Abstract
The personality trait of neuroticism is considered a risk factor for stress vulnerability, putatively via its association with elevated limbic reactivity. Nevertheless, majority of evidence to date that relates neuroticism, neural reactivity and stress vulnerability stems from cross-sectional studies conducted in a “stress-free” environment. Here, using a unique prospective longitudinal design, we assessed personality, stress-related symptoms and neural reactivity at three time points over the course of four and a half years; accounting for prior to, during, and long-time following a stressful military service that included active combat. Results revealed that despite exposure to multiple potentiality traumatic events, majority of soldiers exhibited none-to-mild levels of posttraumatic and depressive symptoms during and following their military service. In contrast, a quadratic pattern of change in personality emerged overtime, with neuroticism being the only personality trait to increase during stressful military service and subsequently decrease following discharge. Elevated neuroticism during military service was associated with reduced amygdala and hippocampus activation in response to stress-related content, and this association was also reversed following discharge. A similar pattern was found between neuroticism and hippocampus-anterior cingulate cortex (ACC) functional connectivity in response to stress-related content. Taken together these findings suggest that stressful military service at young adulthood may yield a temporary increase in neuroticism mediated by a temporary decrease in limbic reactivity, with both effects being reversed long-time following discharge. Considering that participants exhibited low levels of stress-related symptoms throughout the study period, these dynamic patterns may depict behavioral and neural mechanisms that facilitate stress resilience.
Keywords: Personality, Neuroticism, Stress, Resilience, fMRI, Amygdala, Hippocampus, Anterior Cingulate Cortex, Longitudinal
1. Introduction
Personality traits represent a relatively stable set of cognitive and emotional patterns or tendencies that account for individual differences in behavior (Corr and Matthews, 2009; Mischel, 2004; Zelenski and Larsen, 1999). The well-established “Big five” model defines personality based on five broad dimensions or traits: neuroticism, extraversion, agreeableness, openness and conscientiousness (Digman, 1990; McCrae, 2009; McCrae and John, 1992). Among these traits, neuroticism captures the tendency to experience negative emotions or exhibit emotional instability (Barlow et al., 2013; McCrae and John, 1992). As such, elevated levels of neuroticism have been repeatedly associated with enhanced negative affect and physiological hyper-arousal in response to stressful situations among healthy individuals (Aldinger et al., 2014; Bibbey et al., 2013; Leger et al., 2016; Poppelaars et al., 2019; Reynaud et al., 2012), as well as with increased overall risk for stress-related psychopathology (Jeronimus et al., 2016; Kendler et al., 2006; Khan et al., 2005; Kotov et al., 2010; Malouff et al., 2005). In addition to its putative role as a risk factor, longitudinal studies indicate that neuroticism levels may also change in response to stressful environmental circumstances (McGue et al., 1993; Ormel, Riese and Rosmalen, 2012; Robins et al., 2001; Vaidya et al., 2008); implying a reciprocal relation between stress exposure and neuroticism. To this end, exposure to either acute or chronic life stress has been associated with an increase in neuroticism scores (Goldstein et al., 2019; Jeronimus et al., 2014; Metts et al., 2021), and this effect was particularly potent if stress exposure occurred during young adulthood (Leikas and Salmela-Aro, 2015; Ogle et al., 2014; Riese et al., 2014; Roberts et al., 2006; Shiner et al., 2017). It was further suggested that the impact of life events on personality traits might be temporary (Ormel, VonKorff, Jeronimus and Riese, 2017). Whether stress-induced changes in neuroticism persist long-time following stress offset, and whether these dynamic patterns are associated with stress-related psychopathological symptoms or may in fact depict an adaptive response to stress, has yet to be directly assessed.
Compared to the wealth of longitudinal behavioral studies on neuroticism, as reviewed above, neuroimaging literature on neuroticism mostly relied on cross-sectional designs. Majority of studies associated elevated neuroticism with increased reactivity to negatively charged stimuli in limbic brain regions, particularly the hippocampus, amygdala, and anterior cingulate cortex (ACC) (Allen and DeYoung, 2016; Brown et al., 2020; Canli, 2008; Chan et al., 2009; Cunningham et al., 2011; Haas et al., 2007; Hyde et al., 2011; Ormel et al., 2013; Schuyler et al., 2014; Servaas et al., 2013). Though other studies did not find such associations (Bruhl et al., 2011; Cremers et al., 2010; Drabant et al., 2009; Neumann, 2020; Silverman et al., 2019; Thomas et al., 2011). Studies assessing the association between neuroticism scores and functional connectivity patterns also yielded mixed findings, including increased (Cremers et al., 2010) and decreased (Yang et al., 2020) connectivity of the amygdala with the dorsomedial prefrontal (dmPFC), as well as increased amygdala connectivity with the ventromedial prefrontal cortex (vmPFC) (Silverman et al., 2019) and decreased connectivity with the ACC (Cremers et al., 2010; Deng et al., 2019). Additional support for the putative role of the ACC in neuroticism, particularly its dorsal part (dACC), stems from a recent meta-analysis demonstrating a positive relationship between dACC gray matter volume and neuroticism scores among healthy adults (Liu et al., 2021). Taken together, neuroimaging literature highlighted the amygdala, hippocampus, and ACC as neural structures that may relate to neuroticism scores, though multiple inconsistences emerged, potentially implying that the association between neuroticism and limbic reactivity is dynamic and context dependent (Servaas et al., 2013). In support of that, using a within-subject design it was recently demonstrated that neuroticism scores are associated with selectively enhanced amygdala response to fearful faces under stress, but not in stress-free conditions (Everaerd et al., 2015). Longitudinal investigation of the relations between neuroticism and neural reactivity is still missing. Accordingly, it is unclear whether stress-induced dynamics in neuroticism are associated with changes in limbic activation and functional connectivity patterns during stress as well as long-time following its offset.
In order to address these critical gaps, we conducted a prospective longitudinal assessment of personality, stress-related psychopathological symptoms, and neural activation and connectivity patterns, among fifty healthy young adults recently drafted to military service. Three assessment points were completed over the course of four and a half years, accounting for prior to, during, and long-time following a stressful military service that included active combat (Fig. 1). Following previous findings, we hypothesized that exposure to this prolonged period of real-life stress, particularly during young adulthood, would lead to personality changes in the form of elevated neuroticism during military service. We further hypothesized that a subsequent reduction in neuroticism scores long-time following stress offset (i.e. following discharge) may depict an adaptive resilient response to stress, as a sign of recovery or normalization. With respect to limbic reactivity, we hypothesized that stress-induced dynamics in neuroticism will be associated with changes in limbic activation and functional connectivity patterns, particularly in response to negatively charged stress-related content. More specifically, we hypothesized that stress-induced increase in neuroticism during military service will be associated with elevated limbic reactivity, and that an adaptive response to stress will also involve normalization of limbic reactivity long-time following stress offset.
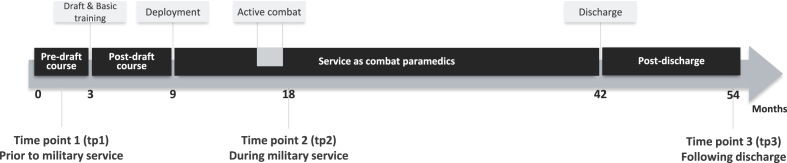
Fig. 1.
Timeline of the prospective longitudinal neuroimaging study. The study involved three time points of assessment, over the course of four and a half years. Each time point included a structural and a functional MRI scan as well as questionnaires assessing personality and posttraumatic and depressive symptoms. The first time point (tp1) was completed following enrollment to combat paramedics pre-draft course, prior to military draft and basic training. After basic training participants were enrolled in the combat paramedics course for six additional months, following which they were deployed in different combat units of the Israeli Defense Forces (IDF). The second time point (tp2) was completed nine months after deployment (about a year and a half after tp1), approximately half-way through the three and a half years of military service. Critically, immediately before tp2 all participants took active combative role during the second Lebanon war (“active combat”). The third time point (tp3) was completed three years after tp2, approximately one year after military discharge.
2. Materials and methods
2.1. Participants
Fifty healthy participants (all 18 years old, 25 males) were recruited to the study. All participants were new draftees to a three-year mandatory military service in the Israeli Defense Forces (IDF). Participants were recruited to the study after entering a pre-draft training course towards becoming combat paramedics. All participants successfully passed a series of physical, cognitive, mental, and sociodemographic tests and examinations prior to their enrollment to this elite training course, as a part of the standard IDF recruitment procedure (see Supplementary methods for more details). In addition, only individuals that reported no history of psychiatric disorders for them and their first-degree relatives, and no traumatic experiences prior to enrollment, were recruited to the study. All participants provided written informed consent approved by the Tel-Aviv Sourasky Medical Center and the IDF ethics committees. The final sample included data of 48 participants at the first time point, 40 participants at the second time point and 28 participants at the third time point (see section 3.1 for more details).
