Abstract
Tumor progression is associated with progressive immunosuppression mediated in part by T regulatory cell(s) (Treg) and/or myeloid-derived suppressor cell(s) (MDSC). Development of strategies to reduce populations of immune cells with suppressive function in cancer patients may enable the induction or recovery of immunity against tumor cells, which may limit or reverse disease progression. With a goal of developing Treg and MDSC neutralizing strategies to treat mycosis fungoides (MF) and Sézary syndrome (SzS), we determined the association between disease stage and suppressor cell populations in patients with MF/SzS, including those responding to therapy. We found elevations in Treg populations, across Treg subtypes, in patients with SzS, and these Treg markedly suppressed proliferation of autologous CD4+CD25− responder T cells. Interestingly, while MDSC numbers were not increased in MF/SzS patients, MDSC from patients with stage IB and above produced significantly more reactive oxygen species than those from stage IA MF patients and control cohorts. Therapy with the CD25-targeting agent denileukin diftitox or IFN-α2b was associated with a reduction in Treg numbers or MDSC function, respectively. These studies identify potential mechanisms of action for these therapies and support the development of coordinated strategies targeting both Treg and MDSC activities in patients with MF/SzS.
Keywords: T regulatory cells, Myeloid-derived suppressor cells, Cutaneous T-cell lymphoma, Sézary syndrome, Mycosis fungoides
Introduction
Mycosis fungoides (MF) and Sézary syndrome (SzS) are T-cell malignancies perpetuated by immune dysregulation. Tumor progression is associated with progressive Th2 skewing [1], decreased antigen-specific T-cell responses [2], and impaired cell-mediated cytotoxicity [3]. Late-stage MF/SzS is associated with declining immune competence resulting in severe, life-threatening infections, and a high incidence of secondary malignancies [4].
Tumor-induced immunosuppression is an important facilitating factor for immune evasion. Several cell types, including Treg and myeloid-derived suppressor cell(s) (MDSC), contribute to tumor-associated immunosuppression. Treg has been shown to suppress the host immune response overall and dampen cytotoxic immune responses (CD8+/NK cells) thought to be critical to tumor immunosurveillance [5]. Over the past two decades, diverse subpopulations of CD4+ Treg have been characterized. They are either induced in the periphery [e.g., CD4+CD25−CTLA4+ T regulatory type 1 (Tr1) and CD4+CD25−Foxp3+ suppressive T-helper 3 (Th3) cells] or naturally occurring with a CD4+CD25+Foxp3+ phenotype [5, 6]. Recent findings indicate that CD4+CD25+Foxp3+ Treg may not only be derived from the thymus (tTreg), but also can be generated from Foxp3− conventional T cells in the periphery (pTreg) during chronic inflammatory conditions [7]. All populations of cells with CD4+CD25+Foxp3+ phenotype will be further designated as nTreg. nTreg induce cytolysis, disrupt metabolic activities, inhibit the maturation of dendritic cells, and secrete IL-10, IL-35, TGF-β1, and Galectin-1 [8]. Tr1 cells secrete IL-10, TGF-β1, and interferon (IFN)-γ [9]. Th3 cells secret TGF-β and IL-4 [10]. Reduction of Treg in cancer patients can enable the recovery or induction of tumor-specific immune responses capable of limiting the disease [11]. Characterization of Treg’ phenotypes in humans is still challenging without concomitant evaluation of their functional state.
MDSC are heterogeneous populations of immature myeloid cells in the mononuclear fraction that mediate immunosuppression in large part because of their Jak3/Stat3-dependent secretion of both reactive oxygen species (ROS) and Arginase-I (ARGI) into the tumor microenvironment [12]. Lack of specific markers has complicated their characterization in humans [13]. Several observations suggest that MDSC are increased in the peripheral blood of patients with hematologic malignancies [14, 15]. Data from patients with solid tumors also indicate direct correlations between metastatic tumor burden and the number of MDSC in peripheral blood [16, 17]. The heterogeneity of these cells presents considerable obstacles for the development of elimination strategies and/or for neutralization of their immunosuppressive effects. In the present study, we evaluate for the first time the impact of IFN-α2b on the immunosuppressive potential of MDSC in patients with MF/SzS.
As the previous studies of immunosuppressive cells in MF/SzS patients have drawn different conclusions [18–20], and no study to date has addressed the role of MDSC in MF/SzS, and we investigated Treg and MDSC populations in MF/SzS patients with different levels of disease activity. We evaluated the effects of strategies directed towards reduction of cell-mediated immunosuppression, including the use of denileukin diftitox (DD), an IL-2-diphtheria recombinant fusion protein (DAB389IL-2) thought to target and eliminate CD25 expressing cells, and IFN-α2b which, we show for the first time, may reduce MDSC activity.
Materials and methods
Settings and participants
A total of 12 patients were treated while enrolled in the study NCT00254332 (IRB#0509084). 65 patients were enrolled in the study NCT00177268 (IRB#0411029). Declaration of Helsinki protocols were followed. All study participants gave written and informed consent before enrollment. All patients were seen in Cutaneous Lymphoma Center of Excellence (University of Pittsburgh Medical Center, Pittsburgh, PA, USA) and followed up prospectively. The average follow-up was 88.9 months (range from 36.1 to 125.0 months). Of the total 77 patients, 26 patients had SzS and 51 patients had MF without peripheral blood involvement. None of the patients were on systemic therapies at for at least 3 weeks before donating blood. The patients with MF were separated into two distinct groups (Table 1). Group 1 was comprised of patients with stage IA MF, with only minor skin involvement present (< 10% patches). Group 2 had patients with stage IB or greater MF, with skin involvement of at least 10% or more of total body surface area. None of these MF patients had extracutaneous disease. All SzS patients comprised Group 3. 22 out 26 (84.6%) patients with SzS had stage B2. The patient with large cell transformation was excluded from the study. Nine healthy volunteers, who were willing to donate 20 mL of peripheral blood, comprised the control group.
