Abstract
Systemically delivered targeted biologics have revolutionized the treatment of moderate to severe psoriasis. For milder forms of psoriasis topical therapies, primarily corticosteroids, remain the mainstay of treatment to reduce the risks and off-target side effects associated with systemic therapies. Most newly developed biologics, including monoclonal antibodies, are structurally complex and are unable to penetrate the skin barrier. Recently developed liposomal spherical nucleic acids overcome this barrier and enable topical delivery of small synthetic antisense oligonucleotides capable of specifically targeting inflammatory pathways underlying psoriasis pathogenesis.
Our improved understanding of the molecular mechanisms underlying a broad range of skin disorders is driving the development of novel therapeutics designed to target specific molecular pathways implicated in disease pathogenesis. Topical application is a particularly appealing delivery approach for these new targeted therapeutics. The skin is readily accessible, and topical delivery can physically target drugs to the site of disease expression. This enables high local drug concentrations to augment efficacy, while increasing safety by reducing systemic exposure and reducing cost. Topical delivery is also less invasive and can typically be self-administered, improving both patient compliance and convenience. In this issue of the JID, Liu et al. [Liu et al., 2019] describe a promising topical oligonucleotide delivery approach that exploits advances in nanotechnology to deliver short anti-sense oligonucleotides specifically targeting the IL-17 receptor as a topical therapy for psoriasis.
The robust barrier function of the hydrophobic stratum corneum and superficial epidermis remains a formidable obstacle to the development of topical therapies. Without physical or chemical penetration enhancers, only molecules with certain physicochemical characteristics (high lipophilicity and molecular weight < 500 Da) can effectively penetrate intact skin [Prausnitz et al., 2008]. Biologic agents used in the treatment of psoriasis including the anti-tumor necrosis factor-α (TNF-α) agents, anti-interleukin antibodies anti-IL-12/IL-23, anti-IL-23/IL-39, and anti-IL-17, and anti-IL-17 receptor antibody have shown considerable efficacy as systemic agents, but fail to penetrate the skin at effective concentrations without penetration enhancers. Several cutaneous drug delivery methods, including thermal ablation (heating up to or above 100 °C for microseconds), iontophoresis (applying electric current), and electroporation (providing high-voltage pulses) have been utilized to temporarily enhance the permeability of skin to macromolecules [Prausnitz et al., 2008]. However, the broad adoption of these methods for skin-targeted delivery of psoriasis therapeutics is limited by their resource-intensive nature. More recently, dissolving microneedle arrays (MNAs) have appeared as a minimally-invasive technology for intercutaneous delivery of micro- and macro-molecules, including proteins and monoclonal antibodies (mAbs) [Prausnitz et al., 2008, Korkmaz et al., 2015]. Although MNAs present unique platforms to quickly and reliably test the therapeutic efficacy of novel biomolecules in pre-clinical psoriasis models, they have yet to be evaluated for safe and prolonged treatment of psoriasis in clinical settings [Lee et al., 2018]). In addition to mAb based therapeutics, gene silencing strategies utilizing synthetic nucleic acids (i.e., antisense DNA and siRNA) are emerging as an attractive alternative for the management of inflammatory skin diseases [Wraight et al., 2001, Zheng et al., 2012]. These approaches hold promise for the treatment of several skin disorders, but oligonucleotides are also relatively large and highly charged molecules. Skin penetration is problematic, and intracellular delivery [Zheng et al., 2012] often requires delivery vehicles (e.g., cationic polymers as transfection agents or recombinant viral vectors) that make topical delivery even more challenging. Electroporation achieves permeabilization of both the stratum corneum and cell membranes, and has been used in pre-clinical settings, but its clinical adaptation has been hampered due to its demanding nature [Wraight et al., 2001].
Liu et al. [Liu et al., 2019] describe the application of liposomal SNAs (L-SNAs) to address the challenges of topical oligonucleotide delivery. Spherical nucleic acids are three-dimensional (3D) nanoparticles coated with highly oriented oligonucleotides [Mirkin et al., 1996]. Unlike traditional gene delivery methods with linear or circular nucleic acids, these unique 3D nano-scale structures have been shown to reliably enter cells (they have been successfully validated on several different cell types) primarily via scavenger receptor-mediated endocytosis, with no cytotoxic effects and without the use of transfection materials, recombinant viral vectors, or other complex technologies [Zheng et al., 2012]. Although their intracellular trafficking has yet to be fully elucidated, SNAs exhibit enhanced cellular uptake, remarkable intracellular stability, and minimal immunogenicity due their high density and geometric configuration [Zheng et al., 2012]. Collectively, SNAs have shown great promise as therapeutic agents capable of simultaneous transfection and gene regulation in a wide range of cell types [Zheng et al., 2012].