2.2. Procedure
The study involved three time points of assessment, over the course of four and a half years. Each time point included a structural and a functional MRI scan as well as questionnaires assessing personality and posttraumatic and depressive symptoms. The first time point (tp1) was completed following enrollment to combat paramedics course, prior to military basic training. The second time point (tp2) was completed a year and a half after tp1 (mean: 18 months, range: 15–20 months), approximately half-way through military service, and immediately following the second Lebanon war, a 34-day military conflict in Lebanon and northern Israel that took part in July–August 2006, during which all participants took active combative role. The third time point (tp3) was completed three years after tp2 (mean: 36 months, range: 32–46 months), approximately one year after military discharge. Taken together, this unique cohort and design enabled prospective longitudinal assessment of personality, stress-related symptoms, and neural activation and connectivity patterns, prior to, during and long-time following exposure to real-life combat stress (Fig. 1).
2.3. Personality and symptomatology questionnaires
Personality was assessed at each time point using the NEO Five-Factor Inventory (NEO-FFI), a 60-items personality inventory that captures the big five personality traits of Neuroticism, Extraversion, Conscientiousness, Openness and Agreeableness with high test-retest and internal reliability (McCrae and Costa Jr, 1989; Murray et al., 2017). Posttraumatic and depressive symptoms were assessed at each time point using the well-established Posttraumatic Stress Diagnostic Scale (PDS) (Foa, Cashman, Jaycox and Perry, 1997) and Beck Depression Inventory (BDI) (Beck et al., 1961), respectively.
2.4. fMRI paradigm
During functional MRI scan at each time point participants completed a backward masking task that included backward masked pictures of either stress-related (military and medical) or neutral (civilian) content, presented for either 33 or 83 ms (Admon et al., 2009). Each picture was followed by a scrambled image presented for 477 or 427 ms respectively (“backward masking”), and a subsequent blank gray background presented for 500 ms. Pictures were presented in blocks of nine, with unified content and presentation duration within each block, for a total of six task conditions: Military33, Military83, Medical33, Medical83, Civilian33 and Civilian83. Overall, the task included four blocks for each condition, four blocks containing only scrambled images, and one block containing pictures of mixed content that was presented at the beginning for practice purposes, for a total of 29 blocks. Blocks were interleaved by a blank gray screen for 6–9 s for a total task duration of 7 min. Throughout the task half of the pictures contained human images and the other half contained objects. Participants were instructed to indicate immediately after each picture presentation whether it included images of humans or objects thus maintaining their attention to the task while implicitly embedding the different task conditions.
2.5. fMRI acquisition
Structural and functional MRI data were obtained using a 3.0 T GE scanner with a standard head coil located at Tel-Aviv Sourasky Medical Center. Scan parameters were identical across all time points. High resolution anatomical images were acquired using a 3D sequence spoiled gradient (SPGR) echo sequence with the following scan parameters: TR/TE = 7.3/3.3 ms, voxel size 1 mm3, FOV = 25 × 18, matrix = 256 × 256. Functional whole-brain scans were conducted using a T2*-weighted gradient echo planner image (EPI) pulse sequence. Scan parameters were as follow: TR/TE = 3000/35 m s, flip angle = 90°, voxel size = 3 mm3, FOV = 20 × 20 mm, slice thickness = 3 mm (no gap), 38–44 descending slices per volume.
2.6. fMRI analysis
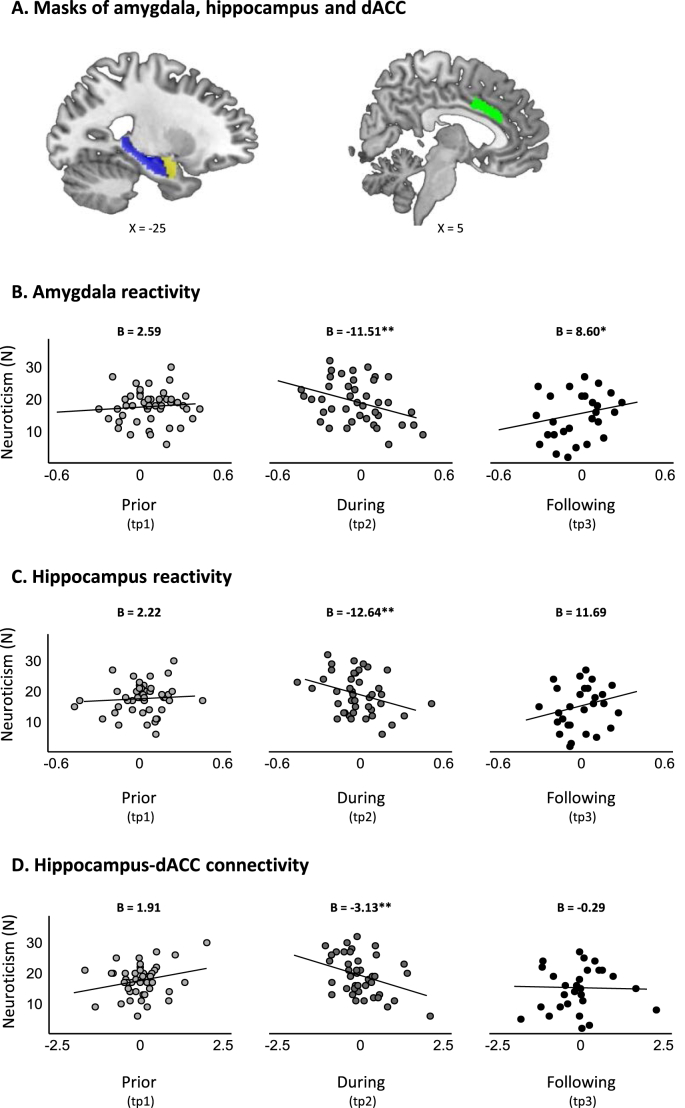
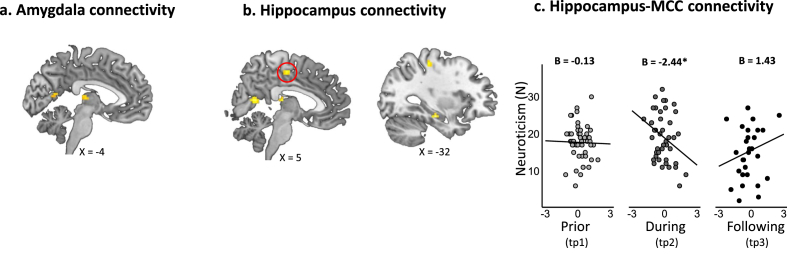
Data preprocessing was completed using SPM12 v.7487 (Wellcome Center for Human Neuroimaging, http://www.fil.ion.ucl.ac.uk) implemented in MATLAB R2018b (www.mathworks.com). Preprocessing steps included slice-timing and motion correction, spatial normalization to Montreal Neurological Institute (MNI) space and smoothing (8 mm FWHM). In addition, Artifact Detection Tool (ART) (http://web.mit.edu/swg/software.htm) was used in order to identify and model outlier TRs due to movement (0.7 mm measured as scan-to-scan separately for translation and rotation) and global signal intensity (3.5 SD from the mean). All preprocessed images were visually inspected for quality control. First-level general linear models (GLMs) were computed using SPM12 separately for each participant and time point. Each model contained the six task regressors of interest (i.e., Military33, Military83, Medical33, Medical83, Civilian33 and Civilian83), alongside regressors for the scrambled and mixed-content blocks, six motion regressors and ART outlier TRs. Region of interest (ROI) analysis of these data were focused on the amygdala, hippocampus and ACC, considering substantial evidence linking these limbic structures with neuroticism and stress (Allen and DeYoung, 2016; Canli, 2008; Chan et al., 2009; Haas et al., 2007; Hyde et al., 2011; Ormel et al., 2013; Schuyler et al., 2014; Servaas et al., 2013). For that, anatomical masks of the bilateral amygdala and bilateral hippocampus were defined using the Automated Anatomical Labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002) (Fig. 3A). Within the ACC, analysis was focused on its dorsal section (dACC) following meta-analyses of structural and functional neuroimaging studies that pointed towards the dACC as the most relevant ACC section in the context of emotional processing and neuroticism (Liu et al., 2021; Servaas et al., 2013). The dACC mask was defined using the Destrieux Atlas (2009) as implemented in neurovault (https://identifiers.org/neurovault.image:23264) (Fig. 3A). MarsBAR toolbox v.044 (http://marsbar.sourceforge.net) was used to extract mean beta-estimates (activations) for all six task regressors from these three ROIs. In addition to activation, functional connectivity patterns between these three limbic ROIs across time points were also assessed. In order to reduce the number of comparisons, functional connectivity analyses were restricted to ROIs and task conditions at which activation levels were related to neuroticism. Accordingly, psychophysiological interaction (PPI) (McLaren et al., 2012) analysis was used to assess changes in amygdala and hippocampus functional connectivity with the dACC during the Medical83 condition (see results section). Following common procedure, PPI models, for each ROI, included all first-level GLM regressors [six task conditions: (Military33, Military83, Medical33, Medical83, Civilian33 and Civilian83), scrambled and mixed-content blocks, six motion regressors and ART outlier TRs], as well as one additional regressor for the ROI time course and one additional regressor for the interaction between ROI time course and the Medical83 regressor, with the latter being the regressor of interest for PPI analysis. Finally, exploratory analyses assessed amygdala and hippocampus functional connectivity during the Medical83 condition at the whole brain level with significance threshold set at p < .05 FWE voxel-wise corrected.
Fig. 3.
