Abstract
From 2014 to 2017, the World Health Organization convened a working group to evaluate influenza disease burden and vaccine efficacy to inform estimates of maternal influenza immunization program impact. The group evaluated existing systematic reviews and relevant primary studies, and conducted four new systematic reviews. There was strong evidence that maternal influenza immunization prevented influenza illness in pregnant women and their infants, although data on severe illness prevention were lacking. The limited number of studies reporting influenza incidence in pregnant women and infants under six months had highly variable estimates and underrepresented low- and middle-income countries. The evidence that maternal influenza immunization reduces the risk of adverse birth outcomes was conflicting, and many observational studies were subject to substantial bias. The lack of scientific clarity regarding disease burden or magnitude of vaccine efficacy against severe illness poses challenges for robust estimation of the potential impact of maternal influenza immunization programs.
Keywords: Influenza, Immunization, Pregnancy, Infants
1. Background and overview of World Health Organization (WHO) working group procedures
Pregnant women and infants under six months of age are among the population subgroups considered to be at high risk for serious influenza-related morbidity and mortality [1–10]. As influenza vaccines are not licensed for use in infants under six months [11], one strategy for preventing influenza illness in this age group is maternal immunization during pregnancy [12,13], an approach that provides both direct protection to pregnant women and indirect protection of their infants through transplacental antibody transfer [14–16]. Evidence primarily from observational studies has additionally suggested that maternal influenza immunization could prevent adverse birth outcomes [17,18]; however, this remains a topic of scientific debate [19–22].
Despite a 2012 WHO recommendation that pregnant women be prioritized for vaccine receipt in countries initiating or expanding influenza immunization programs [12], maternal influenza immunization has not been incorporated into routine immunization programs in many low- and middle-income countries (LMICs) [23]. As part of a formal strategic review In 2013, Gavi, the Vaccine Alliance considered an investment in maternal influenza immunization programs in eligible LMICs but this was not pursued, partly because of limited data regarding the anticipated impact of such a strategy [24]. To address this data gap, the WHO Initiative for Vaccine Research convened a working group in December 2014 [25] with the following objectives: (i) to determine key parameters needed for future influenza vaccine impact and health economic modeling studies, with a focus on immunization of pregnant women in LMICs; (ii) to determine evidence-based estimates for these key parameters; (iii) to evaluate the quality of existing data informing these estimates; and (iv) to recommend future research to address these gaps.
The working group comprised 30 members, representing all WHO regions and with diverse expertise in child health; disease modeling; evidence evaluation methods [26]; health economics; influenza epidemiology; obstetrics; perinatal epidemiology; and vaccinology (Appendix A). Three subgroups evaluated evidence related to influenza epidemiology in pregnant women, infants under six months of age, and the fetus. Evidence assembled included existing systematic reviews, randomized clinical trials (RCTs) of influenza vaccination in pregnancy (three of which were published during the lifespan of this working group [16,27,28]), and other publications (e.g., highly-cited studies relevant to influenza virus infection or immunization during pregnancy). The working group also carried out four new systematic reviews [29–32]. The objective of this report is to summarize the working group evidence evaluation and recommendations for research to inform estimates of maternal influenza immunization program impact.
2. Summary of key findings from WHO working group-initiated systematic reviews
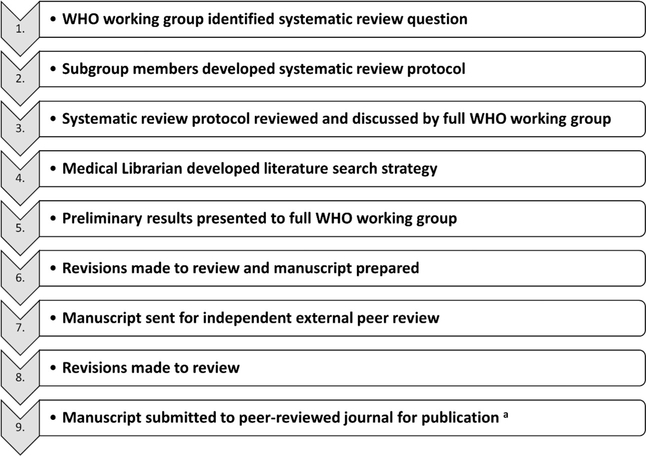
The working group procedures used to design and conduct each new systematic review are summarized in Fig. 1. Briefly, each review included the development of a structured research objective (Table 1); engagement of an experienced medical librarian to design and execute the literature searches; and application of quality assessments to individual studies [33,34] and across studies using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria [26,35]. Specific details about the scope of each review, the date of electronic literature searches, characteristics of included studies, and a quantitative summary of each review’s findings can be found in Tables 1–3, and by consulting the original publications [29–32]. We were unable to standardize incidence rate estimates presented in this report, as the methods used by the primary studies to calculate them differed. As it is possible that our focused systematic reviews could have excluded studies relevant to our working group objectives, we also evaluated highly-cited, but excluded, studies on each particular topic to inform our conclusions and recommendations.
Fig. 1.
Organizational approach for systematic evidence reviews carried out by the WHO working group. aAll manuscripts were prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [86].
Table 1.
Objectives for systematic evidence reviews conducted by the WHO working group.
| Review | A: Incidence of laboratory-confirmed influenza (LCI) outcomes among pregnant women | B: Incidence of LCI outcomes among infants under six months of age | C: Pregnancy as a risk factor for severe outcomes from influenza virus infection | D: Maternal influenza virus infection and adverse birth outcomes |
|---|---|---|---|---|
| Author and citation | Katz et al. BMC Pregnancy and Childbirth 2017;17:155 [29] | Fell et al. In press: BMJ Open [30] | Mertz et al. Vaccine 2017; 35:521–528 [31] | Fell et al. British Journal of Obstetrics and Gynaecology 2016;124:48–59 [32] |
| Objective | To determine evidence-based estimates of the incidence of LCI outcomes among pregnant women | To determine evidence-based estimates of the incidence of LCI outcomes among infants under six months of age | To determine whether pregnancy was a risk factor for severe outcomes of influenza | To assess the association between clinical influenza disease and/or LCI virus infection during pregnancy, compared with no influenza during pregnancy, and adverse birth outcomes |
| Date of electronic literature searches | February 20, 2015 | April 19, 2017 | April 25, 2014 | December 5, 2014 |
| Inclusion criteria | • Studies that, regardless of design, otherwise met criteria in Population, Exposure, and Outcomes (see rows below) • Additionally, only studies that had laboratory confirmation for influenza virus infection and included population-based incidence rates with denominator data were included |
• Studies that, regardless of design, otherwise met criteria in Population, Exposure, and Outcomes (see rows below) • Additionally, only studies that had laboratory confirmation for influenza virus infection and included population-based incidence rates with denominator data were included |
• Observational studies with a comparator arm of non-pregnant patients with evidence of influenza virus infection meeting criteria in Population, Exposure, Comparison, and Outcomes (see rows below) • Ecologic studies, defined as studies that collected data at a group rather than at an individual level, or in which numerators or denominators were imputed or estimated |
• Comparative observational studies in humans (i.e., cohort, case-control, cross-sectional) meeting criteria in Population, Exposure, Comparison, and Outcomes (see rows below) |
| Exclusion criteria | • Editorials, commentaries, opinion pieces, narrative reviews, clinical practice guidelines, conference abstracts, or literature not in peer-reviewed journals • Studies reporting estimated incidence rates of influenza outcomes and excess burden of outcomes due to influenza using ecological approaches and statistical modeling |
• Studies that did not ascertain LCI outcomes either throughout at least one full influenza season in geographic settings with defined seasonality, or otherwise for at least one full year • Studies reporting estimated incidence rates of influenza outcomes and excess burden of outcomes due to influenza using ecological approaches and statistical modeling • Studies where influenza was not examined as a primary outcome, but rather as a co-infection in a study population identified on the basis of another infectious disease |
• Non- English articles • Studies on avian influenza A virus infection in humans |
• Non-English articles, editorials, commentaries, narrative reviews, clinical practice guidelines, conference abstracts or literature not in peer-reviewed journals • Case series and case reports (i.e., no comparison group of uninfected pregnant women) |
| Population | Pregnant women | Infants under six months of age | Pregnant and non-pregnant patients with evidence of LCI virus infection | Pregnant women |
| Exposure | LCI virus infection | LCI virus infection | Pregnancy | Pregnant women with clinical influenza disease and/or LCI virus infection |
| Comparison | N/A | N/A | No pregnancy | Pregnant women with no clinical influenza disease or LCI virus infection |
| Outcomes | • Incidence of LCI infection using serology • Incidence of symptomatic LCI • Incidence of LCI hospitalization • Incidence of LCI ICU admission • Incidence of LCI mortality • LCI disability-adjusted life years lost |
• Incidence of LCI infection • Incidence of LCI hospitalization • Incidence of LCI ICU admission • Incidence of LCI mortality |
Impact of pregnancy on risk of: • LCI community-acquired pneumonia • LCI hospitalization • LCI ICU admission • LCI-associated mechanical ventilator support • LCI mortality |
Impact of influenza during pregnancy on risk of: • Preterm birth (<37 weeks’ gestation) • Small-for-gestational-age birth • Fetal death (miscarriage or stillbirth) |
Abbreviations: ICU: intensive care unit; LCI: laboratory-confirmed influenza.
