Abstract
Objective
Glucagon is secreted by pancreatic α-cells in response to hypoglycemia and its hyperglycemic effect helps to restore normal blood glucose. Insulin and somatostatin (SST) secretions from β- and δ-cells, respectively, are stimulated by glucose by mechanisms involving an inhibition of their ATP-sensitive K+ (KATP) channels, leading to an increase in [Ca2+]c that triggers exocytosis. Drugs that close KATP channels, such as sulfonylureas, are used to stimulate insulin release in type 2 diabetic patients. α-cells also express KATP channels. However, the mechanisms by which sulfonylureas control glucagon secretion are still largely debated and were addressed in the present study. In particular, we studied the effects of KATP channel blockers on α-cell [Ca2+]c and glucagon secretion in the presence of a low (1 mM) or a high (15 mM) glucose concentration and evaluated the role of SST in these effects.
Methods
Using a transgenic mouse model expressing the Ca2+-sensitive fluorescent protein, GCaMP6f, specifically in α-cells, we measured [Ca2+]c in α-cells either dispersed or within whole islets (by confocal microscopy). By measuring [Ca2+]c in α-cells within islets and glucagon secretion using the same perifusion protocols, we tested whether glucagon secretion correlated with changes in [Ca2+]c in response to sulfonylureas. We studied the role of SST in the effects of sulfonylureas using multiple approaches including genetic ablation of SST, or application of SST-14 and SST receptor antagonists.
Results
Application of the sulfonylureas, tolbutamide, or gliclazide, to a medium containing 1 mM or 15 mM glucose increased [Ca2+]c in α-cells by a direct effect as in β-cells. At low glucose, sulfonylureas inhibited glucagon secretion of islets despite the rise in α-cell [Ca2+]c that they triggered. This glucagonostatic effect was indirect and attributed to SST because, in the islets of SST-knockout mice, sulfonylureas induced a stimulation of glucagon secretion which correlated with an increase in α-cell [Ca2+]c. Experiments with exogenous SST-14 and SST receptor antagonists indicated that the glucagonostatic effect of sulfonylureas mainly resulted from an inhibition of the efficacy of cytosolic Ca2+ on exocytosis. Although SST-14 was also able to inhibit glucagon secretion by decreasing α-cell [Ca2+]c, no decrease in [Ca2+]c occurred during sulfonylurea application because it was largely counterbalanced by the direct stimulatory effect of these drugs on α-cell [Ca2+]c. At high glucose, i.e., in conditions where glucagon release was already low, sulfonylureas stimulated glucagon secretion because their direct stimulatory effect on α-cells exceeded the indirect effect by SST. Our results also indicated that, unexpectedly, SST-14 poorly decreased the efficacy of Ca2+ on exocytosis in β-cells.
Conclusions
Sulfonylureas exert two opposite actions on α-cells: a direct stimulation as in β-cells and an indirect inhibition by SST. This suggests that any alteration of SST paracrine influence, as described in diabetes, will modify the effect of sulfonylureas on glucagon release. In addition, we suggest that δ-cells inhibit α-cells more efficiently than β-cells.
Keywords: Pancreatic islets, Sulfonylureas, Ca2+, Glucagon, Somatostatin
Abbreviations: [Ca2+]c, free cytosolic Ca2+ concentration; GIRK channels, G protein-gated inwardly rectifying K+ channels; KATP channels, ATP-sensitive K+ channels; SST, somatostatin; SSTR, somatostatin receptors; TPNQ, tertiapin-Q; VGCC, voltage-gated Ca2+ channels
Highlights
-
•
KATP channel blockers control glucagon secretion by two mechanisms.
-
•
The first one is the direct stimulation of α-cell by a [Ca2+]c rise, as in β-cells.
-
•
The second one is an indirect inhibition mediated by δ-cells releasing somatostatin.
-
•
Somatostatin mainly reduces the efficacy of Ca2+ on exocytosis in α-cells.
-
•
Somatostatin more potently inhibits glucagon than insulin secretion.
1. Introduction
The endocrine pancreas, composed of the islets of Langerhans, plays a major role in glucose homeostasis [1]. Glucagon and insulin secreted by islet α- and β-cells, respectively, are two key hormones assuming this role. When glycemia is high, insulin lowers blood glucose levels by stimulating glucose storage in the tissues. However, when glycemia is reduced, glucagon increases blood glucose levels by promoting hepatic glycogenolysis and gluconeogenesis, thus stimulating glucose release from the liver [2]. In diabetes, this balance is dysregulated towards an impaired insulin secretion/action associated with an excessive glucagon secretion which lead to hyperglycemia. Furthermore, diabetic patients show an impaired glucagon response to hypoglycemia, leading to life-threatening episodes [[3], [4], [5], [6], [7]]. Increased blood glucose also stimulates δ-cells, a third cell type of the islets, which secrete somatostatin (SST) that inhibits both insulin and glucagon secretions [8].
Glucose stimulates insulin secretion from β-cells by increasing the ATP/ADP ratio which closes ATP-sensitive K+ (KATP) channels in the plasma membrane. The closure of these channels induces a membrane depolarization that opens voltage-gated Ca2+ channels (VGCC), leading to a subsequent rise in the free cytosolic Ca2+ concentration ([Ca2+]c) and the triggering of exocytosis [9,10]. The same mechanism has been demonstrated in SST-secreting δ-cells [[11], [12], [13], [14], [15]].
For decades, sulfonylureas have been used as antihyperglycemic agents to treat type 2 diabetic patients because they mimic the effect of glucose on KATP channels. They bind to the SUR1 subunit of KATP channels, thereby closing them and stimulating insulin secretion from β-cells [16,17]. Sulfonylureas also bind to the same KATP channel subunit in α- and δ-cells, and as in β-cells, they also stimulate SST secretion [15,[18], [19], [20]]. However, the effects of sulfonylureas on α-cells are still debated. Some studies report that closing KATP channels decreases [Ca2+]c and glucagon secretion [[21], [22], [23], [24], [25], [26]], while others report an increase [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]] or even no effect [13]. Furthermore, it is unclear whether sulfonylureas control glucagon secretion by a direct effect on α-cells or an indirect effect by stimulating β- or δ-cells [4].
In the present study, we used multiple approaches to decipher the mechanisms by which sulfonylureas control glucagon secretion in conditions where α-cells are stimulated by low glucose or inhibited by high glucose. 1) Using a transgenic mouse model expressing GCaMP6f, a recently developed fluorescent Ca2+ probe, specifically in α-cells, we measured [Ca2+]c in dispersed α-cells and in α-cells within whole islets (by confocal microscopy). We tested whether the [Ca2+]c responses to KATP channel blockers (tolbutamide and gliclazide) were similar between both types of preparations, which is indicative of a direct effect of the drugs on α-cells. 2) By measuring [Ca2+]c changes in α-cells within islets and glucagon secretion using the same perifusion protocols, we tested whether glucagon secretion correlated with changes in [Ca2+]c in response to sulfonylureas. 3) We evaluated the implication of SST in the control of glucagon secretion by sulfonylureas using a novel transgenic mouse model expressing GCaMP6f specifically in α-cells but lacking SST. 4) To decipher the mechanisms activated by SST during sulfonylurea application, we tested the effect of exogenous SST and antagonists of SST receptors (SSTR) in various experimental protocols.
2. Materials and methods
2.1. Animals
Several mouse models were used including Lox-STOP-Lox-GCaMP6f/Sst+/+, GluCreGCaMP6f/Sst+/+, and GluCreGCaMP6f/Sst−/− mice. Lox-STOP-Lox-GCaMP6f/Sst+/+ mice were obtained from Jackson Laboratory (stock No: 024105). They exhibit a floxed-STOP cassette upstream of the sequence of the GCaMP6f Ca2+ probe in the ROSA26 allele. GluCreGCaMP6f/Sst+/+ mice were generated by crossing homozygous Lox-STOP-Lox-GCaMP6f/Sst+/+ mice with GluCre/Sst+/+ mice, as previously described [37], to allow the excision of the STOP cassette and the expression of GCaMP6f, specifically in α-cells. In this model, GCaMP6f was expressed in a single allele. GluCreGCaMP6f/Sst−/− mice were generated by first crossing GluCreGCaMP6f/Sst+/+ mice heterozygous for GluCreGCaMP6f together to obtain homozygous GluCreGCaMP6f/Sst+/+ mice. They were then crossed with Sst−/− mice, as previously described [38], to obtain GluCreGCaMP6f/Sst+/- mice. These mice were finally crossed together to obtain the GluCreGCaMP6f/Sst−/− mouse model. These mice are homozygous for GCaMP6f and do not express SST. Because we did not observe any differences between sexes and ages in our experiments, we used both males and females aged from four months to one year. The study was approved by our ethics committee for animal experimentation (2014/UCL/MD/016 and 2018/UCL/MD/18 projects).
2.2. Solutions and drugs
All perifusion and [Ca2+]c experiments were performed in a medium containing (in mM) 124 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 20 NaHCO3, and 1 mg/ml BSA except for experiments with K30 where NaCl and KCl concentrations were 98.8 mM and 30 mM, respectively, to maintain the osmolarity of the medium. Media were supplemented with a 6 mM mixture of amino acids (2 mM alanine, 2 mM arginine, and 2 mM glutamine) to have a rate of glucagon secretion that is high enough to allow detection of the secreted hormone in perifusion experiments with a reasonable number of islets (150–250 islets) and to reliably detect stimulatory or inhibitory responses. A mixture of amino acids is routinely used by others [39,40]. Test agents were added as indicated in the figures. The medium was gassed O2:CO2 (95:5%), which maintained a pH of 7.4. Adrenaline was obtained from Sterop, CYN154806 from Tocris, tertiapin-Q from Tocris and Alomone, collagenase P from Roche, SST-14 and H6056 from Bachem. Unless otherwise indicated, all other compounds were obtained from Merck.
2.3. Preparation of isolated islets and dispersed cells
Islets were isolated with collagenase P (0.65 mg/ml). For some [Ca2+]c experiments, isolated islets were further dissociated with trypsin–EDTA (0.25%) to obtain dispersed cells, which were then plated on coverslips. Islets and isolated cells were cultured for up to three days (one day for perifusion experiments and one to three days for [Ca2+]c measurements) in RPMI 1640 medium containing 7 mM glucose and 10% heat-inactivated FBS.
2.4. Dynamic secretion experiments with isolated islets
Batches of 150–250 islets were perifused at 37 °C at the flow rate of 0.5 ml/min. They were first perifused for 20 min with the first test solution for equilibration before starting effluent collection every 2.5 min. They were then challenged with various test solutions as indicated in the figures. For experiments of Figure 1G aiming at testing the effect of tolbutamide on glucagon secretion with a better time resolution, the flow rate was set at 0.7 ml/min and the effluent was collected every 30 s between 43 and 50 min (instead of every 2.5 min). Glucagon (Merck Millipore GL-32K) and insulin (home-made assay) were measured by radioimmunoassay. We checked that none of the drugs interfered with the assays.
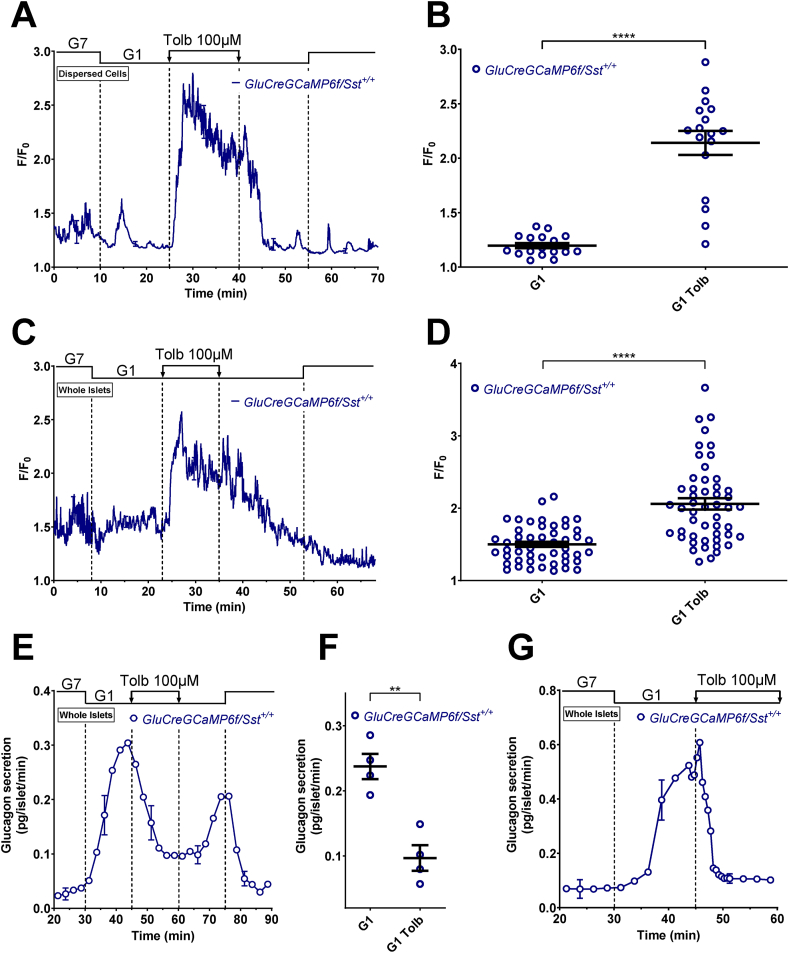
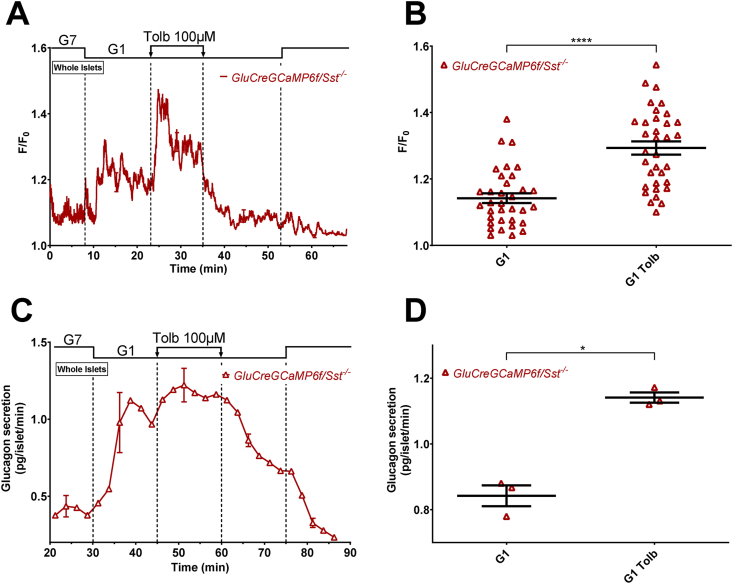
Figure 1.
