Abstract
Introduction
A reliable biomarker is urgently needed in the diagnosis and management of acid sphingomyelinase deficiency (ASMD, also known as Niemann Pick A, A/B, and B). Lyso-sphingomyelin (LSM) has previously been proposed as a biomarker for this disease. However, existing studies have not investigated the relationship between LSM levels and clinical subtype or severity. The purpose of this study is to address this gap in knowledge.
Material and methods
We present a cross-sectional study of 28 patients with ASMD, enrolled in an ongoing natural history study at the Icahn School of Medicine at Mount Sinai and The Children's Hospital at Montefiore. Plasma LSM levels from 28 patients were analyzed, including 7 patients with the infantile neurovisceral phenotype (ASMD type A), 3 patients with chronic neurovisceral disease (ASMD type A/B) and 18 patients with chronic visceral ASMD (ASMD type B). The association between LSM levels and clinical subtype, dichotomized as infantile (type A) or chronic (type A/B and B), was analyzed using the Wilcoxon rank sum test. In secondary analysis, the association between LSM levels and clinical severity among the chronic ASMD patients was analyzed using the Kruskal-Wallis test.
Results
LSM levels were elevated in all patients with ASMD when compared to a reference range of (0.04–3.8 (ng/mL)). Median LSM levels were higher in patients with infantile ASMD (386 ng/mL [314, 605]) compared to chronic ASMD (133 ng/mL [90, 209]), p < .001. Additionally, among individuals with chronic ASMD there was a positive association between LSM level and clinical severity (p = .01, p for trend <0.001).
Conclusion
We identified greater LSM elevations in patients with infantile ASMD compared to those with chronic ASMD. Among patients with chronic ASMD, LSM levels were positively associated with clinical severity. These data support investigation of LSM as a biomarker for ASMD. Future studies are required to determine if LSM levels are predictive of phenotype in pre-symptomatic patients and how such levels correlate in response to treatment.
Keywords: Lyso-sphingomyelin, Nieman pick disease, Acid sphingomyelinase deficiency, Biomarker, Lysosomal storage diseases
Abbreviations: ASMD, acid sphingomyelinase deficiency; LSM, Lyso-sphingomyelin; SMPD1, sphingomyelin phosphodiesterase 1; ASM, acid sphingomyelinase; ERT, enzyme replacement therapy
Highlights
-
•
Lyso-sphingomyelin (LSM) has previously been proposed as a biomarker for acid sphingomyelinase deficiency (ASMD).
-
•
Existing studies have not investigated the relationship between degree of LSM elevation and clinical subtype or severity.
-
•
We present a cross-sectional study of 28 patients with ASMD enrolled in an ongoing natural history study.
-
•
LSM levels were elevated in all patients with ASMD compared to reference ranges.
-
•
Median LSM levels were higher in patients with infantile ASMD compared to chronic ASMD.
-
•
Among individuals with chronic ASMD, there was a positive association between LSM level and clinical severity.
1. Introduction
Acid sphingomyelinase deficiency (ASMD), also known as Niemann-Pick Disease, is a rare, autosomal recessive lysosomal storage disorder arising from ineffective sphingomyelin metabolism. ASMD is caused by pathogenic variants in the sphingomyelin phosphodiesterase 1 (SMPD1) gene, resulting in marked reduction in acid sphingomyelinase (ASM, EC 3.1.4.12) activity. There is a wide clinical spectrum, with varying degrees of primary neurologic and visceral disease [1,2]. Currently, a diagnosis of ASMD is established based on detection of biallelic pathogenic variants in SMPD1 or less than 10% ASM activity in peripheral blood or cultured skin fibroblasts [1,3]. However, phenotype predictions based on genotype may be unreliable due to variants of uncertain significance and unique compound heterozygous combinations. Similarly, the amount of residual enzymatic activity may overlap across the spectrum of ASMD, limiting its use as an accurate predictor of phenotypic severity. As enzyme replacement therapy (ERT) is under development for the non-neurologic manifestations of ASMD, a reliable biomarker with clinical correlate is urgently needed in the diagnosis and management of this disease [4].
