Results |
|---|---|---|---|---|---|---|
| Cai et al. 202044 | 5,276 children at age 8 years, 3,446 children at age 15 years (Avon in southwest England, ALSPAC) | Prospective birth cohort | Prenatal and childhood air pollution | PM10 during each trimester, 0–6 months, 7–12 months, and up to age 15 years | FEV1% predicted and FVC% predicted at ages 8 and 15 years |
|
| Usemann et al. 201963 | 232 children at age 6 years (Bern, Switzerland, BILD) | Prospective birth cohort | Low-to-moderate air pollution levels, from pregnancy until school-age | NO2, O3, PM10 during each pregnancy, 1st year of life, 6th year of life, and birth until follow-up | FEV1, FVC, FEV1/FVC ratio, and FEF25–75 measured at age 6 years |
|
| He et al. 201966 | 2,942 children at age ~17.5 years (Hong Kong, “Children of 1997” cohort) | Prospective birth cohort | Prenatal and childhood air pollution | NO, NO2, PM10, and SO2 during pregnancy, age 0–2 years, and age 2–8 years | FEV1, FVC, FEF25–75, and FEV1/FVC ratio measured at age ~17.5 years |
|
| Milanzi et al. 201848 | 915 children for the age 8–16 years growth analysis, 721 for the age 16 years analysis (The Netherlands, PIAMA) | Prospective birth cohort | Air pollution exposure from birth | NO2, PM2.5, PM10, and PM coarse during preschool, primary school, and secondary school time windows | FEV1 and FVC measured at age 16, and FEV1 and FVC growth from ages 8 to 16 years based on repeated measures at ages 8, 12, and 16 years |
|
| Tsui et al. 201867 | 1,016 children age 6–15 years (Taiwan) | Nationwide, cross-sectional, school-based survey | Lifetime residential exposure to air pollution | PM10, O3, SO2, NO2, and CO during 1st year of life, age 2–6 years, and lifetime | FEV1, FVC, FEF25–75, and FEV1/FVC ratio measured once among children at mean age 11.9 years (standard deviation: 2.4) |
|
| Bougas et al. 201864 | 788 children age 8–9 years (Paris, France, PARIS) | Prospective birth cohort | Traffic-related air pollution | NOx during each trimester and entire pregnancy, 1st year of life, and lifetime | FEV1, FVC, and FEF25–75 measured at age 8–9 years |
|
| Bose et al. 201868 | 191 children age 5–8 years (Boston, USA, ACCESS) | Prospective birth cohort | Prenatal NO3-exposure | NO3- during pregnancy by gestational week | FEV1, FVC, FEF25–75, and FEV1/FVC ratio measured once among children at mean age 7.0 years (standard deviation: 0.89) |
|
| Bergstra et al. 201865 | 424 children age 7–13 years (East Vlissingen in southwest of The Netherlands) | Cross-sectional study | Long-term air pollution from heavy industry | PM2.5 and NOx during prior five years | FVC, FEV1, PEF, FEF25–75, FEV1/FVC ratio measured once among children between the ages of 7 and 13 years |
|
Abbreviations:
CO: Carbon monoxide
FEF25–75: Forced expiratory flow at 25–75% of the pulmonary volume
FEV1 : Forced expiratory volume in 1 second
FVC: Forced vital capacity
NO2: Nitrogen dioxide
NO3-: Nitrate
NOx: Nitrogen oxide
O3: Ozone
PEF: Peak expiratory flow
PM10: Particulate matter <10 μm
SO2: Sulfur dioxide
PM2.5: Particulate matter <2.5 μm
Our review of recent literature highlights the small number of assessments of lung function growth in studies of long-term air pollution at contemporary levels of exposure. As illustrated with hypothetical growth curves in Figure 1, the trajectories of lung function growth can be tracked throughout childhood, with assessment of whether children attain normal peak growth and avoid a steep decline that could lead to COPD or other respiratory deficits shown to be predictive of higher morbidity or mortality (Figure 1). More frequent longitudinal outcome measurements and more precise exposure estimates during critical childhood life stages offer more opportunities for investigators to assess whether and when air pollution perturbs lung function growth, and whether any deficit in growth can be recovered with reduced air pollution exposure (e.g., through regulation to reduce emissions or limit exposure to pollution sources at school, or interventions to purify the air). Single measures of lung function during adolescence are particularly challenging, as estimates of lung function growth during this period need to consider height growth and where the children are in their growth spurt, which differs by sex.69 While challenging to perform, studies with repeated pulmonary function testing across childhood and adolescence would add important data to the literature by enabling evaluation of more nuanced aspects of the pollution-lung function growth relation, such as pollution effects on lung function growth velocity (i.e., time-varying rate of growth); whether effects are fixed or reversible; or whether effects differ by timing of exposure (e.g., during puberty—a period of great somatic growth related to lung development).
Studies evaluating effect of air pollutant mixtures
Continued child lung function research should consider the context of air pollution mixtures when examining health effects. Such considerations can refer to a number of different research questions, such as: Which pollutant is the causal agent in this mixture? Do pollutants interact in their health effect? What is the overall effect of the mixture?70 Air pollution studies often examine one pollutant at a time due to statistical realities regarding multi-collinearity for highly correlated pollutants. Some studies of child lung function have implemented multi-pollutant models examining the effects of multiple pollutants in a single model (e.g., PM2.5 and NO2 together as explanatory variables in a single model), often just two pollutants,25, 71, 72 although some studies have included more.49 This type of approach is often used to answer the first question above, which is the causal agent or dominant mixture/cluster associated with the adverse outcome?70 There have been several recent advancements in mixture model approaches70, 73, 74—a research area of great interest in environmental epidemiology—that could be implemented in studies of child lung function to improve understanding of air pollution effects. Overall, however, disentangling the effects of individual pollutants is challenging and often not possible in observational studies in children, as compared to toxicologic studies and controlled human exposure studies in adults, which themselves have limitations in terms of size, generalizability, and potential to measure long-term as opposed to short-term exposure responses. Nevertheless, complementary studies from these and other fields will continue to be needed to evaluate causality and understanding potential biologic mechanism. In some cases, removing or reducing the exposure and witnessing the improvement in health has been the strongest evidence for causality.
INDOOR SOURCES OF AIR POLLUTION AND CHILD LUNG FUNCTION
Young children often spend most of their time at home, where they are exposed to household air pollution (from household sources such as cooking and heating) and ambient pollution (from outdoor sources) that penetrates inside the home. Here, we review the literature on three major sources of household air pollution—biomass burning, gas stove use, and VOCs—and child lung function.