Tolbutamide increases α-cell [Ca2+]cbut inhibits glucagon secretion in G1. α-cell [Ca2+]c was measured in dispersed islet cells (A and B) and isolated islets (C and D) whereas glucagon secretion was only measured in isolated islets (E–G). Dispersed islet cells and isolated islets from GluCreGCaMP6f/Sst+/+ mice were both perifused sequentially with 7 mM (G7) and 1 mM glucose (G1). Tolbutamide (Tolb 100 μM), a KATP channel blocker, was added in G1 as indicated. Panels A, C, E, and G represent the mean traces ± SEM of 3–4 experiments. Panels B and D represent the scatter plots of individual cells (B, n = 17 cells/3 mice; D, n = 50 cells/5 islets/4 mice; two-tailed paired t-test) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1, mean of 17–25 min and 47–55 min; G1 Tolb, mean of 32–40 min) and C (G1, mean of 15–23 min and 45–53 min; G1 Tolb, mean of 27–35 min), respectively. Panel F represents the scatter plot of individual experiments with the means ± SEM (two-tailed paired t-test) of the average glucagon secretion calculated from panel E (G1, mean of 37–45 min and 67–75 min; G1 Tolb, mean of 55–60 min). Secretion from panel G was collected every 30 s (instead of 2.5 min) with a flow rate set at 0.7 ml/min (instead of 0.5 ml/min) to have a better temporal resolution. ∗∗P < 0.005; ∗∗∗∗P < 0.0001.
2.5. [Ca2+]c measurements
Both isolated islets and dispersed islet cells were perifused at 37 °C at the flow rate of 0.5 ml/min. A Nikon Eclipse TE2000-E inverted microscope equipped with a confocal QLC100 spinning disk (Yokogawa) was used to measure changes in GCaMP6f fluorescence in α-cells within whole isolated islets. This system allows [Ca2+]c measurements in each α-cell of the islet instead of a global fluorescence of the whole islet. For dispersed cells, a confocal system was not needed, and a Zeiss Axiovert 100 inverted microscope was used instead. For confocal imaging, the GCaMP6f probe was excited at 491 nm and emission fluorescence was recorded at 503–552 nm. For epifluorescence imaging, the GCaMP6f probe was excited at 485 nm and the emitted fluorescence was recorded at 510–560 nm. Images were acquired every 1–3 s with an EMCCD QuantEM 512SC or a sCMOS Prime 95B camera (Photometrics) using Metafluor software (Molecular Devices).
2.6. Immunofluorescence
Dispersed cells were fixed for 1 h at room temperature with 4% paraformaldehyde and then permeabilized for 45 min with Triton X-100 (0.025%). Nonspecific binding was blocked with normal goat serum (NGS 10%) before immunostaining. Dispersed cells were immunolabeled overnight at 4 °C for glucagon, GCaMP6f, and SST with a mouse monoclonal anti-glucagon antibody (1:2500, Merck G2654), a rabbit polyclonal anti-GFP antibody (1:2000, Novus Biologicals NB600-308) and a rabbit polyclonal anti-SST antibody (1:400, Abcam ab108456), respectively. After washing the primary antibodies, the cells were treated for 2 h at room temperature with secondary antibodies. Glucagon was revealed in red using Alexa Red 594-conjugated goat anti-mouse IgG (1:500, Thermo Fisher A11020), whereas GCaMP6f and SST were revealed in green using Alexa Green 488-conjugated goat anti-rabbit IgG (1:100, Thermo Fisher A11070).
2.7. Fluorescence-activated cell sorting
Islet cells from GluCreGCaMP6f/Sst+/+ and GluCreGCaMP6f/Sst−/− mice were dispersed into single cells by trypsin. After dissociation, cells were resuspended in the medium used for the perifusion supplemented with the viability dye eFluor 780 (1/1000, Thermo Fisher 65086514) and with 5 mM glucose and 5 mM arginine, to increase the Ca2+-dependent fluorescence of GCaMP6f. Samples were washed, analyzed, and sorted with FACSAriaIII (BD Biosciences) according to the following gating strategy; FSC-A/W and SSC-A/W were used to identify cells and discriminate debris and doublets. Dead cells were excluded using eFluor 780. α-cells were separated from non α-cells by their GCaMP6f fluorescence.
2.8. Quantification of gene expression
Total RNA from minimum 60 000 FACS-sorted cells (50–150 ng) was extracted by Absolutely RNA Nanoprep Kit (Agilent Technologies) and reverse transcribed into cDNA using RevertAid H Minus First Strand cDNA synthesis kit (Thermo Fisher). RNases were inhibited by Ribolock RNase inhibitor (Thermo Fisher). Mouse insulin (Ins), glucagon (Gcg), somatostatin (Sst), somatostatin receptors 1–5 (Sstr1-5), and Gapdh expression were quantified using iQ SYBR Green Supermix (Bio-Rad). The primers used were as follows (5′→3′ forward and reverse sequences): insulin, 5′-TCTTCTACACACCCATGCCC-3′ and 5′-GGTGCAGCACTGATCCAC-3′; glucagon, 5′-ATGAACACCAAGAGGAACCGG-3′ and 5′-CTTCTGGGAAGTCTCGCCTT-3′; somatostatin, 5′-ATGCTGTCCTGCCGTCTC-3′ and 5′-TTCTCTGTCTGGTTGGGCTC-3′; Sstr1, 5′-CTACTGTCTGACTGTGCT-3′ and 5′-ATGGGCAAGATAACCAGTAAT-3′; Sstr2, 5′-TCTTCCGTGTCTGTGGC-3′ and 5′-GGGATTTGTCCTGCTTACT-3′; Sstr3, 5′-CTGGCGAACAGCCTTTAT-3′ and 5′-GGTGCCTGTACCCACTGA-3′; Sstr4, 5′-TGTGCTATTATTCAAACTGGCT-3′ and 5′-GGTGTCAACTTCAGGATTGT-3′; Sstr5, 5′-CTATGTGGTGTTGCGGT-3′ and 5′-GGCACAAGAAGGAGCCAAA-3′; and Gapdh, 5′-ACCCAGAAGACTGTGGATGG-3′ and 5′-ACACATTGGGGGTAGGAACA-3′. The annealing temperature was set to 60 °C for all primers. Gapdh was used as an internal control for qPCR normalization. It was an excellent housekeeping gene because its cycle threshold (Ct), using the same amount of total cDNA, was similar between α-cells (24.6) and non α-cells (23.5), and between GluCreGCaMP6f/Sst+/+ (24.3) and GluCreGCaMP6f/Sst−/− islet cells (23.8). Expression of all genes was normalized to that of Gapdh and calculated using the 2-ΔCt method.
2.9. Statistical analyses and data presentation
Results are presented as representative traces or means ± SEM for islets or dispersed cells from at least three different mice, where n is the number of cells for [Ca2+]c experiments and the number of mice for secretion experiments. Secretion experiments are presented as pg/islet/min. [Ca2+]c measurements are presented as F/F0 where F is the fluorescence intensity at a given time point and F0 the lowest fluorescence intensity. Continuous perifusion with diazoxide + K30 yielded an initial peak of [Ca2+]c followed by a plateau. However, because the signal from the plateau decreased during time, statistics comparing the conditions with and without SST or SSTR antagonists were biased by this spontaneous trend and denoted significance although no effect was observed (Figure 6, Figure 7, and Supplementary Figure S10). A spontaneous decrease in [Ca2+]c also occurred when analyzing the experiments performed in the continuous presence of arginine (Figure 5). Therefore, for those experiments, we corrected the signal using a local linear regression (for smoothing) [41] followed by a cubic spline (for extrapolation) [42]. In a first step, we fitted a linear regression to the mean data in each of the two intervals, before and after the addition of SST or SSTR antagonists. In a second step, a cubic spline was fitted using the two intervals as references to extrapolate an estimated curve that best matches the signal. Finally, we calculated the difference between the actual signal and the estimated curve for each cell at each time point, and we compared, for each cell, the mean of the differences in the condition where SST or SSTR antagonists are present with the mean of the differences in the conditions in their absence. The same strategy was adopted for glucagon/insulin secretion experiments performed under the same conditions because secretion continuously increased and biased the statistical tests without correction for spontaneous trend (refer Supplementary Figure S1 for representative examples). However, the difference was instead calculated between the actual secretion and the estimated curve for each experiment at each time point. A two-tailed paired Student's t-test was used to compare two different conditions from the same cells or islets, whereas two-tailed unpaired Student's t-test was used to compare two conditions from different cells or islets. One-way RM ANOVA or ordinary two-way ANOVA with post hoc Sidak correction was used for multiple comparisons. For qPCR data, a Mann–Whitney test was used instead, because of the logarithmic nature of the results. All statistical tests were calculated using GraphPad Prism 8 (GraphPad Software).
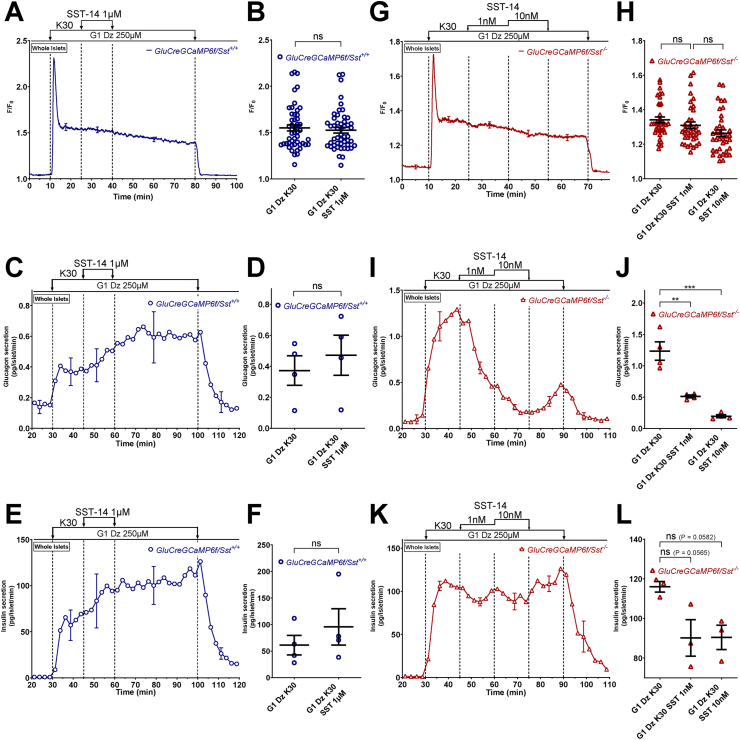
Figure 6.
In a depolarizing condition where KATPchannels and [Ca2+]care clamped, exogenous SST strongly inhibits glucagon secretion of SST-lacking islets but not that of SST-expressing ones. α-cell [Ca2+]c (A, B, G, and H), glucagon (C, D, I, and J) and insulin secretions (E, F, K, and L) were measured in isolated islets from either GluCreGCaMP6f/Sst+/+ (A–F) or GluCreGCaMP6f/Sst−/− mice (G–L) perifused with 1 mM glucose (G1) and the KATP channel opener, diazoxide (Dz 250 μM). The K+ concentration of the medium was increased from 4.8 to 30 mM (K30), and SST (SST-14 1 μM for GluCreGCaMP6f/Sst+/+ and SST-14 1 nM and 10 nM for GluCreGCaMP6f/Sst−/−) was added in the presence of K30 as indicated. Panels A, C, E, G, I, and K represent the mean traces ± SEM of 3–4 experiments. Panels B and H represent the scatter plots of individual cells (B, n = 50 cells/5 islets/3 mice; two-tailed paired t-test; H, n = 38 cells/3 islets/3 mice; one-way RM ANOVA with Sidak correction) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1 Dz K30, mean of 15–25 min; G1 Dz K30 SST 1 μM, mean of 30–40 min) and G (G1 Dz K30, mean of 15–25 min; G1 Dz K30 SST 1 nM, mean of 30–40 min; G1 Dz K30 SST 10 nM, mean of 45–55 min), respectively. Panels D, F, J, and L represent the scatter plots of individual experiments with the means ± SEM (two-tailed paired t-test for D and F, and one-way RM ANOVA with Sidak correction for J and L) of the average glucagon and insulin secretions calculated from panels C, E (G1 Dz K30, mean of 37–45 min; G1 Dz K30 SST 1 μM, mean of 52–60 min), I and K (G1 Dz K30, mean of 37–45 min; G1 Dz K30 SST 1 nM, mean of 55–60 min; G1 Dz K30 SST 10 nM, mean of 67–75 min), respectively. ns, not significant; ∗∗P < 0.005; ∗∗∗P < 0.001.
Figure 7.
