Abstract
It is commonly thought that the optimal method for intracoronary administration of cells is to stop coronary flow during cell infusion, in order to prolong cell/vascular wall contact, enhance adhesion, and promote extravasation of cells into the interstitial space. However, occlusion of a coronary artery with a balloon involves serious risks of vascular damage and/or dissection, particularly in non-stented segments such as those commonly found in patients with heart failure. It remains unknown whether the use of the stop-flow technique results in improved donor cell retention. Acute myocardial infarction was produced in 14 pigs. One to two months later, pigs received 10 million indium-111 oxyquinoline (oxine)-labeled c-kitpos human cardiac stem cells (hCSCs) via intracoronary infusion with (n = 7) or without (n = 7) balloon inflation. Pigs received cyclosporine to prevent acute graft rejection. Animals were euthanized 24 h later and hearts harvested for radioactivity measurements. With the stop-flow technique, the retention of hCSCs at 24 h was 5.41 ± 0.80 % of the injected dose (n = 7), compared with 4.87 ± 0.62 % without coronary occlusion (n = 7), (P = 0.60). When cells are delivered intracoronarily in a clinically relevant porcine model of chronic ischemic cardiomyopathy, the use of the stop-flow technique does not result in greater myocardial cell retention at 24 h compared with non-occlusive infusion. These results have practical implications for the design of cell therapy trials. Our observations suggest that the increased risk of complications secondary to coronary manipulation and occlusion is not warranted.
Keywords: Cardiac stem cells, Retention, Coronary stop flow, Intracoronary, c-kit, CSCs
Introduction
Since their initial discovery and characterization [1], c-kitpos cardiac stem cells (CSCs) have emerged as a promising modality in the treatment of ischemic cardiomyopathy. Preclinical studies conducted over the last decade have reproducibly demonstrated the capacity of in vitro expanded c-kitpos cardiac cells to induce myocardial repair and functional recovery [23]. These observations led to the Cardiac Stem Cell Infusion in Patients with Ischemic CardiOmyopathy (SCIPIO) phase I clinical trial, which demonstrated the safety and feasibility of intracoronary delivery of c-kitpos CSCs in humans [2, 4]. Recently, the safety of even larger doses (up to 20 million in vitro expanded c-kitpos CSCs) has been demonstrated in a porcine model [12].
In SCIPIO [2], as well as in almost every trial of intracoronary cell infusion performed to date, the cells were delivered with the stop-flow technique [5, 15, 17]; that is, an intracoronary balloon was inflated to stop flow within the coronary artery and prevent rapid wash out of the cells, thus, in theory, promoting greater cell retention by enhancing vascular adhesion and extravasation into the surrounding myocardium. This approach is being used in most ongoing and planned clinical trials in which cells are infused intracoronarily. Although theoretically attractive, however, the stop-flow technique has not been shown to be superior to non-occlusive cell delivery in terms of cell product retention. The stop-flow technique is potentially hazardous [14], and therefore constitutes an impediment to the widespread use of cell therapy in patients with cardiovascular disease, particularly when, as is often the case in chronic ischemic cardiomyopathy, the culprit coronary arteries targeted for cell delivery are not stented. Manipulation of a non-stented coronary artery with an intraluminal balloon under pressure carries a significant risk of vascular damage, coronary artery dissection, and even life-threatening arterial perforation and rupture [14]. In addition, the interruption of coronary flow may elicit myocardial injury, either directly from epicardial coronary artery occlusion or by distal microembolization of dislodged atherosclerotic plaque material [9], and may cause arrhythmias in already dysfunctional hearts. Again, this issue is particularly relevant to patients with ischemic cardiomyopathy, whose targeted coronary arteries or bypass grafts are often not protected by stents.
Given the seriousness of the aforementioned complications, objective evidence of improved cell retention is essential to justify subjecting patients to increased procedural risks in future clinical trials involving intracoronary administration of cell-based products. However, as mentioned above, despite the widespread assumption that the stop-flow technique promotes improved extravasation and cardiac retention of cells, no studies have evaluated the utility of the technique using c-kitpos CSCs or any cardiac-derived cell. Accordingly, we addressed this issue in a clinically relevant porcine model of ischemic cardiomyopathy in which we measured the cardiac retention of 10 million indium-111 oxine radiolabeled c-kitpos CSCs infused with or without the stop-flow technique.
Methods
Ethics statement
This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the guidelines of the Animal Care and Use Committee of the University of Louisville (KY) School of Medicine following the guidelines set forth by the 1996 Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville (IACUC number: 12114).
The experimental protocol is illustrated in Fig. 1.
Fig. 1.