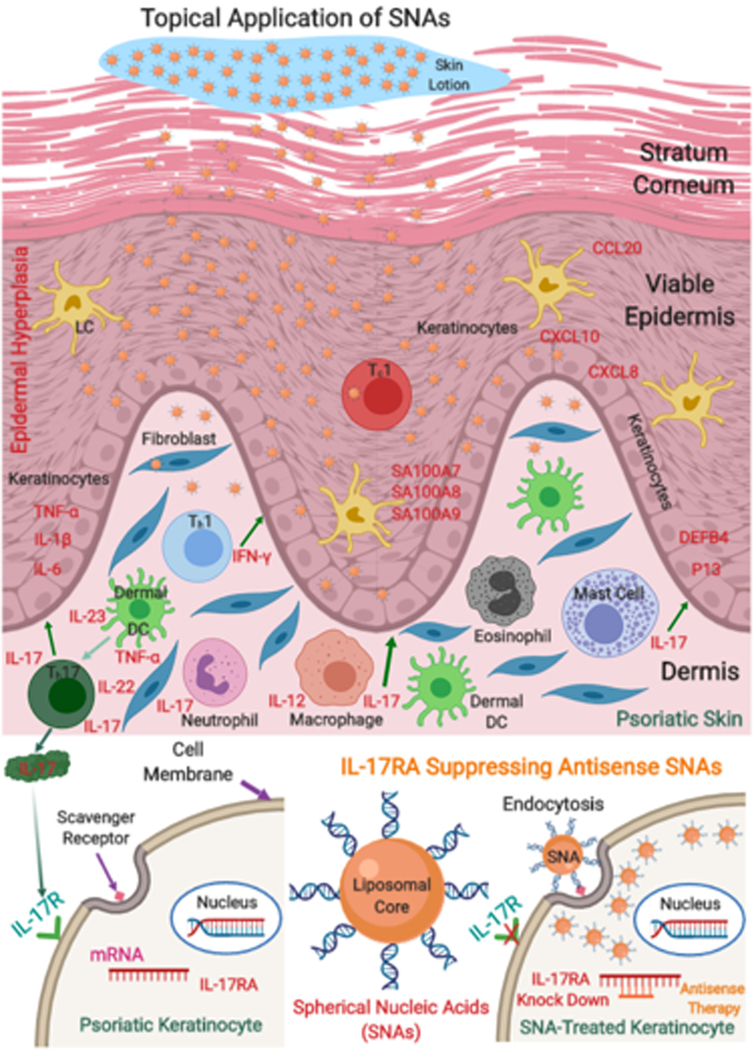
Dr. Paller and collaborators constructed dense particles consisting of short gapmer anti-sense oligonucleotides targeting the IL-17A receptor embedded in a lipid core (IL17RA L-SNAs). Leveraging recent studies demonstrating the critical role of the IL-23/Th-17 signaling pathway in the pathogenesis of psoriasis, they proposed that topically applied liposomal SNAs targeting the gene encoding the IL-17A receptor in keratinocytes would reduce IL-17A receptor expression and the induction of IL-17A receptor activation dependent proinflammatory mediators, thereby preventing and potentially reversing the development of psoriasis (Figure 1) In the imiquimod induced mouse model of psoriasis, they applied these L-SNAs topically with imiquimod to the same skin site throughout the course of psoriasis induction. L-SNAs penetrated the skin and reduced IL-17A receptor expression (confirmed by both qRT-PCR and western blotting). IL-17RA L-SNA treated animals demonstrated significant clinical and histologic reductions in the psoriasis-like phenotype compared to those treated with sham L-SNA controls, and these changes were associated with significant reductions in mRNA expression of psoriasis markers. To evaluate L-SNA delivery and function in human skin, they applied IL17RA L-SNAs to human skin explants. IL17RA L-SNAs successfully penetrated human skin explants and knocked down IL-17RA in a dose-dependent manner. As an in vitro model of human psoriasis, they generated human psoriatic 3D rafts by treating 3D organotypic rafts every other day with a mixture of TNF-α, IL-17 and IL-22. This cytokine-induced 3D raft model exhibited transcriptional changes associated with human psoriasis, confirmed by significantly increased expression of TNF-α, DEFB4, and P13, and downregulation of loricrin (LOR) as compared to non-treated 3D human keratinocyte rafts. Using this model, the authors showed that applying L-SNAs topically before, and continuously during, cytokine delivery reduced expression of IL17RA and the IL-17 induced genes IL17C, DEFB4, TNF-α and P13.
Figure 1.
Topical liposomal spherical nucleic acid (L-SNA) treatment of psoriasis. Topically applied L-SNAs delivering IL-17RA antisense oligonucleotides are internalized by IL-17R expressing keratinocytes in psoriatic skin, reducing IL-17R expression and the expression of IL-17 dependent proinflammatory genes involved in psoriasis pathogenesis.