Infantile neurovisceral disease (Niemann-Pick disease type A or ASMD type A) is the most severe form and is characterized by presentation in early infancy, with death by about age three. This is a rapidly progressive disease with neurodegeneration and severe visceral disease. Patients who survive infancy are said to have chronic disease, which may present along a range of disease burden and progression. Chronic visceral ASMD (Niemann-Pick disease type B or ASMD type B) is the milder form and is characterized by slowly progressive visceral disease with minimal or no neurologic involvement. Somatic symptoms may include a combination of hepatosplenomegaly, liver dysfunction, thrombocytopenia, interstitial lung disease, and dyslipidemia. An intermediate phenotype, referred to as chronic neurovisceral ASMD (Niemann-Pick disease type A/B or ASMD type A/B), is characterized by survival through childhood to adulthood and the presence of progressive somatic and neurologic symptoms [5].
ASM is responsible for conversion of sphingomyelin to ceramide and phosphocholine. In the setting of absent or reduced ASM, as is the case with ASMD, sphingomyelin accumulates. The de-acylated form of sphingomyelin, lyso-sphingomyelin (LSM), is a potential biomarker for this disease. The framework of using lyso-sphingolipids as a biomarker has been successfully applied to other sphingolipidoses, such as Fabry disease (lysoGB3) and Gaucher disease (LysoGL1) [6]. Prior studies have demonstrated that LSM is markedly elevated in the plasma and dried blood spots of ASMD patients [[6], [7], [8], [9], [10]]. Kuchar et al. demonstrated that simultaneous measurement of plasma LSM and its carboxylated counterpart, Lysosphingomyelin 509 (LSM-509), were useful in differentiating ASMD and Niemann-Pick Type C (NPC) Disease, a biochemically distinct disorder of cholesterol transport [8]. However, to the best of our knowledge there have been no prior studies investigating the relationship between LSM levels and ASMD clinical sub-type or severity. The purpose of this study is to fill this gap in knowledge. We hypothesized that LSM levels would be greater in patients with infantile neurovisceral disease when compared to chronic neurovisceral and visceral disease. Additionally, we hypothesized that LSM levels would be positively associated with clinical severity in patients with chronic disease.
2. Materials and methods
2.1. Patient samples
This cross-sectional study was conducted as an extension of an ongoing, Institutional Review Board approved ASMD natural history study at the Icahn School of Medicine at Mount Sinai and The Children's Hospital at Montefiore. The natural history study involves comprehensive clinical evaluation in patients with ASMD, including neurologic and developmental assessments.
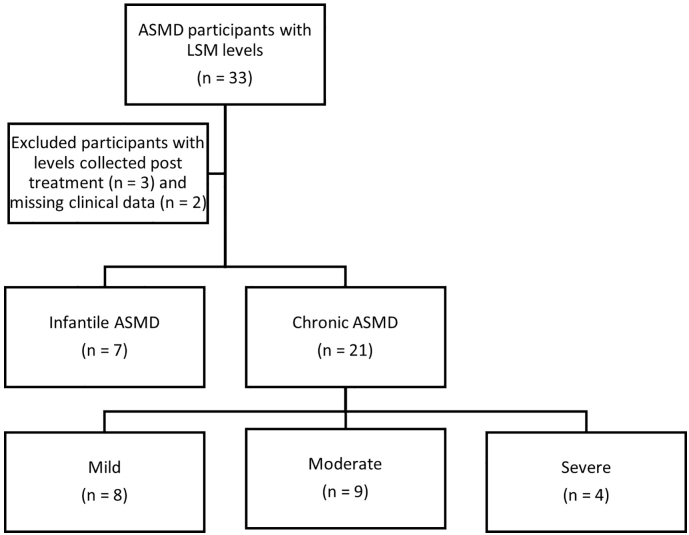
Between February 2015 and February 2019, LSM levels from a total of 33 consecutive patients with ASMD were available for analysis.
2.2. Inclusion/exclusion criteria
Participants were included given a confirmed diagnosis of ASMD based on biallelic pathogenic variants in SMPD1 or less than 10% ASM activity in peripheral blood or cultured skin fibroblasts, active enrollment in the ASMD natural history study and existing plasma LSM measurement.
Three patients were excluded due to LSM levels collected after disease-modifying clinical interventions, defined as: participation in an enzyme replacement therapy clinical trial, liver transplantation or splenectomy. An additional two patients with chronic ASMD were excluded from analysis due to missing clinical data that precluded accurate clinical severity determination. For consistency, we decided to exclude these patients from both primary and secondary analysis.