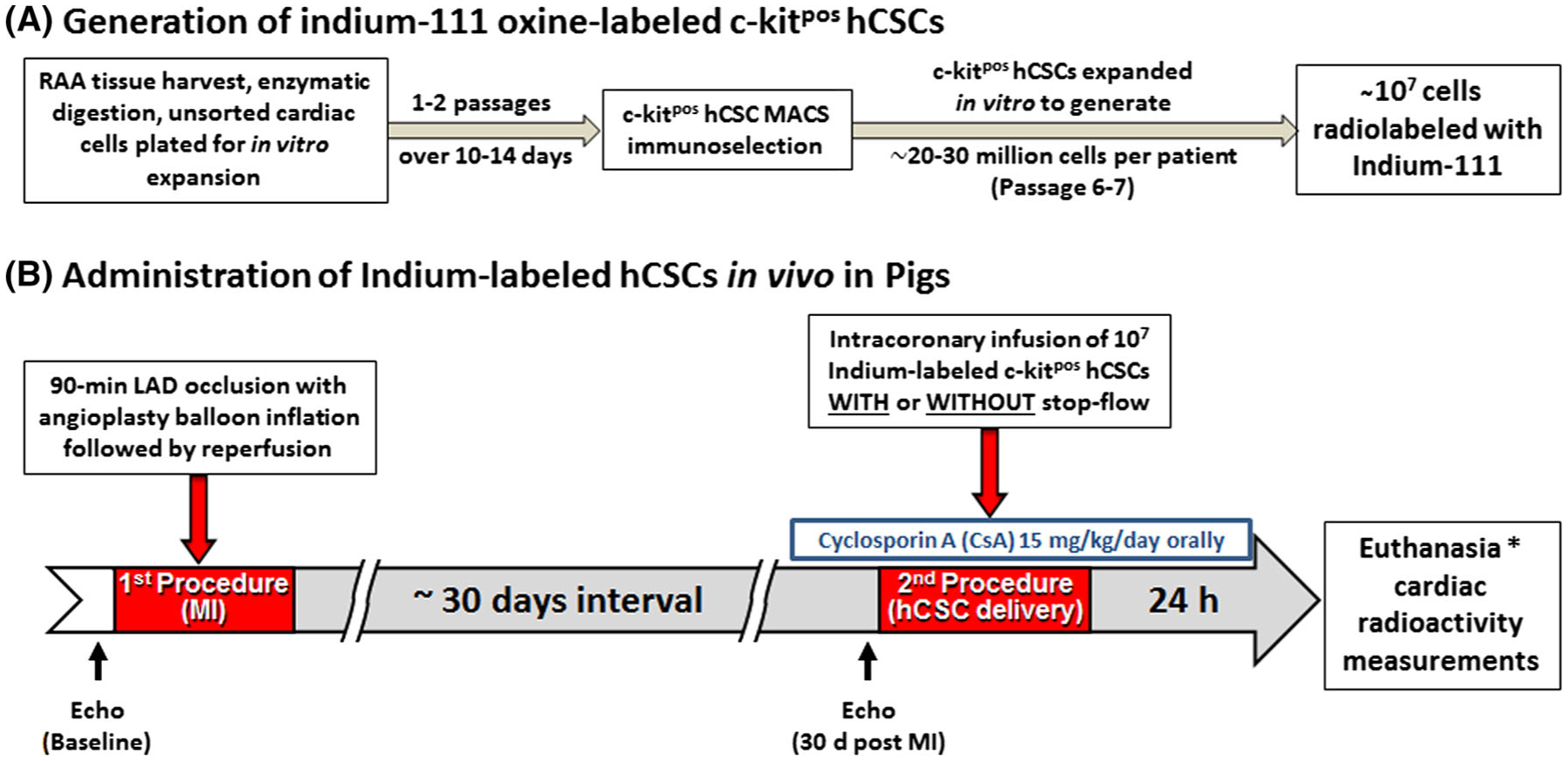
Experimental protocol and timeline
Human c-kitpos CSC isolation and flow cytometry
Isolation, immunomagnetic selection, and flow cytometric analysis of c-kitpos hCSCs were performed as previously described [12]. Briefly, human right atrial appendage specimens (RAAs) were obtained with Jewish Hospital Institutional Review Board (IRB) approval (IRB number 07.0062) from patients undergoing open heart, on pump, coronary artery bypass surgery at Jewish Hospital in Louisville, Kentucky. All patients were between 40 and 80 years of age, so as to approximate the ages of patients in the recently conducted SCIPIO trial [2]. RAAs were washed several times with PBS and were minced to obtain fragments <1 mm3. The tissue fragments were underwent multiple rounds of enzymatic digestion. Isolated cells were plated in a 6-well plate for passage 0 initial expansion. Passage 1 cells were sorted for c-kit with anti-CD117 Miltenyi microbeads and a Miltenyi magnetic sorting apparatus per manufacturer’s specifications. Positively selected cells were expanded exponentially over 3–4 additional passages to obtain 2–3 × 107 cells per patient. Multiple patients’ cells were pooled to obtain a uniform cell product that was radiolabeled and infused intracoronarily into pigs. Cells were assessed for c-kit positivity by flow cytometric analysis at passage 3 after fixation and labeling with c-terminal specific Santa Cruz C19 rabbit polyclonal IgG anti-human c-kit antibody and secondary antibody, a FITC- or APC-conjugated Invitrogen donkey anti-rabbit IgG versus isotype control using a BD LSR flow cytometer. BD LSR DIVA software was used for final analysis of c-kit positivity.