Indoor Biomass and Coal Burning
In low- and middle-income countries, biomass burning for cooking or heating is a major source of air pollution inside the home, and in the neighborhood if vented to the outdoors. The few studies of indoor biomass or coal burning for cooking or heating have generally found that these exposures are associated with lower lung function in children.75–80 The largest of these followed more than 3,000 children aged 6–13 with repeated lung function measures in four Chinese cities and found that use of coal as a household fuel was associated with a 16.5 (95% CI −23.6, to −9.3) mL/year lower annual growth of FEV1, and a 20.5 (95% CI-28.3 to −12.7) mL/year lower growth of FVC.78 This study also found that household ventilation improved lung function growth. A small number of intervention studies in low-income countries have examined the effect of placing clean cookstoves in homes to improve indoor air quality. Most of these have examined childhood pneumonia as the primary outcome, with mixed results.81 The RESPIRE trial placed clean chimney stoves in households with pregnant women and infant children in Guatemala either at baseline or 18 months later. Among the 440 children with longitudinal spirometry testing, delayed (18 month) chimney stove placement was associated with slower PEF growth of 173 mL/min/year (95% CI −341 to −7) and slower FEV1 growth of 44 mL/year (95% CI −91 to 4).82 This suggests an effect of early life indoor air quality on lung function growth that may not be fully reversible with a subsequent air quality intervention, and that household air quality interventions during infancy may have lasting lung function benefits.
Indoor biomass burning is not only a phenomenon in low- and middle-income countries. Wood-burning stoves are a common source of heating in many high-income countries. In the US, wood stove use has been increasing over the past two decades, and it has been estimated that 11 million households and more than three million children are exposed to higher levels of indoor particulate matter due to the use of wood-burning stoves for heating.83 In a recent narrative review of 36 studies conducted in developed countries on the pediatric health effects of wood smoke exposure, estimated community-level wood smoke exposure was consistently associated with child respiratory symptoms, while findings on wood stove use were less consistent.84 There are few studies of wood stove use and child lung function. The recent review identified a single study, which was conducted in Turkey among 617 schoolchildren aged 9–12 years and found lower average child lung function among those living in households using wood stoves.85 Pediatric research on wood stove use has relied on self-reported questionnaire data, and there is a clear need for studies that measure wood smoke pollution in the home to arrive at a more precise estimate of household air pollution exposure.84
Gas Cooking
Gas cooking is a common source of indoor NO2 in low-, middle-, and high-income countries, with peak indoor NO2 levels during cooking far exceeding exposure to NO2 from outdoor sources that penetrates indoors.86 In fact, it has been estimated that more households are exposed to indoor NO2 levels above the 1-hour US EPA national ambient air quality standard for NO2 due to use of unvented gas stoves than NO2 emitted by outdoor pollution sources that enters the home.87 While controlled human exposure studies have yielded inconsistent results regarding the acute effects of NO2 gas alone on lung function at typical outdoor NO2 exposure concentrations (up to 20 ppb), indoor NO2 concentrations can exceed 100 ppb (especially in the absence of outdoor kitchen ventilation),87 and among adults such concentrations have been linked to oxidative stress and acute decrements in lung function.88 Numerous individual studies have examined whether gas stove cooking is associated with lower lung function in children, with inconsistent findings.89, 90 Inconsistencies may vary in part by whether the gas stove has a continuously burning pilot light, whether it is vented to the outdoors, whether it is used for heating the home, and also by the amount of air exchange in the home. A meta-analysis combining data from 24,000 children in Europe and North America concluded that gas stove cooking is associated with a small but significant reduction in FEV1 (0.7%) and FVC (0.6%), with evidence of greater effect sizes among children with atopy.91 This suggests a reduction in average lung function among healthy children with gas stoves in the home, and that children with allergic phenotypes may be more susceptible. If true, this would add to a very long and complicated literature on the effect of gas stove use on risk of childhood asthma and respiratory symptoms among children with asthma or atopy.92, 93
There is also some evidence that gas stove ventilation can mitigate the effect of gas stoves on lung function: in NHANES III girls living in households with gas stoves using ventilation had higher FEV1 and FEV1/FVC than those with gas stoves and no ventilation.94 This suggests an opportunity to improve child lung function through household interventions such as improved ventilation or replacement of gas stoves with electric models.
Volatile Organic Compounds
VOCs include wide range of chemicals, and while emission sources are numerous and varied most are emitted from indoor sources such as paints and floor coverings, building materials and furnishings, and consumer products (e.g., cleaning supplies, air fresheners, hobby supplies).95–97 Outdoor sources (e.g., industrial facilities, dry cleaners, gasoline stations) and attached garages can also contribute to residential VOC exposure.96, 98 Concentrations of many VOCs are often higher indoors compared with outdoors levels.96, 99
Adverse effects of VOCs children’s respiratory health have been reported,100–102 but there are very few studies examining associations with lung function in children.103–106 A Canadian study measured 84 VOCs in residential indoor air and report that among the 709 participants aged 6–16 years there were significant reductions in lung function observed for nine VOCs.103 The largest associations were a 9.0% (95%CI: −15.4, −2.6) decrease in FEV1, associated with an IQR change in nonanal, and a 5.1% (95%CI: −9.2, −1.0) change in FEV1 associated with an IQR change 2-furancarboxaldehyde.103 A study of 772 Mexican preschoolers examined prenatal VOC exposure and lung function measured with forced oscillation tests at 36, 48, and 60 months of age.104 Negative associations between prenatal exposure to xylene and respiratory reactance were reported, but null associations for benzene and toluene were found.104 Data from a South African birth cohort with lung function measured at 6–10 weeks of age among 645 infants demonstrated higher levels of household benzene to be associated with lower early lung function as measured by the forced oscillation technique.105 Higher levels of prenatal exposure to benzene were associated with clinically significant deficits in FEV1 among 620 Spanish preschoolers (aged 4.5 years); however, this study used estimates of outdoor (not indoor) benzene levels at residential address.106 Given the expansive sources of VOCs and their ubiquitous presence in the air, especially indoors, coupled with this small literature, their effects on child lung function merit further research.
WHAT CAN BE DONE?
There are several actions that can be taken to reduce children’s exposure to air pollution, implemented at a range of control levels from individuals and families to national policies (Figure 2). Interventions at higher levels (e.g., national air quality standards) would impact a larger number of people compared with actions taken at smaller scales (e.g., an air filtration unit will only reduce exposure for those within the household). To be clear we are not recommending each of the interventions, instead when there is sufficient or reasonable evidence then such interventions could be considered and implemented.
FIGURE 2.
