Highlights
-
•
Soft tissue sarcomas have traditionally been treated with a one-size fits all approach, despite a wide range of histologies and clinical outcomes.
-
•
The radiosensitivity index has demonstrated that soft tissue sarcomas are in general radioresistant, however exhibit a wide range of radiosensitivity.
-
•
These differences in radiosensitivity are associated with decreased locoregional control in patients with radioresistant histologies.
-
•
Using the radiosensitivity index we identify specific histologies of soft tissue sarcoma that may be more radioresistant, and suggest a genomic-based radiation dosing framework.
Keywords: Radiation therapy, Radiosensitivity, Genomic-adjusted radiation dose
Abstract
Background
Soft-tissue sarcomas (STS) are heterogeneous with variable response to radiation therapy (RT). Utilizing the radiosensitivity index (RSI) we estimated the radiobiologic ratio of lethal to sublethal damage (α/β), genomic-adjusted radiation dose(GARD), and in-turn a biological effective radiation dose (BED).
Methods
Two independent cohorts of patients with soft-tissue sarcoma were identified. The first cohort included 217 genomically-profiled samples from our institutional prospective tissue collection protocol; RSI was calculated for these samples, which were then used to dichotomize the population as either highly radioresistant (HRR) or conventionally radioresistant (CRR). In addition, RSI was used to calculate α/β ratio and GARD, providing ideal dosing based on sarcoma genomic radiosensitivity. A second cohort comprising 399 non-metastatic-STS patients treated with neoadjuvant RT and surgery was used to validate our findings.
Results
Based on the RSI of the sample cohort, 84% would historically be considered radioresistant. We identified a HRR subset that had a significant difference in the RSI, and clinically a lower tumor response to radiation (2.4% vs. 19.4%), 5-year locoregional-control (76.5% vs. 90.8%), and lower estimated α/β (3.29 vs. 5.98), when compared to CRR sarcoma. Using GARD, the dose required to optimize outcome in the HRR subset is a BEDα/β=3.29 of 97 Gy.
Conclusions
We demonstrate that on a genomic scale, that although STS is radioresistant overall, they are heterogeneous in terms of radiosensitivity. We validated this clinically and estimated an α/β ratio and dosing that would optimize outcome, personalizing dose.
Research in context:
Current preoperative radiotherapy in soft-tissue sarcoma (STS) does not take into account potential differences in intrinsic radiosensitivity. The radiosensitivity index (RSI) has identified STS as overall radioresistant. We hypothesize that RSI can be used to distinguish highly radioresistant STS histologies, which may subsequently allow the individualization of radiotherapy dose using the genomic adjusted radiation dose (GARD).
Added value of this study
We demonstrate that RSI can be used to group STS histologies into conventionally and highly radioresistant histologies. We demonstrate a difference in locoregional control between these groups.
Implications
We demonstrate the utility of RSI in identifying radiosensitivity of STS histologies and propose this as the framework for personalized radiotherapy dose in STS.
Alt-text: Unlabelled box
Introduction
Soft tissue sarcomas (STS) constitute a heterogeneous group of rare solid tumors of mesenchymal cell origin with distinct histological and clinical features. Due to the rarity of STS and the diverse histopathological subtypes, the study and treatment of STS has been challenging [1]. STS has generally been classified into subtypes according to their histological resemblance to normal tissue. Common subtypes of STS include undifferentiated pleomorphic sarcoma (UPS), liposarcoma (LPS), and leiomyosarcoma (LMS) [2]. The mainstay in the treatment of STS is surgery, but the addition of perioperative radiation treatment (RT) has allowed for improved limb preservation and improved locoregional control (LRC) [3,4]. The RT volume, dose, and timing of treatment are clinical decisions primarily based on clinical features (e.g. size, tumor location, etc.).
Since there is evidence that favorable pathologic response (fPR≥90–95%) can predict for LRC and OS [5,6], neoadjuvant dose escalation has been considered but is limited by toxicity and long-term sequelae. Currently, retroperitoneal (RP) STS is one of the only subtypes considered for selective dose-escalation, but this decision is based on expected high microscopic residual tumor burden (e.g. anatomic challenges preventing clear margins) rather than specific tumor biology [7], [8], [9]. To date, this is the first exploration of utilizing biology to personalize RT-based treatment for soft tissue sarcomas.
Improvements in understanding the intrinsic radiation sensitivity of STS can potentially guide the evolution of more effective RT. Our group has previously described an algorithm to assess the intrinsic radiosensitivity of tumors using a genome-based approach - the radiosensitivity index (RSI) [10], [11], [12]. RSI is a 10-gene signature that has been clinically validated in over 2200 RT- receiving patients across multiple malignancies, including: glioblastomas, breast, colon, pancreas, and lung tumors [13], [14], [15], [16], [17], [18], [19], [20]. In radiobiology, the intrinsic radiosensitivity of a cell has been commonly referred to as the alpha (α), whereas the beta (β) reflects a cell's ability for cellular repair [21]. Therefore, patients with a low α/β ratio, as seen in sarcoma (2 to 6), require higher radiation dose per fraction to have a biologically equivalent dose [22], [23], [24]. Further, RSI can be used to estimate a tumor specific α value creating a personalized α/β [18,25]. In addition, our group developed a model which incorporates RSI and RT dose to estimate the genomically based effective dose, referred to as the genomic adjusted radiation dose (GARD), which can be utilized to personalize RT dose prescription for individual patients [18].
Although soft-tissue sarcoma (STS) is thought to be resistant to radiation, we hypothesize that there will be significant variability in the gene expression-based radiosensitivity index (RSI). Our goal will be to identify radioresistant subsets of STS and evaluate for differences in α/β ratio, and whether this translates to a difference in a response to neoadjuvant radiation, as measured by favorable pathologic response (FPR) and local control (LC), We will then utilize GARD to identify a biological effective dose required to optimize patient outcome.