Table 3.
Main results from systematic evidence reviews carried out by the WHO working group.
| Review | Results |
|---|---|
| A: Incidence of laboratory-confirmed influenza (LCI) outcomes among pregnant women [29] |
Incidence of LCI infection using serology (3 studies) • Lowest rate: 483 per 10,000 pregnancies (hospital-based; 1975–1977 influenza seasons) • Highest rate: 1097 per 10,000 pregnancies (hospital-based; 1993–1994 influenza seasons) • Modified GRADE assessment [35] of overall evidence quality: very low Incidence of symptomatic LCI (3 studies) • Lowest rate: 0.10 per 10,000 pregnancies (hospital/clinic-based; 2009 H1N1 pandemic) • Highest rate: 486 per 10,000 pregnanciesa(clinic-based; 2011 and 2012 influenza seasons) • Modified GRADE assessment [35] of overall evidence quality: very low Incidence of LCI hospitalization (4 studies) • Lowest rate: 0.04 per 10,000 pregnancies (2009 H1N1 pandemic) • Highest rate: 7.7 per 10,000 pregnancies (2009 H1N1 pandemic) • Modified GRADE assessment [35] of overall evidence quality: very low Incidence of LCI ICU admission (4 studies) • Lowest rate: 0.01 per 10,000 pregnancies (2009 H1N1 pandemic) • Highest rate: 6.8 per 10,000 pregnancies (2009 H1N1 pandemic) • Modified GRADE assessment [35] of overall evidence quality: very low Incidence of LCI mortality (4 studies) • Lowest rate: 0.003 per 10,000 pregnancies (hospital/clinic-based; 2009 H1N1 pandemic) • Highest rate: 0.69 per 10,000 pregnancies (hospital-based; 2009 H1N1 pandemic) • Modified GRADE assessment [35] of overall evidence quality: very low Summary: • Limited number of studies reporting each of the outcomes (no studies reported LCI disability-adjusted life years lost) • Meta-analysis of individual study incidence rates was not possible due to high heterogeneity |
| B: Incidence of LCI outcomes among infants under six months of age [30] |
Incidence of LCI infection (6 studies) • Lowest rate: 0.75 per 100 person-years <6 months (2011–2014 influenza seasons) • Highest rate: 35 per 100 person-years <6 months (community-based active household surveillance; 2009 H1N1 pandemic and 2010–2011 infleunza season) • Modified GRADE assessment [35] of overall evidence quality: low Incidence of LCI hospitalization (23 studies)b • Seasonal influenza: – Lowest rate: 0 per 10,000 infants <6 months – Highest rate: 250 per 10,000 infants <6 months (2010–2012 influenza seasons) • H1N1pdm09 influenza: – Lowest rate: 20.2 per 10,000 infants <6 months – Highest rate: 259 per 10,000 infants <6 months • Modified GRADE assessment [35] of overall evidence quality: moderate (across all influenza seasons) Incidence of LCI ICU admission (7 studies) • Lowest rate: 0 per 10,000 infants <6 months (2000–2001 influenza season) • Highest rate: 3.5 per 10,000 infants <6 months (2001–2004 influenza seasons) • Modified GRADE assessment [35] of overall evidence quality: low Incidence of LCI mortality (9 studies) • Lowest rate: 0 per 100,000 infants <6 months • Highest rate: 5 per 100,000 infants <6 months (hospital-based; 2009 H1N1 pandemic) • Modified GRADE assessment [35] of overall evidence quality: low Summary: • Limited number of studies reporting each of the outcomes, particularly few from non-US settings • Meta-analysis of individual study incidence rates was not possible due to high heterogeneity • There was wide variation in incidence rates for LCI hospitalization • Incidence rates of LCI death among infants under six months were based on few cases (e.g., six out of nine studies did not ascertain any LCI deaths, and two additional studies documented 2–3 LCI deaths) |
| C: Pregnancy as a risk factor for severe outcomes from influenza virus infection [31] |
Risk of community-acquired pneumonia in pregnant vs. non-pregnant patients with influenza (8 studies) • Pooled OR: 1.80 (95% CI: 0.72–4.49) – Among 3 studies that used women of reproductive age as the comparator: Pooled OR: 1.09 (95% CI: 0.29–4.08) • GRADE assessment [26] of overall evidence quality: very low Risk of hospitalization in pregnant vs. non-pregnant patients with influenza (13 studies) • Pooled OR: 2.44 (95% CI: 1.22–4.87) – Among 2 studies that used women of reproductive age as the comparator: Pooled OR: 3.28 (95% CI: 0.52–20.6) • GRADE assessment [26] of overall evidence quality: moderate Risk of ICU admission in pregnant vs. non-pregnant patients with influenza (47 studies) • Pooled OR: 0.85 (95% CI: 0.62–1.17) – Among10 studies that used women of reproductive age as the comparator: Pooled OR: 0.51 (95% CI: 0.42–0.62) • GRADE assessment [26] of overall evidence quality: very low Risk of mechanical ventilator support in pregnant vs. non-pregnant patients with influenza (26 studies) • Pooled OR: 1.21 (95% CI: 0.70–2.08) – Among 8 studies that used women of reproductive age as the comparator: Pooled OR: 0.82 (95% CI: 0.40–1.67) • GRADE assessment [26] of overall evidence quality: very low Risk of mortality in pregnant vs. non-pregnant patients with influenza (94 studies) • Pooled OR: 1.04 (95% CI: 0.81–1.33) – Among 18 studies that used women of reproductive age as the comparator: Pooled OR: 1.02 (95% CI: 0.57–1.84) • GRADE assessment [26] of overall evidence quality: very low Summary: • Quality of evidence across studies was poor, with significant heterogeneity between studies for most analyses • Influenza during pregnancy resulted in a higher risk of hospitalization than influenza infection in non-pregnant patients, but among hospitalized patients, the risk of mortality following influenza was similar in pregnant and non-pregnant individuals • Whether the reason for hospitalization was severity of illness versus precautionary admission for obstetrical reasons is unclear |
| D: Maternal influenza virus infection and adverse birth outcomes [32] |
Maternal influenza during pregnancy and risk of preterm birth (16 studies) • Across all studies, individual-study relative effect estimates ranged from 0.40 (95% CI: 0.11–1.41)to 4.08 (95% CI: 3.56–4.67) – Among the 2 highest-quality studies of seasonal influenza, individual-study relative effect estimates ranged from 0.82 (95% CI: 0.55–1.22) for mild-to-moderate illness, to 1.20 (95% CI: 0.72–2.01) for severe illness – Among the 5 highest-quality studies of H1N1pdm09 influenza, individual-study relative effect estimates ranged from 1.03 (95% CI: 0.85–1.25) for mild-to-moderate illness, to 4.00 (95% CI: 2.71–5.90) for severe illness • Modified GRADE assessment [35] of overall evidence quality: very low Maternal influenza during pregnancy and risk of small-for-gestational-age birth (5 studies) • Pooled OR: 1.24 (95% CI: 0.96–1.59) – Among 3 estimates of seasonal influenza, individual-study relative effect estimates ranged from 0.71 (95% CI: 0.23–2.20) to 1.66 (95% CI: 1.11–2.49) – Among 3 estimates of H1N1pdm09 influenza, individual-study relative effect estimates ranged from 0.98 (95% CI: 0.47–2.05) to 1.14 (95% CI: 0.59–2.22) • Modified GRADE assessment [35] of overall evidence quality: low Maternal influenza during pregnancy and risk of fetal death (10 studies) • Two fetal death studies were of sufficient quality and size to permit meaningful interpretation – Both reported an increased risk of fetal death following maternal H1N1pdm09 influenza: HR = 1.91 (95% CI: 1.07–3.41) for mild-to-moderate illness, and RR = 4.2 (95% CI: 1.42–12.4) for severe illness • Modified GRADE assessment [35] of overall evidence quality: very low Summary: • Meta-analysis of individual study effect estimates was not possible for preterm birth or fetal death due to high heterogeneity • Several of the highest-quality studies suggested an association between severe influenza illness during the 2009 H1N1 pandemic and risk of preterm birth and fetal death • An important limitation of this literature concerns the potential for different clinical thresholds for influenza testing/diagnosis/hospitalization among women with high-risk pregnancies or suspected poor pregnancy outcome, compared with women with low-risk pregnancies. Such differential misclassification of the exposure by outcome would be expected to inflate the magnitude of effect estimates, making influenza disease appear more strongly associated with poor outcome |
Abbreviations: CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HR: hazard ratio; ICU: intensive care unit; LCI: laboratory-confirmed influenza; OR: odds ratio; RR: risk ratio.