Table 1.
Patient characteristics
| Characteristics | All patients (N = 77) | |
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 62.2 | |
| Range | 49–86 | |
| Sex | ||
| Male | 31 | 40.8 |
| Female | 48 | 59.2 |
| CTCL | ||
| MF, IA | 21 | 27.2 |
| MF, > IB | 30 | 39.0 |
| T2 | 17 (56.7%) | |
| T3 | 13 (43.3%) | |
| SzS | 26 | 33.8 |
| ≤ 10,000 Sézary cells/mm3 | 17 (65.4%) | |
| > 10,000 Sézary cells/mm3 | 9 (34.6%) | |
Suppression of T-cell proliferation by freshly separated autologous CD4+CD25+ T cells
CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi Biotec, Auburn, CA, USA) was used to isolate cells. The median purity of isolation procedures was > 90%. CD4+CD25− T cells were stained with 1.5 µM CFSE (Molecular Probes/Invitrogen, Carlsbad, CA, USA) for 10 min at room temperature. After quenching by FBS, cells were washed with PBS. A total of 5 × 104 of CD4+CD25+ cells were cultured in triplicate with 5 × 104 CFSE-labelled autologous CD4+CD25− T cells stimulated with anti-CD3 (1 μg/mL) (ATCC, Rockville, MD, USA) and anti-CD28 Abs (1 μg/mL) (Miltenyi Biotec, Auburn, CA, USA) in AIM V medium supplemented with IL-2 at 150 IU/mL. As negative controls, CFSE-labelled CD4+CD25− cells were stimulated with anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL) in the absence of fresh CD4+CD25+ cells. CFSE expression was measured by flow cytometry on day 5 after stimulation. CFSE data were analyzed using the ModFit software. The percentages of suppression were calculated based on the proliferation index (PI) of responder cells alone compared with the PI of cultures containing responders and Treg. The program determines the percent of cells within each peak, and the sum of all peaks in the control culture is taken as 100% of proliferation and 0% of suppression.
Identification of the TCR-Vβ family of the malignant clone
Staining of PBMCs was performed according to manufacturer instructions (IOTest Beta Mark TCR-Vβ repertoire kit, Beckman Coulter). The Vβ family of the malignant clone was identified as one that was exceeding the average Vβ family size for 2 SD according to the data provided by Beckman Coulter.
Assessment of intracellular ROS
Cells were washed out of culture medium and left for 10 min in PBS, re-suspended at 1.5 × 106 100 µL in pre-warmed Hank’s buffer containing 2% FBS. 1 µM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) (Invitrogen, Carlsbad, CA, USA) was added to the cell suspension at 37 °C for 30 min. Washed cells were re-suspended in flow buffer and fluorescence emitted at 535 nm was read after excitation at 485 nm. Cells stimulated with 3% H2O2 were used as positive control.
Flow cytometry
PBMC from peripheral blood (at least 15 mL of blood collected and processed within 2–4 h) were isolated by Ficoll–Paque gradient (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA). Cell staining was performed with anti-CD4-APC (RPA-T4) from BD Pharmingen (San Diego, CA, USA), anti-CD25-PE-Cy7 (2A3) from Caltag Laboratories (Burlingame, CA, USA), anti-Foxp3-FITC (236A/E7) from eBioscence (San Diego, CA, USA), and anti-CD127-PE (HIL-7R-M21) from BD Pharmingen (San Diego, CA, USA). The following markers were used for MDSC: anti-CD33-PE-Cy5 (WM53), anti-CD14-FITC (M5E2), anti-CD11b-APC (ICRF44), anti-CD86-PE (FUN-1), all from BD Pharmingen (San Diego, CA, USA), and anti- HLA-DR-Pacific Blue (L243) from BioLegend (San Diego, CA, USA). The cells were evaluated using the FACSAria and CellQuest Software (Becton Dickenson, San Jose, CA, USA). The absolute numbers were calculated based on the commercial complete blood count data drawn on the same day. The FlowJo software (Tree Star Inc, Ashland, OR, USA) was used for analysis of flow cytometric data.
Confocal microscopy
Tissue sections were incubated with rat anti-human Foxp3 (236A/E7) from eBioscence (San Diego, CA, USA), mouse anti-human CD4 (L3T4) from Biodesign (Saco, ME, USA), or mouse anti-human CD25 (2A3) from Caltag Laboratories (Burlingame, CA, USA). Sections were then treated with a mixture of goat anti-rat IgG (H + L) Alexa Fluor® 488 from Molecular Probes (Carlsbad, CA, USA) or goat anti-mouse IgG (H + L) Cy3 from Jackson Immuno (West Grove, PA, USA). Sections were counterstained with Hoechst (Sigma-Aldrich, St. Louis, MO, USA). Images were captured on Olympus BX51 fluorescence microscope (Olympus America) using the Magnafire software.