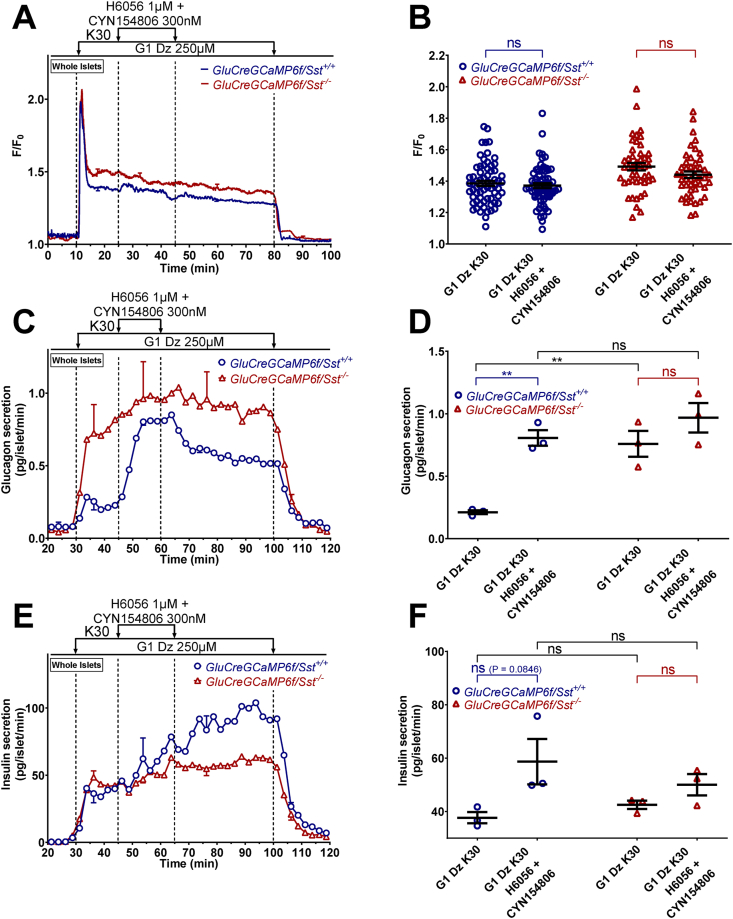
Blocking the endogenous effect of SST stimulates glucagon secretion in a depolarizing condition where KATPchannels and [Ca2+]care clamped, but it does not affect insulin release. α-cell [Ca2+]c (A and B), glucagon (C and D) and insulin secretions (E and F) were measured in isolated islets from either GluCreGCaMP6f/Sst+/+ (blue traces) or GluCreGCaMP6f/Sst−/− (red traces) mice perifused with 1 mM glucose (G1) and the KATP channel opener, diazoxide (Dz 250 μM). The K+ concentration of the medium was increased from 4.8 to 30 mM (K30) as indicated. H6056 (H6056 1 μM), an SSTR2 and SSTR3 antagonist, was added in combination with CYN154806 (CYN154806 300 nM), an SSTR2 antagonist, as indicated, to prevent the endogenous action of SST on α-cells. Panels A, C, and E represent the mean traces ± SEM of 3 experiments. Panel B represents the scatter plots of individual cells (n = 67 cells/5 islets/3 mice for GluCreGCaMP6f/Sst+/+ and n = 46/5 islets/3 mice for GluCreGCaMP6f/Sst−/−, two-tailed paired t-test) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1 Dz K30, mean of 15–25 min; G1 Dz K30 H6056 + CYN154806, mean of 30–40 min). Panels D and F represent the scatter plots of individual experiments with the means ± SEM (two-tailed paired and unpaired t-tests) of the average glucagon and insulin secretions calculated from panel C and E (G1 Dz K30, mean of 37–45 min; G1 Dz K30 H6056 + CYN154806, mean of 52–60 min), respectively. ns, not significant; ∗∗P < 0.005.
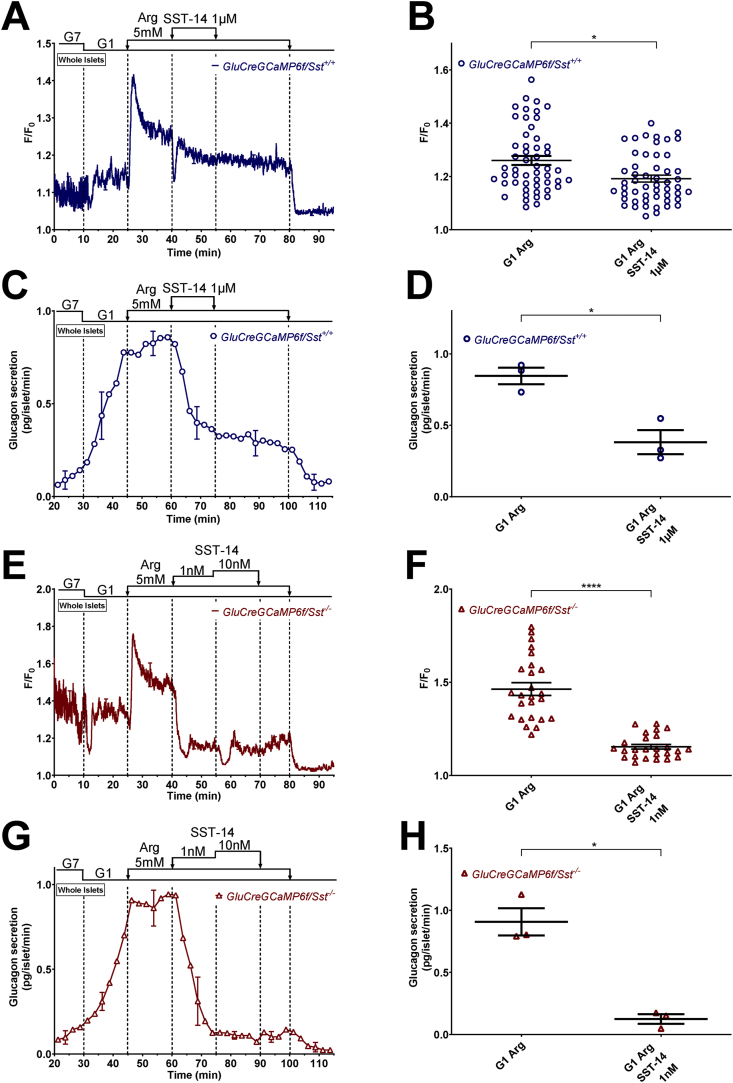
Figure 5.
SST induces a strong and sustained inhibition of glucagon secretion in the presence of arginine that is not strictly associated with a drop in [Ca2+]c. α-cell [Ca2+]c (A, B, E, and F) and glucagon secretion (C, D, G, and H) were measured in isolated islets from either GluCreGCaMP6f/Sst+/+ (A–D) or GluCreGCaMP6f/Sst−/− (E–H) mice perifused with 7 mM (G7) and 1 mM glucose (G1). SST (SST-14 1 μM for GluCreGCaMP6f/Sst+/+ and SST-14 1 nM and 10 nM for GluCreGCaMP6f/Sst−/−) was added in the presence of arginine (Arg 5 mM) as indicated. Panels A, C, E, and G represent the mean traces ± SEM of 3 experiments. Panels B and F represent the scatter plots of individual cells (B, n = 52 cells/7 islets/3 mice; F, n = 25 cells/5 islets/3 mice; two-tailed paired t-test) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1 Arg, mean of 32–40 min; G1 Arg SST-14 1 μM, mean of 47–55 min) and F (G1 Arg, mean of 32–40 min; G1 Arg SST-14 1 nM, mean of 47–55 min), respectively. Panels D and H represent the scatter plots of individual experiments with the means ± SEM (two-tailed paired t-test) of the average glucagon secretion calculated from panel C (G1 Arg, mean of 52–60 min; G1 Arg SST-14 1 μM, mean of 67–75 min) and G (G1 Arg, mean of 52–60 min; G1 Arg SST-14 1 nM, mean of 67–75 min), respectively. ns, not significant; ∗P < 0.05; ∗∗∗∗P < 0.0001.
3. Results
3.1. Validation of the GluCreGCaMP6f/Sst+/+ mouse as a model to study the correlation between α-cell [Ca2+]c and glucagon secretion
To evaluate the effects of sulfonylureas on α-cell [Ca2+]c, we generated a transgenic mouse model expressing the green fluorescent Ca2+ probe, GCaMP6f, specifically in α-cells by crossing GluCre/Sst+/+ mice with Lox-STOP-Lox-GCaMP6f/Sst+/+ mice. For the sake of brevity, we refer to these mice as GluCreGCaMP6f/Sst+/+ mice throughout the manuscript. To evaluate the validity of this new model, we performed three series of experiments. We first tested the effects of arginine and adrenaline which are known to increase α-cell [Ca2+]c even in a medium that contains low glucose concentration [4,26,27,29,43,44]. As expected, both agents elevated [Ca2+]c in α-cells of GluCreGCaMP6f/Sst+/+ islets in the presence of 3 mM glucose (G3). Moreover, the addition of the KATP channel opener diazoxide to hyperpolarize α-cells completely inhibited the rise in [Ca2+]c elicited by arginine (Supplementary Figure S2A and B). We next tested whether the expression of GCaMP6f interferes with glucagon secretion by comparing the glucagon responses to various agents in islets from mice expressing (GluCreGCaMP6f/Sst+/+ mice) or not expressing (Lox-STOP-Lox-GCaMP6f/Sst+/+ mice) GCaMP6f. Changing the glucose concentration from 7 mM (G7) to 1 mM (G1) stimulated glucagon secretion to a similar extent in both types of islets. Subsequent addition of the KATP channel blocker gliclazide induced a similar inhibition of glucagon release in both preparations (Supplementary Figure S2C and D). Finally, we evaluated the specificity of GCaMP6f expression in GluCreGCaMP6f/Sst+/+ mice. Islets from GluCreGCaMP6f/Sst+/+ mice were dispersed, and glucagon, GFP (which is the fluorophore part of GCaMP6f), and SST were immunolabeled. Results revealed that 96.4% of GFP-positive cells were positive for glucagon (396/411 cells), attesting great specificity of the probe for α-cells (Supplementary Figure S2E). However, only 55.4% of cells that were positive for glucagon were also positive for GCaMP6f (396/715 cells). Immunodetections also revealed that the percentages of islet cells corresponding to α-cells and δ-cells were 16.2% (1357/8367 cells) and 5.6% (238/4232 cells), respectively, which is in accordance with what is found in rodent pancreas [2]. All these experiments demonstrate that the GluCreGCaMP6f/Sst+/+ mouse model is a reliable model to study the correlation between α-cell [Ca2+]c and glucagon secretion.
3.2. Sulfonylureas increase α-cell [Ca2+]c but inhibit glucagon secretion at low glucose
We next evaluated the effects of sulfonylureas in a condition where α-cells were stimulated by low glucose. To ensure that only α-cells are selected (because GCaMP6f is also expressed in 3.6% of non α-cells), we added adrenaline at the end of each experiment (see Supplementary Figure S3 for representative traces of [Ca2+]c measurements). The addition of the KATP channel blocker tolbutamide to a medium containing 1 mM glucose (G1) increased [Ca2+]c in both dispersed α-cells (Figure 1A,B, and Supplementary Figure S3A) and α-cells within islets (Figure 1C,D, and Supplementary Figure S3B), suggesting a direct stimulatory effect of tolbutamide on α-cell [Ca2+]c. By contrast, tolbutamide inhibited the rise in glucagon secretion induced by G1 from whole islets under the same conditions (Figure 1E,F). Similar results were obtained with gliclazide: an increase in [Ca2+]c in both dispersed α-cells and in α-cells within islets, but an inhibition of glucagon secretion (Supplementary Figures S3C, D, and S4). Taken together, these results reveal that the closure of KATP channels increases α-cell [Ca2+]c but inhibits glucagon secretion. Because the effects of gliclazide and tolbutamide on α-cells were similar, but the reversibility was slower upon the removal of gliclazide than tolbutamide (compare Figure 1E with Supplementary Figure S4E), all the subsequent experiments were performed with tolbutamide only.
3.3. Sulfonylureas increase α-cell [Ca2+]c and glucagon secretion in the absence of SST influence
Given that sulfonylureas also stimulate SST secretion from δ-cells [8,11,12,15,20] and that SST acts as a strong inhibitor of glucagon secretion [19,32,33,[45], [46], [47], [48]], we hypothesized that inhibition of glucagon secretion by sulfonylureas is mediated by SST. Therefore, we generated a new transgenic mouse model, referred to as GluCreGCaMP6f/Sst−/−, expressing GCaMP6f specifically in α-cells and lacking SST. In these mice, immunodetections of glucagon, GCaMP6f (detected by immunolabelling of GFP), and SST indicated that 93.9% of GFP-positive cells were positive for glucagon (185/197 cells) and that 45.3% of glucagon-positive cells were positive for GCaMP6f (185/408 cells) (Supplementary Figure S5). The percentage of islet cells corresponding to α-cells was 15.5% (819/5281 cells). These results were thus similar to those found in SST-expressing GluCreGCaMP6f/Sst+/+ mice (see Supplementary Figure S2E). More importantly, no SST-positive cells were found in all preparations (0/5281 cells), showing that these mice exhibit a complete ablation of SST (Supplementary Figure S5). In this new model lacking SST, tolbutamide increased α-cell [Ca2+]c within islets (Figure 2A,B, and Supplementary Figure S6) as found in control islets expressing SST (Figure 1C,D). However, it stimulated glucagon secretion (Figure 2C,D) in opposition to what was found in control islets (Figure 1E,F). This suggests that the direct effect of tolbutamide on α-cells is stimulation of glucagon secretion resulting from an increase in α-cell [Ca2+]c, probably by a mechanism that mirrors that of β-cells, i.e., by the closure of KATP channels leading to membrane potential depolarization and the opening of VGCC. However, in control islets expressing SST, the stimulatory effect of the [Ca2+]c increase is counteracted by the inhibitory effect of SST, which leads to a glucagonostatic effect of tolbutamide.
Figure 2.
The glucagonotropic effect of tolbutamide is counteracted by SST. α-cell [Ca2+]c (A and B) and glucagon secretion (C and D) were measured in isolated islets from GluCreGCaMP6f/Sst−/− mice perifused sequentially with 7 mM (G7) and 1 mM glucose (G1). Tolbutamide (Tolb 100 μM), a KATP channel closer, was added in G1 as indicated. Panels A and C represent the mean traces ± SEM of 4 and 3 experiments, respectively. Panel B represents the scatter plots of individual cells (n = 34 cells/5 islets/3 mice; two-tailed paired t-test) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1, mean of 15–23 min and 45–53 min; G1 Tolb, mean of 27–35 min). Panel D represents the scatter plots of individual experiments with the means ± SEM (two-tailed paired t-test) of the average glucagon secretion calculated from panel C (G1, mean of 37–45 min and 67–75 min; G1 Tolb, mean of 55–60 min). ∗P < 0.05; ∗∗∗∗P < 0.0001.
Because it is expected that the direct stimulatory effect of tolbutamide on α-cells precedes the indirect inhibitory effect by SST (because a depolarizing effect is quicker than a paracrine effect), we repeated the same experiments as in Figure 1E except that we increased the temporal resolution by collecting secretion every 30 s (instead of every 2.5 min). Expectedly, tolbutamide induced a transient stimulation of glucagon secretion (resulting from the α-cell [Ca2+]c increase) that preceded the inhibition (induced by the paracrine influence of SST) (Figure 1G).