Taken together, these results demonstrate that the fabricated IL17RA L-SNAs could penetrate mouse and human skin, and deliver anti-sense oligonucleotides to specifically knock down the IL-17A receptor. The results are consistent with the investigators previous studies in which topically delivered TNF-α suppressing antisense L-SNAs reduced TNF-α expression and the psoriatic phenotype in the same models [Lewandowski et al., 2017]. Together, the results establish the general feasibility of the approach, and suggest the intriguing potential of future combination L-SNA therapies for psoriasis and other skin pathologies, including wound healing and inflammatory skin diseases.
It is noteworthy that in both models studied, psoriatic features have been shown to be heavily dependent on IL-17 induced effects on keratinocytes. In the human 3D model, the development of psoriatic features depends on the presence of exogenously added IL-17. In the mouse imiquimod model, it has recently been shown that specific deletion of IL-17RA in keratinocytes protected mice from developing the psoriasiform phenotype [Moos et al., 2019].
While results thus far are encouraging, clinical efficacy will likely depend on L-SNA penetration and delivery efficiency. The knockdown approach is both concentration and time dependent, requiring the sustained presence of relatively high levels of anti-sense oligonucleotides in the target cells. In the models used thus far, L-SNA applications were applied continuously to essentially normal skin to prevent disease development. Changes in the stratum corneum and epidermis that develop with the psoriatic phenotype could have important effects on both of these variables. Prior studies suggested that L-SNAs could penetrate to the dermis in explants of psoriatic skin [Lewandowski et al., 2017], but the efficiency of this process is unknown. In the current models, L-SNA treatment of essentially normal skin in these prevention models reduced IL-17RA mRNA by 57% in mouse skin and as much as 72% in cytokine treated human 3D skin cultures, and the reductions were time dependent. In established psoriasis, the barrier is more pronounced, the epidermis is thicker, target keratinocytes are more numerous, and IL-17A receptor expression is upregulated, all of which suggest greater challenges for effective L-SNA delivery. It is difficult to predict how much and how often topical L-SNA application will be required for clinical efficacy. Limitations in current psoriasis models suggest that these issues can only be addressed through clinical trials, which are now underway.
Despite remaining challenges, the current studies provide important proof of concept for the future development of topical L-SNA therapies for the treatment of psoriasis. They also support the feasibility of L-SNA delivery for the treatment of other skin diseases. In particular, L-SNA delivery has the potential to be effective for skin pathologies in which the skin barrier may be normal or compromised, and thereby presents less of an obstacle to delivery, such as wound healing, immunobullous diseases, and disorders of the hair follicle. L-SNA delivery has the potential to be an enabling technology for the topical delivery of molecularly targeted therapies, dramatically expanding the scope of topical interventions available to dermatologists and other health care providers.
Clinical Implications.
Improved understanding of the molecular mechanisms underlying a broad range of skin disorders is driving the development of novel therapeutics designed to target specific molecular pathways implicated in disease pathogenesis.
In preclinical models, topically applied liposomal spherical nucleic acids deliver short anti-sense oligonucleotides specifically targeting the IL-17 receptor to reduce the severity of psoriasis-like inflammation.
Liposomal spherical nucleic acids could enable topical application of molecularly targeted therapeutics to treat psoriasis and other skin diseases, dramatically expanding the scope of topical interventions available to dermatologists and other health care providers.
ACKNOWLEDGMENTS
LDF is supported by National Institutes of Health (NIH) Grants R01AR074285, R01AR071277, R01AR068249, and P50CA121973.
Footnotes
Conflict of Interest
EK states no conflict of interest. LDF is a scientific advisor for and holds an equity position in BrainStage and SkinJect.
References
- Korkmaz E, Friedrich EE, Ramadan MH, Erdos G, Mathers AR, Ozdoganlar OB, et al. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater, 24 (2015), pp.96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KT, Thiede R, Guido N, Daniel WL, Kang R, Guerrero-Zayas MI, et al. Topically delivered tumor necrosis factor-α-targeted gene regulation for psoriasis. J Investig Dermatol, 137 (2017), pp.2027–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jung YS, Kim GM, Bae JM. A hyaluronic acid-based microneedle patch to treat psoriatic plaques: a pilot open trial. Br J Dermatol, 178 (2018), pp.24–25. [DOI] [PubMed] [Google Scholar]
- Liu H, Kang R, Lewandowski KT, Yu JM, Radecki S, Daniel WL. et al. Targeting the IL-17 receptor using liposomal spherical nucleic acids as topical therapy for psoriasis, J Investig Dermatol, 140 (2019),pp.- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature, 382 (1996), pp. 607–609 [DOI] [PubMed] [Google Scholar]
- Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-Induced Psoriasis in Mice Depends on the IL-17 Signaling of Keratinocytes, J Investig Dermatol, 139 (2019),pp.1110–1117. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol, 26 (2008), pp. 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight CJ, White PJ. Antisense oligonucleotides in cutaneous therapy. Pharmacol Ther, 90 (2001), pp. 89–104. [DOI] [PubMed] [Google Scholar]
- Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Lordanov H, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci USA, 109 (2012), pp.11975–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]