Abstract
Study Objectives
The amount of recovery sleep needed to fully restore well-established neurobehavioral deficits from sleep loss remains unknown, as does whether the recovery pattern differs across measures after total sleep deprivation (TSD) and chronic sleep restriction (SR).
Methods
In total, 83 adults received two baseline nights (10–12-hour time in bed [TIB]) followed by five 4-hour TIB SR nights or 36-hour TSD and four recovery nights (R1–R4; 12-hour TIB). Neurobehavioral tests were completed every 2 hours during wakefulness and a Maintenance of Wakefulness Test measured physiological sleepiness. Polysomnography was collected on B2, R1, and R4 nights.
Results
TSD and SR produced significant deficits in cognitive performance, increases in self-reported sleepiness and fatigue, decreases in vigor, and increases in physiological sleepiness. Neurobehavioral recovery from SR occurred after R1 and was maintained for all measures except Psychomotor Vigilance Test (PVT) lapses and response speed, which failed to completely recover. Neurobehavioral recovery from TSD occurred after R1 and was maintained for all cognitive and self-reported measures, except for vigor. After TSD and SR, R1 recovery sleep was longer and of higher efficiency and better quality than R4 recovery sleep.
Conclusions
PVT impairments from SR failed to reverse completely; by contrast, vigor did not recover after TSD; all other deficits were reversed after sleep loss. These results suggest that TSD and SR induce sustained, differential biological, physiological, and/or neural changes, which remarkably are not reversed with chronic, long-duration recovery sleep. Our findings have critical implications for the population at large and for military and health professionals.
Keywords: sleepiness, vigor, fatigue, psychomotor vigilance test, cognitive, chronic sleep restriction, recovery, total sleep deprivation, MWT
Statement of Significance.
In a large sample, we observed differential recovery profiles to key neurobehavioral responses after two commonly experienced types of sleep loss, chronic sleep restriction (SR; five consecutive nights of 4 hours nightly sleep opportunity) and acute total sleep deprivation (TSD; one night without sleep). After chronic SR, all neurobehavioral functions, except for behavioral attention, returned to baseline after one recovery night. By contrast, after TSD, all neurobehavioral functions, except self-reported vigor, returned to baseline after one recovery night. Remarkably, four consecutive long-duration recovery nights were not enough to reverse these deficits. Our results are relevant for the general population and those in military and health professions and provide critical, novel information for predictive mathematical models of recovery.
Introduction
The average American receives at least 1 hour less than the recommended number of hours of sleep needed to achieve optimal levels of cognitive performance and wakefulness [1, 2]. Sleep deprivation negatively affects emotion regulation/recognition, pain habituation and sensitization, multitasking abilities, cardiovascular and metabolic efficiency, and higher cognitive functioning [3–14]. Sleep loss, including total sleep deprivation (TSD) and chronic sleep restriction (SR), degrades behavioral attention, cognitive throughput, memory, and physiological alertness and increases sleepiness and fatigue [6, 15–21]. However, the number of recovery days required to return and maintain neurobehavioral functioning across a diverse set of tasks to baseline levels after sleep loss remains unknown, as does whether the pattern of recovery and maintenance across multiple days differs for various neurobehavioral measures and differs after two common types of sleep loss, acute TSD and chronic SR.
Little is known about the neurobehavioral patterns of recovery after acute TSD. After one night of TSD and 8- to 10-hour time-in-bed (TIB) recovery sleep, sustained attention performance (measured using the Psychomotor Vigilance Test [PVT] and/or Simple Reaction Time task [SRTT]) recovered to baseline [22–25], as did self-reported sleepiness (measured using the Karolinska Sleepiness Scale [KSS]) [23, 24]. Cognitive throughput performance (as measured by the Digit Symbol Substitution Task [DSST]) following 36- to 62-hour TSD and 10-hour TIB recovery sleep returned to and exceeded baseline levels [22]. However, the time course of physiological sleepiness was slower to return to baseline after acute TSD, requiring 1–2 recovery nights of 8- to 9-hour TIB [23, 24]. Importantly, these studies did not examine the recovery of DSST after multiple nights of recovery sleep to determine if improved performance is maintained or examine other key domains including self-reported fatigue or vigor (as measured by the Profile of Mood States [POMS]), which show marked impairment with acute TSD [21].
By contrast, the recovery of neurobehavioral functioning after chronic SR (ranging from 5 to 14 nights of 4- to 5-hour TIB) may be slower than that after acute TSD for some measures [15, 23, 26–28]. After five nights of 4-hour TIB, chronic SR, one night of 10-hour TIB recovery was insufficient to restore PVT lapses and response speed or self-rated sleepiness and fatigue to baseline levels, though DSST performance and physiological sleepiness returned to baseline [15]. Moreover, while self-reported and/or physiological sleepiness tests recovered after multiple recovery sleep nights (ranging from 2 to 7 nights of 8- to 10-hour TIB), behavioral or sustained attention measures (PVT or SRTT) failed to recover to baseline levels [27–30]. Notably, none of these studies employed a longer recovery sleep opportunity (e.g. 12 hours) across multiple nights to examine whether such an extended recovery paradigm would be sufficient to fully restore behavioral attention performance to baseline. Moreover, it remains unknown whether DSST performance, thought to reflect cognitive instability comparable to that observed for the PVT during sleep loss [22], would show similar recovery patterns across days to that of the PVT and, therefore, reflect similar underlying mechanisms.
The aim of this study is to investigate recovery and maintenance patterns of neurobehavioral measures after acute TSD or after chronic SR using a large sample of participants for each type of sleep loss and a long sleep duration (12-hour TIB) of four consecutive recovery nights. Specifically, we investigated how many nights of 12-hour recovery sleep would be needed to restore and maintain neurobehavioral functioning to baseline levels following sleep loss. We also sought to determine whether four nights of 12-hour recovery sleep following sleep loss would reveal different neurobehavioral recovery and maintenance dynamics after SR versus after acute TSD and whether the recovery and maintenance pattern would differ across various self-rated and objective measures (especially if recovery sleep is insufficient or if there is instability in recovery performance). We hypothesized that one or more nights of recovery would be enough to return and maintain self-reported and objective measures back to baseline levels after acute TSD. We also hypothesized that a longer recovery sleep period of four consecutive nights of 12 hours would return and sustain both objective and self-reported measures back to baseline levels after SR. Finally, we hypothesized that the patterns of recovery would differ across various measures after SR and TSD.
Methods
Participants
In total, 83 healthy adults (mean ± standard deviation [SD], 34.7 ± 8.9 y; 36 females), between the ages of 21 and 50 years were recruited in response to study advertisements. Participants reported habitual nightly sleep durations between 6.5 and 8.5 hours, with habitual bedtimes between 10:00 pm and 12:00 am and habitual awakenings between 06:00 am and 09:30 am; these were confirmed via wrist actigraphy. Chronotype was determined via the Morningness–Eveningness Composite Scale, with extreme morning and evening types excluded [31]. Participants did not engage in habitual napping and did not present with sleep disturbances (i.e. no complaints of daytime sleepiness, insomnia, or other sleep–wake disturbances) [32]. They did not have any acute or chronic psychological and medical conditions, as determined by questionnaires, interviews, physical exams, clinical history, and urine and blood tests (including a fasting blood glucose test). Participants did not take regular medications (except for oral contraceptive use in some females) and were nonsmokers with body mass index (BMI) between 17.3 and 30.9 kg/m2. They did not participate in transmeridian travel or shift work or have irregular sleep–wake routines in the 60 days before the study. Participants were monitored at home with actigraphy, sleep–wake diaries, and time-stamped call-ins to determine bedtimes and waketimes during the 7–14 days before the laboratory phase. Sleep disorders were excluded on the first laboratory night by oximetry and polysomnography (PSG) measurements, if applicable—in our sample, no participants demonstrated a sleep disorder.