Based on incidence in the HIV-uninfected and HIV-infected placebo groups combined.
Four studies reporting LCI hospitalization provided estimates for both seasonal influenza and for H1N1pdm09 influenza.
2.1. Review A: Incidence of laboratory-confirmed influenza (LCI) outcomes among pregnant women [29]
This systematic review of studies published up to February 20, 2015 sought to establish evidence-based incidence estimates of laboratory-confirmed influenza (LCI) outcomes among pregnant women (see Table 1 for list of outcomes and Table 2 for characteristics of included studies) [29]. Among the nine studies that met the inclusion criteria, six reported data exclusively from the 2009 H1N1 pandemic period, two reported exclusively from seasonal influenza epidemics, and one study covered both. Almost all (8/9 studies) were from high-income countries (Table 2). Meta-analysis of incidence rates was not performed due to substantial heterogeneity and the small number of studies reporting any given outcome. Incidence estimates for symptomatic LCI infection ranged from 0.10 per 10,000 pregnant women (95% confidence interval [CI]: 0.07–0.14) to 486 per 10,000 pregnant women (95% CI: 375–630), the latter from the HIV-uninfected and HIV-infected placebo arms, combined, of an RCT of influenza immunization in pregnant women in South Africa [16]. Estimates of LCI hospitalization and intensive care unit (ICU) admission were similarly variable (Table 3), likely due to differences in surveillance methodologies and influenza season variability. LCI mortality in pregnant women was reported by only four studies, all of which were case series conducted during the 2009 H1N1 pandemic. Rates ranged from 0.003 per 10,000 pregnant women (95% CI: 0.000–0.021) to 0.69 per 10,000 pregnant women (95% CI: 0.26–1.51), and were based on very low numerator counts (between one and eight deaths with LCI).
Table 2.
Descriptive characteristics of studies included in systematic evidence reviews carried out by the WHO working group.
| Review | Total number of included studies | World Bank country income groupa | World Health Organization regionb | Type of influenza |
|---|---|---|---|---|
| A: Incidence of laboratory-confirmed influenza (LCI) outcomes among pregnant women [29] | 9 | 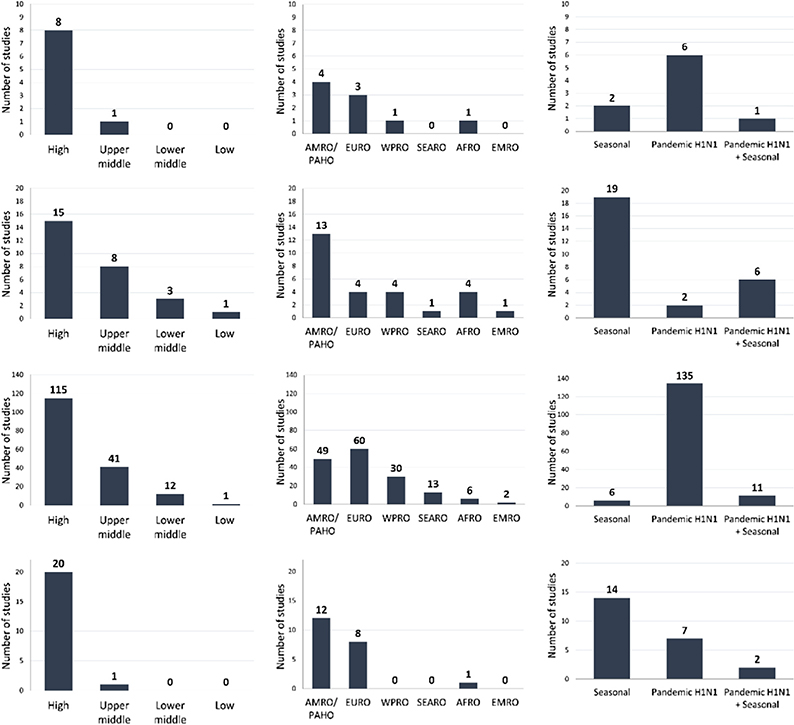 |
||
| B: Incidence of LCI outcomes among infants under six months of age [30] | 27 | |||
| C: Pregnancy as a risk factor for severe outcomes from influenza virus infection [31] | 152c,d | |||
| D: Maternal influenza virus infection and adverse birth outcomes [32] | 21 | |||
Abbreviations: AFRO: WHO Regional Office for Africa; AMRO/PAHO: WHO Regional Office for the Americas/Pan American Health Organization; EMRO: WHO Regional Office for the Eastern Mediterranean; EURO: WHO Regional Office for Europe; SEARO: WHO Regional Office for South-East Asia; WPRO: WHO Regional Office for the Western Pacific; LCI: laboratory-confirmed influenza.
World Bank. World Development Indicators. Accessed: 10 Jan 2017. Available at: http://data.worldbank.org/data-catalog/world-development-indicators
World Health Organization. WHO regional offices. Accessed: 10 Jan 2017. Available at: http://www.who.int/about/regions/en/
9 countries were not specified in one of the included studies and, therefore, do not appear in the first two panels [87].
One of the included studies was a global pooled analysis of 19 countries, which have been individually included in the first two panels [88].
The primary rationale for excluding other commonly-referenced studies reporting influenza outcomes among pregnant women was the lack of laboratory confirmation, which was a pre-specified inclusion criterion for the review. As a general observation, the excluded studies of influenza-associated outcomes in pregnant women tended to report rates that were higher than those described by the studies included in our review, likely explained by the use of a broad group of non-specific cardiopulmonary diagnostic codes from health administrative databases, which could overestimate influenza incidence due to misclassification of other influenza-like illnesses as influenza. For example, a Canadian study estimated that one in 1000 healthy pregnant women had seasonal influenza-associated hospitalizations per year, based on administrative diagnostic codes [36]; this rate was higher than those reported by the studies in our review, all of which described laboratory-confirmed pandemic influenza (Table 3). Two additional highly-cited studies [37,38] not only used administrative diagnostic codes but also focused on trimester-specific incidence alone, precluding direct comparisons with the studies in our review. We excluded studies reporting estimated incidence rates of influenza outcomes and excess burden of outcomes due to influenza [39] using ecological approaches and statistical modeling due to the absence of individual-level laboratory-confirmed data. Typically, these studies used regression models to analyze data over multiple seasons using influenza surveillance or administrative databases. This approach has most commonly been used to estimate excess mortality [40] and respiratory and circulatory hospitalizations in the general US population [41]. One such study of pregnant and non-pregnant women of childbearing age was conducted in South Africa [42], where baseline maternal mortality rates are higher than in high-income countries. Between 1999 and 2009, the mean annual seasonal influenza-associated mortality rate was 12.6 per 100,000 person-years (95% CI: 7.2–18) among pregnant women, while the estimated H1N1pdm09 influenza virus-associated mortality rate was 19.3 per 100,000 person-years (95% CI: 11–27.6) [42], higher than the rates reported by studies in our review.