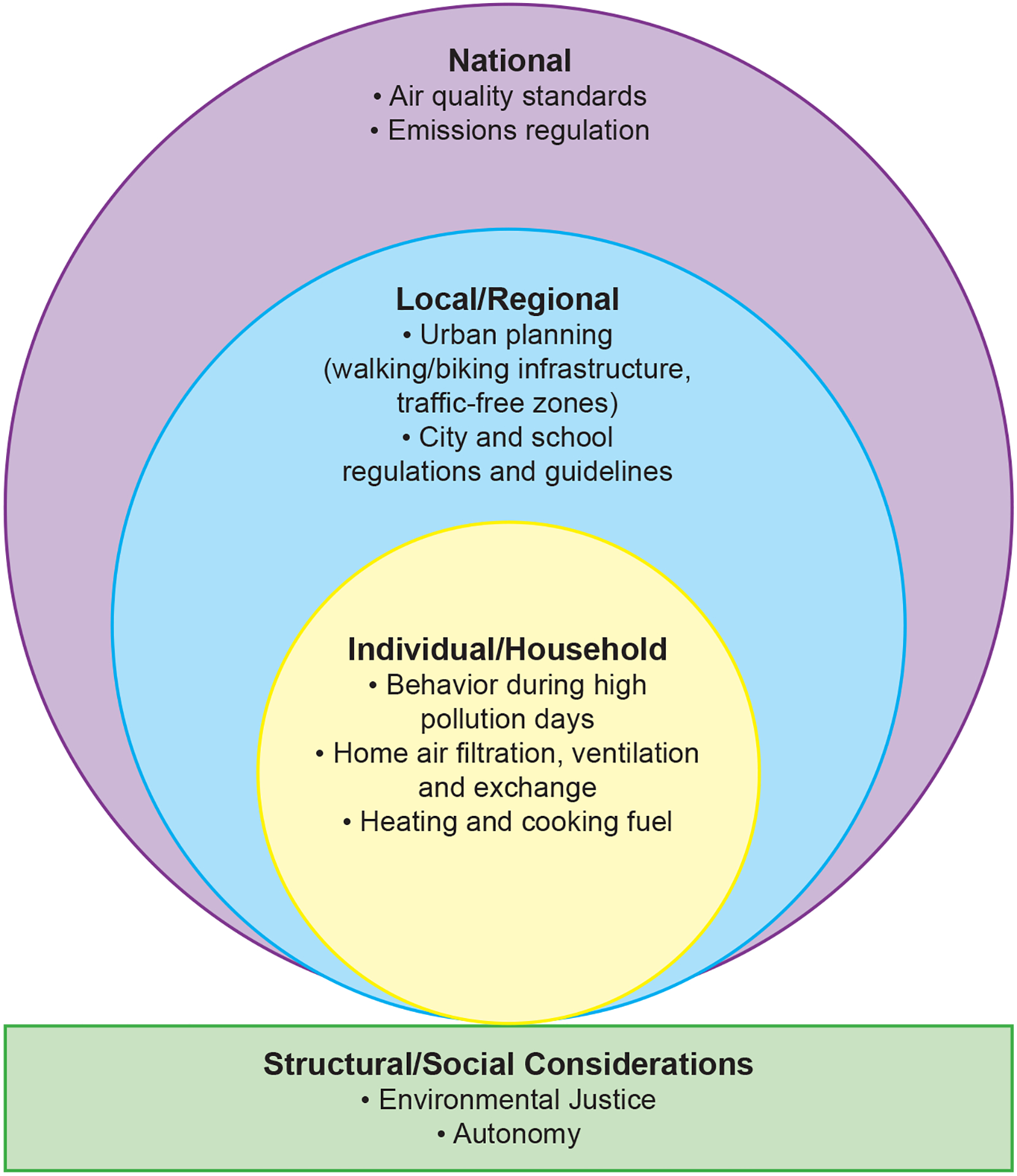
Examples of current or potential air pollution interventions at various intervention levels.
Individual and Household Interventions
Several studies of air pollution interventions at the household and individual levels have identified subsequent improvements in child respiratory health (though not always including lung function as an outcome). As discussed above, improved kitchen ventilation and placement of clean cookstoves appear to benefit child lung function, especially if done early in life. Short-term use of indoor air purifiers has been found to lower airway resistance in adults107 and appears to improve asthma symptoms,108 but any long-term benefits of indoor air filtration on child lung function or lung function growth remains unknown.
National and Local Policies to Improve Outdoor Air Quality
Several studies have taken advantage of changes—usually reductions—in air pollution levels to investigate subsequent impacts on children’s lung function. These studies add to the evidence of a causal relationship between air pollution and respiratory health, directly relating to the “experiment” viewpoint of the Bradford Hill criteria for causality.109 If lung function is adversely impacted by higher air pollution exposure—and these relations are truly causal—then it is expected in situations of improved air quality benefits in lung function would be observed. Here we provide a brief review of studies that have used this approach.
During the 1990s the reunification of Germany saw marked declines in air pollution levels, including for total suspended particles and sulphur dioxide, and several studies of schoolchildren from East and West Germany reported associated improvements in lung function primarily based on consecutive cross-sectional surveys between 1992 and 1999.110, 111 Using multiple prospective cohorts consecutively recruited in the 1990s and early 2000s in the Southern California Children’s Health Study (CHS), studies have reported observed improvements in air quality to be associated with improved lung function in schoolchildren.50, 52, 55, 112 In the most recent of these CHS studies, which combined data from three cohorts spanning a 13-year period of air quality improvement, lower concentrations of NO2, PM2.5, and PM10 across cohorts were related with improved lung function growth between the ages of 11 and 15 years.52 An evaluation of the Oxford Transport Strategy (implemented in 1999 to reduce city center congestion) did find improvements in lung function in schoolchildren as measured by peak expiratory flow (PEF) after its implementation, but these improvements were not related to observed changes in traffic exposure.113 A 5-year study of London’s low emission zones (implemented in 2008, restricts vehicle entry in urban areas and applies penalties on polluting vehicles) found reduced NO2 and NOx concentrations, although not PM10, and that annual NO2 and PM10 exposures were inversely associated with FVC in 8–9 year old children; however, no change was observed in the proportion of children with small lung volumes for their age over the study period.114 Adoption of clean air technologies and fuels in school buses were found to reduce PM2.5 and ultrafine PM in buses, and ultralow-sulfur diesel adoption was associated with improved changes in FVC and FEV1 school bus riders (aged 6–12 yrs).115 Other interventions that have been found to improve health (though child lung function was not a measured outcome) include public guidance for low-pollution walking/biking routes,116–119 idle-free zones,120 and complete streets programs.121 Increasing neighborhood green space122 and the building of affordable “healthy housing”123 may also reduce indoor and outdoor air pollution exposures. Future lung function research should continue to take advantage of “natural experiments” to improve understanding of the air pollution impacts on children’s respiratory health.