Materials and methods
Identification of clinical correlates for RSI in STS: The study was institutional review board (IRB) approved by the University of South Florida and Moffitt Cancer Center (MCC). This cohort was extracted from Total Cancer Care (TCC), a prospective IRB-approved data and tissue collection protocol active at Moffitt and 18 other institutions since 2006[26]. Tumors from patients enrolled in TCC protocol were arrayed on Affymetrix Hu-RSTA-2a520709 (Affymetrix, Santa Clara, CA), which contains approximately 60,000 probesets representing 25,000 genes. Chips were normalized using iterative rank-order normalization (IRON) [27]. Batch-effects were reduced using partial-least squares (PLS). To identify clinical correlates for RSI in STS, we abstracted patient information from the TCC database.
For clinical correlation, we retrospectively reviewed a cohort of 399 STS patients treated with neoadjuvant RT and surgery from 11/1987 to 1/2016. Neoadjuvant therapy consisted of preoperative radiation to a median dose of 50 Gy in 25 fractions in 2 Gy daily fractions – treatment technique used was 3D-conformal RT or intensity modulated RT (IMRT). The patient and clinical characteristics were obtained by the TCC protocol and reviewed from the clinical chart. Osteosarcoma, Ewing sarcoma, and chondrosarcoma histologies were confirmed as extraskeletal in origin. Pathologists specializing in soft tissue sarcoma confirmed all histologies and determined response at the time of surgery, by quantifying percentage of viable cells remaining in concert with tumor necrosis, when possible.
Individual tumor intrinsic radiosensitivity assessment
RSI score was calculated with the previously published algorithm listed below[10], [11], [12] where the lower the RSI, the more radiosensitive the tumor.
RSI = – 0.0098009 * AR + 0.0128283 * cJun + 0.0254552 * STAT1 – 0.0017589 * PKC – 0.0038171 * RelA + 0.1070213 * caBL – 0.0002509 * SUMO1 – 0.0092431 * PAK2 – 0.0204469 * HDAC1 – 0.0441683 * IRF1
A previously described RSI cut-off of ≥0.375 was identified as radioresistant [18]. This cut-point was utilized to determine the proportion of sarcoma histologies deemed radioresistant when compared to other tumor types similarly assessed by RSI. To dichotomize our clinical cohort into highly radioresistant sarcomas (HRR) or conventionally radioresistant sarcomas (CRR), we evaluated the 75th percentile RSI value for each histology, against the RSI distribution for the whole cohort. Histologies with a median at or above the 75th percentile RSI value for the whole cohort were classified as highly radioresistant.
Estimating personalized α/β and genomic-adjusted radiation dose in sarcoma
A patient-specific α was derived by substituting the radiosensitivity index for survival (S) in S= e–nd(α + βd), where dose (d) is 2 Gy, n=1, and β was derived from sarcoma cell line experiments (0.045/Gy) [23,24,28]. The algorithm of GARD has been previously described [18]. GARD was derived using the linear quadratic model and the individual gene-expression-based RSI, defined as GARD=nd(α + βd). GARD was modeled for the neoadjuvant radiation dose in this study (50 Gy in 25 fractions). The calculation for GARD is similar to the biologically effective dose (BED) but is genomically determined with a patient-specific α. A higher GARD predicts a higher radiation therapeutic effect on the tumor.
To identify a GARD value that distinguishes CRR and HRR sarcomas, receiver operator characteristic (ROC) analysis was utilized to guide recommendations for dose which maximized sensitivity and specificity. Using the specific α/β, we determined the dose required for highly radioresistant sarcomas (HRR) to achieve effective therapeutic doses as the conventionally radioresistant sarcomas (CRR), with respect to total number of fractions (n), and daily dose (d).
Statistical analysis
Descriptive statistics were administered to assess the clinical cohort for continuous and categorical variables with comparisons using Pearson Chi Square and Mann-Whitney U test as appropriate. Kaplan-Meier curves with log-rank tests were calculated from the start of RT to the date of event or last follow-up. Locoregional control was defined as absence of failure within gross disease in the primary site. Univariate and multivariate analyses (UVA and MVA, respectively) were conducted with Cox proportional-hazard models. Multivariate analysis included variables associated with locoregional control, such as age (≥50 vs. <50), margin status, primary site, and clinical tumor classification. Grade of disease was omitted from MVA due to difficulties with consistent grading after neoadjuvant therapy.
SPSS25 (IBM corporation, Armonk, NY) and JMP 15(SAS Institute, Cary NC), were used for statistical analyses and generation of figures and graphs. A two-tailed p <0.05 was considered statistically significant. Mathematical calculations were performed using Excel 2016 (Microsoft, Redmond WA).
Results
Highly radioresistant sarcomas as a clinical correlate for radioresistance
There were a total of 217 resected sarcoma samples available. The most common histologies were leiomyosarcoma (n=53, 24.2%) and non-myxoid liposarcoma (n=44, 20.2%) (Table 1). Table 1 depicts the distributions of RSI by histology.
Table 1.