Assessment of serum arginase
Serum arginase was assessed by ELISA according to manufacturer’s instructions (Hycult Biotech, Plymouth Meeting, PA, USA).
Statistical analysis
The statistical analysis was based on the calculation of mean and standard deviation. The difference between two means was compared by a two-tailed unpaired Student’s t test without the assumption of equal variances. Correlation analysis measured the relationship between two or more variables. A p value of less than 0.05 was considered statistically significant.
Results
Treg populations are increased in SzS
Previous investigations showed either no differences in the percentage of nTreg [21, 22] in MF/SzS patients compared to healthy controls, or reduced portions of nTreg in the peripheral blood [23, 24]. When Foxp3 positive cells in skin biopsies were quantified, increases in the absolute number of Foxp3 positive cells were found in the early stage of MF (patch and plaque disease), and not in tumor stage or SzS [19, 20]. In another study, lower numbers of CD4+Foxp3+ cells were observed in lesional skin biopsies of MF patients compared to those of patients with atopic dermatitis [25]. Neither absolute numbers of Treg nor relative changes in Treg subsets over time have been measured in MF/SzS.
To investigate the association of various Treg subsets with tumor burden, we evaluated the percentage and the absolute number of regulatory T lymphocytes with the nTreg, Th3 cell, and Tr1 cell phenotypes in the peripheral blood of MF/SzS patients (Fig. 1a). It has previously been shown that nTreg are particularly enriched in the CD4+CD25highFoxp3+ subset, while CD4+CD25+Foxp3+ phenotype may contain a significant portion of activated cells [26]. Consistently across all samples, the proportion of CD4+CD25highFoxp3+ was 20–25% among CD4+CD25+Foxp3+ population. In addition, to discriminate nTreg from the conventional (activated) CD4+ T cells, a low expression of CD127 (the α-chain of the IL-7 receptor) was proposed as a co-marker together with the expression of FOXP3 and CD25 [27]. We have performed the co-staining and demonstrated the direct correlation between the number of CD4+CD25+CD127− cells and the number of CD4+CD25+Foxp3+ cells in our patients (Fig. 1b). Due to these findings, we consider the phenotype of CD4+CD25+Foxp3+ suitable for further analysis of nTreg. We found a trend towards having a higher proportion of CD25+Foxp3+ cells among CD4+ T cells in advanced disease: 4.1 ± 2.1% of CD25+Foxp3+ among CD4+ T cells in normal volunteers (n = 9), 5.7 ± 2.5% in patients with IA MF (n = 21), and 6.9 ± 2.4% in patients with > IB MF (n = 30). The absolute numbers of nTreg and Th3 cells, but not Tr1 cells, were statistically higher in patients with SzS than in patients with MF (Fig. 1c–e). The highest absolute numbers of nTreg, Tr1 cells, and Th3 cells were found in patients with T4 disease (25 out of 26 patients with stage T4 had SzS) (Fig. 1f). 69.4 ± 14.1% of CD4+Foxp3+ cells in patients with MF co-expressed CD39, a functional marker of regulatory effector/memory-like T cells, compared to only 0.2 ± 0.2% of CD4+Foxp3+ cells co-expressing CD39 in healthy volunteers (p < 0.05) (data not shown). Disease stage comparisons also suggested that the absolute number of nTreg was slightly higher in stage T1 (although not statistically significant), consistent with recent data obtained from the analysis of nTreg in skin samples [19, 20].
Fig. 1.
Characterization of Treg in MF/SzS patients and healthy volunteers. a PBMCs gating strategy: CD25+Foxp3+ (nTreg), CD25-Foxp3+ (Th3 cells), and CTLA-4+CD25− (Tr1 cells) cells were selected within the CD4+ cell population. b Correlation of absolute numbers of CD4+CD25+CD127− and CD4+ CD25+Foxp3+ cells. c Absolute number of nTreg in peripheral blood of controls and patients with IA MF, > IB MF, or SzS (M ± SEM). *p < 0.05; ***p < 0.001. d Absolute number of Th3 cells in peripheral blood of controls and patients with IA MF, > IB MF, or SzS (M ± SEM). ***p < 0.001. e Absolute number of Tr1 cells in peripheral blood of controls and patients with IA MF, > IB MF, or SzS (M ± SEM). f Absolute number of nTreg, Th3 cells, or Tr1 cells in peripheral blood of controls and patients with MF/SzS according to T stage. g nTreg gating strategy in SzS patients: within the tumor subset that is TCR-Vβ2 positive (TS) and the non-tumor cell subset that is TCR-Vβ2 negative (NTS) (a representative patient). h Absolute number of CD4+CD25+Foxp3+ cells in peripheral blood of controls and patients with SzS within a tumor subset (T-SzS) and a non-tumor subset (NT-SzS). *p < 0.05, ***p < 0.001. i Isolated CD4+ CD25+ cells suppress proliferation of CFSE-labelled autologous CD4+CD25− T cells in proliferation assays. A representative experiment (6 performed). j CD4+CD25+ lymphocytes from patients demonstrate significantly higher suppressive activity than normal controls. ***p < 0.001
In a previous Treg analysis [21, 22, 24], malignant and non-malignant cells in the CD4+ population were not discriminated. Without discrimination, the proportion of nTreg in the non-malignant CD4+ population could be underestimated in the presence of high numbers of circulating CD4+ tumor cells. Consistent with this notion, and previous observations [22], we found several SzS patients, whose absolute number of nTreg and Tr1 cells was significantly higher (more than 2.5 times) than in the rest of the SzS patient population (data not shown). Therefore, to control for the presence of malignant CD4+ T cells in SzS patients, we differentially evaluated peripheral lymphocytes by discriminating tumor vs. non-tumor T cells based on specific TCR-Vβ expression by the malignant clone. We found that in general, cells demonstrating the nTreg phenotype resided within the non-malignant T-cell population (Fig. 1g). When accounting for CD4+ tumor cells, it became evident that the proportion of nTreg in the non-malignant CD4+ T-cell subset of SzS patients was consistently elevated (Fig. 1h). Importantly, CD4+CD25+ cells from MF/SzS patients secreted IL-10 (not shown) and suppressed proliferation of autologous CD4+CD25− cells in a dose-dependent manner (representative patient, Fig. 1i). This suppressive function was considerably higher than that observed with CD4+CD25+ cells from normal controls (85 ± 7 vs. 12 ± 6%) (Fig. 1j), indicating that this population in SzS patients are nTreg. These observations provide a possible explanation for the lack of suppressive activity in the normal controls.