It is important to acknowledge that individuals may be limited in their ability to engage in pollution exposure reduction activities and advocacy due structural or social constraints (Figure 2), which could lead to further disparities in pollution exposure. For example, individual families may not have the financial resources to undertake interventions such as replacement of stoves or installation of air purifiers, and instead structural changes (e.g., in public and private housing, insurance coverage) would be needed to bring about such interventions. Some U.S. communities have suffered an overt expression of structural racism through historical “redlining”, leading to the siting of communities, their homes, and their schools nearer to highways and sources of air pollution, which in turn have public health effects.124–126 Lastly, just as people are exposed to air pollutants as a mixture, people are exposed to several factors concurrently when one considers the wider realm of exposures, including other environmental exposure, individual characteristics including heritable factors, diet/nutrition, psychosocial stressors, structural racism, etc., which can interact to either adversely or beneficially impact health outcomes such as lung function.53, 127–130
CONCLUSION
There is a large literature supporting the link between air pollution exposure and adverse effects on children’ lung function level and growth; however, many questions remain, some relating to more nuanced understanding of air pollution effects and others to new or less studied exposures. Studies of repeated measures of pulmonary function in children as they grow—including into adulthood—would provide significant opportunity to expand our understanding of air pollution effects on children’s lung function. Important areas needing further study include identification of windows of increased susceptibility, whether early life effects are fixed or reversible, evaluating the long-term effects of repeated wildfire events on child lung function, and the effect of air quality interventions at the individual, household, and local level on child lung function and respiratory health. As additional studies are conducted to improve scientific knowledge about the adverse effects of air pollutants on children, simultaneous efforts at the individual, local/city, state and national level can be made to improve indoor and outdoor air quality, with assessment of the short and long-term benefits to children and their families. Citizen and community engagement on the need for improved air quality and environmental justice may inspire ground-breaking policy changes with lasting health benefits.
The rate of lung function decline and level of lung function in later adulthood are strong predictors of overall morbidity and mortality. If reduction of air pollution exposures improves lung function growth, maximizes the peak lung function children attain as young adults, and minimizes population decline in lung function in adulthood, this will have significant long-term benefits for the health of the next generation.131
What Do We Know?
Short-term exposure to ozone, nitrogen dioxide, and particle pollution have been associated with lower child lung function
Fine particulate matter levels can reach extreme concentrations during wildfires, and are associated with acute decrements in peak expiratory flow
Long-term early childhood exposure to ambient pollutants, especially nitrogen dioxide and fine particulate matter, are associated with subsequent children’s lung function level
Children worldwide are often highly exposed to smoky household air pollution at a very critical age of lung development
What Is Still Unknown?
Long-term consequences of repeated exposure to wildfire smoke on child lung function
Whether timing or cumulative period of exposure influences if pollution effects are fixed or reversible over time, as children reach adulthood
Long-term consequences of air pollution on reduced lung function development in childhood are not fully understood. Studies need to investigate impacts of childhood exposures on lung function trajectories and later respiratory health in adulthood
Need to understand (i) who is more susceptible to the adverse lung function effects of gas stove pollution and (ii) the extent to which gas stove-related lung function decrements are improved or prevented with improved kitchen ventilation
What factors, in trials for homes with biomass or coal burning cookstoves, are responsible for the inconsistencies in effects of provision of clean stoves and improved indoor ventilation on child lung function
Box 1. Why Study Effects of Ambient Air Pollution on Lung Function in Children?
Why children
More susceptible to pollution effects due to developing physiology
Increased exposure due to size and behavior
Why lung function
Subclinical marker with short- and long-term clinical implications for lung health across the lifespan
Clinical outcome measure in children with asthma, cystic fibrosis, and other chronic lung diseases
Box 2. Short-term Ambient Air Pollution Exposure and Children’s Lung Function.
Summary observations
Short-term exposure to O3 and particle pollution (PM2.5 and PM10) have been associated with lower lung function, including lower FEV1, FVC, and FEV1/FVC, in generally healthy children and in those with asthma
Large wildfires are occurring at increasing frequency and are a major source of PM and other pollutants
PM2.5 levels during wildfires can reach extreme concentrations that far exceed air quality standards and are associated with acute decrements in peak expiratory flow
Short-term elevations NO2 levels outdoors and indoors have been associated with reduction in child lung function
Knowledge gaps
Need to improve understanding of the extent to which observed acute effects of NO2 on child lung function are causal, due to confounding by correlated pollutants (e.g., PM2.5), or represents a pollution mixture that is causal (e.g., traffic pollution)
Need to evaluate the long-term consequences of repeated exposure to wildfire smoke on child lung function
Box 3. Long-term Ambient Air Pollution and Children’s Lung Function.
Summary observations
A large number of studies support a relation between early-life or long-term air pollution exposures and subsequent children’s lung function level, with the most epidemiologic evidence for PM2.5 and NO2
There is inconsistent evidence of an effect of early-life or long-term exposure to O3 on subsequent children’s lung function, although animal model studies suggest pre-natal or early-life O3 effects on lung development
Associations with long-term exposures are more consistent for FEV1 compared with for FVC, which may indicate greater impacts on airway caliber/airflow obstruction than overall lung size or growth
Knowledge gaps
While there is evidence that air pollution exposure at all ages in childhood can have adverse lung function effects, few studies have evaluated whether timing or cumulative period of exposure influences whether pollution effects are fixed or reversible
Long-term consequences of air pollution on reduced lung function development/growth/trajectories in childhood are not fully understood; studies need to investigate impacts of childhood exposures on lung function in adulthood as well as risk for later respiratory health, such as chronic obstructive pulmonary disease, emphysema, fibrosis, or pulmonary vascular disease
Need to further assess the toxicity of specific PM components and sources on lung function
Box 4. Indoor Sources of Air Pollution and Children’s Lung Function.
Summary observations
Worldwide, young children are often highly exposed to smoky household air pollution from biomass burning for heating and cooking at a very critical age when their lungs are developing
There are a few trial studies providing evidence that installation of clean chimney stoves in infancy may lead to more rapid lung function growth
Installation of clean stoves can improve indoor air pollution, but levels of indoor/outdoor pollution may remain elevated (though lower than previously) and these reductions are inconsistently associated with improvements in lung function
Knowledge gaps
- Gas stoves.
- Need to understand who is more susceptible to the adverse lung function effects of gas stove pollution
- Need to understand the extent to which gas stove-related lung function decrements are improved or prevented with improved kitchen ventilation
- Biomass and coal burning cook stoves
- Need to assess what additional exposures or factors in the use of multiple fuel sources or lack of stove maintenance are responsible for the inconsistencies in effects of provision of clean stoves and improved indoor ventilation on child lung function
Additional research is needed on the effects of VOCs on child lung function to corroborate the few existing studies which provide evidence of adverse effects
Funding Statement:
This work was supported in part by NIEHS P30 ES00002 (DG, MR) and P30 ES007048 (EG).