3.4. Sulfonylureas increase α-cell [Ca2+]c and glucagon secretion at high glucose
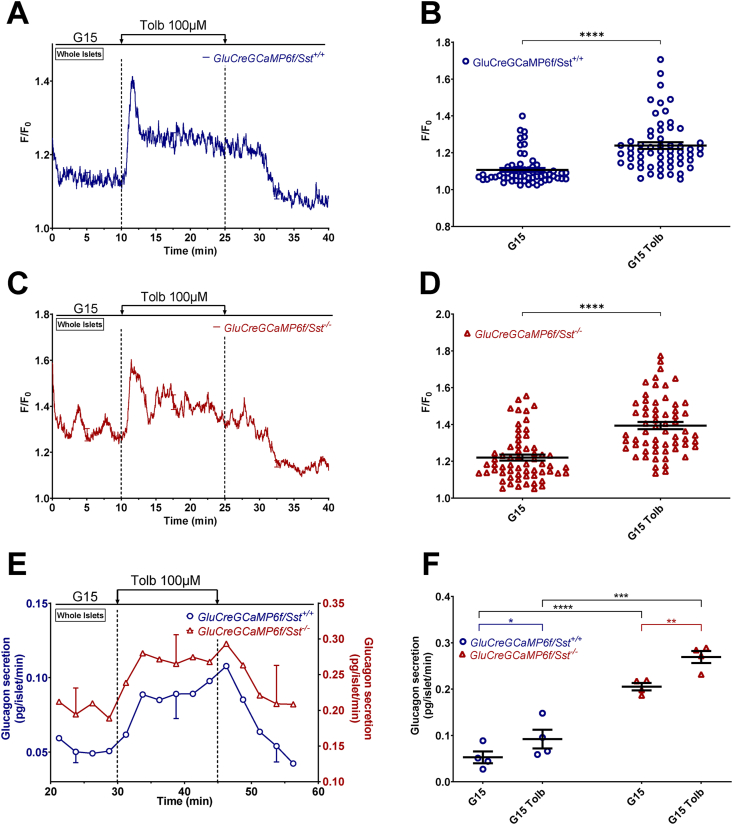
We also tested the effects of tolbutamide at 15 mM glucose (G15), a glucose concentration that strongly stimulates SST secretion in mice [27,49]. Interestingly, tolbutamide increased α-cell [Ca2+]c and glucagon secretion of both Sst+/+ and Sst−/− islets (Figure 3 and Supplementary Figure S7). This suggests that in control islets, at least part of the direct glucagonotropic effect of tolbutamide mediated by the rise in α-cell [Ca2+]c is not counteracted by the glucagonostatic effect of SST, when SST secretion is already strongly stimulated by high glucose.
Figure 3.
Tolbutamide increasesα-cell [Ca2+]cand stimulates glucagon secretion in G15. α-cell [Ca2+]c (A–D) and glucagon secretion (E and F) were measured in isolated islets from GluCreGCaMP6f/Sst+/+ (A, B, E, and F) and GluCreGCaMP6f/Sst−/− (C, D, E, and F) mice perifused with 15 mM glucose (G15). Tolbutamide (Tolb 100 μM) was added in G15 as indicated. Panels A, C, and E represent the mean traces ± SEM of 4, 5, and 4 experiments, respectively. Panels B and D represent the scatter plots of individual cells (B, n = 59 cells/8 islets/4 mice; D, n = 61 cells/9 islets/5 mice; two-tailed paired t-test for B and D) with the means ± SEM of the average [Ca2+]c calculated from panel A and C (G15, mean of 2–10 min and 32–40 min; G15 Tolb, mean of 17–25 min). Panel F represents the scatter plot of individual experiments with the means ± SEM (two-tailed paired and unpaired t-tests) of the average glucagon secretion calculated from panel E (G15, mean of 22–30 min and 52–60 min; G15 Tolb, mean of 37–45 min). ∗P < 0.05; ∗∗P < 0.005; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
Taken together, these data show that the effects of sulfonylureas on α-cells depend on the balance between their direct stimulatory effect on α-cells and their indirect inhibitory effect by SST, and that the glucose concentration is critical in tipping the balance in one way or the other.
3.5. SST-14 inhibits glucagon secretion by decreasing [Ca2+]c and the efficacy of Ca2+ on exocytosis
3.5.1. Experiments in conditions where the membrane potential was not clamped
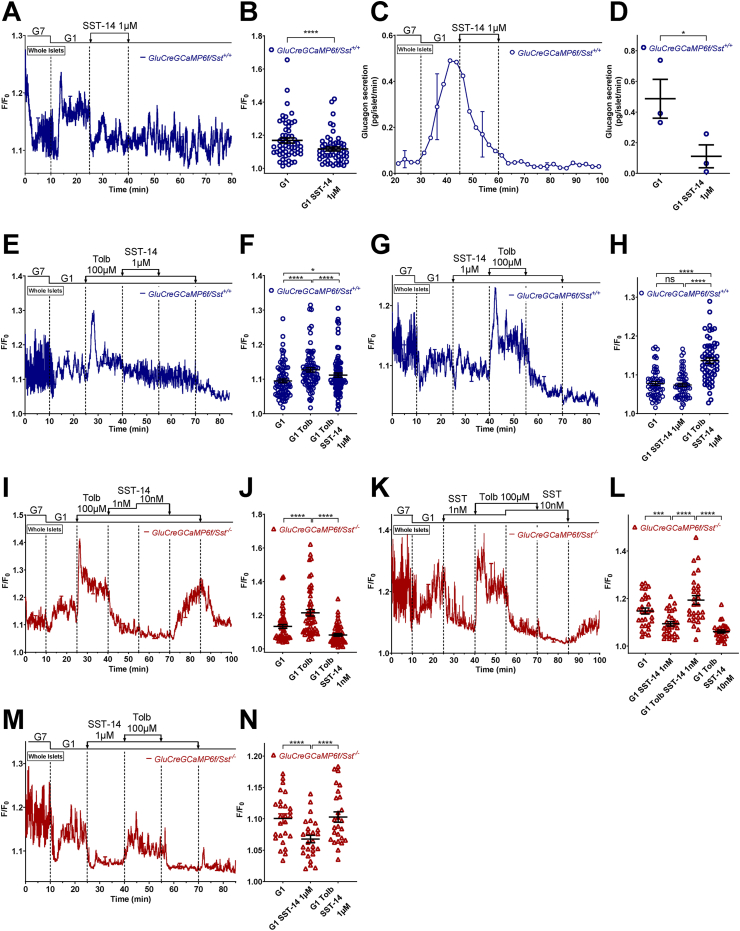
Because our results strongly suggest that SST is involved in the glucagonostatic effect of sulfonylureas, we verified its direct effect on α-cell [Ca2+]c and glucagon secretion. Therefore, we applied SST-14, the principal isoform of SST expressed by δ-cells [8,[50], [51], [52]], on GluCreGCaMP6f/Sst+/+ islets. The addition of 1 μM SST-14 to the medium completely suppressed the increase in glucagon secretion induced by G1 (Figure 4C,D), and decreased α-cell [Ca2+]c (Figure 4A,B), most likely by inhibiting α-cell electrical activity as previously reported [[53], [54], [55]]. The amplitude of the SST-induced [Ca2+]c drop was variable between experiments and sometimes weak (Figure 4G,H). If SST can decrease [Ca2+]c, then it is difficult to explain how in GluCreGCaMP6f/Sst+/+ islets, tolbutamide which is known to strongly stimulate SST release [8,11,12,15,20] did not decrease α-cell [Ca2+]c but instead elevated it (see Figure 1C,D, and Supplementary Figure S3B). We hypothesized that the depolarizing effect of tolbutamide exceeds and counteracts the hyperpolarizing effect of SST. To test this, we applied 1 μM SST-14 in the presence of tolbutamide. As expected, the sulfonylurea increased α-cell [Ca2+]c from GluCreGCaMP6f/Sst+/+ islets, whereas SST-14 applied thereafter very slightly decreased [Ca2+]c to levels that remained higher than in the absence of tolbutamide (Figure 4E,F). Furthermore, performing the reverse protocol, by adding SST-14 first, did not prevent the stimulatory effect of tolbutamide (Figure 4G,H). These data suggest that the depolarizing effect of tolbutamide counteracts the hyperpolarizing effect of SST and explain why we did not observe a decrease of α-cell [Ca2+]c when tolbutamide was applied on control islets. We also tested the effect of SST in the presence of tolbutamide in the GluCreGCaMP6f/Sst−/− mouse model. Interestingly, 1 nM SST-14 already completely inhibited the rise in [Ca2+]c elicited by tolbutamide (Figure 4I,J), suggesting that the islets from these mice are much more sensitive to the inhibition of SST than those from control mice. However, although SST is more potent in GluCreGCaMP6f/Sst−/− mice, it did not prevent the stimulatory effect of tolbutamide, either at a concentration of 1 nM (Figure 4K, L) or at a much higher concentration, 1 μM (Figure 4M, N).
Figure 4.
SST decreases [Ca2+]cwhen α-cells are not depolarized or when SST is absent but does not prevent the effect of tolbutamide. α-cell [Ca2+]c (A, B, and E–N) and glucagon secretion (C and D) were measured in isolated islets from either GluCreGCaMP6f/Sst+/+ (A–H) or GluCreGCaMP6f/Sst−/− (I–N) mice perifused with 7 mM (G7) and 1 mM glucose (G1). SST-14 (1 μM for GluCreGCaMP6f/Sst+/+ and 1–10 nM or 1 μM for GluCreGCaMP6f/Sst−/−) was added in G1 alone or in the presence of tolbutamide (Tolb 100 μM), and tolbutamide was added either in G1 or in the presence of SST-14, as indicated. Panels A, C, E, G, I, K, and M represent the mean traces ± SEM of 2 (M) to 3 (A, C, E, G, I, and K) experiments. Panels B, F, H, J, L, and N represent the scatter plots of individual cells (B, n = 56 cells/7 islets/3 mice; two-tailed paired t-test; F, n = 80 cells/5 islets/3 mice; H, n = 59 cells/6 islets/3 mice; J, n = 54 cells/6 islets/3 mice; L, n = 29 cells/3 islets/3 mice; N, n = 26 cells/2 islets/2 mice; one-way RM ANOVA with Sidak correction for F, H, J, L, N) with the means ± SEM of the average [Ca2+]c calculated from panel A (G1, mean of 17–25 min; G1 SST-14 1 μM, mean of 32–40 min), E (G1, mean of 17–25 min and 77–85 min; G1 Tolb, mean of 32–40 min and 62–70 min; G1 Tolb SST-14 1 μM, mean of 47–55 min), G (G1, mean of 17–25 min and 77–85 min; G1 SST-14 1 μM, mean of 32–40 min and 62–70 min; G1 Tolb SST-14 1 μM, mean of 47–55 min), I (G1, mean of 17–25 min and 92–100 min; G1 Tolb, mean of 32–40 min and 77–85 min; G1 Tolb SST-14 1 nM, mean of 47–55 min), K (G1, mean of 17–25 min and 92–100 min; G1 SST-14 1 nM, mean of 32–40 min; G1 Tolb SST-14 1 nM, mean of 47–55 min; G1 Tolb SST-14 10 nM, mean of 62–70 min) and M (G1, mean of 17–25 min and 77–85 min; G1 SST-14 1 μM, mean of 32–40 min and 62–70 min; G1 Tolb SST-14 1 μM, mean of 47–55 min), respectively. Panel D represents the scatter plot of individual experiments with the means ± SEM (two-tailed paired t-test) of the average glucagon secretion calculated from panel C (G1, mean of 40–45 min; G1 SST-14 1 μM, mean of 55–60 min). ns, not significant; ∗P < 0.05; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
Similar results were obtained with arginine which mimics the depolarizing effect of tolbutamide, but by another mechanism that involves the electrogenic entry of the positively charged amino acid [1]. As expected, arginine (5 mM) increased [Ca2+]c, thereby reproducing the direct stimulatory effect of sulfonylureas on α-cell [Ca2+]c. The subsequent addition of 1 μM SST-14 induced only a transient drop in [Ca2+]c (probably reflecting a transient hyperpolarization as previously described [53]) followed by a small decrease because the hyperpolarizing effect of SST was too small to counteract the depolarizing effect of arginine (Figure 5A,B). Nevertheless, SST-14 strongly inhibited glucagon secretion (Figure 5C,D), suggesting that SST inhibited the efficacy of Ca2+ on exocytosis, which is expected to also occur during sulfonylurea application. In GluCreGCaMP6f/Sst−/− islets which are more sensitive to SST, a concentration of SST-14 as low as 1 nM was sufficient to strongly inhibit the rise in [Ca2+]c induced by arginine and glucagon secretion under the same conditions (Figure 5E–H).
Our experiments detected that α-cells from Sst−/− mice were much more sensitive to SST-14 than α-cells from Sst+/+ mice. To test whether this hypersensitivity reflects or not an increased expression of somatostatin receptors (SSTR), we compared Sstr mRNA expression in α-cells from GluCreGCaMP6f/Sst+/+ and GluCreGCaMP6f/Sst−/− mice. Therefore, we FACS-sorted α-cells from non α-cells of both strains of mice based on their GCaMP6f fluorescence, and then performed qPCR on GCaMP6f+ and GCaMP6f− fractions for insulin (Ins), glucagon (Gcg), Sst, and Sstr1-5 mRNA. As expected, glucagon mRNA was much more expressed in the GCaMP6f+ fraction than in the GCaMP6f− fraction, whereas it was the opposite for insulin mRNA, showing correct cell separation of α- and non-α-cells (Supplementary Figure S8A and B). Sst mRNA was low in α- and non-α-cells from Sst+/+ mice, whereas it was absent in Sst−/− islet cells. Among all Sstr subtypes, Sstr2 gene was the most expressed in α-cells (Supplementary Figure S8C) as previously documented [33,53,[56], [57], [58]]. Importantly, Sstr1-5 gene expression in α- and non-α-cells was similar between Sst+/+ and Sst−/− mice (Supplementary Figure S8C and D), suggesting that the hypersensitivity of Sst−/− α-cells to exogenous SST-14 was not because of a difference in SSTR expression.