Dynamics in neuroticism and limbic reactivity. A) Masks of three anatomically defined ROIs. Anatomical masks of the amygdala (yellow) and hippocampus (blue) were defined using the Automated Anatomical Labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002), and the mask of the dACC (green) was defined using the Destrieux Atlas (2009) as implemented in neurovault (https://identifiers.org/neurovault.image:23264). (B–D) Scatter plots of the association between neuroticism scores and limbic reactivity in response to the Medical83 condition over the three time points of assessment. B) Linear mixed effects (LME) model for amygdala reactivity revealed a significant time by amygdala activation interaction (F(2, 99.84) = 6.21, p = .002), driven by time-dependent relationship between neuroticism and amygdala activation such that elevated neuroticism during military service was associated with reduced amygdala activation at that time (B(tp2) = −11.51, p = .002), while elevated neuroticism following discharge was associated with increased amygdala activation (B(tp3) = 8.60, p = .046). C) LME model for hippocampus reactivity revealed a significant time by hippocampus activation interaction (F(2, 101.12) = 4.81, p = .010), driven by time-dependent relationship between neuroticism and hippocampus activation such that elevated neuroticism during military service was associated with reduced hippocampus activation at that time (B(tp2) = −12.64, p = .005), while the association between elevated neuroticism following discharge and increased hippocampal activation was trending towards significance (B(tp3) = 11.69, p = .061). D) LME model for hippocampus-dACC connectivity revealed a significant time by hippocampus-dACC connectivity interaction (F(2, 103) = 4.14, p = . 018), driven by time-dependent relationship between neuroticism and hippocampus-dACC connectivity such that elevated neuroticism during military service was associated with reduced hippocampus-dACC functional connectivity (B(tp2) = −3.13, p = .008). In all panels, reactivity values were person-mean centered, representing intra-individual change. *indicates p < .05, **indicates p < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.7. Statistical analysis
In order to assess the relation between neuroticism and limbic activation over time, linear mixed effects models (LME) were implemented using the lme 4 package in R v.3.6.3 (Bates et al., 2015), while treating neuroticism as the dependent variable and brain activation estimates as independent variables. First, a null model that contains only neuroticism scores and random intercepts was constructed. This null model yielded an intra-class correlation (ICC) of 45% for the subject's random effect (S2 = 18.69, p < .001), indicating that the degree of individual differences in our data justified the use of LME. Second, the effect of time on neuroticism was assessed with neuroticism levels at each time point as the dependent variable and time as an independent categorical variable. Neuroticism scores at tp2 were regarded as the reference category, such that tp2 minus tp1 and tp2 minus tp3 were both included in the model. The EMAtools package (Kleiman, 2017) was utilized in order to assess the remaining contrast between tp3 and tp1. Similar LME models were implemented in order to assess the effect of time on the four additional NEO personality traits. Third, LME models also assessed the link between neuroticism and amygdala, hippocampus, and dACC activation (i.e. beta estimates extracted from first level analyses) for each task condition separately. In these models, beta-estimates were treated as both time-varying (person-centered for the mean) and time-invariant (group mean-centered) predictors of neuroticism, in order to differentiate the contribution of intra-individual and inter-individual variance, respectively. The full models included time, beta estimates (time varying \ time invariant) and their interaction. Non-significant interactions were removed from analysis. Significance was adjusted for multiple comparisons using Bonferroni correction (α = .05/(3 ROIs) = 0.0167). Simple slopes for each time point were extracted using the interactions package (JA, 2019). Fourth, similarly constructed LME models assessed the link between neuroticism and amygdala-dACC functional connectivity during the Medical83 condition, neuroticism and hippocampus-dACC functional connectivity during the Medical83 condition, and neuroticism and connectivity of the amygdala and hippocampus during the Medical83 condition with clusters that emerged in the exploratory whole brain analyses. P-values for all models were calculated via the Satterthwaite's formula using the lmerTest package in R v.3.6.3 (Kuznetsova et al., 2017; Luke, 2017).
3. Results
3.1. Dynamics in stress exposure
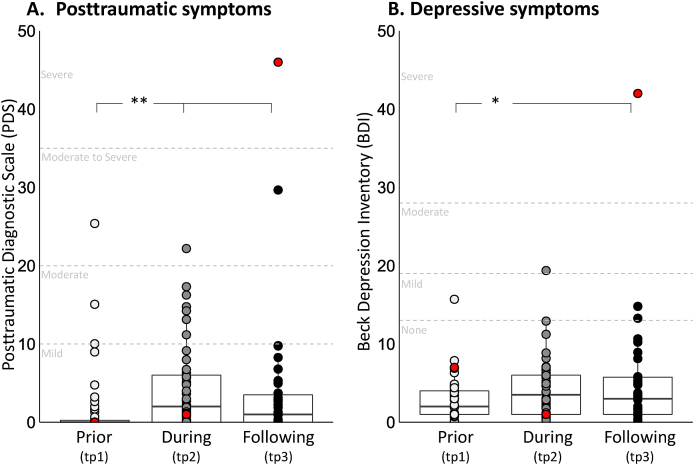
Out of the fifty participants that completed the first time point of the study (tp1), three did not complete the second time point (tp2) and thirteen did not complete the third time point (tp3). Data of seven additional participants from tp2 and eight additional participants from tp3 was partly missing with respect to either personality, stress-related symptoms, or neural reactivity. One additional participant was excluded from the study as an outlier due to extreme neuroticism score at tp3 (Z > 3.3). Anecdotally, this same participant was also the single outlier with respect to levels of posttraumatic and depressive symptoms at tp3, being the only participant to exhibit severe symptoms long-time following discharge (Figure S1, marked in red dot). Hence, the final sample included data of 49 participants at tp1, 39 participants at tp2 and 28 participants at tp3, accounting for prior to, during, and long-time following military service, respectively. Study completers did not differ from non-completers with respect to gender, personality scores and posttraumatic and depressive symptoms at tp1 (see Supplementary results).
Immediately prior to their arrival to tp2, all study participants took active role as combat paramedics in the second Lebanon war. During this period, participants were exposed to multiple potentially traumatic sights of severe causalities; and were often also in charge of providing first-line medical care in the battlefield (Table 1). As expected, these events yielded an increase in posttraumatic and depressive symptoms from prior to during and following military service. Critically however, for both posttraumatic and depressive symptoms, mean group levels remained well below clinical threshold, with majority of soldiers exhibiting none-to-mild symptoms throughout the study three time points (see Supplementary results, Table 1 and Figure S1 for more details).
Table 1.
Dynamics in stress exposure, symptomatology and personality scores over time. Descriptive statistics of exposure to potentially traumatic events (%), posttraumatic and depressive symptom levels (Mean (SD)) and NEO big five personality trait scores (Mean (SD)) over time. Exposure statistics are based on n = 39 (tp1), n = 44 (tp2) and n = 31 (tp3). Symptomatology and personality statistics are based on n = 49 (tp1), n = 39 (tp2) and n = 28 (tp3). Groups with matching superscripts indicate significant difference (p < .05). PDS - posttraumatic stress diagnostic scale; BDI - Beck Depression Inventory. NEO-FFI - NEO Five-Factor Inventory.
| Prior to military service | During military service | Following discharge | |
|---|---|---|---|
| Exposure to potentially traumatic events | |||
| Exposed to harsh scenes of casualties | 36.8 | 97.7 | 51.6 |
| Managed an event with a seriously injured or unstable patient |
23.1 |
95.5 |
58.1 |
| Symptomatology | |||
| PDS | 1.62 (4.52)1,2 | 4.58 (5.55)1 | 3.10 (5.83)2 |
| BDI |
3.05 (3.18)1 |
4.31 (3.84) |
4.2 (4.01)1 |
| Personality (NEO-FFI) | |||
| Neuroticism | 17.71 (4.91)1 | 19.61 (7.00)1,2 | 15.19 (7.00)2 |
| Extraversion | 32.77 (5.41)1,2 | 30.43 (6.02)1 | 30.43 (5.79)2 |
| Conscientiousness | 31.62 (6.75)1 | 32.14 (7.66)2 | 35.10 (6.14)1,2 |
| Agreeableness | 29.85 (4.67) | 28.64 (5.01) | 29.68 (5.15) |
| Openness | 28 (5.35) | 27.70 (6.58) | 27.55 (6.59) |
3.2. Dynamics in neuroticism and additional personality traits
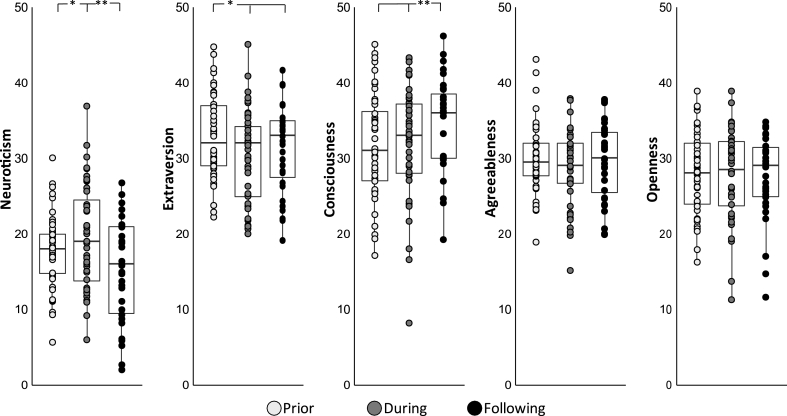
With respect to neuroticism, the unconditional growth model revealed a significant effect of time (F(2,78.19) = 7.22, p = .002), driven by an increase in neuroticism levels from prior to during military service, and a subsequent decrease in neuroticism from during military service to following discharge (B(tp2-tp1) = 2.05, p = .028, 95% CI 0.23 to 3.88; B(tp3-tp1) = 2.00, p = .062, 95% CI -0.11 to 4.10; B(tp3-tp2) = −4.05, p < .001, 95% CI -6.16 to −1.94; Fig. 2). See Figure S2 for the distribution and internal consistency of neuroticism scores across time-points. Importantly, neuroticism was the only personality trait to exhibit such quadratic pattern of change over time. With respect to extraversion, the unconditional growth model revealed a significant effect of time (F(2, 75.378) = 6.28, p = .002), driven by a decrease in extraversion from prior to during military service as well as from prior to following discharge (B(tp2-tp1) = −2.34, p = .001, 95% CI -3.76 to −0.93; B(tp3-tp1) = 2.17, p = .010, 95% CI 0.53 to 3.81), with no change from during military service to following discharge (B(tp3-tp2) = 0.18, p = .830, 95% CI 1.46 to 1.82; Fig. 2). With respect to conscientiousness, the unconditional growth model revealed a significant effect of time (F(2, 74.13) = 4.04, p = .021), driven by an increase in conscientiousness from prior to following discharge as well as from during military service to following discharge (B(tp3-tp1) = −2.48, p = .006, 95% CI -4.24 to −0.71; B(tp3-tp2) = 1.90, p = .034, 95% CI 0.14 to 3.67), with no change from prior to during military service (B(tp2-tp1) = −0.57, p = .461, 95% CI -2.09 to 0.94; Fig. 2). Finally, there was no time effect in agreeableness (F(2, 79.46) = 1.53, p = .222) or openness (F(2, 77.41) = 0.31, p = .729).