ABBREVIATIONS:
- CO
Carbon monoxide
- COPD
Chronic obstructive pulmonary disease
- FEF25–75
Forced expiratory flow at 25–75% of the pulmonary volume
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- ISA
Integrated Science Assessments
- MEF25–75
Mean flow between 25% and 75% of FVC
- MMEF
Maximum mid-expiratory flow
- NO2
Nitrogen dioxide
- NO3-
Nitrate
- NOx
Nitrogen oxide
- O3
Ozone
- PEF
Peak expiratory flow
- PEFR
Peak expiratory flow rate
- PM
Particulate matter
- PM10
Particulate matter <10 μm
- PM2.5
Particulate matter <2.5 μm
- SO2
Sulfur dioxide
- US EPA
United States Environmental Protection Agency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None declared.
References
- 1.Integrated Science Assessments (ISAs).] Available from https://www.epa.gov/isa.
- 2.Spektor DM, Lippmann M, Lioy PJ, Thurston GD, Citak K, James DJ, et al. Effects of ambient ozone on respiratory function in active, normal children. Am Rev Respir Dis 1988; 137:313–20. [DOI] [PubMed] [Google Scholar]
- 3.Raizenne ME, Burnett RT, Stern B, Franklin CA, Spengler JD. Acute lung function responses to ambient acid aerosol exposures in children. Environ Health Perspect 1989; 79:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurston GD, Lippmann M, Scott MB, Fine JM. Summertime haze air pollution and children with asthma. Am J Respir Crit Care Med 1997; 155:654–60. [DOI] [PubMed] [Google Scholar]
- 5.Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol 2000; 151:798–810. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–31. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel Working Group of the National Heart L, Blood Institute a, coordinated National Asthma E, Prevention Program Coordinating C, Cloutier MM, Baptist AP, et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020; 146:1217–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speizer FE, Tager IB. Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev 1979; 1:124–42. [DOI] [PubMed] [Google Scholar]
- 9.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med 2016; 374:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold DR, Sordillo JE, Coull BA. Lung Function Tracking throughout Childhood: Growth Trajectories May Not Be Set in Stone. J Allergy Clin Immunol Pract 2020; 8:1272–4. [DOI] [PubMed] [Google Scholar]
- 11.United States Environmental Protection Agency. Integrated Science Assessment for Oxides of Nitrogen –Health Criteria. Research Triangle Park, NC: Office of Research and Development, National Center for Environmental Assessment, 2016. [Google Scholar]
- 12.United States Environmental Protection Agency. Integrated Science Assessment for Ozone and Related Photochemical Oxidants. Research Triangle Park, NC: Office of Research and Development, Center for Public Health & Environmental Assessment, 2020. [Google Scholar]
- 13.United States Environmental Protection Agency. Integrated Science Assessment for Particulate Matter. Research Triangle Park, NC: Office of Research and Development, National Center for Environmental Assessment, 2019. [Google Scholar]
- 14.Schwartz J Air pollution and children’s health. Pediatrics 2004; 113:1037–43. [PubMed] [Google Scholar]
- 15.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A 2008; 71:238–43. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Williams G, Jalaludin B, Baker P. Panel studies of air pollution on children’s lung function and respiratory symptoms: a literature review. J Asthma 2012; 49:895–910. [DOI] [PubMed] [Google Scholar]
- 17.Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J 2002; 19:699–705. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol 2008; 121:1133–9.e1. [DOI] [PubMed] [Google Scholar]
- 19.Castillejos M, Gold DR, Damokosh AI, Serrano P, Allen G, McDonnell WF, et al. Acute effects of ozone on the pulmonary function of exercising schoolchildren from Mexico City. Am J Respir Crit Care Med 1995; 152:1501–7. [DOI] [PubMed] [Google Scholar]
- 20.Castillejos M, Gold DR, Dockery D, Tosteson T, Baum T, Speizer FE. Effects of ambient ozone on respiratory function and symptoms in Mexico City schoolchildren. Am Rev Respir Dis 1992; 145:276–82. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Chan CC, Chen BY, Cheng TJ, Leon Guo Y. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ Res 2015; 137:40–8. [DOI] [PubMed] [Google Scholar]
- 22.Scarlett JF, Abbott KJ, Peacock JL, Strachan DP, Anderson HR. Acute effects of summer air pollution on respiratory function in primary school children in southern England. Thorax 1996; 51:1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunekreef B, Hoek G. The relationship between low-level air pollution exposure and short-term changes in lung function in Dutch children. J Expo Anal Environ Epidemiol 1993; 3 Suppl 1:117–28. [PubMed] [Google Scholar]
- 24.Linn WS, Shamoo DA, Anderson KR, Peng RC, Avol EL, Hackney JD, et al. Short-term air pollution exposures and responses in Los Angeles area schoolchildren. J Expo Anal Environ Epidemiol 1996; 6:449–72. [PubMed] [Google Scholar]
- 25.Moshammer H, Hutter HP, Hauck H, Neuberger M. Low levels of air pollution induce changes of lung function in a panel of schoolchildren. Eur Respir J 2006; 27:1138–43. [DOI] [PubMed] [Google Scholar]
- 26.Chang YK, Wu CC, Lee LT, Lin RS, Yu YH, Chen YC. The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere 2012; 87:26–30. [DOI] [PubMed] [Google Scholar]
- 27.Altuğ H, Gaga EO, Döğeroğlu T, Brunekreef B, Hoek G, Van Doorn W. Effects of ambient air pollution on respiratory tract complaints and airway inflammation in primary school children. Sci Total Environ 2014; 479–480:201–9. [DOI] [PubMed] [Google Scholar]
- 28.Mentz G, Robins TG, Batterman S, Naidoo RN. Effect modifiers of lung function and daily air pollutant variability in a panel of schoolchildren. Thorax 2019; 74:1055–62. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Li C, Li Y, Liu J, Meng C, Han J, et al. Short-term effects of ambient air pollution exposure on lung function: A longitudinal study among healthy primary school children in China. Sci Total Environ 2018; 645:1014–20. [DOI] [PubMed] [Google Scholar]
- 30.Xu D, Chen Y, Wu L, He S, Xu P, Zhang Y, et al. Acute effects of ambient PM(2.5) on lung function among schoolchildren. Sci Rep 2020; 10:4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasmin S, Ng CFS, Stickley A, Md N, Saroar G, Yasumoto S, et al. Effects of Short-term Exposure to Ambient Particulate Matter on the Lung Function of School Children in Dhaka, Bangladesh. Epidemiology 2019; 30 Suppl 1:S15–s23. [DOI] [PubMed] [Google Scholar]
- 32.McClure CD, Jaffe DA. US particulate matter air quality improves except in wildfire-prone areas. Proc Natl Acad Sci U S A 2018; 115:7901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report on the Environment: Particulate Matter Emissions. United States Environmental Protection Agency.] Available from https://cfpub.epa.gov/roe/indicator.cfm?i=19.