2.3. Measurement of LSM
50 μL of plasma was mixed with 250 μL of methanol containing internal standard LSM-d7 (Avanti Polar Lipids, #860639, USA) and rigorously vortexed to extract LSM. The sample was then centrifuged at 21,000 rcf for 5 min. 200 μL of supernatant was transferred to 96-well plate to be dried under nitrogen at 40 °C for 20 min and was reconstituted in 50 μL methanol for analysis. LSM was measured on the Agilent 6460A LC-MS/MS system in positive electrospray ionization (ESI) mode using ion transition m/z of 465.3/184.0 (m/z of 472.3 > 184.1 for LSM-D7). Reverse phase liquid chromatography was performed using Acquity UPLC BEH C18 column (2.1 mm × 50 mm with 1.7 μm particle size) at 40 °C with a flow rate of 0.4 mL/min. A gradient program started from 50% mobile phase A (0.1% formic acid in 5% acetonitrile), ramped to 100% mobile phase B (0.1% formic acid in acetonitrile/methanol mixture (1:1)) in 3 min and held for additional 1.5 min, then returned to 50% mobile phase A. 4 μL was injected and total run time of 5 min.
The reference interval of LSM is 1.2 ± 0.6 ng/mL (n = 196) irrespective of age. This reference range was determined by measurement of de-identified plasma samples from 196 individuals not known to be affected with ASMD. LSM concentrations in this cohort ranged from 0.04–3.8 ng/mL and was independent of age. The mean and standard deviation of LSM was 1.2 and 0.6 ng/mL, respectively.
2.4. Phenotyping
Chronic ASMD, which included patients with A/B and B phenotypes, was sub-divided by clinical severity (mild, moderate, and severe) based on the opinion of a single clinician with expertise in ASMD (MW). The presence and severity of the following clinical features were considered in severity sorting: interstitial lung disease, transaminitis, hepatosplenomegaly, HDL cholesterol concentration, thrombocytopenia, pediatric growth restriction, and developmental delays or intellectual disability. Chronic ASMD patients considered to have mild disease had the following general features: absent or minimal interstitial lung disease, normal pediatric growth parameters, mild to moderate hepatosplenomegaly, normal platelet counts with a range of HDL cholesterol. Those with moderate disease generally had mild to moderate interstitial lung disease, moderate hepatosplenomegaly, significant pediatric growth restriction, and elevated hepatic transaminases. Patients with chronic neurovisceral ASMD were classified as severe, as was one child with type B disease who had quite significant interstitial lung disease, growth restriction, transaminitis, and hepatosplenomegaly by 3 years of age.
2.5. Statistical methods
Statistical analysis was performed using STATA software, version 16. Clinical subtype was dichotomized into infantile and chronic ASMD, with chronic ASMD consisting of both chronic neurovisceral ASMD, ASMD type A/B, (n = 3) and Chronic visceral ASMD, or ASMD type B (n = 18). Chronic neurovisceral and visceral patients were combined in this phase of analysis due to sample size constraints. The association between LSM levels and clinical subtype was analyzed using the Wilcoxon rank sum test.
The association between LSM levels and clinical severity (grouped as mild, moderate and severe) in chronic ASMD was analyzed using the Kruskal-Wallis test. P for trend was also obtained. Non-parametric tests were used for these bivariate associations because the normality assumption was not satisfied. For all tests, a p value less than 0.05 was considered significant. A power calculation was not performed given the limitation of available cases for this rare disease. All available cases meeting our inclusion criteria were used.
3. Results
In our study cohort of 28 patients, there were 7 participants with infantile ASMD and 21 with chronic ASMD. As expected, infantile ASMD patients were younger than chronic patients. Additional participant characteristics can be seen in Table 1. Of the 21 chronic ASMD patients who underwent clinical severity categorization, 8 patients were considered mild, 9 moderate and 4 severe. The severe patients included all 3 patients in our cohort with chronic neurovisceral disease. (See Fig. 1.)
Table 1.