2.2. Review B: Incidence of LCI outcomes among infants under six months of age [30]
As the strategy of maternal influenza immunization is also expected to reduce influenza among infants under six months of age, we sought to determine incidence rates of LCI outcomes (Table 1) specifically within this age group [30]. Among 27 primary studies published up to April 19, 2017 that met inclusion criteria, 23 assessed incidence of LCI hospitalization. However, meta-analysis of study-specific rates was not possible due to high statistical and methodological heterogeneity. Of the 27 studies, 19 exclusively covered periods of seasonal influenza, two covered the 2009 H1N1 pandemic period, and six covered both (Table 2). Most studies were from high-income (55%) or upper-middle income countries (30%), and no studies originated from low-income countries. Among US studies, the reported incidence of LCI hospitalization for infants under six months from influenza seasons between 2000 and 2012 ranged from 9.3 (95% CI: 7.9–10.9) to 91.2 (95% CI: 67.0–145) per 10,000 infants for seasonal influenza, while the estimate for H1N1pdm09 influenza was 20.2 per 10,000 infants (95% CI: 18.1–22.5). Although two non-US studies did not ascertain any hospitalizations of infants under six months in a few time periods (i.e., an incidence rate of zero [8,43]), most reported rates of LCI hospitalization for seasonal influenza that ranged between 6.2 (95% CI: 3.1–9.3) and 73.0 (95% CI: 40.6–122) per 10,000 infants under six months, with the exception of an estimated rate of 250 per 10,000 infants (95% CI: 213–292) in one study from China [44]. Of the nine studies that proposed to capture LCI deaths (Table 3), only three ascertained any fatal cases among infants under six months. The most precise rate, from the 2003–2004 influenza season, estimated LCI mortality to be 0.88 per 100,000 infants under six months of age (95% CI: 0.52–1.39), based on 18 fatal cases with concurrent laboratory detection of influenza virus [10]. Although the cases were identified through an enhanced national surveillance effort in the United States, some were likely missed as laboratory testing is not always performed [10]. The highest rate was reported from a study of LCI deaths in Buenos Aires during the 2009 H1N1 pandemic; two deaths of infants under six months were recorded, with a corresponding LCI mortality rate of five per 100,000 infants (95% CI: 0.82–16.1) [45].
Most exclusions of primary studies from our review, that may have been included by other evidence reviews [46], were due to non-reporting of data specific to infants under six months of age. We also excluded studies using the proportion-positive method of estimating influenza burden. These studies conduct surveillance for a clinical syndromes such as respiratory hospitalization, often aggregated over many sites, and report the proportion of samples that test positive for influenza. This method is sufficiently different from the primary design in our review (i.e., enumeration of individual laboratory-confirmed cases in a defined population) that a direct comparison of rates was not appropriate.
2.3. Review C: Pregnancy as a risk factor for severe outcomes from influenza virus infection [31]
The aim of this systematic review of published studies up to April 25, 2014 was to quantify the risk of severe influenza outcomes, as listed in Table 1, among pregnant women with LCI illness relative to other population sub-groups with LCI illness [31]. Only 4% of all studies exclusively covered seasonal influenza observational periods, and the majority of studies were from high-income countries (76%; Table 2). Most individual-level studies enrolled hospitalized subjects (118/142; 83.1%). Pregnant women had a higher risk of LCI hospitalization than non-pregnant patients (pooled odds ratio [OR] 2.44; 95% CI: 1.22–4.87), but among those hospitalized, there was no increased risk for more severe outcomes such as LCI ICU admission, mechanical ventilation, or death (Table 3). A sub-group analysis limited to studies using a comparison group of non-pregnant women of reproductive age found the risk of ICU admission to be significantly lower in pregnant women (pooled OR: 0.51, 95% CI: 0.42–0.62), suggesting that LCI hospitalization of pregnant women may be partly for precautionary reasons, given their lower likelihood of ICU admission. There was also no significant difference in LCI mortality of pregnant women once hospitalized (83/94 studies reporting mortality only included hospitalized LCI deaths). It is unclear whether the lack of association between pregnancy and severe LCI outcomes reflects pregnancy conferring similarly increased risk as other prevalent conditions in the comparison groups (e.g., cardiorespiratory disease, obesity, or older age), or whether it was a result of a lower threshold for hospitalization of pregnant women compared with other high-risk groups. Follow-up in the included studies was not initiated before contact with the healthcare system and studies did not account for illness severity or co-morbidity.
The review identified important discrepancies between results from individual-level studies and ecologic studies. The latter generally reported a higher risk of influenza-associated death and ICU admission for pregnant women compared with non-pregnant women [31]. Study designs which estimate risk based on group-level data are prone to a number of biases [39] and, for this reason, were excluded from the systematic review. Other commonly-cited studies were excluded from the review [37,38] as they defined influenza outcomes according to a broad group of non-specific cardiopulmonary diagnostic codes from health administrative databases during the influenza season without laboratory-confirmation.
2.4. Review D: Maternal influenza virus infection and adverse birth outcomes [32]
The aim of this systematic review of published studies up to December 5, 2014 was to evaluate the association between maternal influenza illness during pregnancy and adverse birth outcomes (Table 1) [32]. Among the 21 comparative studies identified, 20 used observational designs and originated from high-income countries, 14 reported exclusively on seasonal influenza periods, seven reported exclusively on the 2009 H1N1 pandemic, and two reported on both (Table 2). Individual-study estimates for preterm birth risk were highly variable (relative risks [RR] ranged from 0.4, 95% CI: 0.11–1.41 to 4.08, 95% CI: 3.56–4.67 [32]). However, when limited to only the highest-quality studies, two reported significantly increased preterm birth risk (RR: 2.39, 95% CI: 1.64–3.49 [47] and RR: 4.0, 95% CI: 2.71–5.90 [48]) associated with severe H1N1pdm09 influenza illness requiring hospitalization, while no association was reported by the three studies assessing mild-to-moderate H1N1pdm09 influenza illness [49–51] nor by the two highest-quality studies of seasonal influenza [50,52]. There were no significant differences in small-for-gestational-age (SGA) birth between women with and without influenza virus infection during pregnancy (pooled OR: 1.24, 95% CI: 0.96–1.59) [32]. Although fetal death (i.e., miscarriage and/or stillbirth) was included as an outcome by 10 studies, no meta-analysis was possible due to high variability in fetal death definitions and quality. The two highest-quality studies reported a significantly increased risk of fetal death following maternal H1N1pdm09 influenza disease (RR 1.91, 95% CI: 1.07–3.41 for mild-to-moderate disease [49] and 4.2, 95% CI: 1.42–12.4 for severe disease [48]). Vaccine RCTs can provide complementary evidence relating disease to non-specific clinical outcomes [53], which is useful to consider in this context given the low-quality, mixed evidence from observational studies. We included one placebo-controlled RCT [16] in our review, in which the risk for preterm birth computed from raw study data did not differ between treatment arms, though the trial was not powered for this secondary outcome.
As this review considered only comparative studies (i.e., comparing birth outcomes among women with and without influenza virus infection during pregnancy), case series studies were excluded. With the exception of a few individual studies [54–56], previous reviews showed that most of these case series studies reported higher than expected rates of pregnancy loss and preterm birth among infected pregnant women [57–59], especially series of hospitalized women [57,60–62]. Notwithstanding the limitations of case series [63], the existing descriptive studies of influenza disease during pregnancy are more geographically diverse and offer additional contextual information about the clinical course of influenza illness during pregnancy, prevalence of maternal comorbid conditions (e.g., asthma, HIV co-infection), and other sociocultural factors that may affect both treatment and prevention. Taken together, the findings from our review and the excluded descriptive studies agree that pregnant women with severe H1N1pdm09 influenza disease requiring hospitalization appeared to have an increased risk of preterm birth and fetal death, though the limited data preclude firm conclusion on the magnitude. We did not find convincing data that mild maternal influenza virus infection was associated with any of the adverse birth outcomes mentioned above.