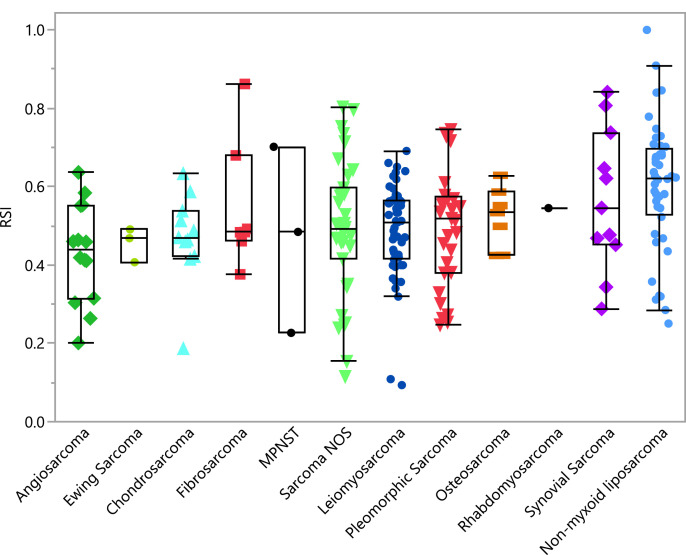
Specimen Cohort Characteristics – the most radiosensitive histologies are angiosarcoma, Ewing sarcoma, and chondrosarcoma. The most radioresistant histology is non-myxoid liposarcoma, with a median RSI value above 0.60 and above the 75th percentile of all RSI values. Abbrev: malignant peripheral nerve sheath tumor (MPNST), not otherwise specified (NOS)
| Count | ||||||
|---|---|---|---|---|---|---|
| Histology | N | % | Mean | Median | RSI Minimum | RSI Maximum |
| Angiosarcoma | 14 | 6.4% | 0.43 | 0.44 | 0.20 | 0.64 |
| Ewing Sarcoma | 3 | 1.4% | 0.46 | 0.47 | 0.41 | 0.49 |
| Chondrosarcoma | 11 | 5.0% | 0.47 | 0.47 | 0.19 | 0.63 |
| MPNST | 3 | 1.4% | 0.47 | 0.48 | 0.23 | 0.70 |
| Sarcoma NOS | 35 | 16.1% | 0.49 | 0.49 | 0.12 | 0.80 |
| Pleomorphic Sarcoma | 29 | 13.3% | 0.50 | 0.52 | 0.25 | 0.75 |
| Leiomyosarcoma | 53 | 24.3% | 0.49 | 0.51 | 0.09 | 0.69 |
| Osteosarcoma | 7 | 3.2% | 0.52 | 0.53 | 0.42 | 0.63 |
| Rhabdomyosarcoma | 1 | 0.5% | 0.54 | 0.54 | 0.54 | 0.54 |
| Fibrosarcoma | 7 | 3.2% | 0.55 | 0.48 | 0.38 | 0.86 |
| Synovial Sarcoma | 11 | 5.0% | 0.57 | 0.55 | 0.29 | 0.84 |
| Non-myxoid Liposarcoma | 44 | 20.2% | 0.60 | 0.62 | 0.25 | 1.00 |
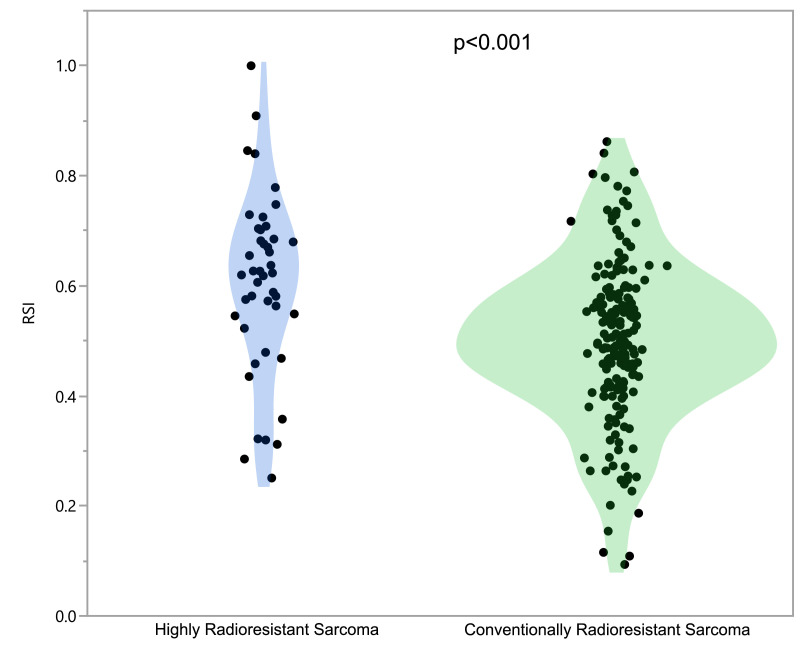
There was a significant overall difference in RSI when comparing all histologies (ANOVA test p=0.011) (Fig. 1a), especially HRR, when compared to CRR. (Fig. 1b). One hundred ninety-five (84%) of the samples had RSI ≥0.375, and would be considered radioresistant when compared to other cancers [15]. The most radiosensitive histologies were angiosarcoma (median 0.44, range 0.2–0.64) and extraskeletal Ewing sarcoma (median 0.47, range 0.41–0.49),whereas the least radiosensitive histology was non-myxoid liposarcoma (median 0.62, 0.25–1.0).
Fig. 1a.
RSI plot by individual sarcoma histology.
Fig. 1b.
Highly Radioresistant Sarcoma and Conventionally Radioresistant sarcoma violin plot for RSI.
3.2. HRR histology is associated with worse pathological response and clinical outcome.
The 25th and 75th percentile of RSI were 0.42 and 0.62 – using the 75th percentile we designated the histologies with median RSI values above this as highly radioresistant sarcoma (HRR), in contrast to histologies which fell below this threshold, termed conventionally radioresistant sarcoma (CRR). Non-myxoid liposarcoma was the sole histology within the HRR cohort (n=44), with the remaining sarcoma histologies comprising the CRR group (n=173).
Based on the RSI data from resected specimens, HRR histology was used as a clinical surrogate for radioresistance in STS. Fig. 1b shows the difference in RSI of STS when dichotomized with this approach (Mann-Whitney U p<0.001).
The clinical characteristics of the STS cohort (n=399) are presented in Table 2. The cohort was most commonly HRR (n=67, 16.7%), age ≥50 (n=313, 78.4%), cT2b (n=280, 70.2%), high grade (n=223, 55.9%), negative surgical margins (n=274, 68.7%), and extremity site (n=241, 60.4%) (Table 2a). There is a significant difference in characteristics by histology with HRR having more unknown grade (p=0.01), retroperitoneal primary site (p<0.01), and higher portion of positive surgical margins (p<0.01) (Table 2a). The histology specific breakdown are illustrated in Table 2b.
Table 2a.
