Abstract
Background
Bezlotoxumab significantly reduces the incidence of recurrent Clostridioides difficile infection (CDI); however, limited data are available in solid organ transplant (SOT) and hematopoietic cell transplant (HCT) recipients.
Methods
We conducted a single-center retrospective analysis comparing recurrent CDI in SOT and HCT recipients receiving standard of care alone (oral vancomycin, fidaxomicin, or metronidazole) or bezlotoxumab plus standard of care. The primary outcome was 90-day incidence of recurrent CDI, and secondary outcomes included 90-day hospital readmission, mortality, and incidence of heart failure exacerbation.
Results
Overall, 94 patients received bezlotoxumab plus standard of care (n = 38) or standard of care alone (n = 56). The mean age was 53 years; patients had a median of 3 prior Clostridioides difficile episodes and 4 risk factors for recurrent infection. Most patients were SOT recipients (76%), with median time to index CDI occurring 2.7 years after transplantation. Ninety-day recurrent CDI occurred in 16% (6/38) in the bezlotoxumab cohort compared to 29% (16/56) in the standard of care cohort (P = .13). Multivariable regression revealed that bezlotoxumab was associated with significantly lower odds of 90-day recurrent CDI (odds ratio, 0.28 [95% confidence interval, .08–.91]). There were no differences in secondary outcomes, and no heart failure exacerbations were observed.
Conclusions
In a cohort of primarily SOT recipients, bezlotoxumab was well tolerated and associated with lower odds of recurrent CDI at 90 days. Larger, prospective trials are needed to confirm these findings among SOT and HCT populations.
Keywords: bezlotoxumab, CDI, Clostridioides difficile infection, transplant recipient
Key Points.
Solid-organ and hematopoietic-cell transplant recipients are at high risk for recurrent Clostridioides difficile infection (CDI) and poor outcomes associated with these infections. On multivariable analysis, bezlotoxumab significantly reduced the incidence of recurrent CDI in this high-risk population.
Clostridioides (formerly Clostridium) difficile infection (CDI) is a common hospital-acquired infection associated with a high incidence of recurrent disease (rCDI) [1]. Solid organ transplant (SOT) and hematopoietic cell transplant (HCT) recipients are at particularly high risk of CDI compared to the general population owing to greater exposure to surgical procedures, antimicrobials, immunosuppression, and health care contact [2–4]. Transplant recipients additionally experience greater severity of disease once infected. Fulminant CDI, for example, is estimated at 15% in SOT recipients, 2-fold higher than the general population [4, 5]. The sequelae of rCDI are more severe among transplant populations as infections within 1 year posttransplantation are associated with graft loss and a significant increase in mortality [2, 6–8]. Additionally, risk of recurrence increases with each successive episode, whereby SOT and HCT patients are at incrementally higher risk for adverse outcomes with each CDI event [9]. Due to the disproportionate burden of CDI on transplant recipients, therapeutic strategies to prevent rCDI are needed to limit morbidity and mortality in this population.
Bezlotoxumab (BEZ) is a monoclonal antibody directed at C. difficile toxin B that has been shown to prevent rCDI [10]. Current American Society of Transplantation (AST) Infectious Diseases Community of Practice guidelines for the treatment of CDI in SOT recipients recommend bezlotoxumab, in combination with standard of care (SoC) CDI antibiotics, for the prevention of rCDI [11]. These recommendations, however, are based on limited data from the Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection (MODIFY I and II) randomized controlled approval trials and retrospective studies conducted in nontransplant populations [12–15]. This study sought to further evaluate the effectiveness and safety of BEZ as an adjunctive therapy to SoC antibiotics for the prevention of rCDI in SOT and HCT recipients.