Fig. 2.
Dynamics in personality across all five NEO personality traits. Boxplots describing all personality traits as a function of time. The lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). The lower and upper whisker extends from the hinge to the smallest\largest value no further than 1.5 * inter quartile range (IQR) from the hinge. *indicates p < .05, **indicates p < .01.
3.3. Dynamics in neuroticism and limbic reactivity
Linear mixed effects (LME) models assessing the link between neuroticism and amygdala activation across time yielded a significant interaction only with respect to time-varying beta-estimates of the Medical83 condition (F(2, 99.84) = 6.21, p = .002; all other p's > 0.12), indicating a time-dependent relationship between neuroticism and intra-individual differences in amygdala activation. Specifically, elevated neuroticism during military service was associated with reduced amygdala activation at that time relative to oneself, while elevated neuroticism following discharge was associated with increased amygdala activation (B(tp2) = −11.51, p = .002, 95% CI -18.66 to −4.40; B(tp3) = 8.60, p = .046, 95% CI 0.19 to 17.00 respectively; Fig. 3B). The association between neuroticism prior to military service and amygdala activation was not significant (B(tp1) = 2.59, p = .479, 95% CI -4.52 to 9.70). Post-hoc comparisons further revealed that the association between neuroticism and amygdala activation during military service was significantly different from the association between neuroticism and amygdala activation prior to (B(tp2-tp1) = −14.19, p = .015, 95% CI -25.56 to −2.68), as well as following discharge (B(tp3-tp2) = −20.04, p < .001, 95% CI -31.68 to −8.58).
LME models assessing the link between neuroticism and hippocampal activation yielded similar results. Here as well only time-varying beta-estimates of the Medical83 condition significantly interacted with neuroticism (F(2, 101.12) = 4.81, p = .010, all other p's > 0.02). Elevated neuroticism during military service was associated with reduced hippocampal activation at that time relative to oneself, while the association between elevated neuroticism following discharge and increased hippocampal activation was trending towards significance (B(tp2) = −12.64, p = .005, 95% CI -21.47 to −3.81; B(tp3) = 11.69, p = .061, 95% CI -0.54 to −23.92, respectively; Fig. 3C). The association between neuroticism prior to military service and hippocampus activation was not significant (B(tp1) = 2.22, p = .619, 95% CI -6.51 to 10.95). Post-hoc comparisons revealed that the association between neuroticism and hippocampal activation during military service was significantly different from the association between neuroticism and hippocampal activation prior to (B(tp2-tp1) = −14.86, p = .042, 95% CI -29.04 to −0.36), as well as following discharge (B(tp3-tp2) = −24.33, p = .002, 95% CI -8.53 to −39.93).
No interactions emerged between neuroticism and dACC activation across conditions (all p's > 0.13). Also, across the three ROIs, no significant effects of time-invariant mean-activation emerged in any of the conditions, implying that current results depict associations between intra-individual differences in amygdala and hippocampus activation and dynamics in neuroticism scores over time.
3.4. Dynamics in neuroticism and limbic functional connectivity
LME models assessing the link between neuroticism and functional connectivity of the amygdala and hippocampus with the dACC in the Medical83 condition yielded a significant effect only for hippocampus-dACC connectivity (F(2, 103) = 4.14, p = .018). This effect was driven by time-dependent relationship between neuroticism and hippocampus-dACC connectivity such that elevated neuroticism during military service was associated with reduced hippocampus-dACC functional connectivity at that time relative to oneself (B(tp2) = −3.13, p = .008, 95% CI -5.49 to −0.94; Fig. 3D). The relation between neuroticism scores and hippocampus-dACC connectivity was not significant prior tomilitary service nor following discharge (B(tp1) = 1.91, p = .105, 95% CI -0.41 to 4.19; B(tp3) = −0.29, p = .789, 95% CI -2.37 to 1.95). Post-hoc comparisons revealed that the association between neuroticism and hippocampus-dACC connectivity during military service was significantly different from the association between neuroticism and hippocampus-dACC functional connectivity prior to but not following discharge (B(tp2-tp1) = 5.04, p = .005, 95% CI 1.65 to 8.44; B(tp3-tp2) = 2.83, p = .099, 95% CI -0.45 to 6.16).
Exploratory whole brain analysis revealed two clusters that exhibited significant functional connectivity with the amygdala during the Medical83 condition, located in the right calcarine sulcus and bilateral thalamus (see Figure S4A and Table S1). Five clusters emerged with respect to hippocampal connectivity during the Medical83 condition, also located in the right calcarine sulcus and bilateral thalamus, as well as in the post-central gyrus, left hippocampus and the left medial section of the cingulate cortex (MCC) (see Figure S4B and Table S1). LME models assessing the link between neuroticism and functional connectivity in these clusters yielded a significant effect only for the hippocampus-MCC functional connectivity (F(2, 95.888) = 3.83, p = .024), such that elevated neuroticism during military service was associated with reduced hippocampus-MCC functional connectivity relative to oneself (B(tp2) = −2.44, p = .009, 95% CI -4.23 to −0.64; Figure S4C), and this association was significantly different from the association between neuroticism and hippocampus-MCC functional connectivity following discharge (B(tp3-tp2) = −3.87, p = . 006, 95% CI -6.63 to −1.12).
4. Discussion
The current prospective longitudinal assessment of personality among healthy young adult soldiers revealed that neuroticism levels increased during a military service that included active combat relative to prior to stress, yet decreased back to pre-stress levels one year following military discharge. Critically, neuroticism was the only personality trait to exhibit such quadratic pattern of stress-induced dynamics. Exposure to multiple potentially traumatic sights of severe causalities during active combat also yielded an expected increase in posttraumatic and depressive symptoms among the soldiers. Nevertheless, these stress-related psychopathological symptoms remained well below clinical levels in the current cohort, with majority of participants exhibiting none-to-mild levels of posttraumatic and depressive symptoms during as well as following their military service, implying that the current cohort mostly include resilient individuals. Taken together, this may suggest that the observed dynamics in their personality scores depict an adaptive response to stress, expressed as elevated neuroticism during stress and subsequent normalization of neuroticism levels long-time following stress offset. In support of that, the resilience framework considers resilience as a dynamic process of successful adaptation to stress that unfolds over time (Kalisch et al., 2017). Stemming from this framework is the idea that an adaptive reaction to stress is not equal to no reaction or no change at all. Accordingly, temporary elevation in neuroticism during periods of prolonged danger or stress, expressed as perceiving the world as more dangerous than before, exhibiting enhanced sensitivity to threat cues (Drabant et al., 2011), or hypervigilance and increased caution (Lommen, Engelhard, & van den Hout, 2010), may reflect this kind of adaptive reaction. In other words, upon exposure to prolonged severe stress, fostering behavioral tendencies that include heightened threat anticipation and anxiety may facilitate coping (King and Trent, 2012; Watson and Casillas, 2003). While these notions are somewhat divergent from the prevalent conceptualization of elevated neuroticism as a risk factor, it is important to keep in mind that majority of evidence to date relied on cross-sectional or longitudinal designs that were conducted in a “stress-free” environment. Our study examined personality and stress-related psychopathological symptoms prior to, during, and long-time following real-life combat stress, and as such may have uncovered novel dynamic resilience patterns. The adaptiveness of these flexible dynamics in behavioral tendencies is also in line with previous demonstrations that cognitive flexibility represents a resilience factor upon exposure to stress (Ben-Zion et al., 2018). Indeed, a critical element in the putative adaptiveness of this dynamic process includes a reduction in neurotic tendencies following stress offset as a sign of recovery or normalization, as demonstrated here. Anecdotally, the single participant in the current cohort with extreme neuroticism scores following military discharge was also the only participant to exhibit severe posttraumatic and depressive symptoms.