Cell product generation and indium-111 oxine radiolabeling
In vitro expanded c-kitpos CSCs were trypsinized and washed with sterile PBS and resuspended in Plasmalyte-A solution with ~750 μCi indum-111 oxine, purchased from Cardinal Health, Nuclear Pharmacy Services in Louisville, Kentucky, for 20 min at 37 °C with 5 % CO2. A Capintec CRC-15R Radioisotope Dose Calibrator was used to conduct all radioactivity measurements. After 20 min, the cells were washed twice in 10 mL of cold Plasmalyte-A to remove unbound radioisotope. The cells were resuspended in 1–2 mL of Plasmalyte-A for final cell count and viability by hemocytometer and Trypan blue. A final radioactivity measurement was made to assess percent radiolabeling efficiency.
After the intracoronary infusions were completed, tubes, catheters, plastic ware, and other materials that had come into contact with the radiolabeled cells were taken back to the dose calibrator for radioactivity measurement. The residual radioactivity was subtracted from that of the labeled cell product to obtain the radioactivity of the cell product that was administered intracoronarily. This number was recorded and utilized for all subsequent calculations of cardiac cellular retention.
Radiolabeling efficiency
Radiolabeling efficiency was defined as the ratio between the final radioactivity of the cell product prior to infusion and the initial dose of Indium to which the cells were exposed.
Adjustments for diffusive loss of indium-111 oxine over the 24 h follow-up and calculation of cardiac radioactivity
Diffusion of unbound indium-111 oxine out of labeled cells continues over time, and this may lead to potential underestimation of cell retention. Similar observations have been made in prior studies [20].
Experiments were performed in triplicate. Ten million cells were radiolabeled with indium-111 oxine as described and placed in 5 mL serum-free Ham’s F12 medium for 24 h with periodic media changes. Media were completely changed every 4 h to maintain a high diffusive gradient similar to that which occurred in vivo after cell infusion. Extreme care was taken to avoid loss of cells. After 24 h, cells were centrifuged and the cell pellet assessed for radioactivity. Diffusive loss was quantified as percent of expected radioactivity after accounting for radioactive decay over the 24 h incubation period. A correction factor was generated by dividing 100 by this observed percent of theoretical maximum radioactivity, so as to account for all infused cells and avoid underestimation of intracardiac retention at 24 h.
The radioactivity measured in all cardiac segments was adjusted according to the correction factor (3.52) to obtain the corrected radioactivity for each cardiac tissue specimen. All radioactivities were then summed to obtain the total radioactivity of each heart. This total corrected cardiac radioactivity was divided by the expected maximum radioactivity of the infusate (adjusted for time dependent decay) to obtain the percent retention of the intracoronarily delivered indium-111 oxine-labeled cell product.
Animal procedures
Female Yorkshire pigs (39.8 ± 5.2 kg, age 12–18 weeks) were sedated using a cocktail of ketamine (20 mg/kg, i.m.) and xylazine (2 mg/kg, i.m.), intubated, and mechanically ventilated with 100 % oxygen. Anesthesia was maintained with 0.8–1.5 % isoflurane. A femoral artery cut down was performed and an 8F arterial sheath was placed; a 7F Hockey stick guide catheter was advanced to the left coronary ostium. An angioplasty balloon catheter was positioned in the left anterior descending (LAD) coronary artery at a site distal to the first diagonal branch, after which the pigs were subjected to a 90-min LAD coronary occlusion followed by reperfusion by inflation and deflation of the balloon, respectively (Fig. 1).
One to two months (45.3 ± 4.5 days) after MI, the pigs were reanesthetized. An angioplasty balloon catheter was placed in the LAD at the site of the previous occlusion. Indium-labeled hCSCs were administered with four infusions, each consisting of 3 ml over 30 s, with or without concurrent 3-min balloon inflation. In the pigs with balloon inflation, five cycles of 3-min balloon inflation/3-min deflation were performed. The aim of the first cycle, which was performed without infusing cells, was to increase microvascular permeability in the distal myocardium; during the following four cycles, hCSCs were infused into the coronary artery as described above. In the pigs without balloon inflation, hCSCs were infused four times; each infusion lasted 30 s and the four infusions were interspaced with intervals of 5 min and 30 s (Fig. 1).
Twenty-four hours after hCSC administration, the pigs were euthanized and various organs (heart, lung, liver, kidney, and spleen) were harvested for analysis.
Immunosuppressive therapy
Pigs received 15 mg/kg/day of oral cyclosporine (Novartis) orally starting 3 days before cell infusion and continuing until euthanasia.