It has been documented that SST hyperpolarizes α-cells by activating G protein-gated inwardly rectifying K+ (GIRK) channels [[53], [54], [55],59]. Therefore, we tested whether pharmacological inhibition of these channels reverses the drop in [Ca2+]c induced by SST-14 in various conditions. Four types of GIRK, encoded by different genes exist; GIRK1 (Kir3.1, Kcnj3), GIRK2 (Kir3.2, Kcnj6), GIRK3 (Kir3.3, Kcnj9), and GIRK4 (Kir3.4, Kcnj5) [60]. Because α-cells strongly express GIRK1 and less importantly GIRK2 [57,58], we used tertiapin-Q (TPNQ), a potent inhibitor of GIRK1/4 (Ki: 13.3 nM) [61] and GIRK1/2 channels (mostly inhibited by 100 nM TPNQ) [62]. We selected 500 nM of TPNQ to massively block GIRK1 and GIRK2 present in α-cells. Unexpectedly, TPNQ did not reverse, even partially, the inhibitory effect of exogenous SST-14 on [Ca2+]c in α-cells of Sst−/− mice (Supplementary Figure S9A and B). Moreover, TPNQ failed to significantly affect [Ca2+]c in α-cells of Sst+/+ mice in the presence of G15 which is expected to strongly stimulate endogenous SST release (Supplementary Figure S9C and D). A similar lack of effect was observed with TPNQ from two different suppliers (Tocris and Alomone). These results suggest that SST decreases [Ca2+]c independently of GIRK channels, unless unexplained experimental conditions affected the sensitivity of GIRK to TPNQ.
3.5.2. Experiments in conditions where the membrane potential was clamped
It has been suggested that SST inhibits VGCC in human α-cells [53]. To indirectly evaluate this possibility, we tested the effect of SST in a condition where the membrane potential was clamped at a depolarized level with 30 mM K+ (K30) in the presence of a high concentration of diazoxide to maintain KATP channels open. As expected, increasing the K+ concentration of the medium from 4.8 to 30 mM in the presence of G1 and diazoxide strongly increased α-cell [Ca2+]c and stimulated glucagon and insulin secretions of GluCreGCaMP6f/Sst+/+ islets (Figure 6A–F). Adding 1 μM SST-14 in this condition affected neither α-cell [Ca2+]c nor glucagon or insulin secretion. K30 also stimulates δ-cells [8,57,[63], [64], [65]], and hence, we hypothesized that the effect of exogenous SST-14 was masked by endogenous SST. We thus increased the SST-14 concentration to 10 μM, but again no effect was observed either on α-cell [Ca2+]c or glucagon and insulin secretions (Supplementary Figure S10). Therefore, we tested the effect of exogenous SST-14 on GluCreGCaMP6f/Sst−/− islets in which the influence of endogenous SST is completely removed. In this model, K30 stimulated glucagon secretion much more potently than in the SST-expressing model (compare Figure 6I (18-fold increase) with Figure 6C (2-fold increase) and Supplementary Figure S10C (3-fold increase)). This confirms that the release of endogenous SST induced by K30 represses glucagon secretion. Moreover, adding a concentration of SST-14 as low as 1 nM already very potently inhibited glucagon secretion in the presence of diazoxide and K30 without affecting α-cell [Ca2+]c (Figure 6G–J). This confirms our hypothesis that SST can also inhibit glucagon secretion by decreasing the efficacy of Ca2+ on exocytosis and indicates that SST does not act through VGCC inhibition. Interestingly, SST-14 did not significantly inhibit insulin secretion from islets of the same mice, suggesting a much weaker effect of SST on insulin release (Figure 6K,L). Because we hypothesized that the failure of SST-14 to inhibit glucagon secretion in GluCreGCaMP6f/Sst+/+ islets could result from a massive SST secretion induced by K30 that could already saturate SSTR, we applied SSTR antagonists to block the effect of endogenous SST. Because mouse α-cells mainly express SSTR2 and SSTR3 [33,57,58], we used a combination of CYN154806 – an SSTR2 antagonist, and H6056 – an SSTR2 and SSTR3 antagonist. As expected, the stimulation of glucagon secretion by K30 was stronger in GluCreGCaMP6f/Sst−/− islets than in GluCreGCaMP6f/Sst+/+ islets (Figure 7C,D). Moreover, adding H6056 + CYN154806 in the presence of diazoxide and K30 potently stimulated glucagon secretion without significantly affecting α-cell [Ca2+]c in GluCreGCaMP6f/Sst+/+ islets (Figure 7A–D, blue traces and circles). This confirms our hypothesis that K30 stimulates so much SST secretion of GluCreGCaMP6f/Sst+/+ islets that the effect of exogenous SST-14 is masked. Expectedly, these antagonists did not affect α-cell [Ca2+]c and glucagon secretion of GluCreGCaMP6f/Sst−/− islets (Figure 7A–D, red traces and triangles). Mouse β-cells mainly express SSTR3 [33,57,58]. However, K30 stimulated insulin secretion to a similar extent in both types of islets, and the addition of H6056 + CYN154806 did not significantly affect insulin secretion (Figure 7E,F). This reinforces our suggestion that SST is not as potent at inhibiting insulin secretion as it is for glucagon release.
4. Discussion
In this study, we evaluated, at two different glucose concentrations (G1 and G15), the effects of sulfonylureas on [Ca2+]c in mouse α-cells by confocal microscopy using a transgenic mouse model expressing a fluorescent Ca2+ indicator specifically in α-cells. We also measured glucagon secretion to test if it is driven by [Ca2+]c changes. Our data indicate that sulfonylureas increase α-cell [Ca2+]c by a direct effect on α-cells, like in β-cells. This is accompanied by a paradoxical inhibition of glucagon secretion at a low glucose concentration. This glucagonostatic effect is mediated by SST because sulfonylureas stimulate glucagon release in islets without the paracrine influence of SST. Thus, in normal islets in which SST is present, the stimulatory effect of sulfonylureas on α-cells is counteracted by SST which inhibits glucagon release by decreasing the efficacy of cytosolic Ca2+ on exocytosis. However, in the presence of high glucose concentration, i.e., under conditions where glucagon secretion is already strongly inhibited, sulfonylureas increase both α-cell [Ca2+]c and glucagon secretion. Our study also suggests that SST much more potently inhibits glucagon than insulin secretion.
4.1. Sulfonylureas control glucagon secretion by a direct stimulatory effect on α-cells (as in β-cells) and an indirect inhibitory effect through δ-cells
Type 2 diabetes (T2D) is characterized by chronic hyperglycemia owing to impaired insulin secretion/action. Recent studies showed that excessive glucagon secretion also contributes to hyperglycemia [3,4,66]. Many drugs have been developed to normalize blood glucose level. They include sulfonylureas which stimulate insulin secretion by closing KATP channels. However, the effects of sulfonylureas on α-cells are still hotly debated. KATP channels have the same subunit composition in α- and β-cells in various species, including rats, mice, and humans [36,57,[67], [68], [69], [70]]. They are octameric heterocomplexes composed of four Kir6.2 (Kcnj11) subunits forming the pore of the K+ channel and four SUR1 (Abcc8) subunits binding sulfonylureas. In humans, Kir6.1 (Kcnj8) is also expressed in α-cells [70] and might be an additional constituent of KATP channels.
Studies testing the effects of KATP channel modulators on α-cell membrane potential or [Ca2+]c reported controversial results. Experiments from Quesada et al., in 1999 on mouse islets did not detect any effect of tolbutamide on α-cell [Ca2+]c [13]. However, one year later, results from Göpel et al. showed a depolarization of α-cells by tolbutamide producing an inhibition of glucagon secretion [24]. They were the first to suggest that depolarizing α-cells induces the inactivation of low-threshold voltage-gated channels (mainly Na+ (Nav) and less importantly T-type Ca2+ channels) leading to the inhibition of glucagon release. These results were confirmed later by experiments from the same group and colleagues [[21], [22], [23]]. When α-cells were already electrically active, such as in the presence of a low glucose concentration, the depolarization induced by tolbutamide was associated with an increase in the frequency of action potentials but also with a reduction in their amplitude, or sometimes, with suppression of action potentials, leading to a drop in α-cell [Ca2+]c [[22], [23], [24], [25]]. Interestingly, high concentrations of diazoxide which strongly open KATP channels decreased [Ca2+]c and inhibited glucagon secretion, mimicking the effect of high concentrations of tolbutamide. By contrast, low concentrations of diazoxide which only slightly activate KATP channels increased [Ca2+]c and stimulated glucagon release [21]. All these observations led to suggest the recently reviewed model that is based on the idea that sulfonylureas modulate glucagon secretion mainly by a direct action on α-cells [71]. In the presence of a low glucose concentration, most KATP channels are already closed (contrary to the situation in β-cells) and α-cells display spontaneous action potentials; the upstroke phase of which involves the activation of Nav and high threshold VGCC (mostly P/Q type). The resulting increase in [Ca2+]c stimulates glucagon secretion. The closure of KATP channels by sulfonylureas induces a depolarization of the plasma membrane which inactivates Nav, reduces Ca2+ influx through high threshold VGCC, and leads to a paradoxical decrease in [Ca2+]c and glucagon release. By contrast, the strong opening of KATP channels by diazoxide keeps the plasma membrane hyperpolarized, which prevents the generation of action potentials, maintaining [Ca2+]c and glucagon secretion at low levels. This model thus predicts that glucagon secretion is stimulated in a narrow range of KATP channel opening, whereas it is inhibited when KATP channels are fully closed or opened. The main reasons why sulfonylureas would exert distinct effects in α- and β-cells are (a) that at low glucose, most KATP channels are closed in α-cells whereas they are opened in β-cells, and (b) that α-cells possess different low-threshold voltage-gated channels (Nav and T-type Ca2+ channels) than those of β-cells. However, this model is not supported by other studies. Thus, Gylfe and collaborators [27,29] reported an increase in [Ca2+]c (instead of a decrease) upon maximal blockade of KATP channels by high tolbutamide concentrations (0.5–1 mM) in mouse α-cells with spontaneous Ca2+ activity or slightly depolarized by 8 mM external K+. The stimulatory effect of tolbutamide was corroborated by studies from our group and others – performed on isolated mouse or rat α-cells or α-cells within mouse islets – indicating that tolbutamide depolarized the plasma membrane [26,36] and increased [Ca2+]c [26,30,34,36]. The sulfonylurea also stimulated glucagon release from isolated α-cells [36]. Furthermore, the effects of sulfonylureas in vivo in humans also yielded divergent results since studies reported a decrease in glucagonemia [[72], [73], [74], [75], [76]], no effect [77,78] or an effect that depended on the type of sulfonylurea [79]. The reasons for all these discrepancies are unknown. It has been speculated that it might be because of species differences as mouse, rat, and human α-cells do not express the same sets of channels and might not have the same KATP channel density [13,22,67,69,71,80]. However, this explanation is not sufficient because divergent results were obtained using the same species, the mouse. Discrepancies might also be related to differences between the experimental conditions or models. In particular, it has been suggested that the expression of channels is different in isolated α-cells and α-cells within islets. Our [Ca2+]c measurements indicated that KATP channel inhibition by tolbutamide or gliclazide exerted similar effects and increased [Ca2+]c in both isolated α-cells and in α-cells within islets. This indicates that sulfonylureas exert a direct stimulatory effect on α-cells, most likely by activating a similar mechanism as in β-cells i.e., a plasma membrane depolarization that opens VGCC. However, in G1, this increase in α-cell [Ca2+]c did not correlate with an increase in glucagon secretion because sulfonylureas inhibited glucagon release from islets. This glucagonostatic effect was attributed to SST because tolbutamide stimulated glucagon secretion of islets from Sst−/− mice. This is fully in agreement with the well-known stimulatory effect of sulfonylureas on SST release [8,11,20,31,46,55,56,[81], [82], [83]]. By contrast, in G15, tolbutamide stimulated glucagon release in both Sst+/+ and Sst−/− mice. Therefore, we propose that the effect of KATP channel blockers on glucagon release involves SST and depends on the glucose concentration present in the medium/plasma, which could explain, at least partly, the discordant interpretations in the literature based on ex vivo studies in rodents and in vivo studies in humans. Taken together, these data indicate that the effects of sulfonylureas on glucagon release result from a balance between their direct stimulatory effect on α-cells and their indirect inhibitory effect through δ-cells activation (Figure 8A). However, this model applies only to KATP channel blockers and not to glucose, for which the involvement of [Ca2+]c in the control of glucagon secretion is highly controverted [1,4,[84], [85], [86]] and was not addressed in the present study.
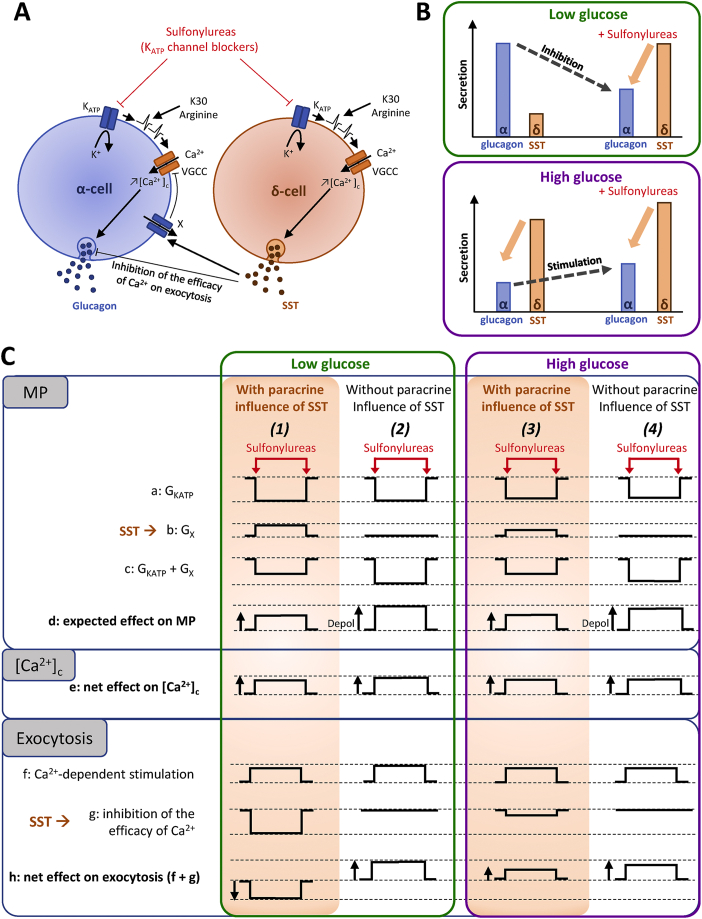
Figure 8.