Characteristicsa of participants by Acid sphingomyelinase deficiency (ASMD) clinical subtype.
| Infantile ASMD (n = 7) | Chronic ASMD (n = 21) | |
|---|---|---|
| Sex | ||
| Male | 5 (71) | 12 (57) |
| Female | 2 (29) | 9 (43) |
| Age in years - median (IQR) | 1.3 (1.1, 1.5) | 15.1 (5.5, 27.3) |
Categorical variables reported as n (%) and continuous variables reported as median (IQR range).
Fig. 1.
Flowchart for inclusion and exclusion and phenotype breakdown.
3.1. LSM level and clinical subtype (infantile versus chronic)
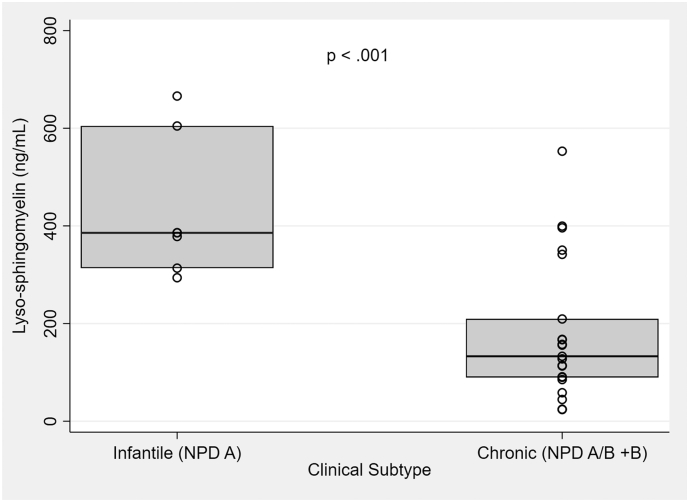
LSM levels were elevated in all patients with ASMD compared to normal references. The range of LSM levels in infantile ASMD patients ranged from 294 to 666 ng/mL, compared to 24–553 ng/mL in chronic ASMD patients. LSM levels were higher in patients with infantile ASMD (median LSM 386 ng/mL [314, 605]) compared to chronic ASMD (156 ng/mL [133, 209]). This difference was statistically significant (p < .001). These results can be seen graphically in Fig. 2.
Fig. 2.
Box plot comparing LSM by ASMD clinical subtype. Median [Q1, Q2] LSM level in patients with infantile ASMD was 386 ng/mL [314, 605]) compared to 156 ng/mL in chronic ASMD [133, 209]. The Wilcoxon rank sum test was utilized to compare these medians. p < .001.
3.2. LSM level and clinical severity among chronic ASMD patients
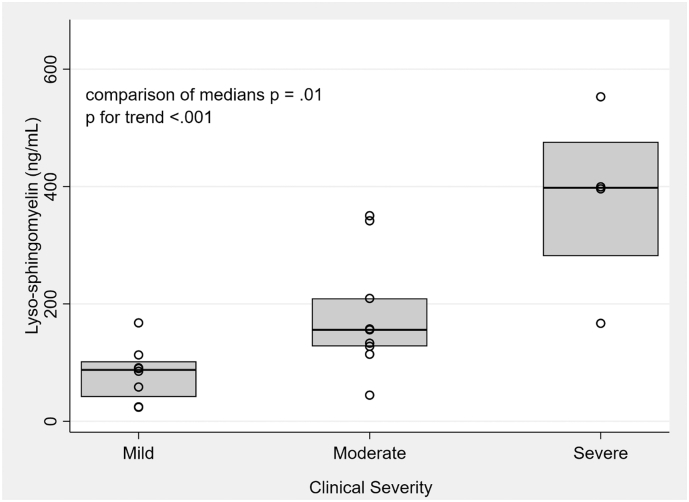
Among individuals with chronic ASMD, there was a positive and statistically significant relationship between LSM level and clinical severity. The median LSM level in the mild patients was 88 ng/mL (42, 102), compared to 156 ng/mL (128, 209) in the moderate group and 398 ng/mL (282, 476) in the severe group. The Kruskal Wallis p was 0.01, with a statistically significant p for trend (<0.001). These results can be seen graphically in Fig. 3. Of note, our cohort included 3 patients with chronic neurovisceral ASMD (ASMD A-B), all of whom were classified as “severe” in our severity sorting.
Fig. 3.