Echocardiography
Echocardiograms were obtained at baseline (before the induction of infarction) and again 30 days later (prior to radiolabeled hCSC intracoronary infusion) using an HP SONOS 7500 ultrasound system (Philips Medical Systems) equipped with a HP 21350A (S8) 3.0–8.0 MHz sector array ultrasound transducer. Briefly, pigs were anesthetized and placed in the left lateral decubitus position. Temperature was kept between 37.0 and 37.5 °C with a heating pad. The parasternal short-axis view was used to obtain 2D and M-mode images [3]. Systolic and diastolic anatomic parameters were obtained from M-mode tracings at the mid-papillary level. Digital images were analyzed off-line by a single-blinded observer using the ComPACS Review Station (version 10.5) image analysis software (Medimatic, Las Cruces, NM 88004, USA) according to the American Society of Echocardiography standards [24].
Nuclear imaging and cardiac radioactivity measurements
Perfusion fixed porcine hearts were subjected to nuclear imaging and radioactivity measurements. After whole heart nuclear imaging with Picker Prism 2000 XP nuclear gamma camera, hearts were bread loafed into five slices of equal thickness from the apex to the base; each slice above the apex was then subdivided into three regions: right ventricle, left ventricular free wall, and septum. The base was divided into right and left sections. The right and left atria were sectioned and placed into independent containers. The proximal portions of the great vessels were removed and placed together into another container. All specimens were measured with a Capintec CRC-15R Radioisotope Dose Calibrator, and the anatomic regions were combined to create a regional distribution of radioactivity. The radioactivity in all regions was then combined to obtain the total radioactivity in each heart. The same dose calibrator was used throughout the study.
Statistics
Student’s t test, one-way ANOVA, or two-way repeated measures ANOVA, as appropriate, was employed for comparisons of echocardiographic parameters, radioactivities, and cell retention between groups. All data are reported as mean ± SEM.