- 34.Balmes JR. Where There’s Wildfire, There’s Smoke. N Engl J Med 2018; 378:881–3. [DOI] [PubMed] [Google Scholar]
- 35.Na K, Cocker DR. Fine organic particle, formaldehyde, acetaldehyde concentrations under and after the influence of fire activity in the atmosphere of Riverside, California. Environ Res 2008; 108:7–14. [DOI] [PubMed] [Google Scholar]
- 36.DeFlorio-Barker S, Crooks J, Reyes J, Rappold AG. Cardiopulmonary Effects of Fine Particulate Matter Exposure among Older Adults, during Wildfire and Non-Wildfire Periods, in the United States 2008–2010. Environ Health Perspect 2019; 127:37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delfino RJ, Brummel S, Wu J, Stern H, Ostro B, Lipsett M, et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med 2009; 66:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirabelli MC, Kunzli N, Avol E, Gilliland FD, Gauderman WJ, McConnell R, et al. Respiratory symptoms following wildfire smoke exposure: airway size as a susceptibility factor. Epidemiology 2009; 20:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson Lda S, Hacon Sde S, de Castro HA, Ignotti E, Artaxo P, Saldiva PH, et al. Acute effects of particulate matter and black carbon from seasonal fires on peak expiratory flow of schoolchildren in the Brazilian Amazon. PLoS One 2014; 9:e104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson Lda S, Hacon Sde S, Castro HA, Ignotti E, Artaxo P, Ponce de Leon AC. Association between fine particulate matter and the peak expiratory flow of schoolchildren in the Brazilian subequatorial Amazon: a panel study. Environ Res 2012; 117:27–35. [DOI] [PubMed] [Google Scholar]
- 41.Shao J, Zosky GR, Hall GL, Wheeler AJ, Dharmage S, Melody S, et al. Early life exposure to coal mine fire smoke emissions and altered lung function in young children. Respirology 2020; 25:198–205. [DOI] [PubMed] [Google Scholar]
- 42.Black C, Gerriets JE, Fontaine JH, Harper RW, Kenyon NJ, Tablin F, et al. Early Life Wildfire Smoke Exposure Is Associated with Immune Dysregulation and Lung Function Decrements in Adolescence. Am J Respir Cell Mol Biol 2017; 56:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz ES, Litonjua AA, Melén E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr Allergy Asthma Rep 2017; 17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Y, Hansell AL, Granell R, Blangiardo M, Zottoli M, Fecht D, et al. Prenatal, Early-Life, and Childhood Exposure to Air Pollution and Lung Function: The ALSPAC Cohort. Am J Respir Crit Care Med 2020; 202:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, et al. Early-Life Exposure to Traffic-related Air Pollution and Lung Function in Adolescence. Am J Respir Crit Care Med 2016; 193:171–7. [DOI] [PubMed] [Google Scholar]
- 46.Strickland MJ. Taking Another Look at Ambient Coarse Particles. American Journal of Respiratory and Critical Care Medicine 2018; 197:697–8. [DOI] [PubMed] [Google Scholar]
- 47.Adar SD, Filigrana PA, Clements N, Peel JL. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Current environmental health reports 2014; 1:258–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milanzi EB, Koppelman GH, Smit HA, Wijga AH, Oldenwening M, Vonk JM, et al. Air pollution exposure and lung function until age 16 years: the PIAMA birth cohort study. Eur Respir J 2018; 52. [DOI] [PubMed] [Google Scholar]
- 49.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med 2007; 176:377–84. [DOI] [PubMed] [Google Scholar]
- 50.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004; 351:1057–67. [DOI] [PubMed] [Google Scholar]
- 51.Breton CV, Salam MT, Vora H, Gauderman WJ, Gilliland FD. Genetic variation in the glutathione synthesis pathway, air pollution, and children’s lung function growth. Am J Respir Crit Care Med 2011; 183:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med 2015; 372:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neophytou AM, White MJ, Oh SS, Thakur N, Galanter JM, Nishimura KK, et al. Air Pollution and Lung Function in Minority Youth with Asthma in the GALA II (Genes-Environments and Admixture in Latino Americans) and SAGE II (Study of African Americans, Asthma, Genes, and Environments) Studies. Am J Respir Crit Care Med 2016; 193:1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilliland F, Avol E, McConnell R, Berhane K, Gauderman WJ, Lurmann FW, et al. The Effects of Policy-Driven Air Quality Improvements on Children’s Respiratory Health. Res Rep Health Eff Inst 2017:1–75. [PMC free article] [PubMed] [Google Scholar]
- 55.Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 2014; 69:540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee D, Wallis C, Van Winkle LS, Wexler AS. Disruption of tracheobronchial airway growth following postnatal exposure to ozone and ultrafine particles. Inhal Toxicol 2011; 23:520–31. [DOI] [PubMed] [Google Scholar]
- 57.Avdalovic MV, Tyler NK, Putney L, Nishio SJ, Quesenberry S, Singh PJ, et al. Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates. Anatomical Record 2012; 295:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee D, Wallis C, Wexler AS, Schelegle ES, Van Winkle LS, Plopper CG, et al. Small particles disrupt postnatal airway development. J Appl Physiol (1985) 2010; 109:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol 2003; 191:74–85. [DOI] [PubMed] [Google Scholar]
- 60.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, et al. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 2006; 291:L644–50. [DOI] [PubMed] [Google Scholar]
- 61.Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol (1985) 2002; 92:1019–28. [DOI] [PubMed] [Google Scholar]
- 62.Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, et al. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol (1985) 2009; 107:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Usemann J, Decrue F, Korten I, Proietti E, Gorlanova O, Vienneau D, et al. Exposure to moderate air pollution and associations with lung function at school-age: A birth cohort study. Environ Int 2019; 126:682–9. [DOI] [PubMed] [Google Scholar]
- 64.Bougas N, Rancière F, Beydon N, Viola M, Perrot X, Gabet S, et al. Traffic-related Air Pollution, Lung Function, and Host Vulnerability. New Insights from the PARIS Birth Cohort. Ann Am Thorac Soc 2018; 15:599–607. [DOI] [PubMed] [Google Scholar]