MATERIALS AND METHODS
Study Design and Population
This was a retrospective cohort study conducted at the University of Colorado Hospital (UCH), an academic tertiary care center with approximately 700 inpatient beds. Patients admitted to UCH between January 2015 and November 2019 meeting the following criteria were eligible for inclusion: (1) age 18–89 years; (2) history of SOT or HCT; (3) CDI diagnosis as documented by positive C. difficile polymerase chain reaction (PCR) results and new onset of clinically significant diarrhea, as defined in AST guidelines [11]; (4) treatment with SoC CDI antibiotics (oral vancomycin [VAN], fidaxomicin [FDX], or metronidazole [MTZ]); and (5) follow-up visit documented ≥90 days after completion of therapy. Patients in the SoC cohort were treated with SoC CDI therapy between January 2015 and June 2017, a period immediately prior to BEZ availability at UCH. Patients in the BEZ cohort were treated with BEZ, in addition to SoC antibiotics, at a UCH-associated facility between November 2017 and November 2019. BEZ was dosed according to an institutional protocol at 10 mg/kg of actual bodyweight (or adjusted bodyweight if actual bodyweight was >30% over ideal bodyweight). Doses were capped at a maximum dose of 1000 mg (1 vial) for all patients. Select vulnerable populations (pregnant, incarcerated, age >89 years, and age <18 years) were excluded in accordance with local institutional review board ethics requirements. This study was approved by the Colorado Multiple Institutional Review Board prior to study initiation.
Clinical Data Extraction
Patient demographic, clinical, and treatment characteristics were abstracted from the electronic medical record using REDCap (Research Electronic Data Capture, Vanderbilt University), a structured data collection tool hosted at the University of Colorado [16]. Infection-related characteristics, such as time from transplant to CDI, presence of prior CDI, CDI complications, and presence of rCDI risk factors, such as age ≥65 years, broad-spectrum antibiotic receipt, proton pump inhibitor use, presence of proteinuria, and severe CDI episode, were also collected. Treatment information collected included antibiotic regimen, antibiotic duration, and adjunctive therapies for rCDI such as probiotics, rifaximin, toxin-binding agents, intravenous immunoglobulin, and fecal microbiota transplant (FMT). Combination therapy for CDI was defined as overlapping receipt of oral (PO) VAN, FDX, or MTZ for any indication. Sequential therapy for CDI was defined as a switch in therapy from one anti-CDI agent to another. Extended-duration CDI therapy was defined as receipt of CDI antibiotics for >14 days [11]. The Charlson Comorbidity Index (CCI) and Zar score were calculated for each patient to estimate mortality risk and CDI severity at time of diagnosis, respectively [17, 18].
Outcomes
The primary endpoint was incidence of rCDI at 90 days after completion of CDI antibiotics. rCDI was defined as new onset of clinically significant diarrhea, as defined in AST guidelines [11], accompanied by initiation of treatment with PO VAN or FDX within 90 days after initial completion of CDI therapy at the discretion of the treating physician. Confirmatory C. difficile PCR was not required due to previously published reports of high rates of repeat positive tests several weeks after completion of initial therapy, even in the absence of active infection [19]. Completion of CDI therapy in patients on tapered/pulsed or prophylactic regimens was defined as the cessation of anti-CDI therapy. Prophylactic PO VAN or FDX was not considered as a criterion for rCDI. Secondary effectiveness outcomes were rCDI at 30 days, and all-cause hospital readmission and all-cause mortality at 90 days. Safety outcomes assessed included the incidence of BEZ infusion-related reactions, and heart failure exacerbations among patients with a preexisting diagnosis of heart failure. Infusion-related reactions were defined as any reaction occurring during BEZ administration that was, in the opinion of the treating physician, related to medication administration requiring discontinuation of the infusion. Heart failure exacerbation was defined as new volume overload after BEZ infusion requiring intervention. Efficacy outcomes included patients who received the entire BEZ infusion, whereas safety analyses included all patients who received any BEZ administration.
Statistical Analysis
We determined that a sample size of 118 patients per group would be required to achieve a statistical power of 80% to detect a 50% relative difference in rCDI at 90 days using an α = .05. We projected an event rate of 30% in the SoC cohort based on previously published literature and an event rate of 15% in the BEZ cohort using data from controlled clinical trials and limited retrospective data [6, 11–15].