Model depicting the direct and indirect (via δ-cells) mechanisms by which sulfonylureas control glucagon release. (A) Direct and indirect effects exerted by sulfonylureas (KATP channel blockers) on α-cells. By depolarizing α-cells, sulfonylureas increase [Ca2+]c which stimulates glucagon secretion. The mechanism is similar to what occurs in β-cells, and any other depolarizing agents (such as high K+ (K30) or arginine) will produce the same effect, i.e., an increase in [Ca2+]c parallel to the stimulation of secretion. However, sulfonylureas also depolarize δ-cells and stimulate SST secretion. The latter then inhibits glucagon release by decreasing the efficacy of Ca2+ on exocytosis and by decreasing α-cell [Ca2+]c (by a hyperpolarizing current carried by a channel of unknown nature (×) but that is different from GIRK channels). Like sulfonylureas, depolarizing agents also stimulate δ-cells so that the net effect on α-cells depends on the depolarizing status of the latter. (B) Sulfonylureas exert two opposite effects on glucagon release depending on the glucose concentration. At low glucose, secretion of glucagon is high whereas that of SST is low. The addition of sulfonylureas stimulates SST secretion which indirectly inhibits glucagon release despite the sulfonylurea-induced increase in α-cell [Ca2+]c because SST mainly inhibits the efficacy of Ca2+ on exocytosis in α-cells. At high glucose, the situation is reversed. SST secretion is high and glucagon release is low because it is already inhibited by SST and other mechanisms. The addition of sulfonylureas stimulates glucagon release because the inhibitory effect by SST is already near its maximum, and therefore, the direct stimulatory effect of sulfonylureas on α-cells is the main visible effect. (C) Effects of sulfonylureas on various parameters (shown as relative changes) affecting the membrane potential (MP), [Ca2+]c and exocytosis in α-cells of islets expressing or not expressing SST, at low and high glucose. (1) At low glucose and in control islets expressing SST, sulfonylureas reduce the KATP channel conductance (GKATP) of α-cells (a) and stimulates SST secretion which activates an unknown hyperpolarizing conductance (GX) in α-cells (b). The net effect (GKATP + GX) is a decrease of hyperpolarizing conductance (c) resulting in the depolarization of the membrane potential (MP) (d). This produces an increase in α-cell [Ca2+]c (e) and a Ca2+-dependent stimulation of exocytosis (f). In addition to activating a hyperpolarizing conductance, SST inhibits the efficacy of Ca2+ on exocytosis (g). The net effect is thus an inhibition of exocytosis (h). (2) At low glucose and in a condition where SST is absent (i.e., in Sst−/− mice), sulfonylureas inhibit the KATP channel conductance (a) but do not affect Gx (b) because SST is not produced. This results in a net decrease of a hyperpolarizing conductance that is stronger than in Sst+/+ mice (c), and therefore, leads to a stronger depolarization (d). This leads to an increase in α-cell [Ca2+]c (e) which strongly stimulates exocytosis because it is not counteracted by the glucagonostatic effect of SST (f–h). (3) At high glucose and in control islets expressing SST, α-cell GKATP is probably lower than at low glucose, so that sulfonylureas probably reduce GKATP relatively less than at low glucose (a). Gx is probably already activated by SST released at high glucose, so that SST secreted in response to sulfonylureas activate relatively less Gx than at low glucose (b). The net effect (GKATP + GX) is a decrease of a hyperpolarizing conductance (c) resulting in a depolarization of the membrane potential (MP) (d). This produces an increase in α-cell [Ca2+]c (e) and a Ca2+-dependent stimulation of glucagon exocytosis (f). SST also decreases the efficacy of Ca2+ on exocytosis, but since glucagon secretion is already inhibited by high glucose (by SST-dependent and -independent mechanisms), this effect is weaker than at low glucose level (g) and does not counteract the direct stimulation of α-cells by sulfonylureas, resulting, infine, in a stimulation of glucagon secretion (h). (4) At high glucose and in Sst−/− islets, sulfonylureas produce almost the same effect as at low glucose level in Sst−/− islets (except for slight differences in the amplitude of some effects), i.e., an inhibition of GKATP of α-cells (a) resulting in an MP depolarization (d), an increase in α-cell [Ca2+]c (e) and stimulation of glucagon secretion (h). KATP, ATP-sensitive K+; VGCC, voltage-gated Ca2+ channels.
4.2. SST-14 inhibits glucagon secretion by decreasing [Ca2+]c and the efficacy of Ca2+ on exocytosis
SST is synthesized from a 116-amino acid precursor, preprosomatostatin, which is converted in a cell-specific pattern into various peptides including two bioactive forms: SST-14 and SST-28. Pancreatic δ-cells mainly secrete SST-14 which contributes to <5% of circulating SST, the rest mainly being secreted by intestinal D-cells in the form of SST-28 [[87], [88], [89], [90], [91]]. Because we hypothesized that SST is responsible for the glucagonostatic effect of sulfonylureas, it was important to test if the effects of SST-14 on [Ca2+]c and glucagon release are compatible with such a role. Several mechanisms of inhibition of glucagon release by SST have been proposed including cAMP reduction [45,[92], [93], [94], [95]], VGCC inhibition [53], activation of GIRK [[53], [54], [55],59], and calcineurin-dependent secretory granules depriming [81]. Our observation that SST-14 very potently inhibits glucagon secretion from Sst−/− islets without affecting α-cell [Ca2+]c in conditions where the membrane potential of α-cells is clamped at a stable depolarized level (i.e., in the presence of high K+ (K30) and diazoxide; Figure 6G–J), suggests that SST-14 does not significantly inhibit VGCC. Since [Ca2+]c remained steadily elevated in this condition, this experiment also demonstrates that SST inhibits the efficacy of Ca2+ on exocytosis. These Ca2+- and membrane potential-independent mechanisms are most likely responsible for the glucagonostatic effect of sulfonylureas despite the rise in α-cell [Ca2+]c that these drugs produce. However, we also observed that SST-14 decreased [Ca2+]c in conditions where the plasma membrane was not clamped (more importantly in Sst−/− than in Sst+/+ islets, discussed below). This effect likely results from the activation of a hyperpolarizing current. Surprisingly, it does not seem to involve GIRK channels since TPNQ did not reverse the effect of exogenous SST-14 or the effect of endogenous SST. An alternative candidate for the hyperpolarizing effect of SST is the big conductance K+ (BK) channel (Kcnma1) and because it is expressed by α-cells [57,58,96], it contributes to the mechanisms controlling glucagon secretion [97], and is activated by SST in neurons and pituitary cells [98,99]. Identification of the channel responsible for the [Ca2+]c lowering effect of SST is, however, beyond the scope of this study.
Sulfonylureas, which also stimulate SST secretion, did not decrease α-cell [Ca2+]c as the membrane potential was not clamped. We hypothesized that, upon sulfonylurea application, the hyperpolarizing effect of SST is counteracted or even surpassed by the depolarizing effect of KATP channel closure (Figure 8C). Several observations support this hypothesis. In Sst+/+ islets, the addition of a high concentration of SST-14 (1 μM) to a medium containing tolbutamide only slightly decreased [Ca2+]c to levels that remained higher than in the absence of sulfonylurea. In both Sst+/+ and Sst−/− islets, SST-14 failed to prevent the stimulatory effect of tolbutamide. To reinforce these conclusions, we used an experimental paradigm in which we depolarized the plasma membrane and increased α-cell [Ca2+]c with arginine (which enters into the cell in a positively charged form). Endogenous SST levels are expected to be lower in the presence of arginine than tolbutamide as previous studies have recognized that arginine less potently stimulates SST secretion than sulfonylurea [19,83,100]. However, the application of exogenous SST-14 in the presence of arginine barely decreased [Ca2+]c. All these results suggest that the hyperpolarizing effect of SST is too small to counteract the depolarizing effect of arginine or tolbutamide.
Our experiments also suggest that the effect of exogenous SST-14 strongly depends on the amount of endogenous SST that is secreted. Indeed, SST-14 much more potently inhibited glucagon secretion and α-cell [Ca2+]c from Sst−/− islets than from Sst+/+ islets. Although we did not find differences in Sstr1-5 mRNA expression between α-cells from Sst−/− and Sst+/+ mice, we cannot rule out the possibility that this hypersensitivity of Sst−/− islets to SST results from a higher expression of SSTR (at the protein level) and/or a lack of SSTR desensitization that is probably present in Sst+/+ islets. However, we also provide indications suggesting that, even in Sst+/+ islets, the level of endogenous SST affects the effectiveness of exogenous SST-14. Thus, exogenous SST-14 more potently inhibited glucagon secretion in G1 than in G1 + 5 mM arginine (compare Figure 4C with Figure 5C). Moreover, exogenous SST-14 was completely ineffective in strong depolarizing conditions (K30 + diazoxide), probably because endogenous SST was already exerting its maximal effect. We did not measure SST secretion in all these conditions because it would not be indicative of the actual level of endogenous SST in islets. However, the potent glucagonotropic effect of SSTR2/3 antagonists (CYN154806 + H6056) in K30 + diazoxide strongly supports our assumption. Interestingly, we previously showed that the same antagonists reversed the glucagonostatic effect of sulfonylureas [33].
4.3. SST-14 inhibits glucagon secretion more efficiently than insulin secretion
The same mechanisms as those documented in α-cells have been identified in β-cells [10] and include cAMP reduction [53,56,93,[101], [102], [103]], VGCC inhibition (in humans [53], but not in mice [104]), GIRK activation [53], and calcineurin-dependent secretory granules depriming [104]. We and others previously showed that endogenous SST or exogenous SST-14 inhibited insulin secretion in conditions where β-cells are stimulated by glucose or KATP channel blockers and without clamping their membrane potential [19,31,48,101,[105], [106], [107], [108]]. However, three observations of the present study suggest a much weaker inhibitory effect of SST-14 on insulin than on glucagon secretion. First, the addition of K30 to a medium containing diazoxide more strongly stimulated glucagon secretion of Sst−/− islets than that of Sst+/+ islets while having a similar effect on insulin secretion from both types of islets (Figure 6, Figure 7). Second, the addition of exogenous SST-14 to a medium containing K30 and diazoxide potently suppressed glucagon secretion of Sst−/− islets while it exerted only a small, but not significant, effect on insulin secretion in the same experiments (Figure 6G-L). Third, SSTR2/3 antagonists strongly stimulated glucagon release of Sst+/+ islets but they had no effect on insulin secretion (Figure 7). Because all these experiments were performed in conditions were the membrane potential was clamped with K30 and diazoxide while [Ca2+]c was steadily elevated in whole islet [109,110], these results suggest that SST-14 very potently inhibits glucagon secretion by its Ca2+-independent mechanism but exerts no or weak effect on insulin release in these conditions. The experiments of the present study were not designed to test the inhibitory effect of SST in conditions where β-cells were stimulated without clamping their membrane potential. They were designed to investigate the effect of SST on glucagon secretion, i.e., in conditions where glucagon secretion was elevated but insulin release was close to basal. Overall, these results suggest a more potent inhibitory effect of SST-14 on glucagon than on insulin secretion. Previous experiments have reported that rat α-cells are much more sensitive than β-cells to SST-14, while they are equally sensitive to SST-28 [95,111]. SST-14 is the endogenous form produced by islets whereas SST-28 is produced outside the islet, and hence, it has been suggested that islet δ-cells are much more potent regulators of glucagon than of insulin secretion. This concept is reinforced by the architecture of mouse islets with a β-cell core surrounded by a mantle of α- and δ-cells [112] and by the existence of frequent contacts between α- and δ-cells [[113], [114], [115], [116]]. It should be remembered that in mice, α-cells mainly express SSTR2 and SSTR3, while β-cells mainly express SSTR3 [8,33,57]. It is possible that SSTR2 more potently inhibits the efficacy of Ca2+ on exocytosis than SSTR3. Other mechanisms downstream of SSTRs could also explain the difference in sensitivity of α- and β-cells to SST.
The critical role of SST in the control of glucagon secretion might be important to avoid excessive glucagon secretion that could lead to hyperglycemia as observed in diabetes [[117], [118], [119], [120]]. Indeed, a recent study showed that human α-cells from T2D patients are insensitive to paracrine control by SST which could lead to the apparition of hyperglucagonemia and exacerbate hyperglycemia [121]. Moreover, T1D and advanced T2D patients exhibit an impaired glucagon response to insulin- or sulfonylurea-induced hypoglycemia, which can lead to life-threatening hypoglycemic episodes [120,122]. The mechanisms are controversial and might result from impaired activation of the central nervous system [[123], [124], [125]], inhibition of glucagon release by excessive insulin [126,127] or SST secretion [128,129], or an impaired direct effect of low glucose on α-cells [1]. Previous studies showed that sulfonylureas blunted the glucagon response to hypoglycemia in hypoglycemic clamps [74,75], raising the possibility that sulfonylureas diminish the normal glucagon response to hypoglycemia, as observed in diabetes. It was suggested that this diminished response was caused by intraislet insulin released in response to sulfonylureas. Our data suggest an alternative mechanism involving an inhibition of the glucagon response to hypoglycemia by SST secreted in response to sulfonylureas.
5. Conclusions
We suggest that sulfonylureas directly stimulate α-cells by similar mechanisms to those in β-cells. By closing α-cell KATP channels, sulfonylureas depolarize α-cells, increase [Ca2+]c, and stimulate glucagon secretion. However, their effects also involve an indirect inhibitory effect via SST secreted by δ-cells so that the net effect depends on the glucose concentration present in the medium (Figure 8). Thus, at a high glucose level, sulfonylureas stimulate glucagon secretion because SST is already secreted in response to glucose, whereas, at a low glucose level, sulfonylureas inhibit glucagon release because SST counteracts the direct stimulatory effect of sulfonylureas on α-cells. This glucagonostatic effect mediated by SST results from an inhibition of the efficacy of cytosolic Ca2+ on exocytosis. Although exogenous SST also inhibits glucagon secretion by decreasing α-cell [Ca2+]c, no decrease in [Ca2+]c occurs during sulfonylurea application because it is largely counterbalanced by the direct stimulatory effect of these drugs on α-cell [Ca2+]c. Experiments with high K+ reveal that endogenous SST also strongly limits glucagon secretion. In addition, our results indicate that, unexpectedly, SST-14 poorly decreases the efficacy of Ca2+ on exocytosis in β-cells.