Dynamics in neuroticism were also found to be associated in a time-dependent manner with intra-individual differences in limbic reactivity. Specifically, elevated neuroticism during military service was associated with reduced amygdala and hippocampus activation in response to stress-related content relative to oneself, with the opposite pattern emerging following stress offset (i.e., one year following discharge). Similar to personality patterns, these results may appear counterintuitive at first, in light of vast literature linking elevated neuroticism scores with increased amygdala and hippocampus activation (Brown et al., 2020; Chan et al., 2009; Haas et al., 2007; Schuyler et al., 2014). In here a positive association between neuroticism and limbic reactivity emerged only in a “stress-free” period, one year following discharge, a period that may resemble the circumstances of previous studies. What could then account for the negative association between limbic reactivity to stress related content and neuroticism scores during stressful military service? First, these results support a dynamic and context-dependent association between neuroticism and limbic reactivity (Everaerd et al., 2015; Servaas et al., 2013), as well as the notion that stress-induced neural changes may be partially persistent and partially reversible over long time periods (van Wingen et al., 2012). But even more specifically, multitude of evidence links limbic, particularly amygdala, reactivity to stress-related and negatively charged stimuli with stress-related psychopathology (Admon et al., 2013b; Etkin and Wager, 2007; Henigsberg et al., 2019). Even within this cohort, elevated amygdala and hippocampus reactivity to stress-related content during stress has been associated with more posttraumatic and depressive symptoms (Admon et al., 2013a; Admon et al., 2009). Following this line, the association between elevated neuroticism and reduced amygdala and hippocampus reactivity to stress-related content during stress further strengthens the putative adaptiveness of these dynamics in personality. In other words, elevated neuroticism in the proper context (i.e., upon exposure to prolonged severe stress) may promote resilience via its association with reduced limbic reactivity to stress-related content. As before, the true adaptiveness of these processes may depend on their reversibility following stress offset, at the behavioral and neural levels. More broadly, these processes may represent protective mechanisms that promote short-term adaptation during stress (allostasis), while their reversibility following stress offset in the current resilient cohort may represent individuals’ ability to avoid a more chronic allostatic load (McEwen and Gianaros, 2010).
Functional connectivity analysis revealed that elevated neuroticism during military service was also associated with reduced hippocampus-cingulate cortex connectivity in response to stress-related content. Interestingly, both the dACC and MCC were found to exhibit such pattern in here, based on ROI and whole brain analysis, respectively. These results further extend the dynamic nature of the association between neuroticism and limbic reactivity towards connectivity patterns. Currently, neuroimaging studies assessing the link between neuroticism and functional connectivity are scarce and have yielded inconsistent results (Cremers et al., 2010; Silverman et al., 2019; Yang et al., 2020). Following the same rationale as for activation results, we can speculate that previous associations that were reported cross-sectionally under non-stressful circumstances may not necessarily emerge under stressful periods, and that the current longitudinal associations between elevated neuroticism and reduced hippocampus-cingulate connectivity in response to stress-related content during military service may have contributed to stress adaptation. Such notion is supported by the well-established role of the dACC in threat appraisal and emotional expression during associative threat learning (Etkin et al., 2011). To this end, in the current sample of combat paramedics, reduced hippocampus-dACC connectivity was only evident in response to a priori neutral medical images that may have gained their stress-context through associative learning processes. Reduced hippocampus-dACC connectivity may therefore reflect diminished emotional association of the a priori neutral medical images during military service, a connectivity pattern that is evident among individuals with elevated neuroticism at that time and may lead to improved coping. Relatedly, the link between hippocampal and dACC activation patterns was also shown to influence threat bias in an independent sample of resilient soldiers (Lin et al., 2015). With respect to MCC, its function has been linked to response selection and feedback-guided decision making in a wide variety of task manipulations (Vogt, 2016, 2019). In the context of stress, MCC activation was found to be positively correlated with posttraumatic stress disorder (PTSD) symptom severity (Etkin and Wager, 2007), and longitudinal reduction in PTSD symptom severity following therapy was associated with decreased MCC activation (Garrett et al., 2019). These results point towards reduced MCC reactivity during stress as a potentially adaptive response, which in here was associated with elevated neuroticism. Whether the dACC and MCC represent two components of a single mechanism for stress adaption via associations with hippocampal connectivity and neuroticism, or whether these cingulate clusters represent two distinct mechanisms, is for future studies to assess.
Although the focus of this study was on neuroticism, we also explored whether the four additional NEO traits changed over the three time points of assessment. Finding include a decrease in extraversion and an increase in conscientiousness scores over time. Critically, unlike the quadratic pattern of change in neuroticism, extraversion decreased from prior to during military service and remained low following discharge, while conscientiousness gradually increased over time from during to following discharge. Considering that our participants were all young adults, a period in which personality is particularly malleable, we cannot attribute these linear changes solely to the effect of stress. In fact, in non-stressful circumstances personality tends to develop in a positive direction with age (i.e., increase in emotional stability, confidence and social vitality) (Roberts et al., 2006; Robins et al., 2001; Vaidya et al., 2008), resembling the pattern of change observed in here. Accordingly, dynamics in extraversion and conscientiousness may (or may not) reflect typical personality changes during young adulthood. These findings further strengthen the notion that the observed dynamics in neuroticism, being the only personality trait to exhibit a quadratic pattern of change, were not driven by age per se but rather by the effect of stress and its offset.
While the unique cohort and design of the current study enabled prospective longitudinal assessment of personality, stress-related psychopathological symptoms, and neural activation and connectivity patterns, prior to, during and long-time following exposure to real-life combat stress, it is not lack of limitations. First, trait questionnaires may be confounded with situational effects. Thus, observed dynamics in neuroticism scores may be partially due to changes in participants' mood state or anxiety levels. In fact, state and trait questionnaires are highly correlated and share overlapping components, with traits questionnaires explaining around 30–50% of the variance in negative affect (Steel et al., 2008). States like depressions and anxiety, however, have shown only small effect on neuroticism levels (Karsten et al., 2012). Additionally, a dynamic perspective of personality suggests that personality itself may change in response to prolonged stress (Metts et al., 2021). Here, participants' levels of neuroticism showed different dynamics then posttraumatic and depression symptoms, suggesting that not all of the effect of neuroticism was due to situational causes. Nevertheless, since we did not obtain data regarding participants' mood or anxiety levels, we cannot completely preclude such confounds. A second limitation of the current study may stem from its relatively homogenous cohort, potentially impacting the generalizability of results. To this end, all study participants were thoroughly screened and passed a series of physical, cognitive, mental, and sociodemographic tests and examinations prior to their enrollment to the military, suggesting that the current cohort may hold multiple resilience factors. Indeed, while all participants were exposed to multiple potentially traumatic sights of severe causalities during active combat, vast majority of them exhibited none-to-mild levels of posttraumatic and depressive symptoms during as well as following their military service. As a consequence of this paucity of symptoms, we were unable to investigate putative relations between the observed personality and neural dynamic patterns and individual variability in symptom levels, and instead we refer to the sample as a homogenously resilient cohort (excluding a single participant).
Participants were also all young adults during the study period, thus might have been particularly malleable to personality and neural changes regardless of stress. It should be noted however, that the five personality traits exhibited different dynamic patterns, with neuroticism being the only trait to exhibit a quadratic pattern of change, supporting the notion that current neuroticism-related results were not driven by age per se but rather by the effect of stress and its offset. Sample homogeneity may have also contributed to the fact that out of the six task conditions, only the response to Medical83 condition was associated with neuroticism scores. Considering that participants were all combat paramedics and that their stressful experiences were in the context of medical care, the response to the medical pictures may be a specific feature of the current sample. Additional limitation may relate to the relatively modest size of our final sample, with 49, 40 and 28 participants prior to, during, and long-time following military service, respectively. While no significant differences emerged between study completers and non-completers with respect to gender, personality scores, and posttraumatic and depressive symptoms prior to their military service, it could still be possible that non-completers exhibited more stress-related symptoms during or following their military service. Nevertheless, as far as we know, the current study represents the first prospective longitudinal assessment of neural reactivity prior to, during, and long-time following military service, due to the tremendous challenges that such design involves. Finally, it should be noted that some of our hypotheses were not met, including no relation between dACC activation and neuroticism across all time points, no relation between reactivity in all limbic region and neuroticism prior to military service and no time-invariant mean-activation effects. It remains open for future investigations whether these null findings stem from the relative homogeneity of the current sample or from its modest size, with both factors potentially limiting our ability to detect inter-individual differences.
5. Conclusions
In conclusion, using a unique prospective longitudinal design, we were able to demonstrate that stressful military service at young adulthood yields a temporary increase in neuroticism as well as a temporary decrease in limbic reactivity, with both effects being reversed long-time following discharge. Considering that most participants maintained functionality and mental health despite exposure to real-life combat stress, these dynamic patterns may depict behavioral and neural mechanisms that facilitate stress resilience.