3. Results from other evidence assembled by the WHO working group
3.1. Vaccine efficacy against LCI infection in pregnant women and newborns
Three RCTs of influenza immunization during pregnancy were, or became, available for review by the working group, originating from Bangladesh [15], South Africa [16] and Mali [27]. A fourth trial from Nepal [28] was published as this report was being prepared, and a Cochrane review [64] is currently being updated to incorporate this new evidence. Table 4 presents vaccine efficacy (VE) estimates for prevention of LCI infection in mothers and infants up to six months of age from the RCTs. VE against LCI infection among pregnant women over six months of follow-up was 70% (95% CI: 42–86) in Mali [27] and 50% in South Africa (95% CI: 14–71) [16], but not statistically significant in the recent RCT from Nepal (VE: 31% for all LCI in the combined cohort, 95% CI: −10 to 56) [28]. Among infants followed from birth to six months of age, VE estimates ranged from 30% (95% CI: 5–48) in Nepal [28] to 63% (95% CI: 5–85) in Bangladesh [15].
Table 4.
Vaccine efficacy (VE) results from randomized controlled trials (RCT) of influenza immunization during pregnancy.
| Location | Bangladesh | South Africaa | Mali | Nepal |
|---|---|---|---|---|
| Reference | Zaman et al. [15] | Madhi et al. [16] | Tapia et al. [27] | Steinhoff et al. [28] |
| NEJM 2008; 359:1555–64. | NEJM 2014; 371:918–31. | Lancet ID 2016; 16:1026–35. | Lancet ID 2017; pii: S1473-3099(17) 30252–9. | |
| [Epub: 2008 Sep17] | [Epub: 2014 Sep 4] | [Epub: 2016 May 31] | [Epub: 2017 May 15] | |
| Type of control | Active (pneumococcal polysaccharide vaccine) | Placebo | Active (quadrivalent meningococcal conjugate vaccine) | Placebo |
| Population | 340 women in the third trimester of pregnancy | 2116 HIV-negative women in the second or third trimester of pregnancy (20–36 weeks of gestation) | 4193 women in the third trimester of pregnancy (≥28 weeks of gestation) | 3693 women in the second or third trimester of pregnancy (17–34 weeks of gestation) |
| Attack rate of symptomatic LCI illness in mothers | ||||
| Vaccine arm | –b | 1.8%c | 0.5%c | 1.7%c |
| Control arm | –b | 3.6%c | 1.9%c | 2.4%c |
| VE against LCI virus illness in mothers | –b | 50% (95% CI: 14–71)c | 70% (95% CI: 42–86)c | 31% (95% CI: −10 to 56)c 23% (95% CI: −43 to 58)d |
| Attack rate of symptomatic LCI illness in infantsc | ||||
| Vaccine arm | 0.7% | 1.9% | 2.5% | 4.1% |
| Control arm | 1.8% | 3.6% | 3.8% | 5.8% |
| VE against LCI virus illness in infants | 63% (95% CI: 5–85) | 49% (95% CI: 12–70) | 33% (95% CI: 4–54) | 30% (95% CI: 5–48) |
Abbreviations: CI: confidence interval; LCI: laboratory-confirmed influenza; RCT: randomized controlled trial; VE: vaccine efficacy.
Summary and results refer to non-HIV infected women.
Outcome measured in mothers was not LCI illness, but influenza-like illness defined as any respiratory illness with fever.
Measured up to 24 weeks post-delivery.
Measured up to the delivery.
3.2. Influenza vaccination and birth outcomes
The majority of studies of maternal influenza immunization and birth outcomes are observational epidemiologic studies and originate from high-income countries. Two published systematic reviews on this subject were available when the WHO working group was convened in 2014 [18,65]. One review included 27 comparative studies assessing the association between maternal influenza immunization and preterm birth and fetal death up to April 2014 [18]. No safety concerns were identified, as none of the studies reported an increased association between influenza vaccine receipt and adverse outcomes. To the contrary, studies generally reported either no association or significant risk reductions for preterm birth (ranging from 14% to 37%) and late fetal death (ranging from 34% to 56%), which the authors cautioned could be attributed to important methodological limitations identified in many of the primary studies [18]. Another review of 19 studies published up to March 2014 [65] also concluded that there was no evidence to suggest any adverse effect of influenza vaccination during pregnancy on congenital anomalies or fetal death, but noted the limited number and quality of available studies.
Two methodological evaluations [19,66] and a WHO expert consultation [22] recently explored issues related to the interpretation of observational studies reporting beneficial effects of maternal influenza immunization on adverse birth outcomes. In brief, existing observational studies have numerous limitations in study design and analytical methods [19]. The most compelling explanation for any large protective effect of influenza vaccination on adverse birth outcomes in observational studies is residual confounding due to preferential selection of vaccination by pregnant women with a more favorable health profile who, in turn, may be less likely to have an adverse birth outcome. This phenomenon has been well documented in observational studies of influenza vaccination in elderly adults [67,68]. Moreover, one study that modeled data using a range of plausible scenarios for rates of influenza illness during pregnancy, vaccine effectiveness, and vaccine uptake, showed that the protective benefits of influenza vaccination for an outcome such as preterm birth would be expected to be very small and, thus, difficult to detect [66]. Taken together with what is known about the multi-factorial etiology of most adverse birth outcomes, it seems unlikely that influenza immunization would produce an improvement in birth outcomes to the magnitude observed by some observational studies [22].
RCT results with respect to birth outcomes have also been mixed. In secondary analyses among a subset of 116 infants born during the influenza season, the trial conducted in Bangladesh found significantly higher mean birth weight (190 g, 95% CI: 93–78) and a lower percentage of SGA infants (37% reduction, 95% CI: 0–60) born to influenza-vaccinated women compared with infants born to women vaccinated with pneumococcal polysaccharide vaccine [69]. The recently-published trial from Nepal, the only RCT to include low birth weight (defined as <2500 g) as a primary outcome [28], also found an increase in mean birth weight (influenza vaccine arm: 2803 g, placebo arm: 2761 g; 42-g increase, 95% CI: 8–76) corresponding to a 15% reduction in low birth weight (95% CI: 3–25) among infants born to influenza-vaccinated mothers compared with infants in the placebo group. However, there were no differences in SGA or preterm birth. In contrast, the two trials from Africa did not detect any differences in preterm birth [16], low birth weight [16,27], or mean birth weight [16,27] between treatment groups overall or when assessed by maternal influenza infection status [70] or birth during the influenza season [27]. It remains unclear whether the divergent findings resulted from protocol differences [71], or due to geographical differences in influenza biology and/or baseline maternal-newborn health status.
4. Interpretation
Across the evidence compiled by this working group, the following general observations can be made:
Apart from the four influenza vaccine RCTs, which were conducted in Bangladesh [15], South Africa [16], Mali [27], and Nepal [28], LMICs were substantially under-represented among the primary publications included in each of the four WHO-initiated reviews, with much of the data originating from the United States in particular;
With the exception of LCI hospitalization among infants under six months of age, limited influenza incidence data were available for seasons outside of the 2009 H1N1 pandemic period;
A low number of studies, combined with high clinical, design and statistical heterogeneity, precluded quantitative meta-analysis in three of the four reviews initiated by the working group; and
The overall quality of evidence in the working group-initiated systematic reviews, as assessed using the GRADE approach, was generally considered low to very low for most outcomes (Table 3).
WHO has noted that policy-makers from LMICs are likely to place higher value on vaccines with demonstrated impact on severe influenza disease [72]. Thus, incidence estimates of influenza disease are essential for informing vaccine policy, investment decisions, and quantifying the potential impact of influenza vaccination programs on important public health outcomes [73,74]. Our systematic reviews of LCI incidence rates in pregnant women [29] and infants under six months [30] found a limited number of studies, a wide range of estimates in incidence rates, and limited representation from LMICs where disease burden and severity may differ due to differences in influenza epidemiology, access to care, testing resources and practices, and background prevalence of underlying comorbid diseases such as HIV infection. A 2015 Bill and Melinda Gates Foundation convening of experts in maternal immunization reached a similar conclusion concerning the lack of robust influenza data to support financial and policy decisions, particularly for LMICs [75]. Given the predominance of high-income country studies, study results may be affected by access to high-quality medical care. Estimates from a systematic review of influenza in young children indicate a higher burden among infants under one year of age in LMICs compared with high-income countries [46]. The lack of more definitive baseline influenza disease burden estimates, including vaccine-preventable disease incidence against severe clinical outcomes such as pneumonia or respiratory disease mortality [53], poses challenges for estimating the public health impact of incorporating maternal influenza immunization into national programs.