- 65.Bergstra AD, Brunekreef B, Burdorf A. The effect of industry-related air pollution on lung function and respiratory symptoms in school children. Environ Health 2018; 17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He B, Huang JV, Kwok MK, Au Yeung SL, Hui LL, Li AM, et al. The association of early-life exposure to air pollution with lung function at ~17.5 years in the “Children of 1997” Hong Kong Chinese Birth Cohort. Environ Int 2019; 123:444–50. [DOI] [PubMed] [Google Scholar]
- 67.Tsui HC, Chen CH, Wu YH, Chiang HC, Chen BY, Guo YL. Lifetime exposure to particulate air pollutants is negatively associated with lung function in non-asthmatic children. Environ Pollut 2018; 236:953–61. [DOI] [PubMed] [Google Scholar]
- 68.Bose S, Rosa MJ, Mathilda Chiu YH, Leon Hsu HH, Di Q, Lee A, et al. Prenatal nitrate air pollution exposure and reduced child lung function: Timing and fetal sex effects. Environ Res 2018; 167:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med 1996; 335:931–7. [DOI] [PubMed] [Google Scholar]
- 70.Gibson EA, Goldsmith J, Kioumourtzoglou MA. Complex Mixtures, Complex Analyses: an Emphasis on Interpretable Results. Curr Environ Health Rep 2019; 6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect 2013; 121:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gold DR, Damokosh AI, Pope CA 3rd, Dockery DW, McDonnell WF, Serrano P, et al. Particulate and ozone pollutant effects on the respiratory function of children in southwest Mexico City. Epidemiology 1999; 10:8–16. [PubMed] [Google Scholar]
- 73.Stafoggia M, Breitner S, Hampel R, Basagaña X. Statistical Approaches to Address Multi-Pollutant Mixtures and Multiple Exposures: the State of the Science. Curr Environ Health Rep 2017; 4:481–90. [DOI] [PubMed] [Google Scholar]
- 74.Zanobetti A, Coull BA, Luttmann-Gibson H, Rossem Lv, Rifas-Shiman SL, Kloog I, et al. Ambient Particle Components and Newborn Blood Pressure in Project Viva. Journal of the American Heart Association 2021; 10:e016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian Z, He Q, Kong L, Xu F, Wei F, Chapman RS, et al. Respiratory responses to diverse indoor combustion air pollution sources. Indoor Air 2007; 17:135–42. [DOI] [PubMed] [Google Scholar]
- 76.Rinne ST, Rodas EJ, Bender BS, Rinne ML, Simpson JM, Galer-Unti R, et al. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural Ecuador. Respir Med 2006; 100:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jedrychowski W, Maugeri U, Jedrychowska-Bianchi I, Flak E. Effect of indoor air quality in the postnatal period on lung function in pre-adolescent children: a retrospective cohort study in Poland. Public Health 2005; 119:535–41. [DOI] [PubMed] [Google Scholar]
- 78.Roy A, Chapman RS, Hu W, Wei F, Liu X, Zhang J. Indoor air pollution and lung function growth among children in four Chinese cities. Indoor Air 2012; 22:3–11. [DOI] [PubMed] [Google Scholar]
- 79.Shen S, Qin Y, Cao Z, Shang J, Liu Y, Yang X, et al. Indoor air pollution and pulmonary function in children. Biomed Environ Sci 1992; 5:136–41. [PubMed] [Google Scholar]
- 80.Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect 2007; 115:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saleh S, Shepherd W, Jewell C, Lam NL, Balmes J, Bates MN, et al. Air pollution interventions and respiratory health: a systematic review. Int J Tuberc Lung Dis 2020; 24:150–64. [DOI] [PubMed] [Google Scholar]
- 82.Heinzerling AP, Guarnieri MJ, Mann JK, Diaz JV, Thompson LM, Diaz A, et al. Lung function in woodsmoke-exposed Guatemalan children following a chimney stove intervention. Thorax 2016; 71:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noonan CW, Ward TJ, Semmens EO. Estimating the number of vulnerable people in the United States exposed to residential wood smoke. Environ Health Perspect 2015; 123:A30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rokoff LB, Koutrakis P, Garshick E, Karagas MR, Oken E, Gold DR, et al. Wood Stove Pollution in the Developed World: A Case to Raise Awareness Among Pediatricians. Curr Probl Pediatr Adolesc Health Care 2017; 47:123–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guneser S, Atici A, Alparslan N, Cinaz P. Effects of indoor environmental factors on respiratory systems of children. J Trop Pediatr 1994; 40:114–6. [DOI] [PubMed] [Google Scholar]
- 86.Nicole W Cooking up indoor air pollution: emissions from natural gas stoves. Environ Health Perspect 2014; 122:A27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Logue JM, Klepeis NE, Lobscheid AB, Singer BC. Pollutant Exposures from Natural Gas Cooking Burners: A Simulation-Based Assessment for Southern California. ; Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States), 2014:Medium: ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol 2009; 39:743–81. [DOI] [PubMed] [Google Scholar]
- 89.Brunekreef B, Houthuijs D, Dijkstra L, Boleij JS. Indoor nitrogen dioxide exposure and children’s pulmonary function. J Air Waste Manage Assoc 1990; 40:1252–6. [DOI] [PubMed] [Google Scholar]
- 90.Jarvis D, Chinn S, Luczynska C, Burney P. Association of respiratory symptoms and lung function in young adults with use of domestic gas appliances. Lancet 1996; 347:426–31. [DOI] [PubMed] [Google Scholar]
- 91.Moshammer H, Fletcher T, Heinrich J, Hoek G, Hruba F, Pattenden S, et al. Gas cooking is associated with small reductions in lung function in children. Eur Respir J 2010; 36:249–54. [DOI] [PubMed] [Google Scholar]
- 92.Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care Med 2006; 173:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am J Respir Crit Care Med 1998; 158:891–5. [DOI] [PubMed] [Google Scholar]
- 94.Kile ML, Coker ES, Smit E, Sudakin D, Molitor J, Harding AK. A cross-sectional study of the association between ventilation of gas stoves and chronic respiratory illness in U.S. children enrolled in NHANESIII. Environ Health 2014; 13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown SK. Occurrence of Volatile Organic Compounds in Indoor Air. In: Organic Indoor Air Pollutants; 1999. p. 170–84. [Google Scholar]
- 96.What are volatile organic compounds (VOCs)?] Available from https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs.