Clinical Cohort Characteristics – patients were balanced with regards to age and clinical tumor stage. Highly radioresistant sarcomas had a large proportion of low grade disease, retroperitoneal primary site, and higher positive margin rate. Institutional practice considers a positive margin to include disease suspicious for well-differentiated liposarcoma at ink. Of the HRR patients with positive margins, only 7 had high-grade/dedifferentiated liposarcoma at the surgical margin.
| Characteristic |
Overall |
Highly Radioresistant (HRR) |
Conventionally Radioresistant (CRR) |
Chi Square | ||||
|---|---|---|---|---|---|---|---|---|
| (p value) | ||||||||
| N | % | N | % | N | % | |||
| Age | Age <50 years | 86 | 21.6% | 15 | 22.4% | 71 | 21.4% | 0.86 |
| Age ≥50 years | 313 | 78.4% | 52 | 77.6% | 261 | 78.6% | ||
| Tumor Stage | cT1a | 7 | 1.8% | 1 | 1.5% | 6 | 1.5% | 0.61 |
| cT1b | 39 | 9.8% | 5 | 7.5% | 34 | 8.5% | ||
| cT2a | 30 | 7.5% | 3 | 4.5% | 27 | 6.8% | ||
| cT2b | 280 | 70.2% | 51 | 76.1% | 229 | 57.4% | ||
| Unknown stage | 43 | 10.8% | 7 | 10.4% | 36 | 9.0% | ||
| Grade | Low grade | 16 | 4.0% | 10 | 14.9% | 6 | 1.8% | <0.01 |
| High grade | 223 | 55.9% | 36 | 53.7% | 187 | 56.3% | ||
| Unknown | 160 | 40.3% | 21 | 31.3% | 139 | 41.9% | ||
| Primary Site | Extremity | 241 | 60.4% | 23 | 34.3% | 218 | 65.7% | <0.01 |
| Retroperitoneal | 88 | 22.1% | 32 | 47.8% | 56 | 16.9% | ||
| Other | 70 | 17.5% | 12 | 17.9% | 58 | 17.5% | ||
| Surgical Margins | Negative | 274 | 68.7% | 31 | 46.3% | 243 | 73.2% | <0.01 |
| Positive | 115 | 28.8% | 36 | 53.7% | 79 | 23.8% | ||
| Unknown | 10 | 2.5% | 0 | 0.0% | 10 | 3.0% | ||
Table 2b.
Sarcoma Histology Subtypes. The most common HRR subtypes were de-differentiated and well-differentiated liposarcoma. The most common CRR subtypes were pleomorphic sarcoma and giant cell sarcoma.
| Non-myxoid liposarcoma histology subtypes (n=67) | N | % |
|---|---|---|
| Liposarcoma, NOS | 11 | 16.4% |
| Well-Differentiated Liposarcoma | 17 | 25.4% |
| Pleomorphic Liposarcoma | 11 | 16.4% |
| De-differentiated Liposarcoma | 28 | 41.8% |
| Other sarcoma histology subtypes (n=332) | N | % |
|---|---|---|
| Alveolar soft part sarcoma | 1 | 0.3% |
| Angiosarcoma | 19 | 5.7% |
| Extraskeletal chondrosarcoma | 9 | 2.7% |
| Dermatofibrosarcoma, NOS | 1 | 0.3% |
| Epitheloid sarcoma | 1 | 0.3% |
| Extraskeletal Ewing sarcoma | 5 | 1.5% |
| Fibromyxosarcoma | 32 | 9.6% |
| Fibrosarcoma, NOS | 7 | 2.1% |
| Leiomyosarcoma | 29 | 8.7% |
| Malignant solitary fibrous tumor | 2 | 0.6% |
| Malignant peripheral nerve sheath tumor | 7 | 2.1% |
| Myxoid sarcoma (NOS) | 2 | 0.6% |
| Extraskeletal osteosarcoma | 3 | 0.9% |
| Undifferentiated pleomorphic sarcoma | 88 | 26.5% |
| Primitive neuroectodermal tumor | 2 | 0.6% |
| Rhabdomyosarcoma | 6 | 1.8% |
| Sarcoma, NOS | 32 | 9.6% |
| Spindle cell Sarcoma | 32 | 9.6% |
| Stromal sarcoma | 9 | 2.7% |
| Synovial sarcoma | 24 | 7.2% |
| Undifferentiated sarcoma | 21 | 6.3% |
Response to therapy
In the entire cohort, the median tumor response to RT was 52%, with 16.4% achieving a ≥95% pathologic response rate. HRR patients had a lower rate of treatment response to RT (median 35% vs. 60%, p=0.01), with a significant difference in favorable pathologic response (≥95%: 2.4% vs. 19.4%, p<0.01).
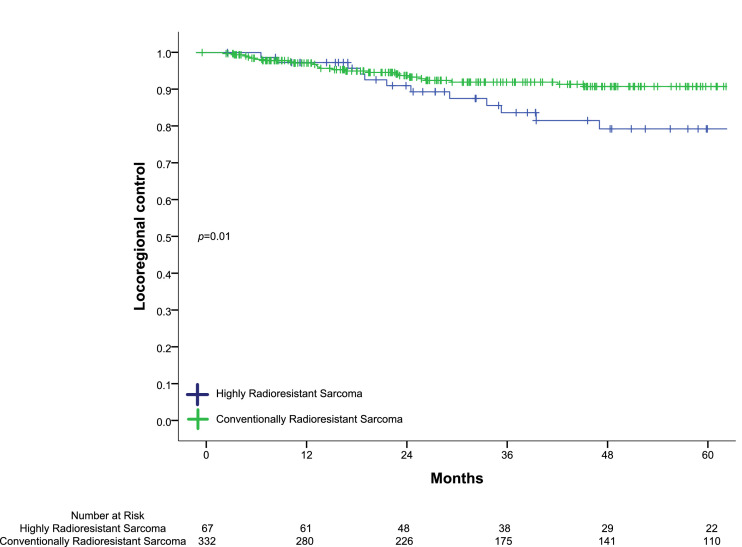
At a median follow-up of 58.9 months in surviving patients, the 5-year LRC was 88.1%. On UVA, HRR had a significant decrease in 5-year LRC (76.5% vs. 90.8%, p=0.01) (Fig. 2) compared to CRR. On MVA, HRR histology was an independent predictor for worse LRC, with an HR of 2.54 (95% CI 1.23-5.22 p=0.01) (Table 3). There was no significant difference in 5-year OS between groups (p=0.08).