Cases of malignant proliferation of Treg in SzS
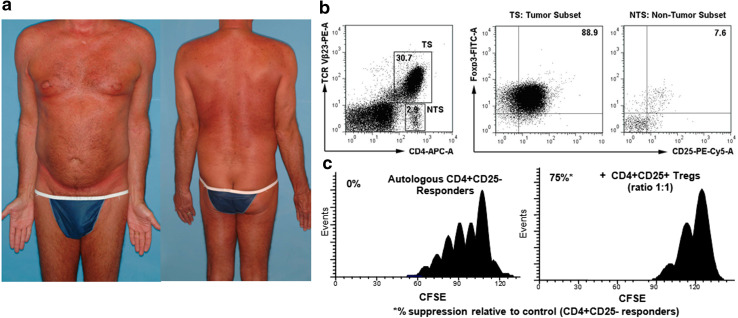
Cases of expression of Foxp3 by a malignant CD4+ T-cell clone have been reported, but the frequency and relevance of these findings remain unclear. One interpretation is that those Foxp3+ tumor cells possessed suppressive function [24]. Alternatively, it has been proposed that acquired expression of Foxp3 by CD4+ tumor cells is a consequence of cell activation, rather than a change in neoplastic phenotype per se [28] or that those cells may not be functional [29]. We identified 2 out of 77 patients with MF/SzS who demonstrated a substantial proportion of cells (> 50%) expressing a Treg phenotype within the tumor. One patient was a 64-year-old male with SzS stage IVA1 (T4N1M0B2) at diagnosis, who had 64% CD25+Foxp3+ cells in his Vβ2+ tumor. Another patient was a 59-year-old male with SzS stage IVA1 (T4N0M0B2) at diagnosis (Fig. 2a), with 89% CD25+Foxp3+ cells in his Vβ23 positive CD4+ tumor (Fig. 2b). Importantly, we found that the malignant cells with a Treg phenotype demonstrated marked suppressive function (Fig. 2c).
Fig. 2.
Malignant proliferation of T regulatory cells may manifest as SzS. a 59-year-old male with SzS stage IVA1 (T4N0M0B2) at diagnosis. b 89% of clonal Vβ23 positive CD4 cells express CD25+Foxp3+. c Malignant cells with nTreg phenotype exhibit cell–cell suppression activity (incubation at 1:1 ratio for 5 days)
Correlation of MDSC numbers and function with MF/SzS tumor burden
To evaluate the association between MDSC and disease activity, we identified MDSC expressing the CD33+CD11b+CD86+CD14−HLA-DR− phenotype in MF/SzS patients (Fig. 3a). We found that in the peripheral blood, the average proportions of CD33+CD14− cells among CD11b+ cells were similar across disease stages [2.5 ± 0.7% in normal volunteers (n = 7), 1.4 ± 0.9% in patients with IA MF (n = 11), 1.0 ± 0.7% in patients with > IB MF (n = 8), and 2.6 ± 1.3% in patients with SzS (N = 26)] (representative patients, Fig. 3b). When analyzing absolute numbers of MDSC, similar results were obtained, with only > IB MF patients demonstrating a significant difference in MDSC numbers (p < 0.05) (Fig. 3c). Importantly, despite similar or decreasing MDSC numbers with increasing tumor burden, we found that ROS production by MDSC was highest in patients with > IB MF (p < 0.01) (Fig. 3d), suggesting that MDSC activity, rather than absolute numbers in peripheral blood, may correlate with disease progression.
Fig. 3.