Results
Flow cytometric analysis
Flow cytometric analysis for c-kit expression was performed for seven cell lines at early passage as described (c-kit positivity ranged from 72.6 to 90.8 %). The mean c-kit positivity of the cells utilized for the study as assessed by standard protocol was 81.6 ± 7.0 % compared with iso-type control (Fig. 2a).
Fig. 2.
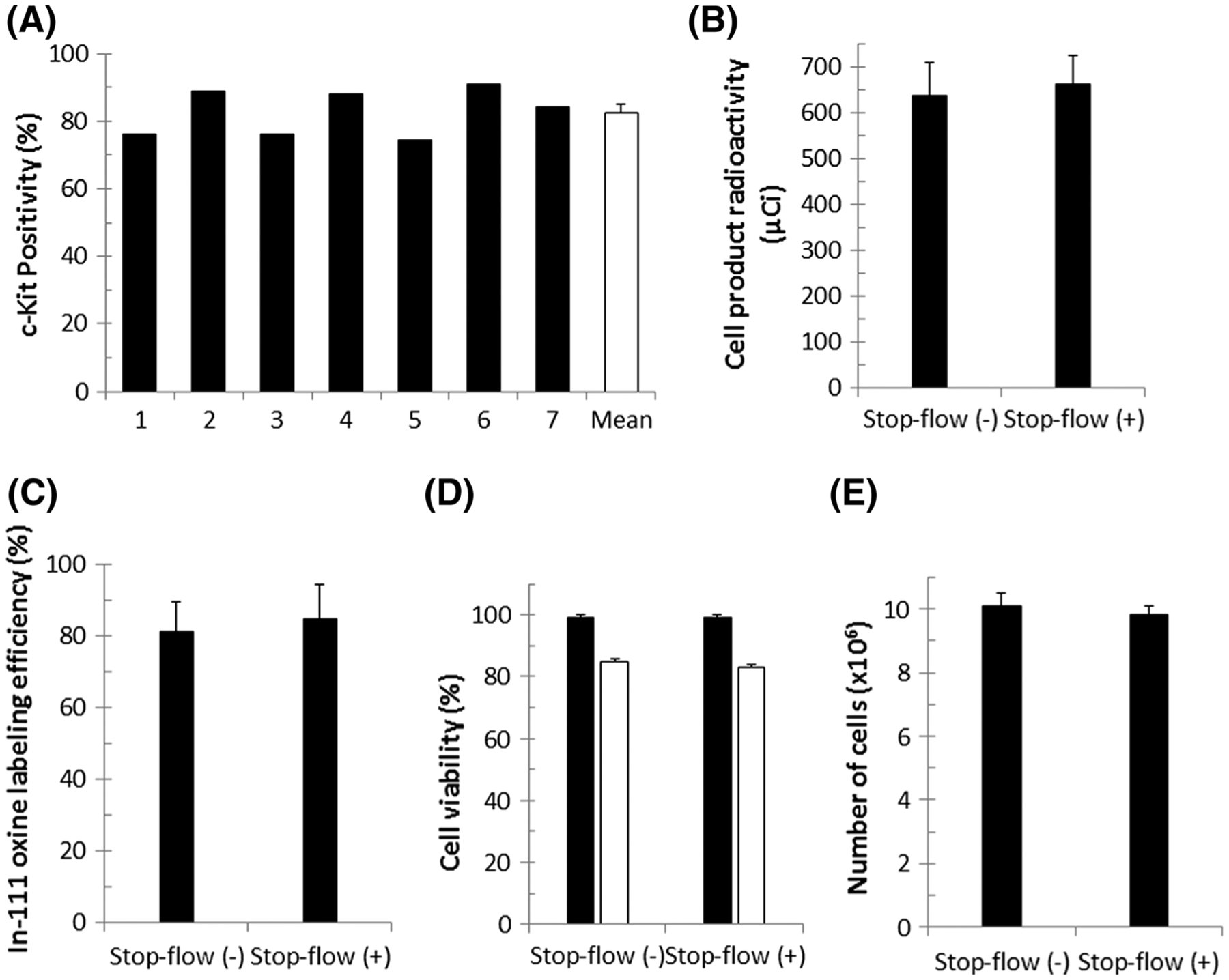
c-kit positivity, cell product radioactivity, radiolabeling efficiency, cell product viability, cell number, and diffusive loss of indium-111 oxine. a c-kit positivity of individual cell lines is illustrated by black bars, with mean positivity shown in white. c-kit positivity ranged from 72.6 to 90.8 % with mean c-kit positivity of 81.6 ± 7.0 %. Cell lines were combined to obtain a homogenous cell product. b Mean final cell product radioactivity of cells infused with continuous flow [Stop-flow (−)] or stop-flow [Stop-flow(+)]. c Indium labeling efficiency of cells administered with or without stop flow. d Mean cell viability, assessed by Trypan blue, before (black bars) and after (white bars) radiolabeling. e Number of radiolabeled hCSCs in each group. All values are mean ± SEM
Final cell product radioactivity, labeling efficiency, cell viability, and cell number
Final radioactivity and radiolabeling efficiency were calculated for all cell products prior to intracoronary infusion. No significant difference between cell products was observed with regard to the final mean radioactivity (Fig. 2b) (635 ± 74.28 μCi in the continuous-flow group vs. 661 ± 63.28 μCi in the stop-flow group) or mean labeling efficiency (Fig. 2c) (81.0 ± 8.4 % in the continuous-flow group, 84.5 ± 9.8 % in the stop-flow group).
Cell viability, measured by hemocytometer and Trypan blue, declined in both groups as a result of indium-111 oxine radiolabeling. However, no significant difference was observed in the viability of the cell products between the continuous-flow (85.0 ± 0.81 %) and the stop-flow (83.0 ± 0.78 %) groups (Fig. 2d). Similarly, there was no significant difference with respect to the final numbers of cells infused intracoronarily between the continuous-flow (10.1 ± 0.41 × 106 cells) and the stop-flow (9.83 ± 0.25 × 106 cells) groups (Fig. 2e).