Fig. 2.
Highly Radioresistant Sarcoma and Conventionally Radioresistant Sarcoma violin plot for RSI.
Table 3.
Multivariate Analysis for locoregional control demonstrated HRR histology as an independent predictor of worse locoregional control, even when evaluating for other features that are traditionally considered as risk factors for recurrence such as age, margin status, clinical T-stage, and primary site.
| Characteristic | Hazard ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Age <50 years | 1.00 | Ref | - |
| Age ≥50 years | 1.35 | 0.59–3.10 | 0.48 |
| Margin status | |||
| Negative surgical margins | 1.00 | Ref | - |
| Positive surgical margins | 1.87 | 0.93–3.80 | 0.08 |
| Clinical T-stage | |||
| T1 | 1.00 | Ref | - |
| T2 | 0.70 | 0.33–2.10 | 0.84 |
| Histology | |||
| Highly Radioresistant | 2.54 | 1.23–5.22 | 0.01 |
| Conventionally Radioresistant | 1.00 | Ref | - |
| Primary site | |||
| Extremity | 1.00 | Ref | - |
| Retroperitoneum/Pelvis | 0.64 | 0.27–1.53 | 0.32 |
| Other | 1.09 | 0.46–2.53 | 0.85 |
GARD modeling
Using specimen specific-RSI to calculate a patient-specific α and sarcoma-specific β (0.045), we calculated the median α/β of the entire cohort as 5.42, which was significantly lower in HRR (3.29 IQR 2.1-5.0) when compared to CRR (5.98 IQR 4.0-7.7, p<0.01).
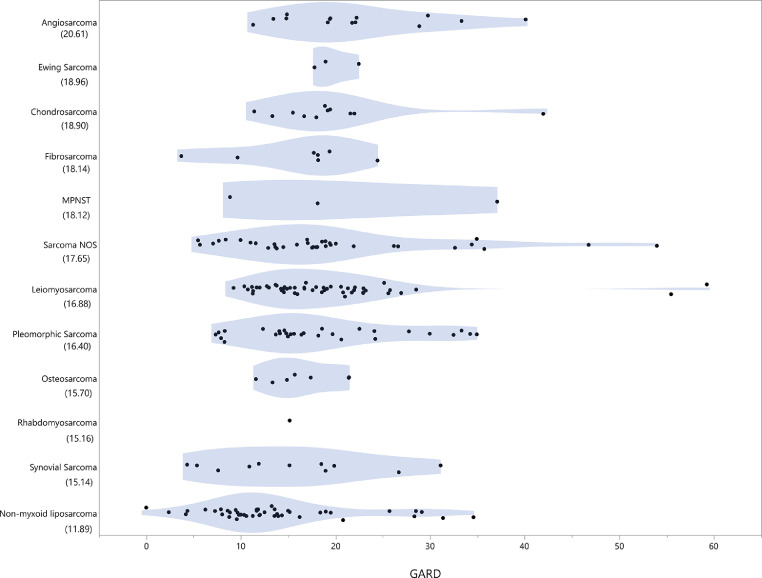
When the GARD was modeled for a total delivered dose of 50 Gy in 2 Gy fractions we observed a wide range of GARD distributed amongst sarcoma histologies. The median delivered GARD was 16.7 (Fig. 3), which was significantly lower for HRR (11.9), when compared to CRR (18.0) (p<0.01).
Fig. 3.
Violin plot of GARD by histology with median GARD values. Sarcoma histologies are listed in decreasing GARD, with increasing GARD representing more effective radiation dose based on individual tumor RSI value. Median GARD of the entire cohort was 16.7, with HRR (non-myxoid liposarcoma) median GARD of 11.9, and CRR histologies median GARD of 18.0.
Given the clinically significant differences in LRC between HRR and CRR, we modeled the radiation dose-escalation required based on the GARD for CRR. This increase in GARD reflects the differences in intrinsic radiosensitivity between HRR and CRR. A threshold GARD of 14.37 was estimated as the ideal cut-point that significantly differentiated between HRR and CRR sarcomas (sensitivity of 70.5%, specificity of 71.7%, and an area under curve (AUC) of 0.71, p<0.01).
A small proportion of HRR patients (7/44, 16%) achieved the GARD threshold (14.37), reflecting a range of BEDα/β=3.29 from 57 to 178 Gy after neoadjuvant radiation (50 Gy) was delivered. In contrast, majority of CRR patients (124/173, 71.6%) achieved above the GARD threshold, with a higher BEDα/β=5.98 range (54.1 to 310.5 Gy).
When modeling GARD, the additional number of fractions needed by HRR (α/β= 3.29) to achieve the GARD threshold (14.37) is 5.2 fractions, at 2 Gy per fraction, totaling 60.4 Gy. Derivation of dose per fraction needed to achieve a GARD of 14.37 in 25 fractions resulted in a daily dose of 2.3 Gy, or 57.3 Gy in 25 fractions. The BEDα/β=3.29 estimated to optimize outcome was ≥97 Gy.
Discussion
Clinically, soft-tissue sarcomas are largely considered radioresistant and treated homogenously. On a genomic level, our study confirms this, but also shows that this resistance can range both within and between histologies. Our study is the first large scale assessment of radiosensitivity in soft tissue sarcomas, utilizing a radiation-specific biomarker to identify a highly radioresistant subset. We demonstrated that the HRR subset has a significantly different treatment response to neoadjuvant radiation, which was independently associated with a detriment in local control. We also genomically estimated an α/β, that although fell into the range of historically reported α/β in sarcoma (2 to 6), was significantly different between radioresistant subsets. This provided the first genomic specific dose personalization, which coincides with the dosing utilized in current prospective studies from Massachussetts General Hospital [9].