Participants were not allowed to use caffeine, alcohol, medications (except oral contraceptives), or tobacco for 7 days before the study, as verified by blood and urine screenings. The protocol was approved by the University of Pennsylvania’s Institutional Review Board. All participants provided written informed consent in accordance with the Declaration of Helsinki. They received compensation for participation.
Procedures
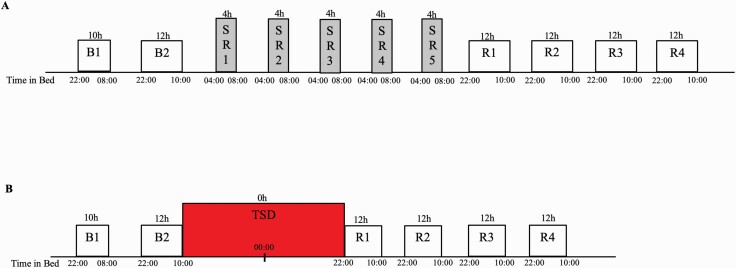
Participants engaged in a 13-day laboratory study in which they were studied continuously and received daily checks of vital signs and symptoms by nurses (with a physician on call). All participants experienced either SR or TSD during the protocol followed by four nights of 12-hour recovery sleep opportunity (10:00 pm to 10:00 am), designed to assess whether there were complete recuperation and return of neurobehavioral variables to baseline. Participants were randomized as a group (N = 4 per group) to one of the two conditions after two initial nights of baseline sleep of 10 hours (10:00 pm to 08:00 am) and 12 hours (10:00 pm to 10:00 am) TIB, respectively, and were blinded to condition assignment until after the second night of baseline sleep (Figure 1). Participants who experienced SR (N = 41) underwent five consecutive nights of 4-hour TIB per night (SR1-5, 04:00 am to 08:00 am) followed by four consecutive nights of 12-hour recovery sleep opportunity (10:00 pm to 10:00 am). Participants who experienced TSD (N = 42) underwent 36 hours of TSD (0 h TIB, wakefulness from 10:00 am to 08:00 pm the following day), followed by four consecutive nights of 12-hour recovery sleep opportunity (10:00 pm to 10:00 am).
Figure 1.
Experimental protocol. Participants were randomly assigned to one of two conditions: (A) SR: two nights of baseline sleep (B1 and B2, 10:00 pm to 08:00/10:00 am) followed by five nights of chronic SR (04:00 am to 08:00 am), followed by four nights of recovery sleep (R1–R4, 10:00 pm to 10:00 am) or (B) TSD: two nights of baseline sleep (B1 and B2, 10:00 pm to 08:00/10:00 am) followed by one night of 36-hour acute TSD followed by four nights of recovery sleep (R1–R4, 10:00 pm to 10:00 am).
Participants were ambulatory and were permitted to perform sedentary activities, such as watching television, reading, and playing video or board games between neurobehavioral test bouts (completed while seated at a computer); however, they were not allowed to exercise. Ambient temperature was maintained between 22 and 24°C. Laboratory light levels remained constant at <50 lux during scheduled wakefulness and <1 lux during scheduled sleep periods. Participants were monitored continuously by trained staff throughout the study to ensure adherence. Participants wore a wrist actigraph continuously and on certain days wore ambulatory electroencephalography (EEG) and electrocardiography (ECG) recording equipment for 24-hour intervals.
Neurobehavioral measures
A precise computer-based neurobehavioral test battery was administered every 2 hours during wakefulness on all five SR days and throughout TSD. The test battery contained the following tasks: the 10-minute PVT [33, 34], the DSST [35], the KSS [36], and the POMS [37]. The number of lapses (reaction times [RT] >500 ms) and the response speed (1/RT) were analyzed for the PVT.
In addition to these test bouts, a modified Maintenance of Wakefulness Test (MWT) [15, 17, 21, 38]—a physiological measure of the ability to resist sleep—was administered at baseline, after five nights of SR or after TSD, and after R1, R3, and R4 (a single trial was conducted between 2:30 pm and 4:00 pm), using a standard recording montage. Before each trial, the lights were dimmed to <10 lux, and participants were instructed, “Keep your eyes open and try not to fall asleep.” Each trial was terminated at the first microsleep (10 s of theta activity) determined by the C3-A2 derivation or at 30 minutes if sleep onset did not occur. MWT scores represented either the time (minutes) to microsleep initiation or 30 minutes (if no microsleep occurred).
Polysomnography
PSG recordings (EC3-A2, Fz-A1, O2-A1; two electroculography [EOG]: left outer canthus [LOC]/right outer canthus [ROC]; two submental electromyography [EMG] were collected using digital ambulatory physiological recorders; Compumedics Profusion PSG3 recording system [128-Hz sampling]; Compumedics Limited, Abbotsford, Victoria, Australia) on B2, R1, and R4. All sleep stages were scored visually in continuous 30-second epochs according to Rechtschaffen and Kales [39] by a trained scorer using commercial software (Profusion PSG 3; Compumedics Limited). The EEGs and EOGs were referenced with A1 or A2 (Fz-A1, C3-A2, O2-A1, LOC-A2, and ROC-A1). A submental EMG was analyzed bipolarly. The sampling rate was 256 Hz. For sleep scoring, high-pass filters were set at 0.3 Hz for EEGs and EOGs and 10 Hz for EMG. Sleep onset latency was defined as three consecutive 30-second epochs of any sleep stage. Recovery PSG sleep was examined since inadequate recovery sleep could affect the recovery of neurobehavioral measures.
Statistical analyses
Two-way mixed analysis of variances (ANOVAs) were used to determine whether changes in cognitive performance, self-reported measures, physiological alertness, and recovery PSG sleep were different between individuals exposed to TSD and SR across all time points and, separately, across only recovery time points following sleep loss (SPSS v26). When mixed ANOVAs revealed a significant interaction term, repeated measures ANOVAs (RMANOVAs) were used to evaluate the time effect in each group, while one-way ANOVAs looked at group differences at each time point. Greenhouse–Geisser corrections for degrees of freedom were applied for all mixed and RMANOVAs to account for sphericity assumption violations as indicated by significant Mauchly’s tests for all mixed ANOVAs and RMANOVAs (χ 2(2–14) = 21.36–525.02, p = 0.000–0.011). Bonferroni adjustment for multiple comparisons was applied to all post hoc pairwise comparisons. Baseline and recovery were assessed using the average values of all test bouts during wakefulness (10:00 am to 08:00 pm). Response to sleep loss was assessed using the average value of data collected every 2 hours from 08:00 am to 08:00 pm during TSD and after the fifth night of SR [21, 40].