Echocardiographic analyses
Baseline left ventricular (LV) ejection fraction (EF) was not significantly different between the continuous-flow group (74.35 ± 4.09 %) and the stop-flow group (71.16 ± 4.11 %) (Fig. 3). At 30 days after infarction, there was a significant decline in both the continuous-flow group (44.57 ± 6.75 %) and the stop-flow group (37.79 ± 6.77 %). The decline in ejection fraction did not differ significantly between the continuous-flow and stop-flow groups (P = 0.49).
Fig. 3.
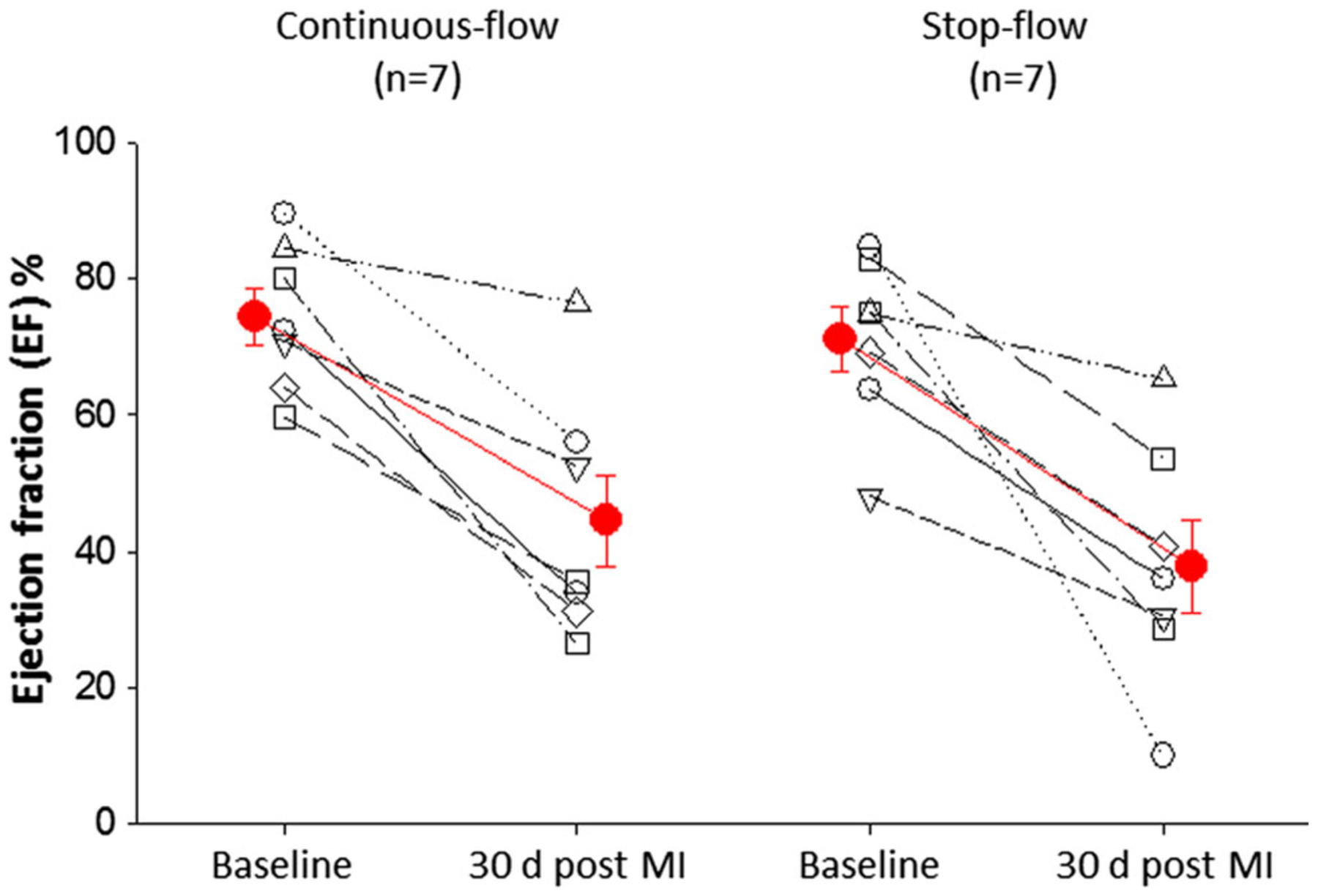
LV ejection fraction. Echocardiographic analyses were performed at baseline and again 30 days after infarction prior to intracoronary radiolabeled hCSC delivery to measure ejection fraction. Ejection fraction at 30 days after infarction was not significantly different between groups indicating similar degree of myocardial injury and functional decline (P = 0.49)
Nuclear imaging
Myocardial nuclear imaging was performed to visualize the distribution of radioactivity and, indirectly, cell retention. As expected, cell (radioactivity) retention was observed in the distribution of the mid-distal LAD within the apical, anteroseptal, and anterolateral regions (Fig. 4).
Fig. 4.
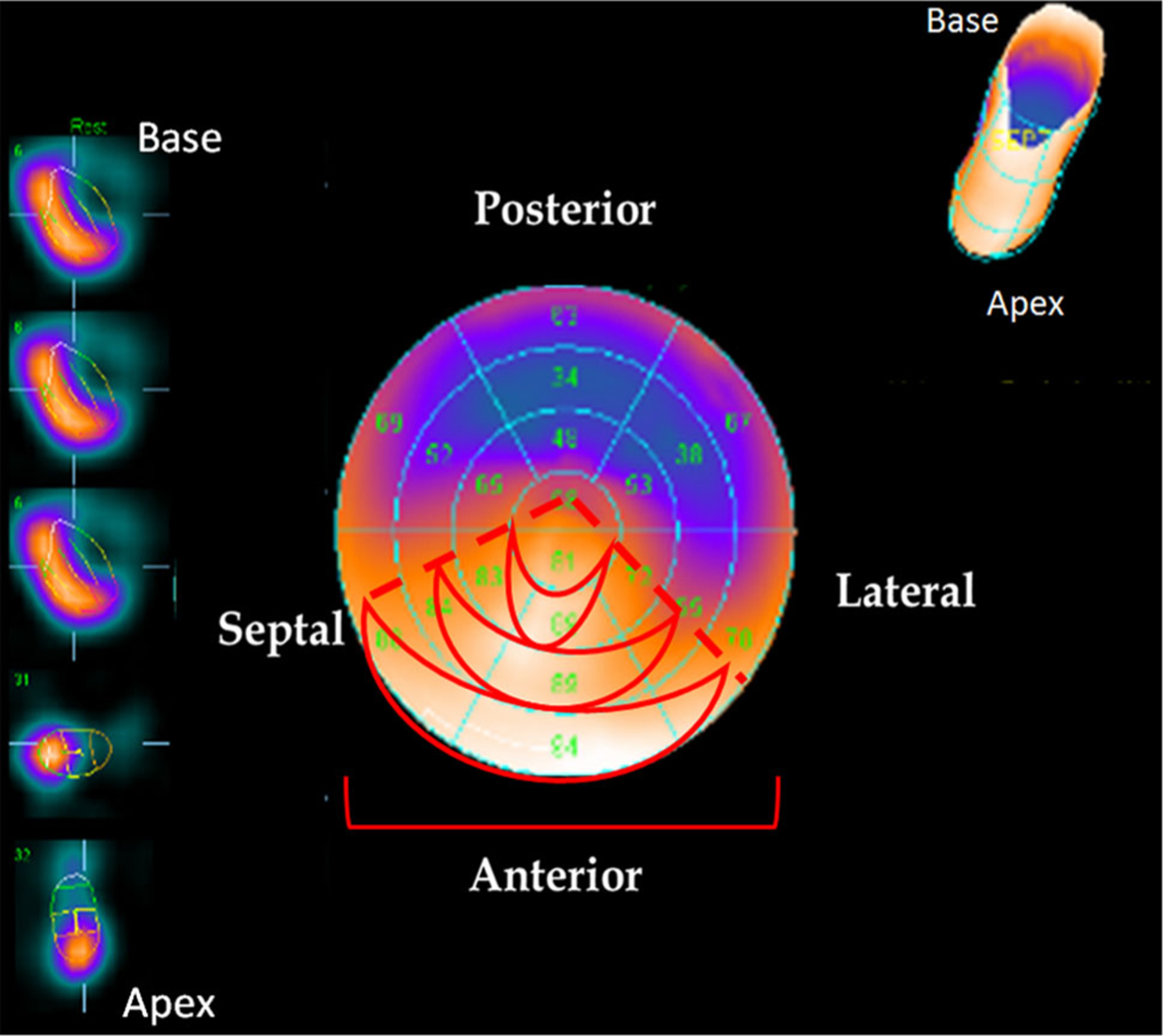
Nuclear imaging of hCSC retention. Cell retention was assessed by radioactivity and visualized by whole heart nuclear imaging. Radioactivity was observed in the distribution of the LAD, specifically, in the anteroseptal, anterior, and anterolateral walls of the left ventricle. Areas of high radioactivity and cell retention are identified by the bright orange coloration, while areas with no or low radioactivity are identified by blue/purple coloration
Cardiac retention of hCSCs