Current national guidelines for STS include preoperative radiotherapy prior to definitive resection as a category one recommendation for operable patients [2], with a trend in its utility over postoperative RT, due to key advantages including: lower radiation dose (50 Gy vs. >60 Gy), smaller treatment volumes, and improved R0 resection rate [29]. These differences may translate to decreased long-term toxicity including fibrosis [30], joint stiffness [30], edema [30,31], and pain [31]. In addition, meta-analysis data suggest that this approach can also lead to improved oncologic outcomes (e.g. LC, DM, an OS) [4,32,33], which may be due to improved tumor oxygenation, radiographically definable lesion with preoperative therapy, prevention of tumor spillage or seeding during resection, and immune modulatory effects.
Favorable pathologic response (fPR) rates in STS, is ~ 8% after standard neoadjuvant radiation [34]. The fPR in our study was markedly different between HRR and CRR (2.4% vs. 19.4%), highlighting genomically identified differences in the innate radiobiology. Achieving a fPR has been proposed as a potential surrogate for response to radiation, as it has been associated with improved R0 resection rate, LC, DM, and OS [5,6,35]. In addition, specific histologies that are commonly treated with definitive RT alone, (e.g. Ewing sarcoma and angiosarcoma), were estimated to have the highest radiosensitivity in our study, further confirming the validity of our genomic signature [36], [37], [38]. This also suggests that there may be a subset of sarcoma patients that could achieve adequate local control with a definitive radiation approach.
Therefore, identifying a population who would selectively benefit from intensified therapy in the form of radiation dose escalation, could potentially improve treatment response and in-turn disease outcome. Based on the GARD, an ideal threshold to improving outcome for HRR subset includes achieving an adequate equivalence dose (BED3.29 ≥97 Gy), which translates to 57.3 Gy in 25 fractions or >60 Gy (2 Gy/fraction). Current dose escalation strategies target areas at high risk for residual microscopic disease (e.g. posterior rim in retroperitoneum sarcoma) [39]. Interestingly, this work and retrospective work leading up to these trials have utilized doses ≥57.5 Gy in 25 fractions [8,40]. In a recent phase I trial, with a high proportion of HRR sarcoma (64%)[9], the 18 month follow up with a dose escalated approach reported no failures in the 9 patients that underwent resection, which surpasses the historic local control rates of 60–80%[41,42]. These preliminary findings may be due to the higher portion of HRR sarcoma with a higher potential for microscopic residual disease. The recently reported STRASS trial utilized a composite abdominal-recurrence free survival endpoint in evaluating preoperative RT in retroperitoneal sarcomas – although this was a negative trial overall, subset analyses focusing on liposarcoma histology appears to support the notion that these tumors may benefit from upfront RT [43].
Our study was the first to genomically estimate the α/β ratio in sarcoma, which coincides with the historic in vitro studies, which showed that the α/β ratio in response to radiation was 2 to 6 [44]. Although our model estimated that both HRR and CRR were within this historic range (3.29 vs. 5.98), they were significantly different. Therefore, determining a personalized α/β ratio can help determine ideal dosing, fractionation, and potentially the ideal therapeutic window to improve disease response and patient toxicity. This is particularly an area of interest as recent studies have investigated more hypofractionated approaches to neoadjuvant radiation [45], and the dosing schema chosen can be tailored to the patient.
As our efforts to identify radiosensitivity in STS evolves, our goal is personalize treatment based on the innate biology of their disease, rendering care histologically agnostic. This evolution could further individualize patients based on their intrinsic tumor genomics and recommend an optimal GARD, dose, and potentially fractionation. Just as landmark prognostic indices have identified which breast cancer patients derive the most benefit from chemotherapy, RSI/GARD can shape how we personalize RT in the future.
Limitations
Despite the novel analysis that RSI and GARD have to offer for sarcoma, our study contains some limitations. The primary limitation remains that the individual RSI values are not available for our clinical correlation cohort, as the RSI was derived from resected surgical tissue from our institution's biorepository. Myxoid liposarcomas were not included in this analysis, given their unusual radiosensitivity and unique genetic features, with over 90% of myxoid liposarcomas containing the 12q13 and 16q11 translocation, leading to FUS-DDITR (TLS-CHOP) fusion [46,47]. This translocation family may be responsible for these unusually radiosensitive characteristics, along with distinct dense vascular patterns in response to radiation [46], which sets this histology apart from all other soft tissue sarcomas and is now an area of interest for treatment de-escalation [48]. Unfortunately, this unique and not yet elucidated mechanism for unusual radiosensitivity is not adequately captured with this genomic signature [48].
The treatment regimens utilized in our study were standard preoperative courses of radiotherapy, and although it offers a homogeneous treatment paradigm, it is difficult to determine varying effects dose-escalation or altered fractionation would have on tumor response. There is no consensus agreement regarding measuring effective neoadjuvant therapy response in STS, especially as necrosis is a component of grading and a potential confounder to treatment response. This limitation was mitigated by using pathologists that specialize in STS, who quantified percent of viable cells remaining in concert with necrosis, when possible. The use of neoadjuvant therapy allowed us to evaluate tumor response but made pathologic grading less reliable. This contributes to the high percentage of unknown grade in our study. There was also a difference in the clinical characteristics between the HRR and CRR cohorts. Namely, there were a higher percentage of retroperitoneal sarcoma and positive margins in the HRR group. Truly negative margins are difficult to obtain in RPS, as our institutional practice considers a positive margin to include disease suspicious for well-differentiated liposarcoma at ink. Of the 67 HRR patient, 32 (47.8%) had RPS, and only 7 had high-grade/dedifferentiated liposarcoma at the surgical margin. Even after accounting for this on the MVA, HRR histology independently predicted for >2 times the risk of developing a locoregional failure. The use of RSI/GARD has been derived from photon-based treatment with unknown correlation to particle therapy. However, further studies investigating the relationship of the relative biological effectiveness (RBE) of proton/particle therapy and RSI could be promising. Due to the limitations of this retrospective study, a large prospective study evaluating tumor response, potentially accounting for tumor volume/heterogeneity and patient outcome utilizing GARD is required. These future trials could help answer further questions on the impact of altered/hypofractionation for normal tissue effects, which are currently unclear and primarily extrapolated from other disease entities [49].