Characterization of MDSC in MF/SzS patients and healthy volunteers. a MDSC gating strategy: MDSC were defined as cells with the CD33+CD14−CD11b+HLA-DR−CD86+ phenotype. b Representative flow of percentage of MDSC among CD11b in controls and patients with MF/SzS. c Absolute number of MDSC in peripheral blood of controls and patients with IA MF, > IB MF, or SzS. *p < 0.05. d ROS production by MDSC in controls and patients with IA MF, > IB MF, or SzS. **p < 0.01; ***p < 0.001
Therapy with DD decreases the number of T lymphocytes and nTreg
DD was an FDA-approved therapy for MF/SzS and is a recombinant IL-2-diphtheria toxin fusion protein targeted to the IL-2-receptor expressed on T cells, and it does not cause myelosuppression. A phase III trial of DD in 73 patients with refractory CTCL who had received three or more prior therapies demonstrated a 30% overall response rate [30]. The clinical response to DD was durable, lasting 277 days on average [31]. We evaluated the effect of DD therapy on nTreg (since only this population of T regulatory cells express a high level of IL-2R) in MF/SzS patients. DD was administered intravenously as a cycle of daily infusions for five consecutive days with 2-week breaks between cycles. After the initial cycle of therapy, we observed immediate decreases in peripheral Treg (Fig. 4a). We found that, on average, a cycle of DD reduces CD4+Foxp3+ cells by median relative intra-patient change of − 29% (94% confidence interval − 83 to − 20%), p = 0.03. 9/12 patients treated with DD had long-term follow-up. The median survival of the responders was 104.9 vs. 20.9 months in nonresponder group (p = 0.07). The response to DD correlated with a statistically significant 20–45% decrease in the absolute number of Treg after the first cycle of DD (r = − 0.6). Two out of three non-responders had an increase in the absolute number of Treg after the first cycle of DD (Fig. 4b). The decrease in the number of Treg in the peripheral blood correlated with a decrease in the number of Treg in cutaneous lesions (Fig. 4c, a representative patient, whose lymphoma was not CD25+Foxp3+). In five patients, we were able to evaluate Treg over multiple courses of DD, and over more than 500 days (Fig. 4d, e). A decrease in the number of Treg after DD was often transient, as the number of Treg rebounded between cycles in some cases. Nevertheless, even in those patients, the number of Treg progressively decreased within a treatment cycle and with increasing treatment cycles to levels well below those initially observed. In those patients, overall reductions in Treg were sustained throughout the study period (> 500 days). As expected, we did not observe significant changes either in the percentage or in the absolute number of MDSC in these patients (Fig. 4f, representative patient, and Fig. 4g): median relative intra-change was + 1.9% with 95% confidence interval ranging from − 119.8 to + 123.5% (p = 0.39).
Fig. 4.
Denileukin diftitox (DD) depletes CD4+CD25+Foxp3+ cells but not CD11b+CD33+CD14− cells in patient peripheral blood and tumors. a Percentage of Treg in the peripheral blood among CD4+ cells of a representative patient before and after a 5-day course of DD. b Absolute number of Treg in the peripheral blood of patients before and after DD (9 patients; 12 different before and after cycles) in responders and non-responders (percent from baseline). c Treg in the skin of MF/SzS patients before and after the first cycle of DD. Skin samples were evaluated for co-expression of CD4 and Foxp3 by fluorescence microscopy. Left: before therapy; right: after therapy. Nuclei stained with Hoechst dye (blue) ×10. d Variation in the percentage of CD4+CD25+Foxp3+ cells during extended DD therapy (patient 3). e Variation in the percentage of CD4+CD25+Foxp3+ cells during extended DD therapy (patient 5). f Percentage of MDSC before and after a 5-day course of DD. Results from a representative patient are shown. g Absolute number of MDSC in peripheral blood of patients before and after a 5-day course of DD in five patients
Efficacy of INF-α2b therapy in MF/SzS correlates with reduction of ROS and arginase production by MDSC
The inhibition of MDSC suppressive function has been associated with significant reductions in tumor spread, even in cases where local tumor growth was not altered [32]. A murine model demonstrated previously that IFN-α was capable of inhibiting the suppressive effect of MDSC by inducing MDSC maturation and differentiation [33]. IFN-α is the most commonly prescribed biological response modifier for MF/SzS [34]. The overall response rate of IFN-α, when used as a single agent, is approximately 50%, with 17% representing complete remission [35] (Fig. 5a). We sought to determine whether the benefit of IFN-α2b therapy in patients with MF correlated with changes in the number and/or function of MDSC.
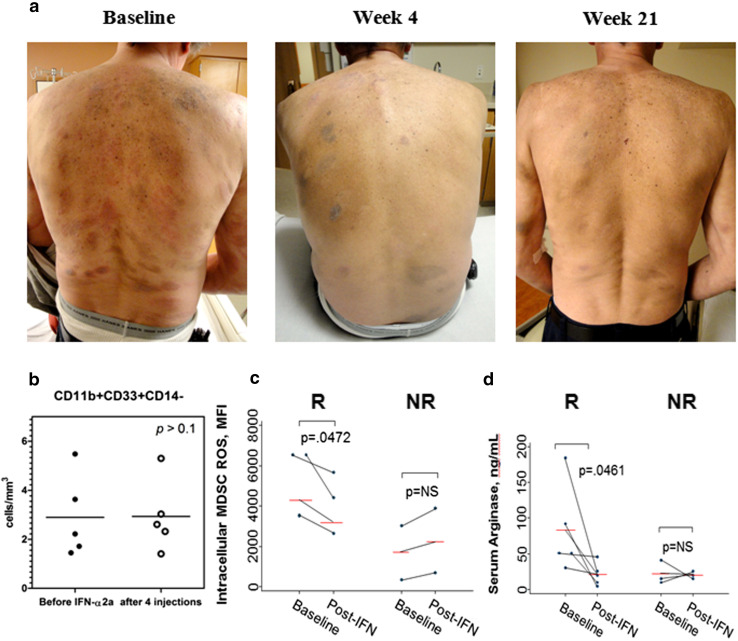
Fig. 5.