Patient characteristics were compared between the BEZ and SoC cohorts using χ 2 /Fisher exact and independent sample t tests/Mann-Whitney U tests based on the nature of the data. P values were considered significant at <.05. A priori subgroup analyses were performed excluding patients who were treated with MTZ monotherapy, in accordance with treatment guideline updates owing to higher incidence of rCDI with MTZ-treated patients compared to VAN- or FDX-treated patients [11]. SOT and HCT recipients were also analyzed individually. Additional subgroup analyses among BEZ recipients investigated timing of BEZ administration, CDI antibiotic treatment duration, and subject bodyweight. To control for confounding, multivariable analysis was performed using binary logistic regression. To identify factors associated with the primary outcome, variables with a P value <.2 on univariate analysis and those with clinical importance were considered for inclusion into a multivariable model using backward stepwise approach. Variables considered of clinical importance to the primary outcome were patient bodyweight, number of prior CDI episodes, SoC antibiotic treatment, duration of CDI antibiotic treatment, number of risk factors for rCDI, age ≥65 years, receipt of concomitant broad-spectrum antibiotics, presence of severe CDI, proton pump inhibitor use, BEZ receipt, and history of a prior CDI episode. Analyses were conducted using JMP Pro, version 15 software (SAS Institute, Cary, North Carolina).
RESULTS
Baseline Characteristics
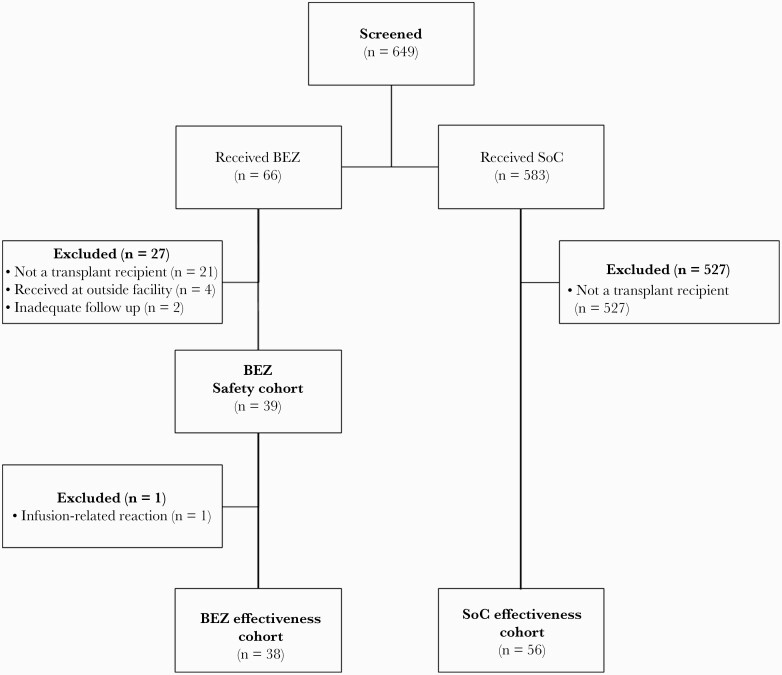
Of 649 screened patients, 39 patients in BEZ and 56 in SoC met study inclusion (Figure 1). The mean age was 53 (standard deviation, 15) years, and the majority were male (56%). Baseline characteristics were well-matched between cohorts with respect to age, sex, race, and comorbid conditions (Table 1). Patients had a median of 3 (interquartile range [IQR], 2–3) prior CDI episodes and the index CDI episode analyzed occurred at a median of 2.7 (IQR, 0.4–7.5) years posttransplantation. Hospitalization within the previous 30 days (55% BEZ vs 36% SoC, P = .09) and antibiotic receipt within the previous 90 days (87% BEZ vs 80% SoC, P = .60) of the index CDI episode were common in both groups. The 2 cohorts had similar number of risk factors for rCDI, with a median of 4 (IQR, 3–5) and 4 (IQR, 3–4) risk factors in the BEZ and SOC cohorts, respectively (P = .36). Among BEZ recipients, there was a higher proportion of patients with prior CDI (71% vs 35%, P < .01) and SOT receipt (92% vs 68%, P < .01), while the SoC cohort had a higher proportion of HCT receipt (8% vs 32%, P < .01). The incidence of severe CDI (Zar score ≥2) was more common among SoC (13% vs 32%, P = .04), mainly due to a higher incidence of intensive care unit admission at time of CDI diagnosis among SoC compared to BEZ.
Figure 1.
Patient selection diagram. Abbreviations: BEZ, bezlotoxumbab; SoC, standard of care.
Table 1.