Measurements of total cardiac radioactivity demonstrated that the stop-flow technique did not result in significantly higher retention of hCSCs at 24 h compared with the continuous-flow technique (5.41 ± 0.80 vs. 4.87 ± 0.62 % of initial radioactivity, respectively, P = 0.61) (Fig. 5). Regional retention was also not significantly different between stop flow vs. continuous flow (right ventricle: 1.60 ± 0.30 vs. 1.02 ± 0.15 %, respectively, P = 0.12; LV septum: 1.88 ± 0.43 vs. 1.81 ± 0.33 %, P = 0.91; LV anterolateral wall: 1.17 ± 0.37 vs. 1.10 ± 0.17 %, P = 0.87; LV apex: 0.23 ± 0.05 vs 0.31 ± 0.11 %, P = 0.56; right atrium: 0.10 ± 0.03 vs. 0.11 ± 0.02 %, P = 0.81; left atrium: 0.10 ± 0.02 vs. 0.12 ± 0.03 %, P = 0.61; base: 0.33 ± 0.38 vs. 0.39 ± 0.13 %, P = 0.71) (Fig. 5).
Fig. 5.
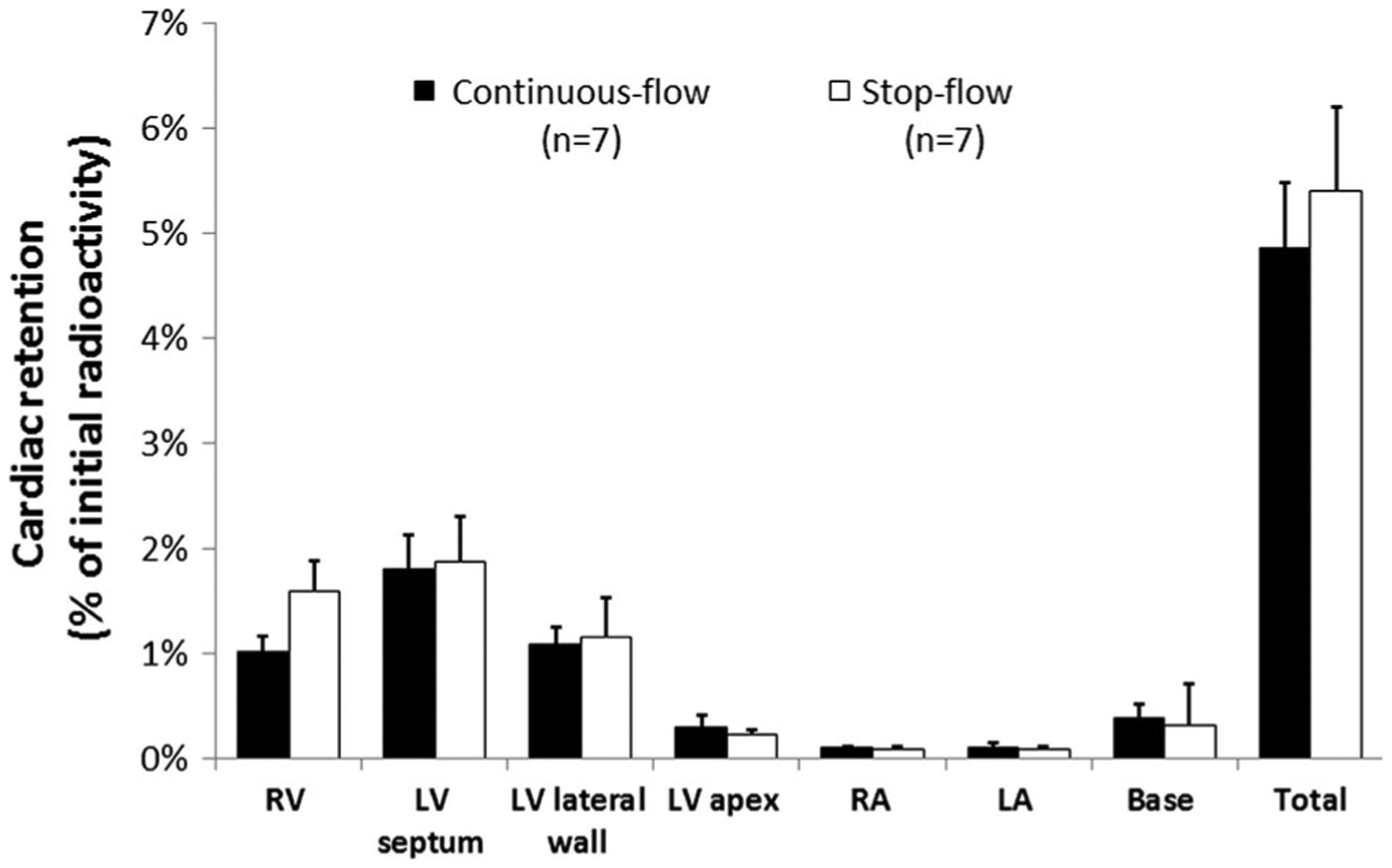
Myocardial hCSC retention at 24 h. Illustrated is the regional distribution of radioactivity in the continuous-flow (black) and stop-flow (white) groups. There was no significant difference in regional or total radioactivity (cell retention) between the continuous-flow (4.87 ± 0.62 %) and the stop-flow (5.41 ± 0.80 %) groups (P = 0.61)
CSC distribution in noncardiac tissues
Three small samples of lung, liver, kidney, and spleen from all pigs were assessed for radioactivity. Radioactivity was highest in the lung (17.48 ± 0.11 % of initial dose), followed by the liver (9.52 ± 0.51 of initial dose), kidney (3.08 ± 0.43 %), and lastly spleen (0.26 ± 0.06 %). Radioactivity was highly variable in different regions of the same organ.
Discussion
This study simultaneously addressed two fundamental questions regarding intracoronary infusion of c-kitpos hCSCs: (1) what fraction of hCSCs is retained in the heart 24 h after intracoronary infusion?, and (2) is the stop-flow technique superior to continuous flow in promoting cardiac retention of hCSCs? That is, is the increased risk of procedural complications associated with balloon inflation justified by increased hCSC retention? Our results indicate that, in pigs with an old myocardial infarction, intracoronary infusion of hCSCs without coronary occlusion is equivalent to intracoronary delivery utilizing the stop-flow technique in terms of cardiac hCSC retention at 24 h. It may also be reasonably inferred that ischemic preconditioning with intermittent balloon inflation/deflation, which