Conclusions
Sarcomas are radioresistant by nature. This study is the first to demonstrate that within this heterogeneous group lies a broad range of radiosensitivities, most notably for non-myxoid liposarcoma histology. With the use of the radiosensitivity index (RSI), we were able to genomically estimate a broad range of α/β within the range previously described in the literature. The innate tumor radiosensitivity is reflected in both the radiation treatment response (favorable pathologic response) and locoregional control. With the use of genomics, we estimate an effective dose (GARD) that could improve patient outcomes. In highly radioresistant subpopulations, the ideal dose needed to optimize outcome was a BED3.29 ≥97 Gy or >60 Gy (2 Gy/fraction).
Declaration of Competing Interest
Javier F. Torres-Roca MD reports stock and leadership in Cvergenx, Inc. and royalty and patents on RSI. Jacob G. Scott MD PhD reports a patent in GARD and stock in Cvergenx, Inc.
All remaining authors have declared no conflicts of interest.
Acknowledgments
Data sharing statement
Data in this study is available upon reasonable request from the corresponding author at Arash.Naghavi@moffitt.org
Contributors
Conceptualization, G.Y, Z.Y, A.N., K.A., J.G. J.T.; methodology, G.Y., E.W., W.J., A.N..; software, G.Y., E.W..; validation, M.B., G.Y., A.N., J.G..; formal analysis, G.Y., E.W., A.N. data curation, G.Y., Z.Y., E.W., A.N..; writing—original draft preparation, G.Y., Z.Y., A.N, .; writing—review and editing, K.A., J.T., J.G., R.G., L.H., J.E, D.L,; funding acquisition, J.G., A.N., J.T.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health [grant numbers R21CA101355, R21CA135620]; US Army Medical Research and Materiel Command [W81XWH-08-2-0101, subaward 12-15479-01-07]; National Functional Genomics Center Award [grant number 170220051]; Bankhead-Coley Foundation Award [grant number 09BB-22]; and the Debartolo Family Personalized Medicine Institute
References
- 1.Crago AM, Brennan MF. Principles in management of soft tissue sarcoma. Adv. Surg. 2015;49:107–122. doi: 10.1016/j.yasu.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Mehren M, Kane JM, Bui MM. NCCN guidelines insights: soft tissue sarcoma, Version 1.2021. J. Natl. Compr. Canc. Netw. 2020;18(12):1604–1612. doi: 10.6004/jnccn.2020.0058. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Chang AE, Baker AR. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan B, Davis AM, Turcotte R. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 5.Eilber FC, Rosen G, Eckardt J. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J. Clin. Oncol. 2001;19(13):3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 6.Shah D, Borys D, Martinez SR. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32(9):3911–3915. [PMC free article] [PubMed] [Google Scholar]
- 7.Baldini E. Retroperitoneal sarcoma: setting the stage for treatment strategies tailored to histologic subtype and other patient and tumor factors. Int. J. Radiat. Oncol. Biol. Phys. 2017;99(2):292–295. doi: 10.1016/j.ijrobp.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Baldini EH, Bosch W, Kane JM., 3rd Retroperitoneal sarcoma (RPS) high risk gross tumor volume boost (HR GTV boost) contour delineation agreement among NRG sarcoma radiation and surgical oncologists. Ann. Surg. Oncol. 2015;22(9):2846–2852. doi: 10.1245/s10434-015-4633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLaney TF, Chen YL, Baldini EH. Phase 1 trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv. Radiat. Oncol. 2017;2(1):85–93. doi: 10.1016/j.adro.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Roca JF, Eschrich S, Zhao H. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res. 2005;65(16):7169–7176. doi: 10.1158/0008-5472.CAN-05-0656. [DOI] [PubMed] [Google Scholar]
- 11.Eschrich S, Zhang H, Zhao H. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(2):497–505. doi: 10.1016/j.ijrobp.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschrich SA, Pramana J, Zhang H. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(2):489–496. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschrich SA, Fulp WJ, Pawitan Y. Validation of a radiosensitivity molecular signature in breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18(18):5134–5143. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed KA, Chinnaiyan P, Fulp WJ, Eschrich S, Torres-Roca JF, Caudell JJ. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget. 2015;6(33):34414–34422. doi: 10.18632/oncotarget.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed KA, Fulp WJ, Berglund AE. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int. J. Radiat. Oncol. Biol. Phys. 2015;92(4):837–842. doi: 10.1016/j.ijrobp.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strom T, Hoffe SE, Fulp W. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiother. Oncol.: J. Eur. Soc. Therapeut. Radiol. Oncol. 2015;117(1):159–164. doi: 10.1016/j.radonc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Roca JF, Fulp WJ, Caudell JJ. Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015;93(3):631–638. doi: 10.1016/j.ijrobp.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JG, Berglund A, Schell MJ. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed KA, Berglund AE, Welsh EA. The radiosensitivity of brain metastases based upon primary histology utilizing a multigene index of tumor radiosensitivity. Neuro Oncol. 2017;19(8):1145–1146. doi: 10.1093/neuonc/nox043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed KA, Scott JG, Arrington JA. Radiosensitivity of lung metastases by primary histology and implications for stereotactic body radiation therapy using the genomically adjusted radiation dose. J. Thorac. Oncol. 2018;13(8):1121–1127. doi: 10.1016/j.jtho.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Leeuwen CM, Oei AL, Crezee J. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018;13(1):96. doi: 10.1186/s13014-018-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int. J. Radiat. Oncol. Biol. Phys. 1985;11(1):87–96. doi: 10.1016/0360-3016(85)90366-9. [DOI] [PubMed] [Google Scholar]