Patient Baseline Characteristics
| Variable | BEZ(n = 38) | SoC(n = 56) | P value |
|---|---|---|---|
| Age, mean years (SD) | 51 (14) | 53 (15) | .70 |
| Male sex, n (%) | 21 (55) | 32 (57) | .99 |
| Weight, mean kg (SD) | 75 (18) | 77 (25) | .70 |
| Obese (BMI ≥ 30), n (%) | 7 (18) | 12 (21) | .80 |
| Race, n (%) | |||
| White | 27 (71) | 39 (70) | .99 |
| African American | 1 (2.6) | 5 (9) | .40 |
| Hispanic | 9 (24) | 9 (16) | .40 |
| Other | 1 (2.6) | 3 (5) | .60 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 14 (37) | 23 (41) | .80 |
| HbA1C, median % (IQR) | 6.9 (5.7–7.9) | 6 (5.6–7.8) | .40 |
| Chronic kidney disease | 4 (11) | 11 (20) | .30 |
| Intermittent hemodialysis | 3 (8) | 3 (5) | .70 |
| Cirrhosis | 6 (16) | 8 (14) | .99 |
| Heart failure | 5 (13) | 7 (13) | .99 |
| Malignancy (solid tumor or hematological) | 6 (16) | 24 (43) | .01 |
| Inflammatory bowel disease | 4 (11) | 6 (11) | .99 |
| Peptic ulcer disease | 3 (8) | 9 (16) | .30 |
| Charlson Comorbidity Index, median (IQR) | 4 (2–5) | 4 (2–6) | .40 |
| Transplant type, n (%) | |||
| Hematopoietic | 3 (8) | 18 (32) | .01 |
| Solid organ | 35 (92) | 38 (68) | < .01 |
| Kidney | 19 (50) | 8 (14) | < .01 |
| Liver | 7 (18) | 17 (30) | .20 |
| Heart | 6 (16) | 5 (9) | .30 |
| Lung | 0 | 3 (5) | .27 |
| Multi–organ | 3 (8) | 5 (9) | .99 |
| Induction with any of the following, n (%) | |||
| Anti-thymocyte globulin | 12 (32) | 9 (16) | .08 |
| Basiliximab | 1 (3) | 3 (5) | .60 |
| Daclizumab | 1 (3) | 0 | .40 |
| Alemtuzumab | 0 | 1 (2) | .99 |
| Time from transplant to index CDI episode, median days (IQR) | 1073 (198–2326) | 1003 (46–3357) | .50 |
| Hospitalization in past 30 days, n (%) | 21 (55) | 20 (36) | .09 |
| Number of lifetime CDI episodes, median (IQR) | 3 (2–4) | 2 (1–3) | .02 |
| Exposure to antibiotics not used in treatment of CDI in preceding 90 days, n (%) | 33 (87) | 45 (80) | .60 |
| Prior fecal microbiota transplant, n (%) | 5 (13) | 3 (5) | .26 |
| Number of treatments, median (IQR) | 2 (1–3) | 2 (1–3) | .90 |
Data are presented as No. (%) unless otherwise indicated. IQR represents values in the 25th to 75th percentile.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; BEZ, bezlotoxumab; IQR, Interquartile Range; SD, standard deviation; SoC, standard of care.
Treatments
PO VAN was the predominant CDI treatment (86%), which was similar between cohorts (P = .99; Table 2). FDX use was more common among BEZ recipients (34% vs 11%, P = .01) while combination therapies (mostly PO VAN + intravenous MTZ) were more common among SoC recipients (13% vs 41%, P = .01). The median duration of CDI therapy was longer in the BEZ cohort (27 days vs 17 days, P = .03) largely due to higher incidence of tapered CDI regimens and prophylactic PO VAN use. Among BEZ recipients, median time to BEZ administration from CDI treatment initiation was 25 (IQR, 13–40) days. Fourteen (37%) patients received BEZ after completing CDI treatment.
Table 2.