Box plot comparing LSM by clinical severity among patients with chronic ASMD. Median [Q1, Q2] LSM level in patients with mild disease was 88 ng/mL [42, 102], compared to 156 ng/mL [128, 209] in moderate disease and 398 [282, 476] in severe disease. The medians were compared with the Kruskal-Wallis test, p 0.01; p for trend <0.001.
4. Discussion
LSM has previously been proposed as a reliable biomarker for ASMD, but prior studies have not investigated the relationship between LSM levels and clinical subtype or severity [11]. The results of this study support the prior finding that LSM levels are increased in patients with ASMD compared to reference values. Furthermore, we present novel data that demonstrate LSM elevations in patients with infantile ASMD are greater than those with chronic ASMD. Among patients with chronic ASMD, a positive relationship was observed between LSM levels and clinical severity (mild > moderate > severe).
The main limitation of this study was the lack of a validated clinical severity score with which to sort chronic ASMD patients. We are aware of a single previously published ASMD scoring system however, this scoring system was not validated and did not take lung disease or neurologic symptomatology into account, so we deemed it inadequate for our purpose [12]. Clinical severity was therefore scored by an expert in the field who was incompletely blinded to LSM levels due to prior clinical contact with the patients and prior review of LSM levels at time of initial clinical encounter (MW). To reduce the impact of such bias, objective clinical characteristics were used to categorize these patients. Sample size was another potential limitation of this study however the identification of a statistically significant association indicates sufficient power. This study involved cross-sectional analysis of diagnosed patients therefore longitudinal extrapolations cannot be made. Another limitation was the enrollment of diagnosed, symptomatic patients, which may have biased the results. In particular, due to the rapidly progressive nature of ASMD type A, all infants in this cohort were seen at a relatively advanced state of disease which likely impacted their LSM levels.
Potential future applications for LSM include differentiating between infantile neurovisceral and chronic forms, predicting clinical severity and need for and response to treatment. Extending our data to such applications will require further study. Although we found that median LSM levels were different at a statistical significance level of 0.05 between infantile and chronic patients, as well as among chronic patients of varying clinical severity, Fig. 2, Fig. 3 demonstrate there is overlap in LSM values among these groups. Adding a second biomarker, such as LSM-509 may increase precision and is an opportunity for further study. Additionally, extension of this analysis to larger, multi-center cohorts that include patients with a wide spectrum of phenotypic severity would increase power to allow for further subgroup analysis.
With existing clinical and laboratory tools, distinguishing infantile and chronic patients in early infancy is a challenge. Early clinical evaluations are limited as patients from both groups can be asymptomatic in the first several months of life before infantile patients declare themselves with rapid clinical deterioration and developmental regression. Furthermore, phenotype estimations based on genotype or enzymatic level are imprecise, making a clinically predictive biomarker of considerable value to the care of pre-symptomatic patients.
Differentiation between infantile and chronic forms of ASMD has prognostic value as well as implications for treatment eligibility. While ERT is currently under development for individuals with chronic ASMD, recombinant enzyme is not indicated for patients with infantile ASMD as it does not cross the blood brain barrier [4].
We anticipate the need for informed phenotype predictions will grow as pre-symptomatic newborns are identified on expanded newborn screening. ASMD is already part of newborn screening in Illinois, and is likely to be added to other states including New York where it is being tested through a pilot newborn screening program, ScreenPlus [13]. LSM will be used as a second-tier analyte as part of this pilot study to inform about its use in the identification of newborns with ASMD. Longitudinal follow-up of these children, including correlation between serial LSM levels and phenotype, will provide critical information about the use of LSM at predicting phenotype in pre-symptomatic individuals.
For individuals already on therapy, a biomarker may have utility in measuring treatment response. Biomarkers have similarly been trended in Gaucher disease and Fabry disease [14,15]. Analysis of LSM levels in five patients at 30 months of ERT with Olipudase alfa has shown decreases in LSM levels with therapy, supporting LSM as a marker of treatment response [4].
In conclusion, this study offers preliminary data demonstrating a positive correlation between LSM levels and clinical severity in ASMD patients. These results support further investigations into LSM as a biomarker for ASMD.