Results
In total, 83 healthy adults (mean ± SD: 34.7 ± 8.9 y; 36 females) (aged 21–50 y, 72.3% African American; 43.4% female) participated in the study, with N = 41 participants randomly assigned to five consecutive nights of SR and N = 42 participants randomly assigned to one night of TSD. There were no significant differences between conditions in age, BMI, chronotype, the percentage of participants who were female or African American, or in pre-study actigraphic sleep duration (overall mean ± SD: 8.00 ± 0.60 h), onset, offset, or midpoint, or in baseline PSG total sleep time (overall mean ± SD: 9.55 ± 1.13 h) or in baseline PSG sleep onset latency (overall mean ± SD: 19.60 ± 18.08 min) (F(1) = 0.009–1.049, p = 0.309–0.925) [21].
Cognitive performance
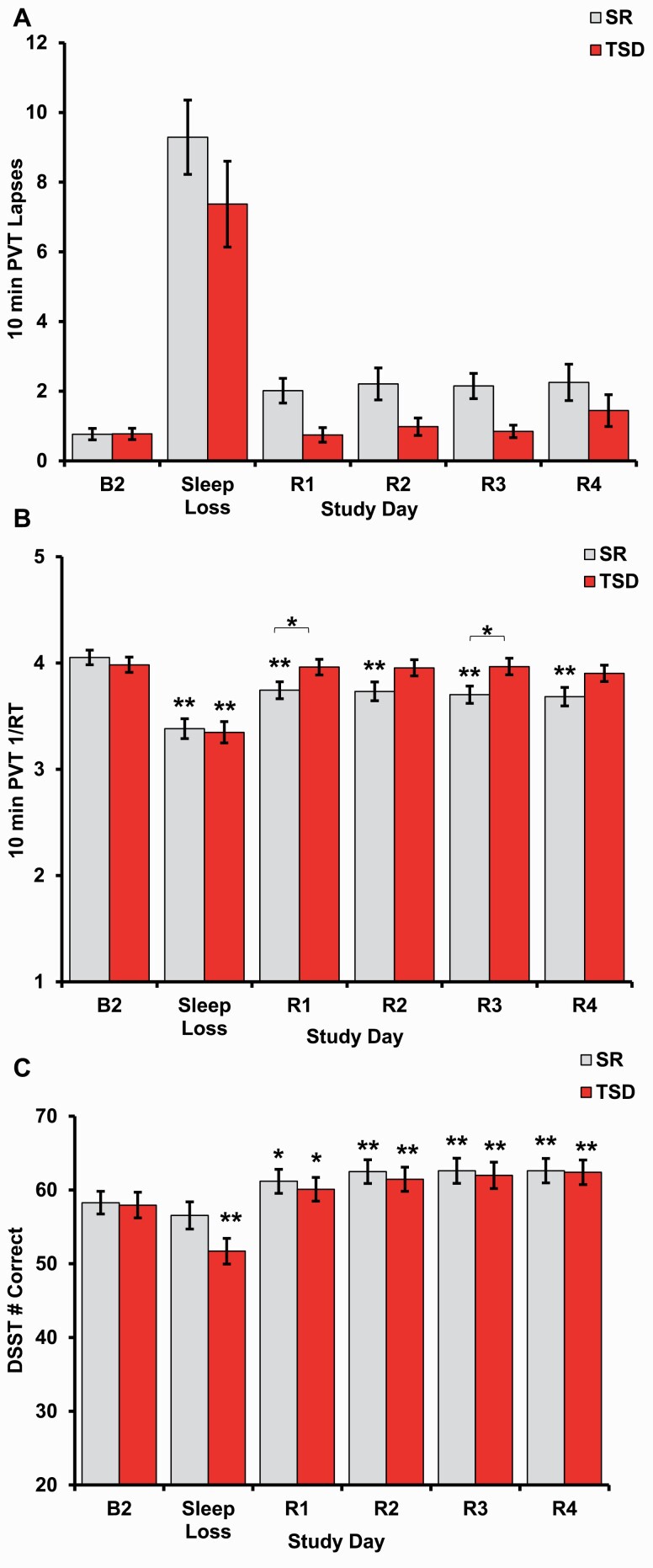
There was no significant interaction between condition (SR vs. TSD) and time for PVT lapses across all study time points (p = 0.337, Figure 2A) or across recovery only (p = 0.504). The main effect of time demonstrated a significant difference in PVT lapses across all study time points (p < 0.000), yet not across recovery (p = 0.144). There was a significant difference in mean PVT lapses between the SR and TSD groups across all time points (p = 0.049), as well as during recovery only (p = 0.014), with the SR group having more lapses than the TSD group across both. Bonferroni-adjusted pairwise comparison results for both conditions combined for all time points and recovery only are presented in Supplementary Table S1.
Figure 2.
Neurobehavioral performance after sleep loss and recovery. (A) Both chronic SR and acute TSD produced performance deficits as measured by more lapses on the 10-min PVT. (B) PVT response speed (1/RT) was impaired after both SR and TSD. 1/RT returned to baseline after R1 and was maintained through R1–R4 in the TSD group but did not recover to baseline after four nights of recovery sleep following SR. 1/RT differed between the SR and TSD groups during R1 and R3. (C) DSST performance was impaired after TSD, but not SR, and subsequently exceeded baseline performance for all nights of recovery sleep for both conditions. Data are mean ± SEM. *p < 0.05; **p < 0.001; asterisks directly above data points are significant paired t-tests using Bonferroni corrections, which compared performance at each time point to baseline performance when the all-time point interaction was significant; asterisks above bars are significant one-way ANOVAs that compared differences between SR and TSD.
For PVT response speed, there was a significant interaction between condition and time across all time points (p < 0.000, Figure 2B), yet not across recovery only (p = 0.485). Response speed was significantly higher at R1 (p = 0.048) and R3 (p = 0.020) in the TSD group relative to the SR group (Table 1). When examined separately by condition across all time points, there was a significant effect of time on response speed for the SR group (F(3.05, 121.98) = 45.20, p < 0.000, partial η2 = 0.530) and the TSD group (F(2.03, 83.23) = 73.90, p < 0.000, partial η2 = 0.643). In the SR condition, response speed differed between B2 and all other study time points (Figure 2B), as well as between SR and all recovery time points (all ps < 0.000). In the TSD condition, response speed differed between B2 and Sleep Loss (p < 0.000, Figure 2B), between TSD and all recovery time points (all p’s < 0.000), and between R3 and R4 (p = 0.013; Supplementary Table S2). Across recovery only, the main effect of time demonstrated a significant difference in PVT response speed (p = 0.027), and there was a significant difference in mean PVT response speed between conditions (p = 0.040), with the TSD group having faster response speed relative to the SR group. Bonferroni-adjusted pairwise comparison results for both conditions combined for recovery only are presented in Supplementary Table S1.
Table 1.