Similarly, evidence on the association between maternal influenza virus infection and adverse birth outcomes is necessary for clarifying expectations for improvement in these outcomes following influenza immunization [19,76]. Despite a small number of high-quality studies suggesting an association between severe H1N1pdm09 influenza disease and preterm birth and fetal death, the magnitude of increased risk is unclear [32]. It also remains unclear whether adverse events were specific to H1N1pdm09 virus exposure or more generally to influenza virus exposure. Moreover, the observational evidence that maternal influenza immunization reduces the risk of such adverse birth outcomes is inconsistent and of limited quality. Recent examinations of the plausibility of this phenomenon recommend that studies suggesting that maternal vaccination has a substantial beneficial effect on adverse birth outcomes such as preterm birth or fetal death should be interpreted with caution [19,22,66]. The clinical significance of the 42-g increase in birth weight found in the recent Nepal study [28] deserves further investigation. Despite uncertain data on vaccine impact on adverse birth outcomes, there is nevertheless strong and consistent evidence that influenza immunization during pregnancy prevents influenza virus infection both in pregnant women and their infants, and thus should continue to serve as a primary guide for vaccine policy and investment strategies.
5. Recommendations
This WHO working group was initiated to review and interpret the evidence base used for global policy and funding decisions concerning maternal influenza immunization programs. The working group synthesized the current evidence on this topic (Table 3) and identified numerous evidence gaps (Table 5). Specific recommendations relating to each of the four systematic reviews carried out by the working group can be found in Table 5. Finally, we offer the following general research recommendations:
Best practices standards for the design, conduct, analysis, and reporting of influenza surveillance among pregnant women and infants under six months, along with uniformly-defined outcome measures and safety measures (e.g., recent Brighton Collaboration and STROBE recommendations [77,78]), would help improve study quality and comparability.
Global standards for influenza surveillance recommend specific age groupings for reporting of data which include 0 to <2 years [79]. Recommendations to report disease data for infants under six months of age would facilitate systematic reviews and comparisons of results most relevant to maternal influenza immunization strategies.
More geographically diverse and higher-quality studies are needed. The literature is dominated by case reports and case series published during the 2009 H1N1 pandemic. While these are important to alert medical and public health professionals about groups with unexpected risks, they are extremely limited in their ability to quantify disease risk or incidence, especially outside of pandemic time periods. Well-designed, multi-year comparative studies (disease risk) or population-based surveillance with systematic case ascertainment (disease incidence) are required, particularly from LMICs.
As data on LCI outcomes from resource-poor countries may exist but remain unpublished [46], and many studies of LCI outcomes among infants have not reported data on infants under six months of age separately, efforts should be directed toward aggregating these existing data before embarking on new studies in this age group.
Phase IV clinical trials conducted to inform WHO policy recommendations should consider more severe illness endpoints, as these are the most important outcomes for many decision makers in LMICs [72]. Vaccine probe studies that include severe illness endpoints among pregnant women and young infants as well as measurement of all potentially important outcomes regardless of influenza confirmation (e.g., all-cause acute lower respiratory tract infection hospitalization [80]) would be especially useful to quantify the public health benefit of influenza vaccine programs [53,81]. However, we acknowledge that variable vaccine effectiveness and the large sample size required for studies of severe outcomes could make this approach unfeasible for logistic and financial reasons.
Given limitations in the evidence base, we were unable to generate definitive pooled estimates for most of the key parameters pursued by this working group. Moreover, expected high heterogeneity across influenza seasons, geographies, and populations would suggest that no single estimate of each parameter will sufficiently inform impact and economic models. Our evidence-based reviews can, however, be used to provide a range of inputs for future impact and economic models, which should employ a probabilistic approach to generate outputs, tailored to the different context-specific geographical and socioeconomic factors of interest to policy makers.
Table 5.
Summary of remaining gaps in the evidence and WHO working group recommendations for future research.
| Review | Evidence gaps and recommendations |
|---|---|
| A: Incidence of laboratory-confirmed influenza (LCI) outcomes among pregnant women [29] |
Evidence gaps: • Few studies describe incidence rates of LCI outcomes in pregnant women, particularly from seasonal epidemics • No studies were identified from low-income countries Recommendations: • Future incidence studies should be conducted using consistent, high-quality surveillance methods • Studies from LMICs and during seasonal epidemics should be a priority |
| B: Incidence of LCI outcomes among infants under six months of age [30] |
Evidence gaps: • Limited number of incidence studies in this policy-relevant age group • Limited number of studies were identified from LMICs • Few studies evaluated LCI mortality, and few events were ascertained in studies assessing this outcome Recommendations: • High-quality population-based surveillance of LCI outcomes in infants under six months should be conducted, particularly in LMICs |
| C: Pregnancy as a risk factor for severe outcomes from influenza virus infection [31] |
Evidence gaps: • Limited evidence from influenza seaons other than 2009 H1N1 pandemic period • Limited evidence from LMICs • As a whole, current studies could not distinguish between whether pregnant women with influenza were hospitalized due to disease severity or for precautionary reasons related to concerns for fetal well-being Recommendations: • A longitudinal study where a community-derived cohort of pregnant and non-pregnant women of reproductive age with LCI influenza infection are prospectively followed for severe LCI outcomes |
| D: Maternal influenza virus infection and adverse birth outcomes [32] |
Evidence gaps: • Evidence is imbalanced with RCTs coming from LMICs, while observational studies are from high-income countries • Lack of evidence on the possible interaction between stage of pregnancy and influenza infection on adverse birth outcomes • Limited evidence of the impact of influenza other than H1N1pdm09 influenza on birth outcomes • Limited evidence on the impact of influenza serotype on fetal death Recommendations: • Future high-quality studies should be conducted to assess the impact of maternal seasonal influenza infection during pregnancy on birth outcomes – A multi-site/multi-national study should be considered to be able to identify less common adverse birth outcomes, such as fetal death or congenital anomalies – Ideally, LCI illness should be used as the study exposure, with ascertainment of the gestational timing of the infection |
Abbreviations: LCI: laboratory-confirmed influenza; LMICs: low- and middle-income countries.
6. Conclusion
This working group conducted an extensive review of disease burden related to maternal influenza immunization and concluded that available data are currently insufficient to estimate the potential impact of maternal immunization programs on severe influenza illness, particularly from LMICs. However, it is worth noting that there are broader potential benefits of an influenza vaccine program in pregnant women not considered by the working group. Influenza epidemics result in substantial economic costs and prevention of influenza illness can decrease ambulatory care visits, inappropriate antibiotic prescribing, and work/school absenteeism [12]. We did not attempt to quantify these additional impacts of influenza but they are important considerations for policy makers reviewing influenza programs. For most LMICs, maternal influenza immunization programs are the most programmatically feasible given the relative strength of antenatal care as an immunization platform [82]. Moreover, integration of immunization and health care services can improve healthcare delivery overall [83,84], suggesting that a Gavi investment in maternal immunization may have additional benefits to antenatal care overall [24]. Lastly, countries that have systems to regulate, procure, and distribute seasonal influenza vaccines will be better able to respond to a future influenza pandemic [85]. There are many potential benefits to a maternal influenza immunization program and more effort is needed to quantify their full public health value to inform vaccine policy and investment decisions.
Supplementary Material
Acknowledgments
The WHO taskforce to evaluate influenza data to inform vaccine impact and economic modeling had the following additional members: Simon Cauchemez (Institute Pasteur, France), Nathorn Chaiyakunapruk (Monash University, Malaysia), Brenda Coleman (University of Toronto, Canada), Michael Kramer (McGill University, Canada), Anand Krishnan (All India Institute of Medical Sciences), and Yot Teerawattananon (Health Intervention and Technology Assessment Program, Thailand). The authors thank them for their contributions to the Taskforce. The authors also thank Chiara Gerardi and Marc Perut of WHO for administrative and technical support. We are grateful to Ashley Charbonneau (Children’s Hospital of Eastern Ontario Research Institute) for assistance producing tables and figures and to Lindsay Wilson (Ottawa Hospital Research Institute) for editorial and formatting assistance for this manuscript. The authors acknowledge the contributions of the Centers for Disease Control and Prevention (CDC), which provides financial support to the World Health Organization Initiative for Vaccine Research (U50 CK000431).
Potential conflicts of interest:
Deshayne B. Fell: Has received travel support from WHO to attend WHO meetings.