Clostridioides difficile Infection Characteristics
| Variable | BEZ(n = 38) | SoC(n = 56) | P value |
|---|---|---|---|
| Zar score, median (IQR) | 0 (0–1) | 1 (0–2) | .08 |
| Complicated infection, n (%) | |||
| ICU admission | 1 (3) | 12 (21) | .01 |
| Ileus | 1 (3) | 2 (4) | .99 |
| Toxic megacolon | 0 | 0 | .99 |
| Shock | 1 (3) | 6 (11) | .20 |
| Pseudomembranous colitis | 1 (3) | 1 (2) | .99 |
| Surgical intervention for CDI episode, n (%) | 0 | 0 | .99 |
| Index CDI episode treatment, n (%)a | |||
| Vancomycin | 33 (87) | 48 (86) | .99 |
| Fidaxomicin | 13 (34) | 6 (11) | .01 |
| Metronidazole | 4 (11) | 26 (46) | < .01 |
| Combination (> 1 treatments above) | 5 (13) | 22 (39) | .01 |
| Duration of CDI treatment, median (IQR) | 27 (15–57) | 17 (14–31) | .03 |
| Extended CDI treatment (> 14 days), n (%) | 28 (74) | 29 (52) | .05 |
| Tapering treatment regimen, n (%) | 22 (58) | 13 (23) | < .01 |
| Prophylaxis, n (%) | 8 (21) | 4 (7) | .06 |
| Adjunctive CDI therapies, n (%) | |||
| Probiotics | 2 (5) | 2 (4) | .99 |
| Rifaximin | 0 | 0 | .99 |
| Toxin-binding agent | 0 | 0 | .99 |
| Intravenous Immunoglobulin | 0 | 0 | .99 |
| Fecal Microbiota Transplant | 4 (11) | 5 (9) | .99 |
| Risk factors for recurrence, n (%) | |||
| Immunocompromised | 38 (100) | 56 (100) | .99 |
| Age ≥ 65 years | 10 (26) | 12 (21) | .58 |
| Concomitant antibiotic use after CDI episode | 27 (71) | 36 (64) | .66 |
| Proton pump inhibitor use | 15 (40) | 34 (61) | .04 |
| Proteinuria | 17 (45) | 31 (55) | .31 |
| Severe CDI (Zar > 2) | 5 (13) | 18 (32) | .04 |
| Prior CDI | 30 (79) | 29 (52) | .01 |
| Number of risk factors for recurrence, median (IQR) | 4 (3–5) | 4 (3–4) | .36 |
Data are presented as No. (%) unless otherwise indicated. IQR represents values in the 25th to 75th percentile.
Abbreviations: CDI, Clostridioides difficile Infection; BEZ, bezlotoxumab; IQR, interquartile range; SoC, standard of care.
aPatients may have received more than one agent, either sequentially or concomitantly (combination).
Outcomes
In unadjusted analysis, no difference was observed in the primary outcome of 90-day rCDI between BEZ recipients, compared to SoC recipients (16% vs 29%, P = .13). Neither 30-day nor 90-day incidence of all-cause mortality or all-cause hospital readmission were different between cohorts (Table 3). Median time to rCDI also did not differ between cohorts (40 days vs 36 days, P = .80). Overall, BEZ was well-tolerated with 1 (2.6%) instance of infusion-related nausea and vomiting that required cessation of BEZ administration; this patient was not included in the effectiveness analysis. Among those with underlying heart failure, the incidence of heart failure exacerbation was rare, and similar between cohorts (0% BEZ vs 8% SoC, P = .50).
Table 3.
Outcomes
| Outcome | BEZ (n = 38) | SoC (n = 56) | P Value |
|---|---|---|---|
| CDI recurrence, No. (%) | |||
| 30 d | 4 (11) | 8 (14) | .76 |
| 90 d | 6 (16) | 16 (29) | .13 |
| Death, No. (%) | |||
| 30 d | 0 | 3 (5) | .27 |
| 90 d | 0 | 3 (5) | .27 |
| Hospital readmission, No. (%) | |||
| 30 d | 9 (24) | 19 (34) | .29 |
| 90 d | 18 (47) | 28 (50) | .67 |
| Heart failure exacerbation among those with baseline heart failure diagnosis, No. (%) | 0 | 2 (8) | .49 |
Abbreviations: BEZ, bezlotoxumab; CDI, Clostridioides difficile infection; SoC, standard of care.