One-way ANOVA results evaluating simple main effects for condition at each time point on variables that demonstrated significant interactions in between-group RMANOVAs
| Measure | Time | df | F | P | Partial η 2 |
|---|---|---|---|---|---|
| Ten-minute PVT 1/RT | B2 | 1, 81 | 0.491 | 0.486 | 0.006 |
| Sleep Loss | 1, 81 | 0.061 | 0.805 | 0.001 | |
| R1* | 1, 81 | 4.026 | 0.048 | 0.047 | |
| R2 | 1, 81 | 3.65 | 0.060 | 0.043 | |
| R3* | 1, 81 | 5.658 | 0.020 | 0.065 | |
| R4 | 1, 81 | 3.530 | 0.064 | 0.042 | |
| DSST number correct | B2 | 1, 81 | 0.021 | 0.886 | 0.000 |
| Sleep Loss | 1, 81 | 3.65 | 0.060 | 0.043 | |
| R1 | 1, 81 | 0.231 | 0.632 | 0.003 | |
| R2 | 1, 81 | 0.201 | 0.655 | 0.002 | |
| R3 | 1, 81 | 0.064 | 0.801 | 0.001 | |
| R4 | 1, 81 | 0.009 | 0.925 | 0.000 | |
| KSS score | B2 | 1, 81 | 0.35 | 0.556 | 0.004 |
| Sleep Loss* | 1, 81 | 6.959 | 0.010 | 0.079 | |
| R1 | 1, 81 | 0.171 | 0.681 | 0.002 | |
| R2 | 1, 81 | 0.04 | 0.842 | 0.000 | |
| R3 | 1, 81 | 0.494 | 0.484 | 0.006 | |
| R4 | 1, 81 | 0.084 | 0.773 | 0.001 | |
| MWT sleep latency | B2 | 1, 81 | 0.979 | 0.325 | 0.012 |
| Sleep Loss | 1, 81 | 2.226 | 0.140 | 0.027 | |
| R1* | 1, 81 | 5.678 | 0.020 | 0.066 | |
| R3* | 1, 81 | 5.521 | 0.021 | 0.064 | |
| R4* | 1, 81 | 4.008 | 0.049 | 0.047 |
B2, baseline day; R1–R4, recovery days one through four.
*p < 0.05.
On the DSST, there was a significant interaction between condition assignment and time across all time points (p = 0.002, Figure 2C), yet not across recovery only (p = 0.559). DSST scores were not significantly different between the groups at any time point (p = 0.060–0.925, Table 1). When examined separately by condition across all time points, there was a significant effect of time on DSST performance for the SR group (F(2.95, 117.88) = 20.85, p < 0.000, partial η2 = 0.343) and for the TSD group (F(2.66, 109.04 = 64.52, p < 0.000, partial η2 = 0.611). In the SR condition, DSST scores differed between B2 and all recovery time points (Figure 2C), as well as between SR and all recovery time points (p = 0.000–0.039). In the TSD condition, DSST scores differed between B2 and all study time points (Figure 2C), between TSD and all recovery time points, and between R1 and all other recovery time points (p = 0.000–0.006; Supplementary Table S2). Across recovery only, the main effect of time demonstrated a significant difference in DSST performance (p < 0.000), yet there was no significant difference in mean DSST scores between conditions (p = 0.748). Full results for two-way mixed ANOVAs discussed in this section are presented in Table 2. Bonferroni-adjusted pairwise comparison results for both conditions combined for recovery only are presented in Supplementary Table S1.
Table 2.
Two-way mixed ANOVA results comparing conditions across all time points and recovery only with time and between-subjects effect test results presented for nonsignificant interaction terms
| Measure | TIME | Test | df | F | P | Partial η 2 |
|---|---|---|---|---|---|---|
| Ten-minute PVT lapses | All | Interaction | 1.37, 110.70 | 1.03 | 0.337 | 0.012 |
| Time** | 80.03 | 0.000 | 0.497 | |||
| Condition* | 1, 81 | 4.00 | 0.049 | 0.047 | ||
| Recovery | Interaction | 1.93, 156.30 | 0.68 | 0.504 | 0.008 | |
| Time | 1.98 | 0.144 | 0.024 | |||
| Condition* | 1, 81 | 6.34 | 0.014 | 0.073 | ||
| Ten-minute PVT 1/RT | All | Interaction** | 2.83, 229.17 | 11.80 | 0.000 | 0.127 |
| Recovery | Interaction | 2.05, 166.30 | 0.73 | 0.485 | 0.009 | |
| Time* | 3.66 | 0.027 | 0.043 | |||
| Condition* | 1, 81 | 4.36 | 0.040 | 0.051 | ||
| DSST number correct | All | Interaction* | 2.98, 241.32 | 5.30 | 0.002 | 0.061 |
| Recovery | Interaction | 2.50, 202.08 | 0.65 | 0.559 | 0.008 | |
| Time** | 10.95 | 0.000 | 0.119 | |||
| Condition | 1, 81 | 0.10 | 0.748 | 0.001 | ||
| KSS score | All | Interaction* | 2.30, 186.43 | 5.85 | 0.002 | 0.067 |
| Recovery | Interaction | 2.15, 174.42 | 0.22 | 0.821 | 0.003 | |
| Time | 0.77 | 0.471 | 0.009 | |||
| Condition | 1, 81 | 0.20 | 0.658 | 0.002 | ||
| POMS Fatigue score | All | Interaction | 1.55, 125.79 | 1.01 | 0.349 | 0.012 |
| Time** | 59.48 | 0.000 | 0.423 | |||
| Condition | 1, 81 | 0.50 | 0.481 | 0.006 | ||
| Recovery | Interaction | 2.11, 170.69 | 0.60 | 0.559 | 0.007 | |
| Time | 1.95 | 0.143 | 0.024 | |||
| Condition | 1, 81 | 0.16 | 0.687 | 0.002 | ||
| POMS Vigor score | All | Interaction | 3.65, 295.86 | 0.51 | 0.711 | 0.006 |
| Time** | 31.13 | 0.000 | 0.278 | |||
| Condition | 1, 81 | 0.02 | 0.898 | 0.000 | ||
| Recovery | Interaction | 2.43, 196.81 | 1.25 | 0.291 | 0.015 | |
| Time* | 5.37 | 0.003 | 0.062 | |||
| Condition | 1, 81 | 0.02 | 0.904 | 0.000 | ||
| MWT sleep latency | All | Interaction* | 3.19, 258.32 | 4.41 | 0.004 | 0.052 |
| Recovery | Interaction | 1.51, 122.42 | 0.16 | 0.789 | 0.002 | |
| Time | 2.12 | 0.137 | 0.026 | |||
| Condition* | 1, 81 | 9.31 | 0.003 | 0.103 |
Greenhouse–Geisser corrected values reported for all tests excluding between-subject effect (condition). Time main effect test results are reported when the interaction term was not significant. Refer to Figures 2–4 for mean and standard error values.
*p < 0.05; **p < 0.001.