Authors’ contribution
P.G. conceived and supervised the study. B.S. designed and performed experiments, and analyzed results. F.K. and H.C. performed experiments and analyzed results. B.S. and P.G. wrote the manuscript. L.D. performed some statistical analyses. P.L.H. provided materials. All coauthors approved the manuscript. P.G. and B.S. are guarantors of this study, and as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by a grant from the Actions de Recherche Concertées (18/23–094) from the Communauté française de Belgique, by a CDR grant (J.0178.17) and PDR grants (T.0124.15 and T.0110.20) from the Fonds National de la Recherche Scientifique (Brussels), by a grant from the Société Francophone du Diabète (Paris), by a grant 1912-03555 from the Leona M. & Harry B. Helmsley Charitable Trust, and by a Diatype grant from Innoviris (Brussels). P.G. is Research Director of the Fonds National de la Recherche Scientifique, (Brussels). F.K. is holder of a fellowship from the FRIA/FNRS, Brussels, Belgium. B.S. was partly supported by a fellowship from UCLouvain, Belgium.
Prior presentation
Parts of this study were presented at the 55th EASD annual meeting, Barcelona, Spain, September 16–20, 2019, and at the SFD annual meeting, Brussels, Belgium, September 8–11, 2020.
Acknowledgments
We thank F. Knockaert and N. Antoine for help with radioimmunoassays and genotyping, respectively. This study benefited from statistical advices from UCLouvain's technological platform for Methodology and Statistical Computing Support - SMCS/LIDAM, UCLouvain.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101268.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gilon P., Cheng-xue R., Lai B.K., Chae H., Gomez-Ruiz A. Physiological and pathophysiological control of glucagon secretion by pancreatic α-cells. In: Islam M.S., editor. Islets of Langerhans. 2nd ed. Springer Science+Business Media; Dordrecht: 2015. pp. 175–247. [Google Scholar]
- 2.Müller T.D., Finan B., Clemmensen C., Dimarchi R.D., Tschöp M.H. The new biology and pharmacology of glucagon. Physiological Reviews. 2017;97(2):721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- 3.Gromada J., Chabosseau P., Rutter G.A. The α-cell in diabetes mellitus. Nature Reviews Endocrinology. 2018;14(12):694–704. doi: 10.1038/s41574-018-0097-y. [DOI] [PubMed] [Google Scholar]
- 4.Gilon P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. Journal of Molecular Biology. 2020;432(5):1367–1394. doi: 10.1016/j.jmb.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation. 2012;122(1):4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryer P.E. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3):1039–1048. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hædersdal S., Lund A., Knop F.K., Vilsbøll T. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clinic Proceedings. 2018;93(2):217–239. doi: 10.1016/j.mayocp.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Rorsman P., Huising M.O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nature Reviews Endocrinology. 2018;14(7):404–414. doi: 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilon P., Chae H., Rutter G.A., Ravier M.A. Calcium signaling in pancreatic β-cells in health and in type 2 diabetes. Cell Calcium. 2014;56(5):340–361. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Rorsman P., Ashcroft F.M. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiological Reviews. 2018;98(1):117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efendic S., Enzmann F., Nylen A., Uvnas-wallensten K., Luft R. Effect of glucose/sulfonylurea interaction on release of insulin, glucagon, and somatostatin from isolated perfused rat pancreas. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(11):5901–5904. doi: 10.1073/pnas.76.11.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Göpel S.O., Kanno T., Barg S., Rorsman P. Patch-clamp characterisation of somatostatin-secreting δ-cells in intact mouse pancreatic islets. Journal of Physiology. 2000;528(3):497–507. doi: 10.1111/j.1469-7793.2000.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quesada I., Nadal A., Soria B. Different effects of tolbutamide and diazoxide in α-, β-, and δ-cells within intact islets of langerhans. Diabetes. 1999;48(12):2390–2397. doi: 10.2337/diabetes.48.12.2390. [DOI] [PubMed] [Google Scholar]
- 14.Nadal A., Quesada I., Soria B. Homologous and heterologous asynchronicity between identified α-, β- and δ-cells within intact islets of Langerhans in the mouse. Journal of Physiology. 1999;517(1):85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun M., Ramracheya R., Amisten S., Bengtsson M., Moritoh Y., Zhang Q. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52(8):1566–1578. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 16.Sola D., Rossi L., Piero G., Schianca C., Maffioli P., Bigliocca M. Sulfonylureas and their use in clinical practice. Archives of Medical Science. 2015;11(4):840–848. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colagiuri S., Matthews D., Leiter L.A., Pheng S., Sesti G., Marre M. The place of gliclazide MR in the evolving type 2 diabetes landscape: a comparison with other sulfonylureas and newer oral antihyperglycemic agents. Diabetes Research and Clinical Practice. 2018;143:1–14. doi: 10.1016/j.diabres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Meulen T., Donaldson C.J., Cáceres E., Hunter A.E., Cowing-zitron C., Pound L.D. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 2015;21(7):769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauge-evans A.C., King A.J., Carmignac D., Richardson C.C., Robinson I.C.A.F., Low M.J. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58(2):403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ipp E., Dobbs R.E., Arimura A., Vale W., Harris V., Unger R.H. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. Journal of Clinical Investigation. 1977;60(3):760–765. doi: 10.1172/JCI108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald P.E., Marinis YZ De, Ramracheya R., Salehi A., Ma X., Johnson P.R.V. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biology. 2007;5(6):e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramracheya R., Ward C., Shigeto M., Walker J.N., Amisten S., Zhang Q. Membrane potential-dependent inactivation of voltage-gated ion channels in α-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59(9):2198–2208. doi: 10.2337/db09-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q., Ramracheya R., Lahmann C., Tarasov A., Bengtsson M., Braha O. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metabolism. 2013;18(6):871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Göpel S.O., Kanno T., Barg S., Weng X.-G., Gromada J., Rorsman P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. Journal of Physiology. 2000;528(3):509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gromada J., Ma X., Høy M., Bokvist K., Salehi A., Berggren P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1-/- mouse α-cells. Diabetes. 2004;53(Suppl. 3):181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 26.Früh E., Elgert C., Eggert F., Scherneck S., Rustenbeck I. Glucagonotropic and glucagonostatic effects of KATP channel closure and potassium depolarization. Endocrinology. 2021;162(1):1–13. doi: 10.1210/endocr/bqaa136. [DOI] [PubMed] [Google Scholar]
- 27.Vieira E., Salehi A., Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50(2):370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 28.Olsen H.L., Theander S., Bokvist K., Buschard K., Wollheim C.B., Gromada J. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology. 2005;146(11):4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Vieira E., Gylfe E. A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell. Cell Calcium. 2004;35(4):357–365. doi: 10.1016/j.ceca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Quoix N., Cheng-xue R., Mattart L., Zeinoun Z., Guiot Y., Beauvois C. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes. 2009;58(2):412–421. doi: 10.2337/db07-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng-xue R., Gómez-ruiz A., Antoine N., Noël L.A., Chae H., Ravier M.A. Tolbutamide controls glucagon release from mouse islets differently than glucose. Diabetes. 2013;62(5):1612–1622. doi: 10.2337/db12-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellman B., Dansk H., Grapengiesser E. Somatostatin promotes glucose generation of Ca2+ oscillations in pancreatic islets both in the absence and presence of tolbutamide. Cell Calcium. 2018;74:35–42. doi: 10.1016/j.ceca.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Lai B., Chae H., Gómez-ruiz A., Cheng P., Gallo P., Antoine N. Somatostatin is only partly required for the glucagonostatic effect of glucose but is necessary for the glucagonostatic effect of KATP channel blockers. Diabetes. 2018;67(11):2239–2253. doi: 10.2337/db17-0880. [DOI] [PubMed] [Google Scholar]
- 34.Le Marchand S.J., Piston D.W. Glucose decouples intracellular Ca2+ activity from glucagon secretion in mouse pancreatic islet alpha-cells. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Marchand S.J., Piston D.W. Glucose suppression of glucagon secretion: metabolic and calcium responses from α-cells in intact mouse pancreatic islets. Journal of Biological Chemistry. 2010;285(19):14389–14398. doi: 10.1074/jbc.M109.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin I., Gromada J., Gjinovci A., Theander S., Wollheim C.B. β-cell secretory products activate α-cell ATP-dependant potassium channels to inhibit glucagon release. Diabetes. 2005;54(6):1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 37.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 38.Low M.J., Patel Y.C., Rubinstein M., Low M.J., Otero-corchon V., Parlow A.F. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. Journal of Clinical Investigation. 2001;107(12):1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz A., Hu M., Hussain K., Bryan J., Aguilar-Bryan L., Rajan A.S. Regulation of glucagon secretion at low glucose concentrations: evidence for adenosine triphosphate-sensitive potassium channel involvement. Endocrinology. 2005;146(12):5514–5521. doi: 10.1210/en.2005-0637. [DOI] [PubMed] [Google Scholar]
- 40.Ørgaard A., Holst J.J. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017;60(9):1731–1739. doi: 10.1007/s00125-017-4315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wand M.P., Jones M.C. 1st ed. Chapman and Hall; London: 1995. Kernel smoothing. [Google Scholar]
- 42.Hastie T.J. Generalized additive models. In: Chambers J.M., Hastie T.J., editors. Statistical models. Wadsworth & Brooks/Cole; California: 1992. [Google Scholar]
- 43.Vieira E., Liu Y., Gylfe E. Involvement of α1 and β-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse α-cell. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2004;369(2):179–183. doi: 10.1007/s00210-003-0858-5. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton A., Zhang Q., Salehi A., Willems M., Knudsen J.G., Ringgaard A.K. Adrenaline stimulates glucagon secretion by Tpc2-dependent Ca2+ mobilization from acidic stores in pancreatic α-cells. Diabetes. 2018;67(March):1128–1139. doi: 10.2337/db17-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott A.D., Ustione A., Piston D.W. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. American Journal of Physiology Endocinology Metabolism. 2015;308(2):E130–E143. doi: 10.1152/ajpendo.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vergari E., Knudsen J.G., Ramracheya R., Salehi A., Zhang Q., Adam J. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nature Communications. 2019;10(1):139. doi: 10.1038/s41467-018-08193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu S., Andersen D., Izarzugaza J., Kuhre R., Holst J. In the rat pancreas, somatostatin tonically inhibits glucagon secretion and is required for glucose-induced inhibition of glucagon secretion. Acta Physiologica. 2020;229(3) doi: 10.1111/apha.13464. [DOI] [PubMed] [Google Scholar]
- 48.Svendsen B., Holst J.J. Paracrine regulation of somatostatin secretion by insulin and glucagon in mouse pancreatic islets. Diabetologia. 2020;64(1):142–151. doi: 10.1007/s00125-020-05288-0. [DOI] [PubMed] [Google Scholar]
- 49.Walker J.N., Ramracheya R., Zhang Q., Johnson P.R.V., Braun M., Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsec or both? Diabetes, Obesity and Metabolism. 2011;13(Suppll. 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 50.Luft R., Efendic S., Hökfelt T., Johansson O., Arimura A. Immunohistochemical evidence for the localization of somatostatin--like immunoreactivity in a cell population of the pancreatic islets. Medical Biology. 1974;52(6):428–430. [PubMed] [Google Scholar]
- 51.Dubois P.M. Immunoreactive somatostatin is present in discrete cells of the endocrine pancreas. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(4):1340–1343. doi: 10.1073/pnas.72.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arimura A., Sato H., Dupont A., Nishi N., Andrew V.S. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- 53.Kailey B., Van De Bunt M., Cheley S., Johnson P.R., Macdonald P.E., Gloyn A.L. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β- and α-cells. American Journal of Physiology Endocinology Metabolism. 2012;303(9):E1107–E1116. doi: 10.1152/ajpendo.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimoto Y., Fukuyama Y., Horio Y., Inanobe A., Gotoh M., Kurachi Y. Somatostatin induces hyperpolarization in pancreatic islet α cells by activating a G protein-gated K+ channel. FEBS Letters. 1999;444(2–3):265–269. doi: 10.1016/s0014-5793(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 55.Gromada J., Høy M., Olsen H.L., Gotfredsen C.F., Buschard K., Rorsman P. Gi2 proteins couple somatostatin receptors to low-conductance K+ channels in rat pancreatic α-cells. Pflügers Archiv. 2001;442(1):19–26. doi: 10.1007/s004240000474. [DOI] [PubMed] [Google Scholar]
- 56.Braun M. The somatostatin receptor in human pancreatic β-cells. Vitamins & Hormones. 2014;95:165–193. doi: 10.1016/B978-0-12-800174-5.00007-7. [DOI] [PubMed] [Google Scholar]
- 57.Digruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-zitron C. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Molecular Metabolism. 2016;5(7):449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adriaenssens A.E., Svendsen B., Lam B.Y.H., Yeo G.S.H., Holst J.J., Reimann F. Transcriptomic profiling of pancreatic alpha , beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Briant L., Salehi A., Vergari E., Zhang Q., Rorsman P. Glucagon secretion from pancreatic α-cells. Upsala Journal of Medical Sciences. 2016;121(2):113–119. doi: 10.3109/03009734.2016.1156789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh K.B. Targeting GIRK channels for the development of new therapeutic agents. Frontiers in Pharmacology. 2011;2:64. doi: 10.3389/fphar.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin W., Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1999;38(43):14286–14293. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- 62.Kanjhan R., Coulson E.J., Adams D.J., Bellingham M.C. Tertiapin-Q blocks recombinant and native large conductance K+ channels in a use-dependent manner. Journal of Pharmacology and Experimental Therapeutics. 2005;314(3):1353–1361. doi: 10.1124/jpet.105.085928. [DOI] [PubMed] [Google Scholar]