In a subgroup analysis of the primary outcome excluding 5 patients treated with MTZ monotherapy (all from the SoC cohort), 90-day rCDI was 16% (6/38) BEZ vs 31% (16/51) SoC (P = .15). Among SOT recipients, 90-day rCDI was 14% (5/35) BEZ vs 25% (9/36) SoC, whereas 90-day rCDI among HCT recipients was 33% (1/3) BEZ vs 39% (7/18) SoC (P = .26 and P = .99, respectively). Among BEZ recipients, there was no difference in rCDI when comparing BEZ administration during (5/24 [21%]) or after (1/14 [7%]) CDI treatment (P = .40). Similarly, no difference was demonstrated with standard (3/10 [30%]) or extended-duration (3/28 [11%]) CDI treatment (P = .30). With respect to institutional practices of capping doses to a maximum of 1000 mg, subject bodyweight >100 kg (0/3) or <100 kg (6/35 [17%]) did not impact BEZ effectiveness (P = .99).
In a multivariable analysis of 90-day rCDI incidence in the total population, BEZ was associated with 72% lower odds of rCDI compared to those who did not receive BEZ (odds ratio, 0.28 [95% CI, .08–.91]; P = .03). Number of prior CDI episodes was also independently associated with greater odds of rCDI (Table 4).
Table 4.
Results of Multivariable Analysis of Factors Associated With 90-Day Clostridioides difficile Infection Recurrence
| Variable | Odds Ratio | (95% CI) | P Value |
|---|---|---|---|
| No. of prior CDI episodes | 1.48 | (1.12–1.95) | .01 |
| Bezlotoxumab receipt | 0.28 | (.08–.91) | .03 |
| Receipt of concomitant antibiotics | 1.82 | (.57–5.84) | .31 |
Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval.
DISCUSSION
This real-world analysis of BEZ represents the first analysis conducted in a transplant patient population consisting of SOT and HCT recipients. In unadjusted analysis, this study observed a nonsignificant trend toward lower 90-day rCDI with the addition of BEZ to SoC and upon multivariable analysis, BEZ was associated with 72% lower odds of rCDI at 90 days. rCDI incidence at 90 days in BEZ-treated and SoC-treated patients were similar to rates published in previous trials [12, 13]. Immunocompromised patient populations are underrepresented in previously published reports, constituting approximately 20% and 40% of the MODIFY I/II and 2 real-world experience studies [12–15], respectively. Previous studies also do not delineate specific immunocompromising conditions, so it is unclear how many of these patients were SOT or HCT recipients. Overall, findings herein suggest that high-risk SOT/HCT recipients may derive benefit from BEZ.
Patients in this study possessed more risk factors for rCDI than previous trials, with >50% of patients having 4 or more risk factors for recurrence. Additionally, many had multiple prior rCDI episodes and had failed numerous CDI treatment strategies (PO VAN, FDX, tapered CDI regimens, FMT) prior to BEZ administration. Our findings in a cohort of patients with a median of 3 prior CDI episodes are promising given comparable rates of rCDI to the MODIFY trials, where a substantial percentage of patients (28%) were treated with BEZ during the initial CDI episode and would be considered lower risk for recurrence than a patient with multiple recurrences [12]. These data suggest benefit of BEZ during either an initial or subsequent episode of CDI in SOT/HCT recipients, a population in which CDI is associated with increased morbidity and mortality.
No difference was seen in all-cause mortality or all-cause hospital readmission. Hospital readmission is difficult to attribute to CDI alone, and rates of all-cause hospital readmission may have been unrelated to CDI, which may have limited our ability to detect a difference in this outcome if one was present. Nevertheless, it is possible that CDI-associated mortality and CDI-related hospital readmissions could be reduced by preventing subsequent CDI episodes with the use of BEZ, although neither of these outcomes have been reported in previous publications. Additionally, given poor graft outcomes for SOT recipients in association with CDI, it is plausible that rCDI prevention through BEZ use could impact graft survival as well. These hypotheses warrant further investigation.
In the MODIFY trials, patients were administered BEZ at a median of 3 days after initiation of CDI-directed antibiotics. In contrast, in this study patients were administered BEZ at a median of 25 days after CDI treatment initiation due to relatively long median hospital length of stay, reimbursement concerns with inpatient administration, and insurance prior authorization requirements and scheduling considerations for outpatient infusion. Additionally, many patients were administered BEZ after CDI treatment had been completed, which is outside of the dosing window studied in the MODIFY trials and dosing recommendations provided in the BEZ package insert [10]. BEZ administration within or after the typical CDI treatment period did not appear to affect BEZ effectiveness in prevention of rCDI (21% during vs 7% after). This demonstrates the need for further study in BEZ administration timing, secondary to the multiple challenges that accompany timely BEZ use in the real world.