Funding
This work was supported by the Brain & Behavior Research Foundation (Formerly NARSAD) Young Investigator Award (Grant ID: 25993) awarded to R.A. The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare no competing interests or conflict of interest.
Acknowledgments
We would like to thank Dr. Gad Lubin and Dr Eyal Fructer from the Israel Defense Forces (IDF) medical corps for their support of this work. Special thanks to all the volunteering soldiers that took part in the study. This work is dedicated to the memory of staff sergeant Yotam Gilboa who volunteered for this study and was later killed during his military service at the age of 21.
Appendix A.
Supplementary methods
Recruitment procedure
Prior to their enrollment to a pre-draft course of combat paramedics, participants went through a series of tests and examinations in order to determine their suitability for the paramedic course. Since military service is mandatory in Israel, there is a regular recruitment process that maps each soldier's status and abilities across several domains in order to determine his\her role and unit of assignment. This process is comprised of several parts: First, a quality index is given to each soldier based on socio demographic information such as education level and on primary psycho-technical rating index. The primary psycho-technical rating index is based on performance in psycho-technical tests that measure cognitive abilities (quantitative, verbal and formal thinking abilities). Additionally, each soldier is given a medical profile score based on a physical exam. Lastly, each soldier is interviewed by a trained psychotechnical diagnostician on order to assess their mental capability to serve in a combat military service. These scores serve as a threshold for recruitment to elite units and roles such as the combat paramedics in our sample.
Supplementary results
Comparing study completers and non-completers
Study completers (n = 28) did not differ from non-completers (n = 22) with respect to gender (Χ2(1, 50) = 0.01, p = .905), personality at the first time point MNeuroticism_t1(completers) = 17.34, MNeuroticism_t1(non completers) = 18.15, t(47) = −0.56, p = .57, 95% CI -3.671 to 2.060; MExtraversion_t1(completers) = 33.51, MExtraversion_t1(non completers) = 31.60, t(47) = 1.236, p = .222, 95% CI -1.202 to 5.037; MConciousness_t1(completers) = 32.37, MConciousness_t1(non completers) = 30.65, t(47) = 0.886, p = .379, 95% CI -2.193 to 5.652; MAgreeblness_t1(completers) = 30.03, MAgreeblness_t1(non completers) = 29.60, t(47) = 0.319, p = .75, 95% CI -2.297 to 3.166; MOpenness_t1(completers) = 27.48, MOpenness_t1(non completers) = 29.00, t(47) = −0.975, p = .33, 95% CI -4.645 to 1.611), and posttraumatic and depressive symptoms at the first time point (Mann-Whiteny UPDS_t1 = 309.5, p = .608; Mann-Whiteny UBDI_t1 = 144.5, p = .314, respectively).
Dynamics in psychopathological symptoms
The effect of time on posttraumatic and depressive symptoms was analyzed using poisson generalized linear mixed effect models (GLMM) to account for skewed distribution, with either posttraumatic or depressive symptoms at each time point as the dependent variables and time as an independent factor. Unconditional growth models of posttraumatic symptom levels revealed a significant effect of time (χ2(2) = 63.63, p < .001), due to an increase in posttraumatic symptoms at the group level from prior to during military service (IRRPDS(tp2-tp1) = 2.73, Z = 7.57, p < .001). Following discharge, posttraumatic symptoms slightly declined back yet were still significantly different from symptom levels prior to, but not during military service (IRRPDS(tp3-tp1) = 2.18, Z = 4.95, p < .001; IRRPDS(tp3-tp2) = 0.80, Z = −1.66, p = .096; Figure S1A and Table 1). Depressive symptoms also exhibited a pattern of change over time (χ2(2) = 6.69, p = .03), with a trend towards significant increase in depressive symptoms during military service, and a significant increase following discharge compared to prior to military service (IRRBDI(tp2-tp1) = 1.26, Z = 1.88, p = .060; IRRBDI(tp3-tp1) = 1.43, Z = 2.54, p = .010; IRRBDI(tp3-tp2) = 1.13, Z = 1.00, p = .316; Figure S1B and Table 1).
Fig. S1.
Dynamics in post-traumatic and depressive symptoms. Boxplots describing posttraumatic and depressive symptom over the three time points of assessment, accounting for prior to, during, and long-time following military service, respectively. A) Unconditional growth models of posttraumatic symptom levels revealed a significant effect of time (χ2(2) = 63.63, p < .001), driven by an increase in symptoms from prior to during military service and to following discharge (IRRPDS(tp2-tp1) = 2.73, Z = 7.57, p < .001; IRRPDS(tp3-tp1) = 2.18, Z = 4.95, p < .001). B) Unconditional growth models of depressive symptoms levels revealed a significant effect of time (χ2(2) = 6.69, p = .03), driven by a trend towards increase in symptoms from prior to during military service and a significant increase from prior to following discharge (IRRBDI(tp2-tp1) = 1.26, Z = 1.88, p = .060; IRRBDI(tp3-tp1) = 1.43, Z = 2.54, p = .010). Critically, for both posttraumatic and depressive symptoms, mean levels remained well below clinical threshold throughout all three time points, with majority of participants exhibiting none-to-mild symptoms. A single participant exhibited severe posttraumatic and depressive symptoms long-time following discharge and was excluded from analysis as an outlier (marked in red dot). Levels of severity norms are labeled in gray, based on (Foa, 1995) and (Beck et al., 1988) respectively.
Fig. S2.
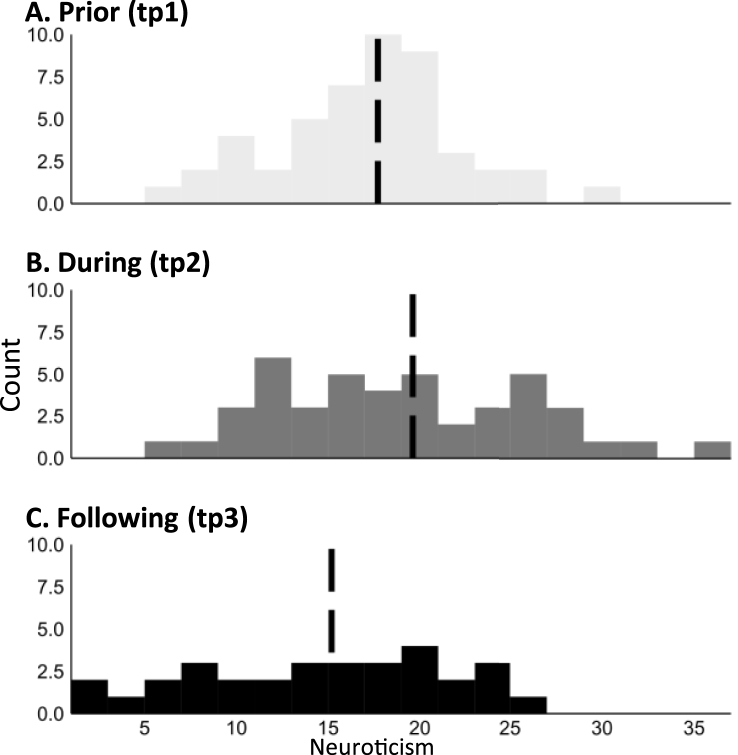
Distribution of neuroticism scores across time-points. Dashed line represents group mean. Also, Neuroticism scores showed internal consistency (Cronbach's alpha) of: αtp1 = .82, αtp2 = .77, and αtp3 = .83 across time points, representing acceptable consistency scores.
Fig. S3.
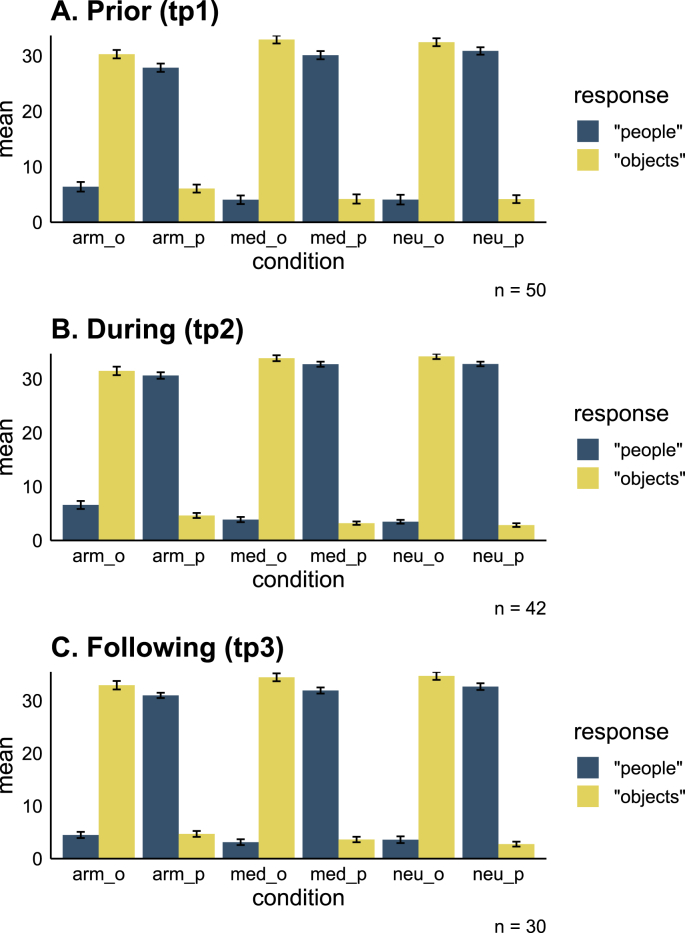
Task performance. Accuracy during the backward masking task across conditions and time points (A-C). During the task participants were instructed to indicate immediately after each picture presentation whether it included images of people or objects. A total of (9 × 4) 36 pictures of people and 36 pictures of objects were presented per condition per time point. Y axis – mean number of times participants indicated that the picture was of people\objects. arm = army, med = medical, neu = neutral, p = people, o = objects. Error bars represent standard errors.