- 63.Hermansen K., Christensen S.E., Orskov H. Characterization of somatostatin release from the pancreas: the role of potassium. Scandinavian Journal of Clinical & Laboratory Investigation. 1979;39(8):717–722. doi: 10.3109/00365517909108162. [DOI] [PubMed] [Google Scholar]
- 64.Berts A., Ball A., Dryselius S., Gylfe E., Hellman B. Glucose stimulation of somatostatin-producing cells involves oscillatory Ca2+ signaling. Endocrinology. 1996;137(2):693–697. doi: 10.1210/endo.137.2.8593819. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q., Bengtsson M., Partridge C., Salehi A., Braun M., Cox R. R-type Ca2+-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nature Cell Biology. 2007;9(4):453–460. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 66.Albrechtsen N.J.W., Kuhre R.E., Pedersen J., Knop F.K., Holst J.J. The biology of glucagon and the consequences of hyperglucagonemia. Biomarkers in Medicine. 2016;10(11):1141–1151. doi: 10.2217/bmm-2016-0090. [DOI] [PubMed] [Google Scholar]
- 67.Bokvist K., Olsen H.L., Høy M., Gotfredsen C.F., Holmes W.F., Buschard H.K. Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic A-cells. Pflügers Archiv. 1999;438(4):428–436. doi: 10.1007/s004249900076. [DOI] [PubMed] [Google Scholar]
- 68.Shiota C., Rocheleau J.V., Shiota M., Piston D.W., Magnuson M.A. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. American Journal of Physiology Endocinology Metabolism. 2005;289(4):E570–E577. doi: 10.1152/ajpendo.00102.2005. [DOI] [PubMed] [Google Scholar]
- 69.Leung Y.M., Ahmed I., Sheu L., Gao X., Hara M., Tsushima R.G. Insulin regulates islet α-cell function by reducing KATP channel sensitivity to adenosine 5’-triphosphate inhibition. Endocrinology. 2006;147(5):2155–2162. doi: 10.1210/en.2005-1249. [DOI] [PubMed] [Google Scholar]
- 70.Segerstolpe A., Palasantza A., Eliasson P., Kasper M., Ammala C., Sandberg R. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q., Dou H., Rorsman P. ‘Resistance is futile?’ – paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells. Journal of Physiology. 2020;598(21):4765–4780. doi: 10.1113/JP279775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landstedt-Hallin L., Adamson U., Lins P.-E. Oral glibenclamide suppresses glucagon secretion during insulin-induced hypoglycema in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 1999;84(9):3140–3145. doi: 10.1210/jcem.84.9.6002. [DOI] [PubMed] [Google Scholar]
- 73.Pfeifer M.A., Beard J.C., Halter J.B., Judzewitsch R., Best J.D., Porte D. Suppression of glucagon secretion during a tolbutamide infusion in normal and noninsulin-dependent diabetic subjects. Journal of Clinical Endocrinology & Metabolism. 1983;56(3):586–591. doi: 10.1210/jcem-56-3-586. [DOI] [PubMed] [Google Scholar]
- 74.Banarer S., McGregor V.P., Cryer P.E. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51(4):958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- 75.Peacey S.R., Rostami-Hodjegan A., George E., Tucker G.T., Heller S.R. The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. Journal of Clinical Endocrinology & Metabolism. 1997;82(5):1458–1461. doi: 10.1210/jcem.82.5.3910. [DOI] [PubMed] [Google Scholar]
- 76.Ter Braak E.W.M.T., Appelman A.M.M.F., Van Der Tweel I. The sulfonylurea glyburide induces impairment of glucagon and growth hormone responses during mild insulin-induced hypoglycemia. Diabetes Care. 2002;25(1):107–112. doi: 10.2337/diacare.25.1.107. [DOI] [PubMed] [Google Scholar]
- 77.Szoke E., Gosmanov N.R., Sinkin J.C., Nihalani A., Fender A.B., Cryer P.E. Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism. 2006;55(1):78–83. doi: 10.1016/j.metabol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Holstein A., Plaschke A., Hammer C., Ptak M., Kuhn J., Kratzsch C. Hormonal counterregulation and consecutive glimepiride serum concentrations during severe hypoglycaemia associated with glimepiride therapy. European Journal of Clinical Pharmacology. 2003;59(10):747–754. doi: 10.1007/s00228-003-0697-9. [DOI] [PubMed] [Google Scholar]
- 79.Joy N.G., Tate D.B., Davis S.N. Counterregulatory responses to hypoglycemia differ between glimepiride and glyburide in non diabetic individuals. Metabolism. 2015;64(6):729–737. doi: 10.1016/j.metabol.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spigelman A.F., Dai X., Macdonald P.E. Voltage-dependent K+ channels are positive regulators of alpha cell action potential generation and glucagon secretion in mice and humans. Diabetologia. 2010;53(9):1917–1926. doi: 10.1007/s00125-010-1759-z. [DOI] [PubMed] [Google Scholar]
- 81.Gromada J., Høy M., Buschard K., Salehi A., Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic α-cells by Gi2-dependent activation of calcineurin and depriming of secretory granules. Journal of Physiology. 2001;535(2):519–532. doi: 10.1111/j.1469-7793.2001.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vergari E., Denwood G., Salehi A., Zhang Q., Adam J., Alrifaiy A. Somatostatin secretion by Na+-dependent Ca2+-induced Ca2+ release in pancreatic delta cells. Natural Metabolism. 2020;2(1):32–40. doi: 10.1038/s42255-019-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sako Y., Wasada T., Umeda F., Ibayashi H. Effect of glibenclamide on pancreatic hormone release from isolated perfused islets of normal and cysteamine-treated Rats. Metabolism. 1986;35(10):944–949. doi: 10.1016/0026-0495(86)90059-4. [DOI] [PubMed] [Google Scholar]
- 84.Gromada J., Franklin I., Wollheim C.B. Alpha-cells of the endocrine pancreas : 35 years of research but the enigma remains. Endocrine Reviews. 2007;28(February):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 85.Gylfe E., Gilon P. Glucose regulation of glucagon secretion. Diabetes Research and Clinical Practice. 2013;103(1):1–10. doi: 10.1016/j.diabres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 86.Gylfe E. Glucose control of glucagon secretion —‘ There's a brand-new gimmick every year ’. Upsala Journal of Medical Sciences. 2016;121(April):120–132. doi: 10.3109/03009734.2016.1154905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benoit R., Bohlen P., Brazeau P., Ling N., Guillemin R. Isolation and characterization of rat pancreatic somatostatin. Endocrinology. 1980;107(6):2127–2129. doi: 10.1210/endo-107-6-2127. [DOI] [PubMed] [Google Scholar]
- 88.Francis B.H., Baskin D.G., Saunders R., Ensinck J.W. Distribution of somatostatin-14 and somatostatin-28 gastrointestinal-pancreatic cells of rats and humans. Gastroenterology. 1990;99(5):1283–1291. doi: 10.1016/0016-5085(90)91151-u. [DOI] [PubMed] [Google Scholar]
- 89.Strowski M.Z., Blake A.D. Function and expression of somatostatin receptors of the endocrine pancreas. Molecular and Cellular Endocrinology. 2008;286(1–2):169–179. doi: 10.1016/j.mce.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 90.Taborsky G.J., Jr., Ensinck J.W. Contribution of the pancreas to circulating somatostatin-like immunoreactivity in the normal dog. Journal of Clinical Investigation. 1984;73(1):216–223. doi: 10.1172/JCI111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutniak M., Grill V., Wiechel Se K.L. Basal and meal-induced somatostatin-like immunoreactivity in healthy subjects and in IDDM and totally pancreatectomized patients. Effects of acute blood glucose normalization. Diabetes. 1987;36(7):802–807. doi: 10.2337/diab.36.7.802. [DOI] [PubMed] [Google Scholar]
- 92.Tian G., Sandler S., Gylfe E., Tengholm A. Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes. 2011;60(5):1535–1543. doi: 10.2337/db10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tengholm A., Gylfe E. cAMP signalling in insulin and glucagon secretion. Diabetes, Obesity and Metabolism. 2017;19(Suppl 1):42–53. doi: 10.1111/dom.12993. [DOI] [PubMed] [Google Scholar]
- 94.Yu Q., Shuai H., Ahooghalandari P., Gylfe E., Tengholm A. Glucose controls glucagon secretion by directly modulating cAMP in alpha cells. Diabetologia. 2019;62(7):1212–1224. doi: 10.1007/s00125-019-4857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schuit F.C., Derde M.-P., Pipeleers D.G. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia. 1989;32(3):207–212. doi: 10.1007/BF00265096. [DOI] [PubMed] [Google Scholar]
- 96.Benner C., Meulen T Van Der, Cacéres E., Tigyi K., Donaldson C.J. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dickerson X.M.T., Dadi P.K., Altman M.K., Verlage K.R., Thorson A.S., Jordan X.K.L. Glucose-mediated inhibition of calcium-activated potassium channels limits α-cell calcium influx and glucagon secretion. American Journal of Physiology Endocinology Metabolism. 2019;316(4):E646–E659. doi: 10.1152/ajpendo.00342.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.White R.E., Schonbrunn A., Armstrong D.L. Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature. 1994;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- 99.Galarraga E., Vilchis C., Tkatch T., Salgado H., Tecuapetla F., Perez-Rosello T. Somatostatinergic modulation of firing pattern and calcium-activated potassium currents in medium spiny neostriatal neurons. Neuroscience. 2007;146(2):537–554. doi: 10.1016/j.neuroscience.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K., Yamatani K., Hara M., Sasaki H. Gliclazide directly suppresses arginine-induced glucagon secretion. Diabetes Research and Clinical Practice. 1994;24(3):143–151. doi: 10.1016/0168-8227(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 101.Strowski M.Z., Parmar R.M., Blake A.D., Schaeffer J.M. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141(1):111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 102.Claro A., Grill V., Efendic S., Luft R. Studies on the mechanisms of somatostatin action on insulin release. IV. Effect of somatostatin on cyclic AMP levels and phosphodiesterase activity in isolated rat pancreatic islets. Acta Endocrinologica. 1977;85(2):379–388. [PubMed] [Google Scholar]
- 103.Schuit F.C., Pipeleers D.G. Regulation of adenosine 3’,5’-monophosphate levels in the pancreatic B cell. Endocrinology. 1985;117(3):834–840. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- 104.Renström E., Ding W.-G., Bokvist K., Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting β cells by activation of calcineurin. Neuron. 1996;17(3):513–522. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 105.Tirone T.A., Norman M.A., Moldovan S., DeMayo F.J., Wang X.P., Brunicardi F.C. Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas. 2003;26(3):e67–e73. doi: 10.1097/00006676-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 106.Robert K., Ohning G., Wong H., Watt P., Walsh J., Brunicardi F.C. Regulatory role of intraislet somatostatin on insulin secretion in the isolated perfused human pancreas. Pancreas. 1994;9(2):172–178. doi: 10.1097/00006676-199403000-00006. [DOI] [PubMed] [Google Scholar]
- 107.Zambre Y., Ling Z., Chen M., Hou X., Woon C., Culler M. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochemical Pharmacology. 1999;57(10):1159–1164. doi: 10.1016/s0006-2952(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 108.Singh V., Brendel M.D., Zacharias S., Mergler S., Jahr H., Wiedenmann B. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. Journal of Clinical Endocrinology & Metabolism. 2007;92(2):673–680. doi: 10.1210/jc.2006-1578. [DOI] [PubMed] [Google Scholar]
- 109.Gembal M., Gilon P., Henquin J.C. Evidence that glucose can control insulin release independently from its action on ATP- sensitive K+ channels in mouse B cells. Journal of Clinical Investigation. 1992;89(4):1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gembal M., Detimary P., Gilon P., Gao Z.Y., Henquin J.C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. Journal of Clinical Investigation. 1993;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mandarino L., Stenner D., Blanchard W., Nissen S., Gerich J., Ling N. Selective effects of somatostatin-14, -25 and -28 on in vitro insulin and glucagon secretion. Nature. 1981;291(5810):76–77. doi: 10.1038/291076a0. [DOI] [PubMed] [Google Scholar]
- 112.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orci L., Stefan Y., Bonner-Weif S., Perrelet A., Unger R. “Obligatory” association between A and D cells demonstrated by bipolar islets in neonatal pancreas. Diabetologia. 1981;21(1):73–74. doi: 10.1007/BF03216229. [DOI] [PubMed] [Google Scholar]
- 114.Arrojo R., Jacob S., García-prieto C.F., Zheng X., Fukuda M., Tran H. Structural basis for delta cell paracrine regulation in pancreatic islets. Nature Communications. 2019;10(3700) doi: 10.1038/s41467-019-11517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brereton M.F., Vergari E., Zhang Q., Clark A. Alpha- , Delta- and PP-cells: are they the architectural cornerstones of islet structure and co-ordination? Journal of Histochemistry and Cytochemistry. 2015;63(8) doi: 10.1369/0022155415583535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grube D., Bohn R. The microanatomy of human islets of Langerhans, with special reference to somatostatin. Archivum Histologicum Japonicum. 1983;46(3):327–353. doi: 10.1679/aohc.46.327. [DOI] [PubMed] [Google Scholar]
- 117.Müller W.A., Faloona G.R., Unger R.H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Americas Journal of Medicine. 1973;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 118.Hare K.J., Vilsbøll T., Holst J.J., Knop F.K. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2010;298(4):E832–E837. doi: 10.1152/ajpendo.00700.2009. [DOI] [PubMed] [Google Scholar]
- 119.Knop F.K., Vilsbøll T., Madsbad S., Holst J.J., Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia. 2007;50(4):797–805. doi: 10.1007/s00125-006-0566-z. [DOI] [PubMed] [Google Scholar]
- 120.Ohneda A., Watanabe K., Horigome K., Sakai T., Kai Y., Oikawa S.I. Abnormal response of pancreatic glucagon to glycemic changes in diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 1978;46(3):504–510. doi: 10.1210/jcem-46-3-504. [DOI] [PubMed] [Google Scholar]
- 121.Omar-Hmeadi M., Lund P.-E., Gandasi N., Tengholm A., Barg S. Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes. Nature Communications. 2020;11(1896):1–11. doi: 10.1038/s41467-020-15717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gerich J.E., Langlois M., Noacco C., Karam J.H., Forsham P.H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 123.Chan O., Paranjape S., Czyzyk D., Horblitt A., Zhu W., Ding Y. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes. 2011;60(5):1582–1589. doi: 10.2337/db10-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cryer P., Heller S. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 125.Davis S.N., Shavers C., Costa F., Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. Journal of Clinical Investigation. 1996;98(3):680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dagogo-Jack S.E., Craft S., Cryer P.E. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. Journal of Clinical Investigation. 1993;91(3):819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Segel S.A., Paramore D.S., Cryer P.E. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 128.Karimian N., Qin T., Liang T., Osundiji M., Huang Y., Teich T. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes. 2013;62(8):2968–2977. doi: 10.2337/db13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yue J.T.Y., Burdett E., Coy D.H., Giacca A., Efendic S., Vranic M. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes. 2012;61(1):197–207. doi: 10.2337/db11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.