- 97.Finlayson-Pitts B, Pitts J Jr. Indoor air pollution: Sources, levels, chemistry and fates. Chemistry of the Upper and Lower Atmosphere 2000:844–70. [Google Scholar]
- 98.Batterman S, Jia C, Hatzivasilis G. Migration of volatile organic compounds from attached garages to residences: a major exposure source. Environ Res 2007; 104:224–40. [DOI] [PubMed] [Google Scholar]
- 99.Billionnet C, Gay E, Kirchner S, Leynaert B, Annesi-Maesano I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ Res 2011; 111:425–34. [DOI] [PubMed] [Google Scholar]
- 100.Mendell MJ. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: a review. Indoor Air 2007; 17:259–77. [DOI] [PubMed] [Google Scholar]
- 101.Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 2004; 59:746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diez U, Kroessner T, Rehwagen M, Richter M, Wetzig H, Schulz R, et al. Effects of indoor painting and smoking on airway symptoms in atopy risk children in the first year of life results of the LARS-study. Leipzig Allergy High-Risk Children Study. Int J Hyg Environ Health 2000; 203:23–8. [DOI] [PubMed] [Google Scholar]
- 103.Cakmak S, Dales RE, Liu L, Kauri LM, Lemieux CL, Hebbern C, et al. Residential exposure to volatile organic compounds and lung function: results from a population-based cross-sectional survey. Environ Pollut 2014; 194:145–51. [DOI] [PubMed] [Google Scholar]
- 104.Gutiérrez-Delgado RI, Barraza-Villarreal A, Escamilla-Núñez MC, Hernández-Cadena L, Cortez-Lugo M, Sly P, et al. Prenatal exposure to VOCs and NOx and lung function in preschoolers. Pediatr Pulmonol 2020; 55:2142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gray D, Willemse L, Visagie A, Czövek D, Nduru P, Vanker A, et al. Determinants of early-life lung function in African infants. Thorax 2017; 72:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morales E, Garcia-Esteban R, de la Cruz OA, Basterrechea M, Lertxundi A, de Dicastillo MD, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015; 70:64–73. [DOI] [PubMed] [Google Scholar]
- 107.Cui X, Li F, Xiang J, Fang L, Chung MK, Day DB, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: A double-blind randomized crossover study. Environ Int 2018; 114:27–36. [DOI] [PubMed] [Google Scholar]
- 108.McDonald E, Cook D, Newman T, Griffith L, Cox G, Guyatt G. Effect of air filtration systems on asthma: a systematic review of randomized trials. Chest 2002; 122:1535–42. [DOI] [PubMed] [Google Scholar]
- 109.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sugiri D, Ranft U, Schikowski T, Krämer U. The influence of large-scale airborne particle decline and traffic-related exposure on children’s lung function. Environ Health Perspect 2006; 114:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frye C, Hoelscher B, Cyrys J, Wjst M, Wichmann HE, Heinrich J. Association of lung function with declining ambient air pollution. Environ Health Perspect 2003; 111:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007; 369:571–7. [DOI] [PubMed] [Google Scholar]
- 113.MacNeill SJ, Goddard F, Pitman R, Tharme S, Cullinan P. Childhood peak flow and the Oxford Transport Strategy. Thorax 2009; 64:651–6. [DOI] [PubMed] [Google Scholar]
- 114.Mudway IS, Dundas I, Wood HE, Marlin N, Jamaludin JB, Bremner SA, et al. Impact of London’s low emission zone on air quality and children’s respiratory health: a sequential annual cross-sectional study. Lancet Public Health 2019; 4:e28–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adar SD, D’Souza J, Sheppard L, Kaufman JD, Hallstrand TS, Davey ME, et al. Adopting Clean Fuels and Technologies on School Buses. Pollution and Health Impacts in Children. Am J Respir Crit Care Med 2015; 191:1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glazener A, Khreis H. Transforming Our Cities: Best Practices Towards Clean Air and Active Transportation. Curr Environ Health Rep 2019; 6:22–37. [DOI] [PubMed] [Google Scholar]
- 117.Good N, Molter A, Ackerson C, Bachand A, Carpenter T, Clark ML, et al. The Fort Collins Commuter Study: Impact of route type and transport mode on personal exposure to multiple air pollutants. J Expo Sci Environ Epidemiol 2016; 26:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodrigues PF, Alvim-Ferraz MCM, Martins FG, Saldiva P, Sa TH, Sousa SIV. Health economic assessment of a shift to active transport. Environ Pollut 2020; 258:113745. [DOI] [PubMed] [Google Scholar]
- 119.Rojas-Rueda D, de Nazelle A, Teixido O, Nieuwenhuijsen MJ. Health impact assessment of increasing public transport and cycling use in Barcelona: a morbidity and burden of disease approach. Prev Med 2013; 57:573–9. [DOI] [PubMed] [Google Scholar]
- 120.What You Can Do to Reduce Air Pollution Near Schools. Department of Environmental Quality.] Available from oregon.gov/deq/aq/Pages/Pollution-Near-Schools.aspx.
- 121.Transport for London. Healthy Streets for London: Prioritising Walking, Cycling and Public Transport to Create a Healthy City. London, 2017. [Google Scholar]
- 122.Qiu L, Liu F, Zhang X, Gao T. Difference of Airborne Particulate Matter Concentration in Urban Space with Different Green Coverage Rates in Baoji, China. Int J Environ Res Public Health 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Underhill LJ, Fabian MP, Vermeer K, Sandel M, Adamkiewicz G, Leibler JH, et al. Modeling the resiliency of energy-efficient retrofits in low-income multifamily housing. Indoor Air 2018; 28:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nardone A, Casey JA, Morello-Frosch R, Mujahid M, Balmes JR, Thakur N. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: an ecological study. Lancet Planet Health 2020; 4:e24–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krieger N, Van Wye G, Huynh M, Waterman PD, Maduro G, Li W, et al. Structural Racism, Historical Redlining, and Risk of Preterm Birth in New York City, 2013–2017. Am J Public Health 2020; 110:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cusick D Past Racist “Redlining” Practices Increased Climate Burden on Minority Neighborhoods. Scientific American. New York, NY: Springer Nature America, 2020. [Google Scholar]
- 127.Sordillo JE, Rifas-Shiman SL, Switkowski K, Coull B, Gibson H, Rice M, et al. Prenatal oxidative balance and risk of asthma and allergic disease in adolescence. J Allergy Clin Immunol 2019; 144:1534–41.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhong J, Karlsson O, Wang G, Li J, Guo Y, Lin X, et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci U S A 2017; 114:3503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong J, Speck M, Urch B, Silverman F, Gold DR, Koutrakis P, et al. Reply to Lucock et al.: Significance of interpretation and misinterpretation of a small mechanistic study. Proc Natl Acad Sci U S A 2017; 114:E3880–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moreno-Macías H, Dockery DW, Schwartz J, Gold DR, Laird NM, Sienra-Monge JJ, et al. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: a cohort study. Respir Res 2013; 14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ambient (outdoor) air pollution. World Health Organization.] Available from https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.