Overall, BEZ was well-tolerated in this cohort. Out of 39 BEZ recipients, 1 recipient experienced nausea and vomiting during BEZ administration leading to discontinuation. In the MODIFY trials, infusion-related reactions occurred in 10% of patients, yet necessitated stopping medication administration in only 0.1% of recipients. The BEZ package insert additionally lists a precaution for increased incidence of heart failure exacerbation. In patients with a history of congestive heart failure, 12.7% of BEZ-treated patients and 4.8% of placebo-treated patients experienced a heart failure exacerbation during the 12-week MODIFY study period and BEZ recipients experienced higher rates of death (19.5%) compared to placebo-treated patients (12.5%). No cases of heart failure exacerbation or death were identified in this cohort at 90 days and similarly, no cases have been reported in the 2 previously published real-world studies in nontransplant populations [14, 15]. The safety profile of BEZ compared to FMT positions the agent as a preferred treatment option for prevention of rCDI in immunocompromised populations, particularly given recent US Food and Drug Administration warnings concerning lethal donor-derived infections [20]. Overall, the small sample size and relatively low incidence of safety events likely limited our ability to detect a difference between cohorts if one was present.
Our retrospective study has important limitations that must be considered. First, the study was underpowered for the primary and secondary outcomes, and these findings should be considered exploratory, although analyses of immunocompromised patients from prior studies demonstrated comparable rates of rCDI as described herein [12, 13]. Second, the BEZ cohort was studied at a time period after the SoC cohort to avoid both therapy options being available to limit selection bias, and it is plausible that improvements in the management of CDI occurred in the latter period. Certainly, updates made to the Infectious Diseases Society of America practice guidelines in 2017 affected MTZ and FDX use between our cohorts [21]; however, only 9% of the SoC cohort received MTZ monotherapy and subgroup analysis excluding these patients revealed similar findings as the overall population analysis. Additionally, subgroup analyses of MODIFY I/II demonstrated similar BEZ efficacy regardless of the SoC antibiotic used, and previous data in the HCT population suggest similar benefit among MTZ, PO VAN, and FDX use for the prevention of rCDI [22–24]. Third, BEZ-treated patients received a longer duration of CDI therapy, which was a result of patients receiving prophylaxis during broad-spectrum antibiotic courses and tapering regimens with multiple recurrent CDI. Nevertheless, incidence of rCDI did not differ between standard-duration or extended-duration CDI therapy. Fourth, neither confirmatory enzyme immunoassay or PCR was required to diagnose rCDI and this distinction was based solely on new onset of clinically significant diarrhea and initiation of CDI-active antibiotics. While this may have overcalled rCDI incidence, this represents real-world practice where clinicians may wish to treat based on high clinical suspicion in a vulnerable patient population. Fifth, only 3 HCT patients were treated with BEZ in this study, and therefore these findings may not be generalizable to the HCT population. Indeed, differences in the degree of immunosuppression and disease-specific factors between SOT and HCT populations could lead to differential treatment effects. Further studies, specific to this population, are needed.
BEZ appeared effective and well-tolerated in a high-risk transplant cohort and could be considered adjunctively for prevention of rCDI, particularly for those with previous failure of other preventive strategies. These data are promising and warrant further investigation of BEZ among SOT/HCT recipients, as well as transplant-specific outcomes, including the impact on rejection and graft survival. Important questions remain regarding the optimal timing of BEZ administration, repeat dosing, prophylaxis, and adjunctive treatment for refractory CDI.
Notes
Author contributions. All authors were involved in conceptualization of the research idea, methodology and study design, and writing–review and editing of the final manuscript. K. C. M., M. M. A., and V. B. were responsible for funding acquisition. T. M. J., A. H. H., L. L. A., and V. B. performed data collection. M. M. A. performed data curation and analysis and prepared the figures. T. M. J. wrote the original manuscript draft. Project supervision was provided by M. M. A. and V. B.
Patient consent statement. This study was approved by the Colorado Multiple Institutional Review Board (IRB) prior to study initiation. Patient informed consent was not required due to the retrospective nature of data collection, as determined by the local IRB.