Declaration of Competing Interest
Margo Breilyn, Wenyue Zhang and Chunli Yu have no financial disclosures or conflicts of interest. Melissa Wasserstein has received consultant fees, travel reimbursement, and research support from Sanofi Genzyme, consultant fees from Takeda, and research support from Alexion, the Ara Parseghian Medical Research Fund, BioMarin, Cure Sanfilippo Foundation, Firefly Fund, National Niemann Pick Disease Foundation, Orchard Therapeutics, PassageBio, Takeda, Travere Therapeutics, and Ultragenyx.
Acknowledgements
The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number UL1TR001073 and the National Niemann Pick Disease Foundation (NNPDF).
References
- 1.Wasserstein M.P., Schuchman E.H. Acid sphingomyelinase deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews((R)). Seattle (WA) 1993. [Google Scholar]
- 2.Schuchman E.H., Desnick R.J. Types a and B Niemann-pick disease. Mol. Genet. Metab. 2017;120(1–2):27–33. doi: 10.1016/j.ymgme.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGovern M.M., Dionisi-Vici C., Giugliani R. Consensus recommendation for a diagnostic guideline for acid sphingomyelinase deficiency. Genet Med. 2017;19(9):967–974. doi: 10.1038/gim.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserstein M.P., Diaz G.A., Lachmann R.H. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): safety and efficacy in adults treated for 30 months. J. Inherit. Metab. Dis. 2018;41(5):829–838. doi: 10.1007/s10545-017-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern M.M., Avetisyan R., Sanson B.J., Lidove O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD) Orphanet J Rare Dis. 2017;12(1):41. doi: 10.1186/s13023-017-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polo G., Burlina A.P., Kolamunnage T.B. Diagnosis of sphingolipidoses: a new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin. Chem. Lab. Med. 2017;55(3):403–414. doi: 10.1515/cclm-2016-0340. [DOI] [PubMed] [Google Scholar]
- 7.Chuang W.L., Pacheco J., Cooper S. Lyso-sphingomyelin is elevated in dried blood spots of Niemann-pick B patients. Mol. Genet. Metab. 2014;111(2):209–211. doi: 10.1016/j.ymgme.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Kuchar L., Sikora J., Gulinello M.E. Quantitation of plasmatic lysosphingomyelin and lysosphingomyelin-509 for differential screening of Niemann-pick A/B and C diseases. Anal. Biochem. 2017;525:73–77. doi: 10.1016/j.ab.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Voorink-Moret M., Goorden S.M.I., van Kuilenburg A.B.P. Rapid screening for lipid storage disorders using biochemical markers. Expert center data and review of the literature. Mol. Genet. Metab. 2018;123(2):76–84. doi: 10.1016/j.ymgme.2017.12.431. [DOI] [PubMed] [Google Scholar]
- 10.Polo G., Burlina A.P., Ranieri E. Plasma and dried blood spot lysosphingolipids for the diagnosis of different sphingolipidoses: a comparative study. Clin. Chem. Lab. Med. 2019;57(12):1863–1874. doi: 10.1515/cclm-2018-1301. [DOI] [PubMed] [Google Scholar]
- 11.Eskes E.C.B., Sjouke B., Vaz F.M. Biochemical and imaging parameters in acid sphingomyelinase deficiency: potential utility as biomarkers. Mol. Genet. Metab. 2020;130(1):16–26. doi: 10.1016/j.ymgme.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Wasserstein M.P., Desnick R.J., Schuchman E.H. The natural history of type B Niemann-pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114(6):e672–e677. doi: 10.1542/peds.2004-0887. [DOI] [PubMed] [Google Scholar]
- 13.Burton B.K., Charrow J., Hoganson G.E. Newborn screening for lysosomal storage disorders in Illinois: the initial 15-month experience. J. Pediatr. 2017;190:130–135. doi: 10.1016/j.jpeds.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Kritzer A., Siddharth A., Leestma K., Bodamer O. Early initiation of enzyme replacement therapy in classical Fabry disease normalizes biomarkers in clinically asymptomatic pediatric patients. Mol Genet Metab Rep. 2019;21:100530. doi: 10.1016/j.ymgmr.2019.100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murugesan V., Chuang W.L., Liu J. Glucosylsphingosine is a key biomarker of Gaucher disease. Am. J. Hematol. 2016;91(11):1082–1089. doi: 10.1002/ajh.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]