Eduardo Azziz-Baumgartner: None to declare.
Michael G. Baker: None to declare.
Maneesh Batra: None to declare.
Julien Beauté: None to declare.
Philippe Beutels: A university chair in CHERMID at the University of Antwerp was supported by a (unrestricted) gift from Pfizer (2009–2016) and GSK (since 2016). There is no connection between either Pfizer, GSK or the research of the chair holder (Niel Hens) and this article.
Niranjan Bhat: None to declare.
Zulfiqar A. Bhutta: None to declare.
Cheryl Cohen: Has received travel support from WHO to attend WHO meetings. Has received travel support from Parexel to attend meetings. Has received grant support from Sanofi.
Bremen De Mucio: None to declare.
Bradford D. Gessner: For work on this manuscript, BDG was employed by AMP, which receives or received during the past 2 years grant support from Hilleman Laboratories, Merck, GSK, Pfizer, and Sanofi-Pasteur; none of these grants were for work on influenza.
Michael G. Gravett: None to declare.
Mark A. Katz: None to declare.
Marian Knight: Has received travel support from WHO to attend a WHO meeting, and has received grant support from UK National Institute for Health Research for a surveillance study of seasonal influenza in pregnancy.
Vernon J. Lee: Has received travel support from WHO to attend WHO meetings.
Mark Loeb: Has received research grants from Sanofi and Sequris related to influenza vaccines.
Johannes M. Luteijn: None to declare.
Helen Marshall: Investigator on clinical vaccine trials sponsored by Industry. Institution receives grant funding for Investigator initiated studies from Industry including GlaxoSmithKline, Pfizer, Novavax and Sequiris.
Harish Nair: Has received research support from WHO to estimate the influenza disease burden in children.
Rehana A. Salam: None to declare.
David A. Savitz: Has received support from PATH to evaluate methodologies of studies assessing influenza vaccine association with adverse birth events.
Suzanne Jacob Serruya: None to declare.
Becky Skidmore: Was employed as an independent consultant for work on this project.
Kevin Pottie: None to declare.
Justin R. Ortiz: None to declare.
Footnotes
Disclaimer
Julien Beauté is an employee of the European Centre for Disease Prevention and Control. Justin R. Ortiz is an employee of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the European Centre for Disease Prevention and Control, the World Health Organization, or the United States Centers for Disease Control and Prevention.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.08.037.
References
- [1].Centers for Disease Control. Influenza. In: Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiol. Prev. Vaccine-Preventable Dis. Washington, D.C.: Public Health Foundation; 2012. [Google Scholar]
- [2].Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008;121:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Steinhoff MC. Epidemiology and Prevention of Influenza. In: Nelson K, Masters Williams C, editors. Infect. Dis. Epidemiol. Theory Pract.. Sudbury, MA: Jones and Bartlett Publishers; 2007. p. 577–600. [Google Scholar]
- [4].Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poehling KA, Edwards KM, Griffin MR, Szilagyi PG, Staat MA, Iwane MK, et al. The burden of influenza in young children, 2004–2009. Pediatrics 2013;131:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006;355:31–40. [DOI] [PubMed] [Google Scholar]
- [7].Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225–31. [DOI] [PubMed] [Google Scholar]
- [8].Montes M, Vicente D, Perez-Yarza EG, Cilla G, Perez-Trallero E. Influenza-related hospitalisations among children aged less than 5 years old in the Basque Country, Spain: A 3-year study (July 2001–June 2004). Vaccine. 2005;23:4302–6. [DOI] [PubMed] [Google Scholar]
- [9].Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000;342:232–9. [DOI] [PubMed] [Google Scholar]
- [10].Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005;353:2559–67. [DOI] [PubMed] [Google Scholar]
- [11].Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59:1–62. [PubMed] [Google Scholar]
- [12].World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012;47:461–76. [PubMed] [Google Scholar]
- [13].Ortiz JR, Neuzil KM, Ahonkhai VI, Gellin BG, Salisbury DM, Read JS, et al. Translating vaccine policy into action: a report from the Bill & Melinda Gates Foundation Consultation on the prevention of maternal and early infant influenza in resource-limited settings. Vaccine 2012;30:7134–40. [DOI] [PubMed] [Google Scholar]
- [14].Kay AW, Blish CA. Immunogenicity and clinical efficacy of influenza vaccination in pregnancy. Front Immunol 2015;6:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zaman K, Roy E, Arifeen Se, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555–64. [DOI] [PubMed] [Google Scholar]
- [16].Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N Engl J Med 2014;371:918–31. [DOI] [PubMed] [Google Scholar]
- [17].Steinhoff MC, MacDonald N, Pfeifer D, Muglia LJ. Influenza vaccine in pregnancy: policy and research strategies. Lancet 2014;383:1611–3. [DOI] [PubMed] [Google Scholar]
- [18].Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG 2015;122:17–26. [DOI] [PubMed] [Google Scholar]
- [19].Savitz DA, Fell DB, Ortiz JR, Bhat N. Does influenza vaccination improve pregnancy outcome? Methodological issues and research needs. Vaccine 2015;33:6430–5. [DOI] [PubMed] [Google Scholar]
- [20].Vazquez-Benitez G, Kharbanda EO, Naleway AL, Lipkind H, Sukumaran L, McCarthy NL, et al. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol 2016;184:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hutcheon JA, Savitz DA. Invited commentary: influenza, influenza immunization, and pregnancy-it’s about time. Am J Epidemiol 2016;184:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fell DB, Bhutta ZA, Hutcheon JA, Karron RA, Knight M, Kramer MS, et al. Report of the WHO technical consultation on the effect of maternal influenza and influenza vaccination on the developing fetus: Montreal, Canada, September 30-October 1, 2015. Vaccine 2017;35:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM, et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF joint reporting form on immunization. Vaccine 2016;34:5400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Global Alliance for Vaccines and Immunisation (GAVI). Final vaccine investment strategy analysis 2013: maternal influenza [Internet]; 2013. p. 12. Available from: <http://www.gavi.org/about/strategy/vaccine-investment-strategy/>.