Effect of INF-α2b is attributed to a reduction of ROS production by MDSC in patients with MF. a Clinical benefit of therapy with INF-α2b in a representative patient with IB MF. Significant improvement after four injections of IFN-α2b (150 mcg weekly) with sustainable effect through 21 doses. b Absolute number of CD11b+CD33+CD14− in peripheral blood of patients before and after IFN-α2b (five patients). c Response to IFN-α2b in patients with MF is associated with a decrease in ROS production by MDSC. R responder, NR nonresponder. d Response to IFN-α2b in patients with MF is associated with a reduction in serum arginase. R responder, NR nonresponder
To evaluate the effect of IFN-α2b therapy, we followed seven patients with MF who were treated weekly with subcutaneous injection of PEGylated (PEG)-IFN α2b (PEG-Intron) at 1.25 μg/kg. MDSC were detected in peripheral blood before the beginning of therapy and after four injections. In spite of all patients developed neutropenia after four injections of PEG-Intron, MDSC numbers remained unchanged (Fig. 5b). Similar results were seen in patients, whose disease progressed while being treated with PEG-Intron (data not shown). In sharp contrast, we found that ROS production by MDSC was reduced significantly in responders (p < 0.05), while the level of ROS remained unchanged in the patients who did not benefit from therapy with PEG-Intron (Fig. 5c). Similarly, serum arginase levels decreased significantly in responders (Fig. 5d). Taken together, these results suggest that the benefit of INF-α2b therapy in patients with MF is mediated in part by inhibition of MDSC suppressive function.
Discussion
Reduction in the numbers of immunosuppressive cells in cancer patients could enable immune surveillance and/or facilitate the induction of tumor-specific immune responses when used as an adjunct for tumor-specific immunotherapy [36, 37]. Treg and MDSC have been shown to be involved in tumor-associated immunosuppression. Here, we sought to evaluate the number of Treg and MDSC in peripheral blood of MF/SzS patients across stages of the disease and to directly assess the effects of CD25-targeted cell depletion on Treg, and IFN-α treatment on MDSC in MF/SzS patients.
Our results differ from the previous investigations that showed either no difference in the percentage of nTreg [21, 22] or a reduced percentage of nTreg in peripheral blood of SzS patients [23, 24]. Rather, we observed significant increases in both nTreg and Th3 cell populations in SzS patients compared to patients with earlier stage disease or normal controls (in light of the recent emerging genetic studies on the heterogeneity of MF/SzS [38, 39], we would expect to observe different degrees of elevation of these cell populations among the genetically defined groups of MF/SzS). Phenotypically identified Treg from these patients demonstrated strong IL-10 secretion and suppressive function in in vitro proliferation assays. It is worth mentioning that because cells were purified using only the surface markers CD4+CD25+ (Foxp3 marker being intracellular), it will be of interest to examine the purity of this cell transfer population, since effector T cells (that also express CD25+) could also be producing IL-10.
Our studies suggest that the proportion of Treg in SzS patients may be underestimated due to the expansion of the malignant clone. Exclusion of the tumor cell clone showed significant increases in the proportion of Treg (among all T cells) in SzS patients, and these Treg maintained their suppressive function. Interestingly, Berger et al. [40] have reported that in MF/SzS, the malignant clone can adopt characteristics of Treg, including expression of CD25, CTLA-4, and Foxp3, secretion of IL-10 and TGF-beta, and the ability to functionally suppress production of IL-2 and IFN-γ by T cells [40]. Consistent with these observations and other studies [22, 24], we have found that in some patients, the malignant T cells can have a Treg phenotype. However, in the vast majority of our patients, Treg were observed only within the non-malignant CD4+ population.
We have demonstrated that CD25 depletion leads to a significant decrease in the absolute number of Treg. In concordance with recent studies [41], we have also observed some rebound in Treg numbers following a 5-day course of DD therapy (Fig. 4d, e). Since only activated T cells develop late endosomes [42] that are required for pH unfolding of the toxin, it was proposed that resting T cells survive DD treatment [41]. In this way, CD25-mediated depletion may be followed by de novo expansion of resting Treg resulting in the rebound phenomenon [41]. In this regard, it is interesting to note that consecutive cycles of DD therapy can lead to sustained depletion of Treg. The reduction in Treg presents a theoretical “window of opportunity” during which tumor-specific effectors could be induced, expanded, and activated. This could enable auto-immunization, in which tumor cells in the patient could serve as a source of antigen [43]. The delivery of immune stimulating agents or vaccines during this period of Treg depletion in the absence of drug could enable the induction of efficacious tumor-specific immune responses [44]. This is also consistent with reported correlations between Treg depletion and improved immunogenicity in patients with renal cell carcinoma and melanoma receiving tumor-specific immunizations [45].
Paradoxically, the number of circulating MDSC in patients with advanced MF was relatively unchanged compared to controls. Nonetheless, we did observe increased MDSC activation with increasing tumor burden. Using surrogate markers as a measure of MDSC activation, (i.e., ROS [46] and serum arginase [47] produced by MDSC), we found that intracellular ROS was significantly elevated in MDSC patients with > IB MF. Importantly, treatment with IFN-a2b resulted in a reduction of both ROS and arginase production by MDSC, suggesting that inhibition of MDSC function may be related to clinical responses to IFN.