Self-reported sleepiness, fatigue, and vigor
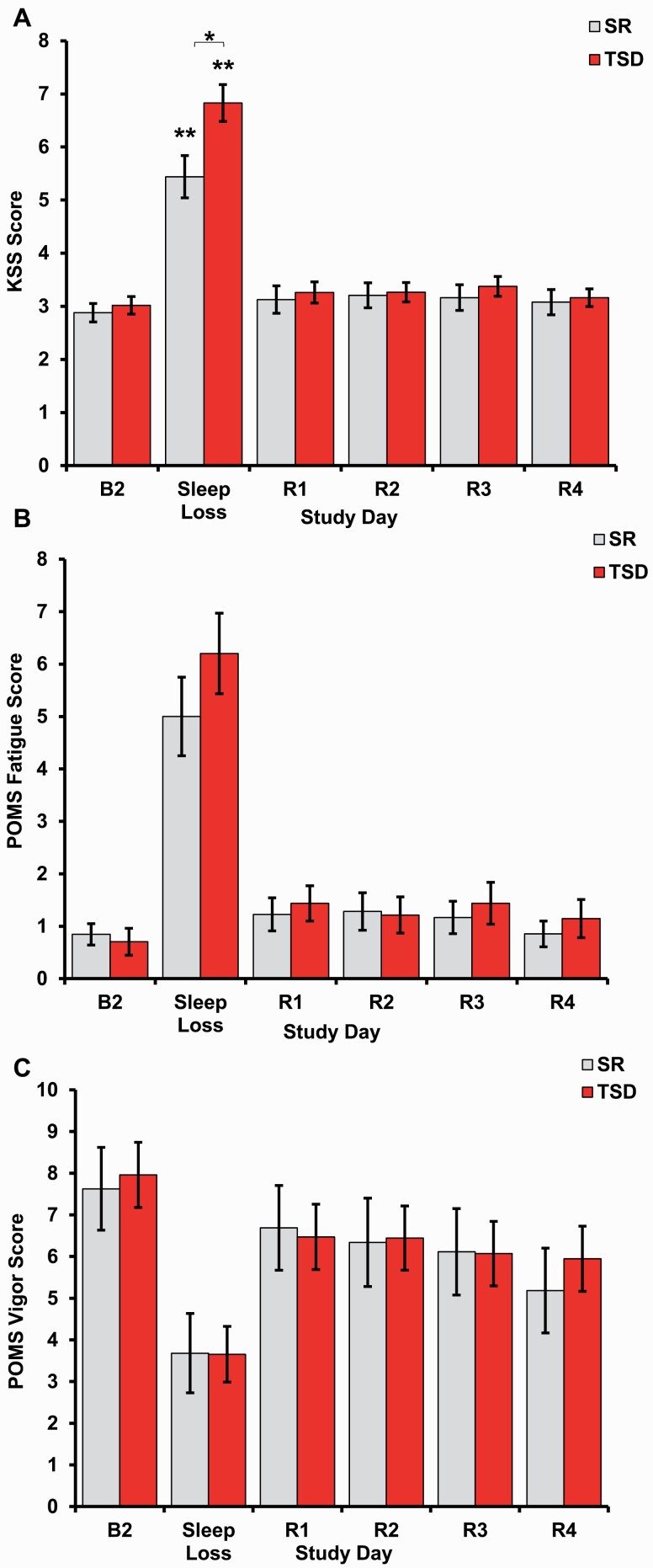
For self-reported sleepiness on the KSS, there was a significant interaction between condition and time across all time points (p = 0.002, Figure 3A), yet not across recovery only (p = 0.821). KSS scores were significantly higher in the TSD condition relative to the SR condition at Sleep Loss (p = 0.010) yet were not significantly different at any other time point (Table 1). When examined separately by condition across all time points, there was a significant effect of time on KSS score for the SR group (F(2.60, 104.12) = 37.63, p < 0.000, partial η2 = 0.485) and for the TSD group (F(1.94, 79.40) = 103.06, p < 0.000, partial η2 = 0.715). In both conditions, KSS scores differed between B2 and Sleep Loss (Figure 3A) as well as between Sleep Loss and all recovery time points (all ps < 0.000, Supplementary Table S2). Across recovery only, the main effect of time did not demonstrate a significant difference in KSS scores (p = 0.471), and there was no significant difference in mean KSS scores between conditions (p = 0.658). Bonferroni-adjusted pairwise comparison results for both conditions combined for recovery only are presented in Supplementary Table S1.
Figure 3.
Self-rated sleepiness, fatigue, and vigor after sleep loss and recovery. Both chronic SR and acute TSD produced impairments in all self-rated measures. (A) Self-reported sleepiness, as measured by the KSS, differed between the SR and TSD groups during sleep loss, with greater sleepiness in the TSD group. Sleepiness returned to baseline levels following one night of recovery sleep (R1) after both SR and TSD, and levels were maintained throughout 4 days of recovery (R1–R4). (B) POMS Fatigue scores significantly increased with both SR and TSD. (C) POMS Vigor scores significantly decreased in both the SR and TSD groups. Data are mean ± SEM. *p < 0.05; **p < 0.001; asterisks directly above data points are significant paired t-tests using Bonferroni corrections, which compared self-report scores at each time point to baseline scores when the all-time point interaction was significant; asterisks above bars are significant one-way ANOVAs that compared differences between SR and TSD.
There was no significant interaction between condition and time for self-reported fatigue on the POMS Fatigue across all study time points (p = 0.349, Figure 3B) or across recovery only (p = 0.559). Across all study time points, the main effect of time demonstrated a significant difference in POMS Fatigue scores (p < 0.000), and there was no significant difference in mean POMS Fatigue scores between conditions (p = 0.481). Across recovery only, the main effect of time did not demonstrate a significant difference in POMS Fatigue scores (p = 0.143), and there was no significant difference in mean POMS Fatigue scores between conditions (p = 0.687). Bonferroni-adjusted pairwise comparison results for both conditions combined for all time points and recovery only are presented in Supplementary Table S1.
There was no significant interaction between condition and time for self-reported vigor on the POMS Vigor across all study time points (p = 0.711, Figure 3C) or across recovery only (p = 0.291). The main effect of time demonstrated a significant difference in POMS Vigor scores across all study time points (p < 0.000) and across recovery only (p = 0.003). There was no significant difference in mean POMS Vigor scores between conditions across all time points (p = 0.898) or across recovery only (p = 0.904). Full results for two-way mixed ANOVAs discussed in this section are presented in Table 2. Bonferroni-adjusted pairwise comparison results for both conditions combined for all time points and recovery only are presented in Supplementary Table S1.
Physiologic alertness
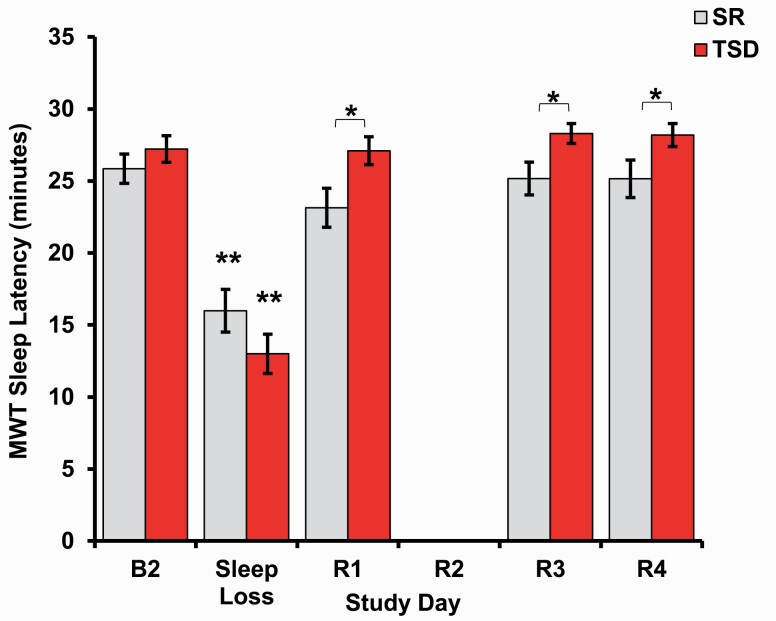
Physiologic alertness was measured using the MWT. There was a significant interaction between condition and time across all time points (p = 0.004, Figure 4), yet not across recovery only (p = 0.789). MWT scores were significantly higher in the TSD group relative to the SR group at R1 (p = 0.020), R3 (p = 0.021), and R4 (p = 0.049, Table 1). When examined separately by condition across all time points, there was a significant effect of time on MWT performance for the SR group (F(3.19, 127.71) = 15.69, p < 0.000, partial η2 = 0.282) and the TSD group (F(2.66, 108.95) = 61.67, p < 0.000, partial η2 = 0.601). In both conditions, MWT performance differed between B2 and Sleep Loss (Figure 4), as well as between Sleep Loss and all recovery time points (all ps < 0.000, Supplementary Table S2). Across recovery only, the main effect of time did not demonstrate a significant difference in MWT performance (p = 0.137), yet there was a significant difference in mean MWT scores between conditions (p = 0.003), with the TSD group having higher scores relative to the SR group. Full results for two-way mixed ANOVAs discussed in this section are presented in Table 2. Bonferroni-adjusted pairwise comparison results for both conditions combined for recovery only are presented in Supplementary Table S1.