- [25].World Health Organization. WHO taskforce to evaluate influenza data to inform vaccine impact and economic modelling [Internet]. Geneva: Switzerland; 2014. [Google Scholar]
- [26].Guyatt G, Oxman AD, Akl Ea, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- [27].Tapia MD, Sow SO, Tamboura B, Teguete I, Pasetti MF, Kodio M, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016;16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017. pii: S1473–3099 (17)30252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Katz MA, Gessner BD, Johnson J, Skidmore B, Knight M, Bhat N, et al. Incidence of influenza virus infection among pregnant women: a systematic review. BMC Preg Childbirth 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fell DB, Johnson J, Mor Z, Katz MA, Skidmore B, Neuzil KM, et al. Incidence of laboratory-confirmed influenza disease among infants under six months of age: a systematic review. BMJ Open 2017:e016526. 10.1136/bmjopen-2017-016526 [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine 2017;35:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fell DB, Savitz DA, Kramer MS, Gessner BD, Katz MA, Knight M, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG 2017;124:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos MTP. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. [Google Scholar]
- [34].Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huguet A, Hayden JA, Stinson J, McGrath PJ, Chambers CT, Tougas ME, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev 2013;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schanzer DL, Langley JM, Tam TWS. Influenza-attributed hospitalization rates among pregnant women in Canada 1994–2000. J. Obstet Gynaecol Can 2007;29:622–9. [DOI] [PubMed] [Google Scholar]
- [37].Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 2007;176: 463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998;148:1094–102. [DOI] [PubMed] [Google Scholar]
- [39].Jackson ML. Confounding by season in ecologic studies of seasonal exposures and outcomes: examples from estimates of mortality due to influenza. Ann Epidemiol 2009;19:681–91. [DOI] [PubMed] [Google Scholar]
- [40].Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179–86. [DOI] [PubMed] [Google Scholar]
- [41].Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012;54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tempia S, Walaza S, Cohen AL, von Mollendorf C, Moyes J, McAnerney JM, et al. Mortality associated with seasonal and pandemic influenza among pregnant and nonpregnant women of childbearing age in a high-HIV-prevalence setting-South Africa, 1999–2009. Clin. Infect. Dis. 2015;61:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McMorrow ML, Emukule GO, Njuguna HN, Bigogo G, Montgomery JM, Nyawanda B, et al. The Unrecognized burden of influenza in young Kenyan Children, 2008–2012. PLoS One 2015;10:2008–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yu H, Huang J, Huai Y, Guan X, Klena J, Liu S, et al. The substantial hospitalization burden of influenza in central China: surveillance for severe, acute respiratory infection, and influenza viruses, 2010–2012. Influenza Other Respi Viruses 2014;8:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med 2010;362:45–55. [DOI] [PubMed] [Google Scholar]
- [46].Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011;378:1917–30. [DOI] [PubMed] [Google Scholar]
- [47].Doyle TJ, Goodin K, Hamilton JJ. Maternal and neonatal outcomes among pregnant women with 2009 pandemic influenza A(H1N1) illness in Florida, 2009–2010: a population-based cohort study. PLoS One 2013;8:e79040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 2011;342: d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hâberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013;368:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hansen C, Desai S, Bredfeldt C, Cheetham C, Gallagher M, Li D-K, et al. A large, population-based study of 2009 pandemic Influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. J Infect Dis 2012;206:1260–8. [DOI] [PubMed] [Google Scholar]
- [51].Naresh A, Fisher BM, Hoppe KK, Catov J, Xu J, Hart J, et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J Perinatol 2013;33:939–43. [DOI] [PubMed] [Google Scholar]
- [52].McNeil SA, Dodds LA, Fell DB, Allen VM, Halperin BA, Steinhoff MC, et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol 2011;204:S54–7. [DOI] [PubMed] [Google Scholar]
- [53].Feikin DR, Scott JAG, Gessner BD. Use of vaccines as probes to define disease burden. Lancet 2014;383:1762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Widelock D, Csizmas L, Klein S. Influenza, pregnancy, and fetal outcome. Public Health Rep 1963;78:1–11. [PMC free article] [PubMed] [Google Scholar]
- [55].Gérardin P, El Amrani R, Cyrille B, Gabrièle M, Guillermin P, Boukerrou M, et al. Low clinical burden of 2009 pandemic influenza A (H1N1) infection during pregnancy on the island of La Réunion. PLoS One 2010;5:e10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Malinowski AK, McGeer A, Robertson J, Sermer M, Farine D, Lapinsky SE, et al. H1N1 in pregnancy: a tertiary care centre experience. J Obstet Gynaecol Can 2011;33:698–704. [DOI] [PubMed] [Google Scholar]
- [57].Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011;205:10–8. [DOI] [PubMed] [Google Scholar]
- [58].Harris J Influenza occurring in pregnant women. J Am Med Assoc 1919;72:978–80. [Google Scholar]
- [59].Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959;78:1172–5. [DOI] [PubMed] [Google Scholar]
- [60].Liu S-L, Wang J, Yang X-H, Chen J, Huang R-J, Ruan B, et al. Pandemic influenza A(H1N1) 2009 virus in pregnancy. Rev Med Virol 2013;23:3–14. [DOI] [PubMed] [Google Scholar]
- [61].Bhalerao-Gandhi A, Chhabra P, Arya S, Simmerman JM. Influenza and pregnancy: a review of the literature from India. Infect Dis Obstet Gynecol 2015;2015:867587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Meijer WJ, van Noortwijk AGA, Bruinse HW, Wensing AMJ. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand 2015;94:797–819. [DOI] [PubMed] [Google Scholar]
- [63].Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol 2011;205. 473.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Salam RA, Das JK, Dojo Soeandy C, Lassi ZS, Bhutta ZA. Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes. Cochrane Database Syst Rev 2015:CD009982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. [DOI] [PubMed] [Google Scholar]
- [66].Hutcheon JA, Fell DB, Jackson ML, Kramer MS, Ortiz JR, Savitz DA, et al. Detectable risks in studies of the fetal benefits of maternal influenza vaccination. Am J Epidemiol 2016;184:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol 2009;62:687–94. [DOI] [PubMed] [Google Scholar]
- [68].Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine 2009;27:6300–4. [DOI] [PubMed] [Google Scholar]
- [69].Steinhoff MC, Omer SB, Roy E, El Arifeen S, Raqib R, Dodd C, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ 2012;184:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Simoes EAF, Cutland C, Carosone-Link P, Hugo A, Jones S, Madimabe R, et al. Lack of efficacy of trivalent influenza vaccination of HIV-negative pregnant women against adverse fetal outcomes: a randomized clinical trial. Open Forum Infect Dis 2015;2. 10.1093/ofid/ofv133.1446. [DOI] [Google Scholar]
- [71].Omer SB, Richards JL, Madhi s A, Tapia mD, Steinhoff MC, Aqil AR, et al. Three randomized trials of maternal influenza immunization in Mali, Nepal, and South Africa: Methods and expectations. Vaccine 2015;33:3801–12. [DOI] [PubMed] [Google Scholar]
- [72].World Health Organization. WHO Preferred Product Characteristics for Next-Generation Influenza Vaccines. Geneva; 2017. [Google Scholar]
- [73].Ortiz JR, Englund JA, Neuzil KM. Influenza vaccine for pregnant women in resource-constrained countries: a review of the evidence to inform policy decisions. Vaccine 2011;29:4439–52. [DOI] [PubMed] [Google Scholar]
- [74].Lambach P, Hombach J, Ortiz JR. A global perspective of maternal influenza immunization. Vaccine 2015;33:6376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sobanjo-ter Meulen A, Abramson J, Mason E, Rees H, Schwalbe N, Bergquist S, et al. Path to impact: a report from the Bill and Melinda Gates Foundation convening on maternal immunization in resource-limited settings; Berlin – January 29–30, 2015. Vaccine 2015;33:6388–95. [DOI] [PubMed] [Google Scholar]
- [76].Fell DB, Sprague AE, Liu N, Yasseen AS, Wen S-W, Smith G, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health 2012;102:e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bonhoeffer J, Kochhar S, Hirschfeld S, Heath PT, Jones CE, Bauwens J, et al. Global alignment of immunization safety assessment in pregnancy – the GAIA project. Vaccine 2016;34:5993–7. [DOI] [PubMed] [Google Scholar]
- [78].Fitchett EJA, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, et al. Strengthening the reporting of observational studies in epidemiology for newborn infection (sTrOBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis 2016;16:e202–13. [DOI] [PubMed] [Google Scholar]
- [79].World Health Organization. A manual for estimating disease burden associated with seasonal influenza. Geneva: World Health Organization; 2015. [Google Scholar]
- [80].Nunes MC, Cutland CL, Jones S, Downs S, Weinberg A, Ortiz JR, et al. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis 2017. 10.1093/cid/cix497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gessner BD, Brooks WA, Neuzil KM, Vernet G, Bright Ra, Tam JS, et al. Vaccines as a tool to estimate the burden of severe influenza in children of low-resourced areas (November 30-December 1, 2012, Les Pensieres, Veyrier-du-Lac, France). Vaccine 2013;31:3222–8. [DOI] [PubMed] [Google Scholar]
- [82].Meeting of the Strategic Advisory Group of Experts on immunization, April 2015: conclusions and recommendations. Wkly Epidemiol Rec 2015;90:261–78. [PubMed] [Google Scholar]
- [83].Anand A, Luman ET, O’Connor PM. Building on success–potential to improve coverage of multiple health interventions through integrated delivery with routine childhood vaccination. J Infect Dis 2012;205(Suppl):S28–39. [DOI] [PubMed] [Google Scholar]
- [84].Health P for MN and C. PMNCH Knowledge Summary # 25: Integrating Immunization and Other Services for Women and Children [Internet]. 2013. Available from: <http://www.who.int/pmnch/knowledge/publications/summaries/ks25/en/> [cited 2017 Jul 14].
- [85].World Health Organization. Global pandemic influenza action plan to increase vaccine supply. Geneva: Switzerland; 2006. [Google Scholar]
- [86].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Riquelme R, Jiménez P, Videla AJ, Lopez H, Chalmers J, Singanayagam A, et al. Predicting mortality in hospitalized patients with 2009 H1N1 influenza pneumonia. Int J Tuberc Lung Dis 2011;15:542–6. [DOI] [PubMed] [Google Scholar]
- [88].Van Kerkhove MD, Vandemaele KAH, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 Influenza A (H1N1) Infection: a global pooled analysis. PLoS Med 2011;8: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.