Recent studies suggest that cell-mediated immunosuppression may not be an isolated function of a particular cell subset, but rather a result of a complex network of cross-talking cellular suppressors. For example, MDSC were shown to modulate and induce Treg, suggesting common pathways that could be targeted for therapy [41]. Simultaneous removal of Treg and MDSC has been demonstrated to provide synergistic antitumor effect [48]. While our results are consistent with these observations, further studies performed in larger series of subjects are needed to confirm these results.
In conclusion, these studies revealed that Treg numbers increased in patients with SzS, and increased disease activity was associated with increases in both Treg number and MDSC activity. Furthermore, these results demonstrate that DD therapy can deplete Treg and that this reduction is significant and can be sustained following sequential cycles of treatment. Furthermore, clinical responses in patients receiving to IFN-α2b were associated with decreased MDSC activation. Taken together, these results support the development of combination therapies targeting multiple suppressive mechanisms to overcome immunosuppression, used alone or as adjuncts to other destructive or immunostimulatory therapies.
Acknowledgements
The authors would like to thank Sean M. Alber (University of Pittsburgh Department of Cell Biology) for assisting with images capture and analysis, Laura Strauss and Mary Jo Buffo for technical assistance with Treg functionality assay. We thank Dan Ilkovitch, MD, Ph.D. for the critical reading of the manuscript. We would like to thank the patients who volunteered their time and made this effort possible.
Abbreviations
- ARGI
Arginase-I
- DD
Denileukin diftitox
- IFN
Interferon
- MDSC
Myeloid-derived suppressor cell(s)
- MF
Mycosis fungoides
- nTreg
Naturally occurring T regulatory cells, CD4+CD25+Foxp3+ cells
- PEG
Polyethylene glycol (PEG)ylated
- ROS
Reactive oxygen species
- SzS
Sézary syndrome
- Th3 cells
T helper 3 cells, CD4+CD25−Foxp3+ cells
- Tr1 cells
T regulatory 1 cells, CD4+CD25−CTLA4+ cells
Compliance with ethical standards
Funding
This work was supported by Research Center Grants for Specialized Programs of Research of Excellence (P50CA121973) of National Institute of Health, Clinical and Translational Science and M01 Award of National Center for Research Resources.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA. Th2 cytokines from malignant cells suppress Th1 responses and enforce a global Th2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19(14):3755–3763. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo EK, Cassin M, Lessin SR, Rook AH. Complete molecular remission during biologic response modifier therapy for Sezary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. J Am Acad Dermatol. 2001;45(2):208–216. doi: 10.1067/mjd.2001.116345. [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, Rook AH. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Investig. 2005;115(4):798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodak E, Lessin S, Friedland R, Freud T, David M, Pavlovsky L, Shapiro J, Cohen AD. New insights into associated co-morbidities in patients with cutaneous T-cell lymphoma (mycosis fungoides) Acta Derm Venereol. 2013;93(4):451–455. doi: 10.2340/00015555-1496. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J Clin Investig. 2015;125(6):2250–2260. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol. 2012;40(2):186–204. doi: 10.1177/0192623311430693. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Konkel JE. Development of thymic Foxp3(+) regulatory T cells: TGF-beta matters. Eur J Immunol. 2015;45(4):958–965. doi: 10.1002/eji.201444999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Investig. 2013;123(3):939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujio K, Okamura T, Yamamoto K. The family of IL-10-secreting CD4+ T cells. Adv Immunol. 2010;105:99–130. doi: 10.1016/S0065-2776(10)05004-2. [DOI] [PubMed] [Google Scholar]
- 10.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Investig Dermatol. 2006;126(1):15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer Immunol Immunother. 2012;61(2):283–288. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tadmor T, Attias D, Polliack A. Myeloid-derived suppressor cells—their role in haemato-oncological malignancies and other cancers and possible implications for therapy. Br J Haematol. 2011;153(5):557–567. doi: 10.1111/j.1365-2141.2011.08678.x. [DOI] [PubMed] [Google Scholar]
- 13.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig. 2015;125(9):3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Li Y, Zhang ZF, Ju Y, Li L, Zhang BC, Liu B. Increase in myeloid-derived suppressor cells (MDSCs) associated with minimal residual disease (MRD) detection in adult acute myeloid leukemia. Int J Hematol. 2015;102(5):579–586. doi: 10.1007/s12185-015-1865-2. [DOI] [PubMed] [Google Scholar]
- 15.Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, Eckart MJ, Krause SW, Oefner PJ, Le Blanc K, Mackensen A, Mougiakakos D. CLL-cells induce IDOhi CD14+ HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote. Blood. 2014;124(5):750–760. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 16.Florcken A, Takvorian A, Singh A, Gerhardt A, Ostendorf BN, Dorken B, Pezzutto A, Westermann J. Myeloid-derived suppressor cells in human peripheral blood: optimized quantification in healthy donors and patients with metastatic renal cell carcinoma. Immunol Lett. 2015;168(2):260–267. doi: 10.1016/j.imlet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Zhang G, Li G, Ma H, Zhang X. Circulating CD14(+)HLA-DR(−/low) myeloid-derived suppressor cell is an indicator of poor prognosis in patients with ESCC. Tumour Biol. 2015;36(10):7987–7996. doi: 10.1007/s13277-015-3426-y. [DOI] [PubMed] [Google Scholar]