Data availability. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclaimer. The contents of this are the authors’ own and do not necessarily represent the views of the National Institutes of Health (NIH). The funding source was not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Financial support. This work was supported by the NIH/National Center for Research Resources Colorado Clinical and Translational Science Institute (grant number UL1 RR025780) and by an investigator-initiated research grant from Merck & Co, Inc.
Potential conflicts of interest. Outside this study, M. M. has received investigator-initiated research support from Allergan for unrelated projects. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Mu Y, Bamberg WM, et al. . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ilett EE, Helleberg M, Reekie J, et al. . Incidence rates and risk factors of Clostridioides difficile infection in solid organ and hematopoietic stem cell transplant recipients. Open Forum Infect Dis 2019; 6:ofz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogala BG, Malat GE, Lee DH, et al. . Identification of risk factors associated with Clostridium difficile infection in liver transplantation recipients: a single-center analysis. Transplant Proc 2016; 48:2763–8. [DOI] [PubMed] [Google Scholar]
- 4. Dallal RM, Harbrecht BG, Boujoukas AJ, et al. . Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002; 235:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paudel S, Zacharioudakis IM, Zervou FN, et al. . Prevalence of Clostridium difficile infection among solid organ transplant recipients: a meta-analysis of published studies. PLoS One 2015; 10:e0124483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schluger A, Rosenblatt R, Knotts R, et al. . Clostridioides difficile infection and recurrence among 2622 solid organ transplant recipients. Transpl Infect Dis 2019; 21:e13184. [DOI] [PubMed] [Google Scholar]
- 7. Cusini A, Béguelin C, Stampf S, et al. ; Swiss Transplant Cohort Study . Clostridium difficile infection is associated with graft loss in solid organ transplant recipients. Am J Transplant 2018; 18:1745–54. [DOI] [PubMed] [Google Scholar]
- 8. Bunnapradist S, Neri L, Wong W, et al. . Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis 2008; 51:478–86. [DOI] [PubMed] [Google Scholar]
- 9. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–40. [DOI] [PubMed] [Google Scholar]
- 10. Zinplava (bezlotoxumab) [prescribing information]. Whitehouse Station, NJ: Merck & Co; 2016. [Google Scholar]
- 11. Mullane KM, Dubberke ER; AST ID Community of Practice . Management of Clostridioides (formerly Clostridium) difficile infection (CDI) in solid organ transplant recipients: guidelines from the American Society of Transplantation community of practice. Clin Transplant 2019; 33:e13564. [DOI] [PubMed] [Google Scholar]
- 12. Gerding DN, Kelly CP, Rahav G, et al. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 14. Hengel RL, Ritter TE, Nathan RV, et al. . Real-world experience of bezlotoxumab for prevention of Clostridioides difficile infection: a retrospective multicenter cohort study. Open Forum Infect Dis 2020; 7:ofaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oksi J, Aalto A, Säilä P, et al. . Real-world efficacy of bezlotoxumab for prevention of recurrent Clostridium difficile infection: a retrospective study of 46 patients in five university hospitals in Finland. Eur J Clin Microbiol Infect Dis 2019; 38:1947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 18. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45: 302–7. [DOI] [PubMed] [Google Scholar]
- 19. Fekety R, Silva J, Kauffman C, et al. . Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med 1989; 86:15–9. [DOI] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration. Fecal microbiota for transplantation: safety alert—risk of serious adverse events likely due to transmission of pathogenic organisms. 2020. https://www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-alert-risk-serious-adverse-events-likely-due-transmission. Accessed 23 March 2021.
- 21. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubberke ER, Gerding DN, Kelly CP, et al. . Efficacy of bezlotoxumab in participants receiving metronidazole, vancomycin, or fidaxomicin for treatment of Clostridioides (Clostridium) difficile infection. Open Forum Infect Dis 2020; 7:ofaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prohaska L, Mahmoudjafari Z, Shune L, et al. . Retrospective evaluation of fidaxomicin versus oral vancomycin for treatment of Clostridium difficile infections in allogeneic stem cell transplant. Hematol Oncol Stem Cell Ther 2018; 11:233–40. [DOI] [PubMed] [Google Scholar]
- 24. Parmar SR, Bhatt V, Yang J, et al. . A retrospective review of metronidazole and vancomycin in the management of Clostridium difficile infection in patients with hematologic malignancies. J Oncol Pharm Pract 2014; 20:172–82. [DOI] [PubMed] [Google Scholar]