- 23.Weichselbaum RR, Beckett MA, Vijayakumar S. Radiobiological characterization of head and neck and sarcoma cells derived from patients prior to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1990;19(2):313–319. doi: 10.1016/0360-3016(90)90539-v. [DOI] [PubMed] [Google Scholar]
- 24.Weichselbaum RR, Rotmensch J, Ahmed-Swan S, Beckett MA. Radiobiological characterization of 53 human tumor cell lines. Int. J. Radiat. Biol. 1989;56(5):553–560. doi: 10.1080/09553008914551731. [DOI] [PubMed] [Google Scholar]
- 25.Joiner MC. A simple alpha/beta-independent method to derive fully isoeffective schedules following changes in dose per fraction. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):871–875. doi: 10.1016/j.ijrobp.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer J. 2011;17(6):528–536. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh EA, Eschrich SA, Berglund AE, Fenstermacher DA. Iterative rank-order normalization of gene expression microarray data. BMC Bioinform. 2013;14:153. doi: 10.1186/1471-2105-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong J, Shoghi KI, Deasy JO. Modelling the interplay between hypoxia and proliferation in radiotherapy tumour response. Phys. Med. Biol. 2013;58(14):4897–4919. doi: 10.1088/0031-9155/58/14/4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MH, Ball AB, Schofield J, Fisher C, Harmer CL, Thomas JM. Preoperative radiotherapy for initially inoperable extremity soft tissue sarcomas. Clin. Oncol. (R. Coll. Radiol.) 1992;4(1):36–43. doi: 10.1016/s0936-6555(05)80772-1. [DOI] [PubMed] [Google Scholar]
- 30.Davis AM, O'Sullivan B, Turcotte R. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol.: J. Eur. Soc. Therapeut. Radiol. Oncol. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Stinson SF, DeLaney TF, Greenberg J. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1991;21(6):1493–1499. doi: 10.1016/0360-3016(91)90324-w. [DOI] [PubMed] [Google Scholar]
- 32.Al-Absi E, Farrokhyar F, Sharma R. A systematic review and meta-analysis of oncologic outcomes of pre- versus postoperative radiation in localized resectable soft-tissue sarcoma. Ann. Surg. Oncol. 2010;17(5):1367–1374. doi: 10.1245/s10434-009-0885-7. [DOI] [PubMed] [Google Scholar]
- 33.Sampath S, Schultheiss TE, Hitchcock YJ, Randall RL, Shrieve DC, Wong JY. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2):498–505. doi: 10.1016/j.ijrobp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 34.Bonvalot S, Rutkowski PL, Thariat J. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 35.MacDermed DM, Miller LL, Peabody TD. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(4):1147–1153. doi: 10.1016/j.ijrobp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhomberg W. The radiation response of sarcomas by histologic subtypes: a review with special emphasis given to results achieved with razoxane. Sarcoma. 2006;2006(1):87367. doi: 10.1155/SRCM/2006/87367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ihara H, Kaji T, Katsui K. Single institutional experience of radiation therapy for angiosarcoma of the scalp without cervical lymph node metastases: Impact of concurrent chemoradiation with maintenance chemotherapy using taxanes on patient prognosis. Mol. Clin. Oncol. 2019;11(5):498–504. doi: 10.3892/mco.2019.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Indelicato DJ, Keole SR. Radiation treatment for Ewing family of tumors in adults: the University of Florida experience. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(4):1140–1145. doi: 10.1016/j.ijrobp.2008.02.071. [DOI] [PubMed] [Google Scholar]
- 39.McBride SM, Raut CP, Lapidus M. Locoregional recurrence after preoperative radiation therapy for retroperitoneal sarcoma: adverse impact of multifocal disease and potential implications of dose escalation. Ann. Surg. Oncol. 2013;20(7):2140–2147. doi: 10.1245/s10434-013-2868-y. [DOI] [PubMed] [Google Scholar]
- 40.Tzeng CW, Fiveash JB, Popple RA. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107(2):371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 41.Bonvalot S, Gronchi A, Pechoux CL. STRASS (EORTC 62092): a phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcoma. J. Clin. Oncol. 2019;37(15_suppl):11001. -11001. [Google Scholar]
- 42.Gronchi A, Strauss DC, Miceli R. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann. Surg. 2016;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 43.Bonvalot S, Gronchi A, Le Pechoux C. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–1377. doi: 10.1016/S1470-2045(20)30446-0. [DOI] [PubMed] [Google Scholar]
- 44.Joiner MC. Oxford University Press; New York: 1997. Basic Clinical Radiobiology - the Linear-Quadratic Approach to Fractionation and Calculation of Isoeffect Relationships. [Google Scholar]
- 45.Kalbasi A, Kamrava M, Chu FI. A phase 2 trial of five-day neoadjuvant radiation therapy for patients with high-risk primary soft tissue sarcoma. Clin. Cancer Res.: Offi. J. Am. Assoc. Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vreeze RS, de Jong D, Haas RL, Stewart F, van Coevorden F. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(5):1480–1487. doi: 10.1016/j.ijrobp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Abbas Manji G, Singer S, Koff A, Schwartz GK. Application of molecular biology to individualize therapy for patients with liposarcoma. Am. Soc. Clin. Oncol. Educ. Book. 2015:213–218. doi: 10.14694/EdBook_AM.2015.35.213. [DOI] [PubMed] [Google Scholar]
- 48.Lansu J, Bovee J, Braam P. Dose reduction of preoperative radiotherapy in myxoid liposarcoma: a nonrandomized controlled trial. JAMA Oncol. 2021;7(1) doi: 10.1001/jamaoncol.2020.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naghavi AO, Yang GQ, Latifi K, Gillies R, McLeod H, Harrison LB. The future of radiation oncology in soft tissue sarcoma. Cancer Control. 2018;25(1) 1073274818815504. [Google Scholar]