- 18.Krejsgaard T, Odum N, Geisler C, Wasik MA, Woetmann A. Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia. 2012;26(3):424–432. doi: 10.1038/leu.2011.237. [DOI] [PubMed] [Google Scholar]
- 19.Zhang QA, Chen ZQ, Chen MH, Xu ZD. The number of regular T cells and immature dendritic cells involved in mycosis fungoides is linked to the tumor stage. Eur Rev Med Pharmacol Sci. 2014;18(4):553–558. [PubMed] [Google Scholar]
- 20.Shareef MM, Elgarhy LH, Wasfy Rel S. Expression of granulysin and Foxp3 in cutaneous T cell lymphoma and Sezary syndrome. Asian Pac J Cancer Prev. 2015;16(13):5359–5364. doi: 10.7314/APJCP.2015.16.13.5359. [DOI] [PubMed] [Google Scholar]
- 21.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+ CD25+ FOXP3+ T cells in advanced stages of primary cutaneous T-cell lymphoma. J Investig Dermatol. 2006;126(10):2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiue LH, Couturier J, Lewis DE, Wei C, Ni X, Duiv M. The effect of extracorporeal photopheresis alone or in combination therapy on circulating CD4(+) Foxp3(+) CD25(−) T cells in patients with leukemic cutaneous T-cell lymphoma. Photodermatol Photoimmunol Photomed. 2015;31(4):184–194. doi: 10.1111/phpp.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barath S, Aleksza M, Keresztes K, Toth J, Sipka S, Szegedi G, Illes A. Immunoregulatory T cells in the peripheral blood of patients with Hodgkin’s lymphoma. Acta Haematol. 2006;116(3):181–185. doi: 10.1159/000094678. [DOI] [PubMed] [Google Scholar]
- 24.Heid JB, Schmidt A, Oberle N, Goerdt S, Krammer PH, Suri-Payer E, Klemke CD. FOXP3+ CD25− tumor cells with regulatory function in Sezary syndrome. J Investig Dermatol. 2009;129(12):2875–2885. doi: 10.1038/jid.2009.175. [DOI] [PubMed] [Google Scholar]
- 25.Hanafusa T, Matsui S, Murota H, Tani M, Iqawa K, Katayama I. Increased frequency of skin-infiltrating FoxP3+ regulatory T cells as a diagnostic indicator of severe atopic dermatitis from cutaneous T cell lymphoma. Clin Exp Immunol. 2013;172(3):507–512. doi: 10.1111/cei.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Fazekas, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumor-associated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leuk Lymphoma. 2008;49(6):1190–1201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wada DA, Pittelkow MR, Comfere NI, Gibson LE, Ansell SM, Wilcox RA. CD4(+)CD25(+)FOXP3(+) malignant T cells in Sezary syndrome are not necessarily functional regulatory T cells. J Am Acad Dermatol. 2013;69(3):485–489. doi: 10.1016/j.jaad.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19(2):376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 31.Duvic M, Geskin L, Prince HM. Duration of response in cutaneous T-cell lymphoma patients treated with denileukin diftitox: results from 3 phase III studies. Clin Lymphoma Myeloma Leuk. 2013;13(4):377–384. doi: 10.1016/j.clml.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174(2):636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 33.Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17(7):1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 34.Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3. London: Elsevier; 2012. pp. 2017–2036. [Google Scholar]
- 35.Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16(4):311–321. doi: 10.1111/j.1396-0296.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 36.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39(11):3121–3133. doi: 10.1002/eji.200939453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silva Almeida AC, Abate F, Khiabanian H, Martinez-Escala E, Guitart J, Tensen CP, Vermeer MH, Rabadan R, Ferrando A, Palomero T. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat Genet. 2015;47(12):1465–1470. doi: 10.1038/ng.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Doorn R, van Kester MS, Dijkman R, Vermeer MH, Mulder AA, Szuhai K, Knijnenburg J, Boer JM, Willemze R, Tensen CP. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood. 2009;113(1):127–136. doi: 10.1182/blood-2008-04-153031. [DOI] [PubMed] [Google Scholar]
- 40.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, Edelson RL. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105(4):1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 41.Baur AS, Lutz MB, Schierer S, Beltrame L, Theiner G, Zinser E, Ostalecki C, Heidkamp G, Haendle I, Erdmann M, Wiesinger M, Leisgang W, Gross S, Pommer AJ, Kampgen E, Dudziak D, Steinkasserer A, Cavalieri D, Schuler-Thurner B, Schuler G. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122(13):2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 42.Muratori C, Cavallin LE, Kratzel K, Tinari A, De Milito A, Fais S, D’Aloja P, Federico M, Vullo V, Fomina A, Mesri EA, Superti F, Baur AS. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine. 2009;27(5):684–689. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castano E, Glick S, Wolgast L, Naeem R, Sunkara J, Elston D, Jacobson M. Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. J Cutan Pathol. 2013;40(11):924–934. doi: 10.1111/cup.12217. [DOI] [PubMed] [Google Scholar]
- 45.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4+ CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120(12):2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss JM, Subleski JJ, Back T, Chen X, Watkins SK, Yagita H, Sayers TJ, Murphy WJ, Wiltrout RH. Regulatory T cells and myeloid-derived suppressor cells in the tumor microenvironment undergo fas-dependent cell death during IL-2/alphaCD40 therapy. J Immunol. 2014;192(12):5821–5829. doi: 10.4049/jimmunol.1400404. [DOI] [PMC free article] [PubMed] [Google Scholar]