Figure 4.
Physiologic alertness after sleep loss and recovery. Both chronic SR and acute TSD produced decreases in physiological alertness as measured by sleep latency onset during a modified MWT. Participants in both the TSD and SR conditions showed a return of sleep latency onset to baseline levels throughout 4 days of recovery. Sleep latency differed between the SR and TSD groups at R1, R3, and R4 (note: data were not collected for the second night of recovery [R2] after SR or TSD). Data are mean ± SEM. *p < 0.05; **p < 0.001; asterisks directly above data points are significant paired t-tests using Bonferroni corrections, which compared sleep latency at each time point to baseline latency when the all-time point interaction was significant; asterisks above bars are significant one-way ANOVAs that compared differences between SR and TSD.
Recovery PSG sleep
Table 3 presents R1 and R4 PSG sleep data for the SR and TSD groups (mean ± SD). There was a significant time effect for total sleep time (F(1, 80) = 158.760, p < 0.000, partial η2 = 0.665) and sleep efficiency (F(1, 80) = 268.389, p < 0.000, partial η2 = 0.770), with both decreasing from R1 to R4. By contrast, there were no significant interactions or between-subjects effects (F(1, 80) = 0.030–2.778, p = 0.099–0.864, partial η2 = 0.000–0.034). There was a significant time effect for sleep onset latency (F(1, 80) = 78.402, p < 0.000, partial η2 = 0.495): participants took longer to fall asleep on R4 compared with R1. There was no significant interaction or between-subjects effects (F(1, 80) = 0.318–3.002, p = 0.087–0.575, partial η2 = 0.004–0.036).
Table 3.
Mean ± SD recovery PSG sleep measures after SR or TSD
| Recovery night 1 | Recovery night 4 | |||
|---|---|---|---|---|
| PSG sleep measure | After SR | After TSD | After SR | After TSD |
| Total sleep time (min) | 626.13 ± 59.20 | 619.33 ± 107.25 | 500.50 ± 91.75 | 499.41 ± 84.60 |
| Sleep efficiency (%) | 87.07 ± 8.03 | 89.21 ± 7.88 | 71.07 ± 12.15 | 69.59 ± 11.40 |
| Sleep onset latency (min) | 12.00 ± 11.99 | 8.62 ± 5.92 | 35.20 ± 23.82 | 43.11 ± 37.75 |
| Stage 1 sleep time (min) | 37.76 ± 19.16 | 28.51 ± 21.17 | 43.38 ± 21.61 | 38.94 ± 23.42 |
| Stage 2 sleep time (min) | 327.21 ± 52.00 | 304.30 ± 56.48 | 264.06 ± 56.06 | 251.35 ± 54.83 |
| SWS time (min) | 95.52 ± 49.06 | 143.38 ± 56.10 | 72.62 ± 39.20 | 90.16 ± 42.09 |
| REM onset latency | 52.03 ± 26.26 | 74.57 ± 34.06 | 61.00 ± 27.21 | 71.54 ± 31.30 |
| REM sleep time (min) | 163.24 ± 35.55 | 157.68 ± 32.25 | 119.16 ± 35.80 | 118.96 ± 26.88 |
| No. of awakenings | 27.17 ± 15.73 | 20.86 ± 8.61 | 28.13 ± 15.68 | 24.12 ± 10.82 |
| Total wake time (min) | 91.53 ± 58.37 | 77.17 ± 57.09 | 205.76 ± 89.29 | 217.76 ± 80.89 |
| WASO (min) | 77.63 ± 57.05 | 67.08 ± 57.69 | 168.83 ± 90.25 | 173.93 ± 81.12 |
Stage 1 and stage 2 sleep duration showed significant time effects (F(1, 80) = 24.215–74.910, ps < 0.000, partial η2 = 0.232–0.484), but no significant interactions or between-subjects effects (F(1, 80) = 0.578–3.110, p = 0.082–0.449, partial η2 = 0.007–0.037). Both the SR and TSD groups spent more time in stage 1 sleep and less time in stage 2 sleep on R4 compared with R1. Slow-wave sleep (SWS) duration showed significant time, interaction, and between-subjects effects (F(1, 80) = 10.599–197.769, p = 0.000–0.002, partial η2 = 0.116–0.709): SWS was higher during recovery in the TSD condition and also showed a sharper decline from R1 to R4. Neither a significant time nor interaction effect was found for rapid-eye movement (REM) onset latency (F(1, 80) = 0.617–2.745, p = 0.101–0.415, partial η2 = 0.008–0.033). There was, however, a significant between-subjects effect whereby the TSD group had a longer average REM onset latency than the SR group in R1 and R4 (F(1, 80) = 8.928, p = 0.004, partial η2 = 0.100). REM sleep duration showed a significant time effect with the amount of time spent in REM decreasing from R1 to R4 (F(1, 80) = 127.531, p < 0.000, partial η2 = 0.615), but no significant interaction or between-subjects effects (F(1, 80) = 0.214–0.537, p = 0.466–0.645, partial η2 = 0.003–0.007). Finally, there was a significant time effect for number of awakenings, total wake time, and wake after sleep onset (WASO) (F(1, 80) = 4.410–261.289, p = 0.000–0.039, partial η2 = 0.052–0.766), with participants having more awakenings, more total time awake, and more WASO on R4 than R1, but no significant interactions or between-subjects effects (F(1, 80) = 0.007–3.672, p = 0.052–0.933, partial η2 = 0.000–0.044).
Discussion
For the first time, this study presents compelling evidence of residual and differential neurobehavioral deficits following chronic SR and acute TSD that were not restored after multiple, consecutive 12-hour TIB recovery nights. Following SR, although self-reported deficits and physiological sleepiness were reversed, PVT impairments after SR failed to be restored after four nights of recovery. Conversely, recovery after TSD for cognitive performance, physiological and self-rated sleepiness, and self-reported fatigue occurred rapidly, but perceptions of decreased vigor lingered after four recovery nights. These results indicate that both SR and TSD produce chronic and sustained biological, physiological, and/or neural changes reflected in neurobehavioral measures; 12-hour TIB recovery across four consecutive nights is, notably, not enough to reverse these alterations.