Fig. S4.
Dynamics in neuroticism and limbic functional connectivity. Results of exploratory whole brain analysis assessing amygdala and hippocampus connectivity during the Medical83 condition with significance threshold set at p < .05 FWE voxel-wise corrected. A) Two clusters emerged with respect to amygdala connectivity, located in the right calcarine sulcus and bilateral thalamus (pulvinar nucleus). B) Five clusters emerged with respect to hippocampal connectivity, also located in the right calcarine sulcus and bilateral pulvinar nucleus of the thalamus, as well as in the post-central gyrus, left hippocampus and the left medial section of the cingulate cortex (MCC; marked with a red circle). C) The LME model for hippocampus-MCC functional connectivity revealed a significant time by hippocampus-MCC connectivity interaction (F(2, 95.888) = 3.83, p = .024), driven by time-dependent relationship between neuroticism and hippocampus-MCC connectivity such that elevated neuroticism during military service was associated with reduced hippocampus-MCC functional connectivity (B(tp2) = −2.44, p = .009). *indicates p < .05.
Table S1.
Clusters that emerged from whole brain functional connectivity analysis. Results of exploratory whole brain analysis assessing functional connectivity with the amygdala and hippocampus during the Medical83 condition. XYZ Coordinates reported in Montreal Neurological Institute (MNI) space. Statistical threshold for search volume: p < .05 FWE voxel-wise corrected, cluster >10 voxels.
| X,Y,Z | Z-score | Cluster size | |
|---|---|---|---|
| Amygdala as seed | |||
| Left Thalamus | −6, −22, 10 | 5.21 | 18 |
| Right Thalamus | 9, −19, 7 | 4.95 | 11 |
| Left Calcarine | 0, −61, 13 | 5.70 | 22 |
| Hippocampus as seed | |||
| Left Hippocampus | −30, −19, −17 | 5.97 | 24 |
| Right Calcarine | 3, −58, 13 | 5.34 | 49 |
| Middle Cingulate Cortex (MCC) | 3, −16, 52 | 5.13 | 12 |
| Left post-central gyrus | −33, −28, 55 | 5.00 | 14 |
| Left Thalamus | 0, −22, 13 | 5.01 | 11 |
References
- Admon R., Lubin G., Rosenblatt J.D., Stern O., Kahn I., Assaf M., Hendler T. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebr. Cortex. 2013;23(1):28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- Admon R., Lubin G., Stern O., Rosenberg K., Sela L., Ben-Ami H., Hendler T. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Milad M.R., Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cognit. Sci. 2013;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Aldinger M., Stopsack M., Ulrich I., Appel K., Reinelt E., Wolff S. Neuroticism developmental courses—implications for depression, anxiety and everyday emotional experience; a prospective study from adolescence to young adulthood. BMC Psychiatr. 2014;14:210. doi: 10.1186/s12888-014-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T.A., DeYoung C.G. Personality neuroscience and the five factor model. In: Widiger T.A., editor. Oxford Handbook of the Five Factor Model. 2016. pp. 319–352. [Google Scholar]
- Barlow D.H., Sauer-Zavala S., Carl J.R., Bullis J.R., Ellard K.K. The nature, diagnosis, and treatment of neuroticism. Clinical Psychological Science. 2013;2(3):344–365. doi: 10.1177/2167702613505532. [DOI] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models Usinglme4. J. Stat. Software. 2015;67(1) doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Ben-Zion Z., Fine N.B., Keynan N.J., Admon R., Green N., Halevi M. Cognitive flexibility predicts PTSD symptoms: observational and interventional studies. Front. Psychiatr. 2018;9:477. doi: 10.3389/fpsyt.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbey A., Carroll D., Roseboom T.J., Phillips A.C., de Rooij S.R. Personality and physiological reactions to acute psychological stress. Int. J. Psychophysiol. 2013;90(1):28–36. doi: 10.1016/j.ijpsycho.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Brown N., Wojtalik J.A., Turkel M., Vuper T., Strasshofer D., Sheline Y.I., Bruce S.E. Neuroticism and its associated brain activation in women with PTSD. J. Interpers Violence. 2020;35(1–2):341–363. doi: 10.1177/0886260516682519. [DOI] [PubMed] [Google Scholar]
- Bruhl A.B., Viebke M.C., Baumgartner T., Kaffenberger T., Herwig U. Neural correlates of personality dimensions and affective measures during the anticipation of emotional stimuli. Brain Imaging Behav. 2011;5(2):86–96. doi: 10.1007/s11682-011-9114-7. [DOI] [PubMed] [Google Scholar]
- Canli T. Toward a neurogenetic theory of neuroticism. Ann. N. Y. Acad. Sci. 2008;1129:153–174. doi: 10.1196/annals.1417.022. [DOI] [PubMed] [Google Scholar]
- Chan S.W., Norbury R., Goodwin G.M., Harmer C.J. Risk for depression and neural responses to fearful facial expressions of emotion. Br. J. Psychiatry. 2009;194(2):139–145. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- Corr P.J., Matthews G. 2009. The Cambridge Handbook of Personality Psychology. [Google Scholar]
- Cremers H.R., Demenescu L.R., Aleman A., Renken R., van Tol M.J., van der Wee N.J. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49(1):963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Arbuckle N.L., Jahn A., Mowrer S.M., Abduljalil A.M. Reprint of: aspects of neuroticism and the amygdala: chronic tuning from motivational styles. Neuropsychologia. 2011;49(4):657–662. doi: 10.1016/j.neuropsychologia.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Deng Y., Li S., Zhou R., Walter M. Neuroticism modulates the functional connectivity from amygdala to frontal networks in females when avoiding emotional negative pictures. Front. Behav. Neurosci. 2019;13:102. doi: 10.3389/fnbeh.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman J.M. Personality structure: emergence of the five-factor model. Annu. Rev. Psychol. 1990;41(1):417–440. [Google Scholar]
- Drabant E.M., Kuo J.R., Ramel W., Blechert J., Edge M.D., Cooper J.R. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55(1):401–410. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant E.M., McRae K., Manuck S.B., Hariri A.R., Gross J.J. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol. Psychiatr. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognit. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., van Wingen G., Tendolkar I., Fernandez G. Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage. 2015;112:218–224. doi: 10.1016/j.neuroimage.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Foa E. National Computer Systems; Minneapolis, MN: 1995. The Posttraumatic Diagnostic Scale (PDS) Manual; pp. 1–5. [Google Scholar]
- Foa E.B., Cashman L., Jaycox L., Perry K. The validation of a self-report measure of PTSD: the Posttraumatic Diagnostic ScaleTM (PDSTM) Psychol. Assess. 1997;9(4):445–451. [Google Scholar]
- Garrett A., Cohen J.A., Zack S., Carrion V., Jo B., Blader J. Longitudinal changes in brain function associated with symptom improvement in youth with PTSD. J. Psychiatr. Res. 2019;114:161–169. doi: 10.1016/j.jpsychires.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B.L., Perlman G., Eaton N.R., Kotov R., Klein D.N. Testing explanatory models of the interplay between depression, neuroticism, and stressful life events: a dynamic trait-stress generation approach. Psychol. Med. 2019:1–10. doi: 10.1017/S0033291719002927. [DOI] [PubMed] [Google Scholar]
- Haas B.W., Omura K., Constable R.T., Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci. 2007;121(2):249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Henigsberg N., Kalember P., Petrovic Z.K., Secic A. Neuroimaging research in posttraumatic stress disorder - focus on amygdala, hippocampus and prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;90:37–42. doi: 10.1016/j.pnpbp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Hyde L.W., Gorka A., Manuck S.B., Hariri A.R. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49(4):651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja L. Interactions: comprehensive, user-friendly toolkit for probing interactions (version R package version 1.1.0) 2019. https://cran.r-project.org/package=interactions Retrieved from.
- Jeronimus B.F., Kotov R., Riese H., Ormel J. Neuroticism's prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol. Med. 2016;46(14):2883–2906. doi: 10.1017/S0033291716001653. [DOI] [PubMed] [Google Scholar]
- Jeronimus B.F., Riese H., Sanderman R., Ormel J. Mutual reinforcement between neuroticism and life experiences: a five-wave, 16-year study to test reciprocal causation. J. Pers. Soc. Psychol. 2014;107(4):751–764. doi: 10.1037/a0037009. [DOI] [PubMed] [Google Scholar]
- Kalisch R., Baker D.G., Basten U., Boks M.P., Bonanno G.A., Brummelman E. The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav. 2017;1(11):784–790. doi: 10.1038/s41562-017-0200-8. [DOI] [PubMed] [Google Scholar]
- Karsten J., Penninx B.W., Riese H., Ormel J., Nolen W.A., Hartman C.A. The state effect of depressive and anxiety disorders on big five personality traits. J. Psychiatr. Res. 2012;46(5):644–650. doi: 10.1016/j.jpsychires.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Gatz M., Gardner C.O., Pedersen N.L. Personality and major depression: a Swedish longitudinal, population-based twin study. Arch. Gen. Psychiatr. 2006;63(10):1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Jacobson K.C., Gardner C.O., Prescott C.A., Kendler K.S. Personality and comorbidity of common psychiatric disorders. Br. J. Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- King L.A., Trent J. second ed. 2012. Personality Strengths Handbook Of Psychology. [Google Scholar]
- Kleiman E. EMAtools: data management tools for real-time monitoring/ecological momentary assessment data. 2017. https://CRAN.R-project.org/package=EMAtools (Version R package version 0.1.3). Retrieved from.
- Kotov R., Gamez W., Schmidt F., Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull. 2010;136(5):768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. J. Stat. Software. 2017;82(13) doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Leger K.A., Charles S.T., Turiano N.A., Almeida D.M. Personality and stressor-related affect. J. Pers. Soc. Psychol. 2016;111(6):917–928. doi: 10.1037/pspp0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikas S., Salmela-Aro K. Personality trait changes among young Finns: the role of life events and transitions. J. Pers. 2015;83(1):117–126. doi: 10.1111/jopy.12088. [DOI] [PubMed] [Google Scholar]
- Lin T., Vaisvaser S., Fruchter E., Admon R., Wald I., Pine D.S. A neurobehavioral account for individual differences in resilience to chronic military stress. Psychol. Med. 2015;45(5):1011–1023. doi: 10.1017/S0033291714002013. [DOI] [PubMed] [Google Scholar]
- Liu X., Lai H., Li J., Becker B., Zhao Y., Cheng B., Wang S. Gray matter structures associated with neuroticism: a meta-analysis of whole-brain voxel-based morphometry studies. Hum. Brain Mapp. 2021 doi: 10.1002/hbm.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen M.J.J., Engelhard I.M., van den Hout M.A. Neuroticism and avoidance of ambiguous stimuli: better safe than sorry? Pers. Indiv. Differ. 2010;49(8):1001–1006. doi: 10.1016/j.paid.2010.08.012. [DOI] [Google Scholar]
- Luke S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods. 2017;49(4):1494–1502. doi: 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- Malouff J.M., Thorsteinsson E.B., Schutte N.S. The relationship between the five-factor model of personality and symptoms of clinical disorders: a meta-analysis. J. Psychopathol. Behav. Assess. 2005;27(2):101–114. doi: 10.1007/s10862-005-5384-y. [DOI] [Google Scholar]
- McCrae R.R. The Five-Factor Model of personality traits: consensus and controversy. In: Corr P.J., Matthews G., editors. The Cambridge Handbook of Personality Psychology. 2009. pp. 148–161. [Google Scholar]
- McCrae R.R., Costa P.T., Jr. More reasons to adopt the five-factor model. Am. Psychol. 1989;44(2):451–452. doi: 10.1037/0003-066X.44.2.451. [DOI] [Google Scholar]
- McCrae R.R., John O.P. An introduction to the five‐factor model and its applications. J. Pers. 1992;60(2):175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M., Bacon S., Lykken D.T. Personality stability and change in early adulthood: a behavioral genetic analysis. Dev. Psychol. 1993;29(1):96–109. doi: 10.1037/0012-1649.29.1.96. [DOI] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metts A., Yarrington J., Enders C., Hammen C., Mineka S., Zinbarg R., Craske M.G. Reciprocal effects of neuroticism and life stress in adolescence. J. Affect. Disord. 2021;281:247–255. doi: 10.1016/j.jad.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W. Toward an integrative science of the person. Annu. Rev. Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- Murray G., Rawlings D., Allen N.B., Trinder J. NEO five-factor inventory scores: psychometric properties in a community sample. Meas. Eval. Counsel. Dev. 2017;36(3):140–149. doi: 10.1080/07481756.2003.11909738. [DOI] [Google Scholar]
- Neumann C.S. Structural equation modeling of the associations between amygdala activation, personality, and internalizing, externalizing symptoms of psychopathology. Personal Neurosci. 2020;3:e8. doi: 10.1017/pen.2020.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle C.M., Rubin D.C., Siegler I.C. Changes in neuroticism following trauma exposure. J. Pers. 2014;82(2):93–102. doi: 10.1111/jopy.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J., Bastiaansen A., Riese H., Bos E.H., Servaas M., Ellenbogen M. The biological and psychological basis of neuroticism: current status and future directions. Neurosci. Biobehav. Rev. 2013;37(1):59–72. doi: 10.1016/j.neubiorev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ormel J., Riese H., Rosmalen J.G. Interpreting neuroticism scores across the adult life course: immutable or experience-dependent set points of negative affect? Clin. Psychol. Rev. 2012;32(1):71–79. doi: 10.1016/j.cpr.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ormel J., VonKorff M., Jeronimus B.F., Riese H. 2017. Set-Point Theory and Personality Development; pp. 117–137. [DOI] [Google Scholar]
- Poppelaars E.S., Klackl J., Pletzer B., Wilhelm F.H., Jonas E. Social-evaluative threat: stress response stages and influences of biological sex and neuroticism. Psychoneuroendocrinology. 2019;109:104378. doi: 10.1016/j.psyneuen.2019.104378. [DOI] [PubMed] [Google Scholar]
- Reynaud E., El Khoury-Malhame M., Rossier J., Blin O., Khalfa S. Neuroticism modifies psychophysiological responses to fearful films. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0032413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese H., Snieder H., Jeronimus B.F., Korhonen T., Rose R.J., Kaprio J., Ormel J. Timing of stressful life events affects stability and change of neuroticism. Eur. J. Pers. 2014;28(2):193–200. doi: 10.1002/per.1929. [DOI] [Google Scholar]
- Roberts B.W., Walton K.E., Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol. Bull. 2006;132(1):1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Robins R.W., Fraley R.C., Roberts B.W., Trzesniewski K.H. A longitudinal study of personality change in young adulthood. J. Pers. 2001;69(4):617–640. doi: 10.1111/1467-6494.694157. [DOI] [PubMed] [Google Scholar]
- Schuyler B.S., Kral T.R., Jacquart J., Burghy C.A., Weng H.Y., Perlman D.M. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc. Cognit. Affect Neurosci. 2014;9(2):176–181. doi: 10.1093/scan/nss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas M.N., van der Velde J., Costafreda S.G., Horton P., Ormel J., Riese H., Aleman A. Neuroticism and the brain: a quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neurosci. Biobehav. Rev. 2013;37(8):1518–1529. doi: 10.1016/j.neubiorev.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Shiner R.L., Allen T.A., Masten A.S. Adversity in adolescence predicts personality trait change from childhood to adulthood. J. Res. Pers. 2017;67:171–182. doi: 10.1016/j.jrp.2016.10.002. [DOI] [Google Scholar]
- Silverman M.H., Wilson S., Ramsay I.S., Hunt R.H., Thomas K.M., Krueger R.F., Iacono W.G. Trait neuroticism and emotion neurocircuitry: functional magnetic resonance imaging evidence for a failure in emotion regulation. Dev. Psychopathol. 2019;31(3):1085–1099. doi: 10.1017/S0954579419000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel P., Schmidt J., Shultz J. Refining the relationship between personality and subjective well-being. Psychol. Bull. 2008;134(1):138–161. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- Thomas E.J., Elliott R., McKie S., Arnone D., Downey D., Juhasz G. Interaction between a history of depression and rumination on neural response to emotional faces. Psychol. Med. 2011;41(9):1845–1855. doi: 10.1017/S0033291711000043. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaidya J.G., Gray E.K., Haig J.R., Mroczek D.K., Watson D. Differential stability and individual growth trajectories of big five and affective traits during young adulthood. J. Pers. 2008;76(2):267–304. doi: 10.1111/j.1467-6494.2007.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen G.A., Geuze E., Caan M.W., Kozicz T., Olabarriaga S.D., Denys D. Persistent and reversible consequences of combat stress on the mesofrontal circuit and cognition. Proc. Natl. Acad. Sci. U. S. A. 2012;109(38):15508–15513. doi: 10.1073/pnas.1206330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A. Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 2016;74:28–46. doi: 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Cingulate cortex in the three limbic subsystems. Handb. Clin. Neurol. 2019;166:39–51. doi: 10.1016/B978-0-444-64196-0.00003-0. [DOI] [PubMed] [Google Scholar]
- Watson D., Casillas A. 2003. Neuroticism: Adaptive and Maladaptive Features Virtue, Vice, and Personality: The Complexity of Behavior; pp. 145–161. [Google Scholar]
- Yang J., Mao Y., Niu Y., Wei D., Wang X., Qiu J. Individual differences in neuroticism personality trait in emotion regulation. J. Affect. Disord. 2020;265:468–474. doi: 10.1016/j.jad.2020.01.086. [DOI] [PubMed] [Google Scholar]
- Zelenski J.M., Larsen R.J. Susceptibility to affect: a comparison of three personality taxonomies. J. Pers. 1999;67(5) doi: 10.1111/1467-6494.00072. [DOI] [PubMed] [Google Scholar]