We observed that SR and TSD had differential effects on the recovery of sustained attention. PVT response speed was significantly slower in the SR group compared with the TSD group at R1 and R3. During the recovery period, TSD participants’ response speed returned to baseline, while the response speed of the SR group remained significantly slower than baseline. Similarly, although post hoc analyses for PVT lapses were not conducted in the formal analysis presented in the results, the SR group also had significantly more lapses than the TSD group during R1–R3. Recovery-only analyses found a significant difference between SR and TSD for both average PVT lapses and response speed across R1–R4, supporting the fact that the SR group had a worse recovery phase. These findings agree with other studies that found decreased behavioral or sustained attention performance during SR, which subsequently never returned to baseline levels following recovery [15, 27–30, 41]. The findings are also in line with studies that found that behavioral attention performance following TSD returned to baseline levels after one night of recovery sleep [22–25].
The failure of PVT performance to return to baseline in the SR group was accompanied by significantly shorter MWT sleep latency in the SR group relative to the TSD group during R1, R3, and R4. The significant interaction for MWT sleep latency across all time points and the significant difference between SR and TSD groups for recovery-only analyses support that although both groups recovered to baseline, the TSD group had significantly better recovery of physiological alertness. Our MWT results agree with other studies that found latencies decreased with SR and returned to baseline after recovery [15, 27, 29, 30]. However, our TSD recovery results contrast with previous results in which Lamond et al. [23] found that two 9-hour TIB recovery opportunities were needed for complete restoration of physiological alertness, while a single night of 12-hour TIB restored physiological alertness in our study. These varying recovery timelines may be due to differing TIB recovery opportunities (9-hour TIB vs. 12-hour TIB) and indicate a longer initial recovery episode should be employed for rapid restoration of physiological alertness. Importantly, the significantly faster and more stable recovery by the TSD group, compared with the SR group, in both the PVT and MWT suggests that the mechanisms involved in behavioral attention and physiological alertness recovery may be linked.
When POMS Fatigue and Vigor were examined across all time points with the TSD and SR groups combined, there was a significant difference between baseline and all recovery days for POMS Vigor, but not POMS Fatigue. Since the two measures did not have a significant interaction across all time points or recovery only, we did not formally present post hoc analyses; however, exploratory post hoc analyses showed that POMS Fatigue returned to baseline in both the TSD and SR groups at R1 and remained stable throughout the recovery period. Conversely, in exploratory analyses of self-reported vigor in the SR group, we detected a return to baseline levels throughout three recovery nights and a significant decrease on the fourth night of recovery. The resurgence of decreased vigor after an initial return to baseline may be attributed to lingering instability of vigor caused by sleep loss, as has been shown for other measures [5, 22]. Moreover, individuals may have initially reported increased vigor due to comparing their perceptions to those during sleep loss. However, as recovery progressed, individuals may have altered their perception comparison anchor to baseline and thus reported decreased vigor. For the first time, we found that the TSD group had decreased vigor, compared with baseline, throughout all 4 days of recovery. Further exploration is necessary to support the idea that full recovery consists of multiple components requiring different timelines and mechanisms.
There were no significant differences in recovery of sleepiness and cognitive throughput between the TSD and SR groups, yet the SR group reported being significantly less sleepy relative to the TSD group during the sleep loss period. Similar to past studies [27–29, 41], but unlike the Banks et al. [15] study, sleepiness returned to baseline after one night of recovery in both sleep loss conditions. Our DSST results extend those of Banks et al. [15], by showing deficits in DSST performance from sleep loss and no lingering effects following recovery and a sustained improvement for DSST from baseline throughout recovery. DSST performance remained above baseline for all four recovery days after both sleep loss conditions, indicating any possible learning effects had plateaued following the first night of recovery.
Interestingly, the recovery of DSST differed from that of behavioral attention, likely due in part to a learning effect. However, PVT performance recovery also differed from the recovery of self-reported vigor, which showed greater variability during recovery than the PVT. The recovery differences between PVT performance, an objective measure, and in POMS Vigor scores, a self-report measure, reflect the relationships seen during sleep loss, where individuals have consistent performance on objective measures and on self-report measures, but an individual’s response to an objective measure may not reflect their response to a subjective measure [21, 40]. Our recovery data suggest that this relationship continues throughout recovery, whereby the mechanisms of perceived vigor recovery are different from behavioral attention recovery. Further exploration is needed to understand how the recovery of perceived sleepiness and fatigue differs from perceived vigor.
In our study, recovery sleep architecture at R1 and R4 was similar to that of previous studies [23, 24, 27, 29, 30, 42–44]. After both TSD and SR, the profiles of recovery sleep revealed higher efficiency and better-quality sleep during R1 relative to R4 [24, 42]. At R4, participants in both groups were sleeping a little over 8 hours; as such, insufficient recovery sleep does not account for the failure of PVT performance to return to baseline after SR or for the failure of self-rated vigor to recover after TSD. Participants in the TSD condition had more SWS during recovery and showed a sharper decline from R1 to R4; they also had a longer overall REM onset latency compared with those in the SR condition. The increased SWS during R1 may have facilitated the recovery of behavioral attention and physiological alertness in the TSD group and impaired the recovery of their perceived vigor.
Another possible mechanism underlying incomplete behavioral attention recovery might be cerebral A1 adenosine receptor (A1AR) availability, which increases with severe TSD and decreases with recovery [45]. The relationship between SR and A1AR availability in the human brain is an important question that needs to be explicitly examined [46]; it is possible that PVT measures may not have returned to baseline in the SR condition due to an incomplete decline in A1AR availability in the brain areas, such as the thalamus, striatum, or insula with recovery [45]. Since the SR group cumulatively lost slightly more sleep than the TSD group, and A1AR availability increases as a function of time awake [47], this might have differentially affected PVT performance; however, additional studies are needed to explicitly examine this possibility. Future studies also should extend 12-hour TIB recovery beyond four nights to determine whether such decrements in vigor and behavioral or sustained attention measures remain and explore their underlying mechanisms.
Besides neurobehavioral measures, various biological, physiological, and neural indices are impacted by TSD or SR [44, 45, 48–53]. These measures, including A1AR availability resting metabolic rate, caloric intake, heart rate variability, and microsaccades, returned to baseline following one to five recovery nights [45, 49, 50, 52, 53]. Similar to our findings that some neurobehavioral measures take longer to recover after TSD or SR, the timing of caloric intake variables [51] and metabolomic markers [48] recovery after TSD and/or SR also differ. Since recovery following SR and/or TSD is highly varied across measures, this underscores the need to employ longer recovery periods for a global return to homeostasis.
There are a few limitations to the current study. All participants were healthy, thus making it difficult to generalize our findings to individuals with sleep or mood disorders or with medical conditions. Similarly, our participants were between the ages of 21 and 50. Adolescents, middle-aged, and older individuals may show different recovery responses, particularly since these periods are often characterized by numerous neurobehavioral changes and differences in response to sleep loss. Additionally, our study did not employ a control group, thus the stability of performance without sleep loss in this highly controlled study environment remains unknown. Our findings are also limited in generalizability to the neurobehavioral tests we employed. It is possible that other measures of neurobehavioral performance linked to real-world situations, such as driving or operating heavy machinery, may not show the same recovery profiles following TSD or SR.
Although these data were collected in a laboratory setting, our results have implications for the general population and for military, health care workers, truck drivers, and personnel in other applied settings where sleep loss is common and recovery opportunities are often limited. For the first time, we show evidence of residual and differential neurobehavioral deficits following chronic SR and acute TSD, two common types of sleep loss, after multiple, consecutive 12-hour recovery nights. Further studies are needed to determine why sustained performance fails to recover completely after SR, while self-rated vigor fails to recover completely after TSD. Investigation into the biological, physiological, and neural mechanisms underlying apparent harboring of neurobehavioral vulnerability to further sleep loss are also needed.
Supplementary Material
Acknowledgments
This work was conducted at the Division of Sleep and Chronobiology, Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, 1017 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104, USA. We thank the faculty and staff of the Unit of Experimental Psychiatry for their contributions to this study. N.G. designed the overall study; C.A. and C.L. conducted statistical analyses of the data; and N.G. provided financial support. C.A., C.L., E.Y., and N.G. prepared the manuscript, and all authors reviewed and approved the final manuscript.
Conflict of interest statement. None declared.
Funding
This work was primarily supported by the Department of the Navy, Office of Naval Research (Award No. N00014-11-1-0361 to N.G). Other support provided by the National Aeronautics and Space Administration (NASA) NNX14AN49G and grant 80NSSC20K0243 (to N.G.), National Institutes of Health (NIH) grant R01DK117488 (to N.G.), and Clinical and Translational Research Center grant (UL1TR000003). None of the sponsors had any role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1. National Sleep Foundation. 2005 Sleep in America Poll – adult sleep habits and styles. Sleep Health. 2015;1(2):e4. [Google Scholar]
- 2. Bonnet MH, et al. We are chronically sleep deprived. Sleep. 1995;18(10):908–911. [DOI] [PubMed] [Google Scholar]
- 3. Balkin TJ, et al. Sleep loss and sleepiness: current issues. Chest. 2008;134(3):653–660. [DOI] [PubMed] [Google Scholar]
- 4. Tartar JL, et al. Sleep restriction and delayed sleep associate with psychological health and biomarkers of stress and inflammation in women. Sleep Health. 2015;1(4):249–256. [DOI] [PubMed] [Google Scholar]
- 5. Goel N, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29(4):320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banks S, et al. Behavioral and physiological consequences of sleep restriction in humans. J Clin Sleep Med. 2007; 3(5): 519–528. [PMC free article] [PubMed] [Google Scholar]
- 7. Lim J, et al. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowe CJ, et al. The neurocognitive consequences of sleep restriction: a meta-analytic review. Neurosci Biobehav Rev. 2017;80:586–604. [DOI] [PubMed] [Google Scholar]
- 9. van der Helm E, et al. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33(3):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson NS, et al. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haavisto ML, et al. Sleep restriction for the duration of a work week impairs multitasking performance. J Sleep Res. 2010;19(3):444–454. [DOI] [PubMed] [Google Scholar]
- 12. Minkel JD, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandner MA, et al. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knutson KL, et al. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banks S, et al. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33(8):1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goel N, et al. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75(17):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goel N, et al. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011;6(12):e29283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goel N, et al. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goel N. “Omics” approaches for sleep and circadian rhythm research: biomarkers for identifying differential vulnerability to sleep loss. Curr Sleep Med Rep. 2015;1:38–46. [Google Scholar]
- 20. Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 21. Yamazaki EM, et al. Robust stability of trait-like vulnerability or resilience to common types of sleep deprivation in a large sample of adults. Sleep. 2020;43(6). doi: 10.1093/sleep/zsz292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honn KA, et al. New insights into the cognitive effects of sleep deprivation by decomposition of a cognitive throughput task. Sleep. 2020:43(7). doi:org/10.1093/sleep/zsz319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamond N, et al. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16(1):33–41. [DOI] [PubMed] [Google Scholar]
- 24. Philip P, et al. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness. Sleep. 2012;35(7):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreno-Villanueva M, et al. The degree of radiation-induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep. 2018;41(7). doi:org/10.1093/sleep/zsy067 [DOI] [PubMed] [Google Scholar]
- 26. McCauley P, et al. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256(2):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belenky G, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 28. Axelsson J, et al. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int. 2008;25(2–3):297–308. [DOI] [PubMed] [Google Scholar]
- 29. Pejovic S, et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am J Physiol Endocrinol Metab. 2013;305(7):E890–E896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rupp TL, et al. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32(3):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith CS, et al. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 32. Douglass AB, et al. The Sleep Disorders Questionnaire. I: creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–167. [DOI] [PubMed] [Google Scholar]
- 33. Lim J, et al. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. [DOI] [PubMed] [Google Scholar]
- 34. Basner M, et al. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartman DE. Wechsler Adult Intelligence Scale IV (WAIS IV): return of the gold standard. Appl Neuropsychol. 2009;16(1):85–87. [DOI] [PubMed] [Google Scholar]
- 36. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 37. Bourgeois A, et al. Full-scale and short-form of the Profile of Mood States: a factor analytic comparison. J Sport Behav. 2010;33(4):355–376. [Google Scholar]
- 38. Goel N, et al. Cognitive workload and sleep restriction interact to influence sleep homeostatic responses. Sleep. 2014;37(11):1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rechtschaffen A, et al. , eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service; 1968. [Google Scholar]
- 40. Dennis LE, et al. Healthy adults display long-term trait-like neurobehavioral resilience and vulnerability to sleep loss. Sci Rep. 2017;7(1):14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. St Hilaire MA, et al. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40(1). doi:org/10.1093/sleep/zsw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skorucak J, et al. Response to chronic sleep restriction, extension, and total sleep deprivation in humans: adaptation or preserved sleep homeostasis? Sleep. 2018;41(7). doi:org/10.1093/sleep/zsy078 [DOI] [PubMed] [Google Scholar]
- 43. Harrison Y, et al. Long-term extension to sleep–are we really chronically sleep deprived? Psychophysiology. 1996;33(1):22–30. [DOI] [PubMed] [Google Scholar]
- 44. Motomura Y, et al. Recovery from unrecognized sleep loss accumulated in daily life improved mood regulation via prefrontal suppression of amygdala activity. Front Neurol. 2017;8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elmenhorst D, et al. Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain. Proc Natl Acad Sci U S A. 2017;114(16):4243–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elmenhorst EM, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115(31):8009–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elmenhorst D, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27(9):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weljie AM, et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci U S A. 2015;112(8):2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spaeth AM, et al. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spaeth AM, et al. Resting metabolic rate varies by race and by sleep duration. Obesity (Silver Spring). 2015;23(12):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dennis LE, et al. Phenotypic stability of energy balance responses to experimental total sleep deprivation and sleep restriction in healthy adults. Nutrients. 2016;8(12):823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang H, et al. Heart rate variability rebound following exposure to persistent and repetitive sleep restriction. Sleep. 2019;42(2). doi:org/10.1093/sleep/zsy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abe T, et al. Tracking intermediate performance of vigilant attention using multiple eye metrics. Sleep. 2020;43(3). doi:org/10.1093/sleep/zsz219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.