has been shown to confer cardioprotective effects [8], does not affect cardiac retention of CSCs, thus eliminating this confounder in studies in which cell therapy has shown benefit when true stop flow was utilized in the treatment group but was withheld in the vehicle control group to minimize risk of complication. With either method, we found that only ~4–5 % of the infused hCSCs remained in the heart 24 h after intracoronary delivery. To our knowledge, this is the first head-to-head comparison of cardiac retention after intracoronary delivery of hCSCs (or any cardiac-derived cell) using continuous flow vs. stop flow.
Previous studies have examined the retention of various stem/progenitor cell types using various routes of administration, such as intravenous, intracoronary, and transmyocardial injections, in clinical and preclinical models [6, 10, 11, 13, 16, 18, 19, 21, 26–29]. However, studies that specifically compared cell product retention under continuous-flow and stop-flow conditions have been limited to bone marrow cells, and most have infused cells after acute myocardial infarction [6, 19, 26]. Doyle et al. [6] infused 18F-FDG-labeled BMCs intracoronarily in pigs after acute myocardial infarction and observed that a single dose of cells given with continuous flow was superior to repeated infusions with stop flow with respect to cardiac retention 1 h later. Tussios et al. [26] infused indium-111 oxine-labeled BMCs intracoronarily into pigs and found no difference in cardiac retention at both 1 h and 24 h after infusion comparing the two techniques. Perhaps of greater relevance, Musialek et al. infused 99Tc-extametazime-labeled bone marrow CD34+ cells in patients 6–14 days after acute myocardial infarction under continuous and stop-flow conditions; they found equivalent cardiac retention (~5 %) with both techniques at 36–48 h after infusion [19], which is similar to the results of the present study. Our study differs from the previous preclinical studies [6, 19, 26] in that we evaluated cardiac-derived cells and used a model of old myocardial infarction (scar), which is more relevant to the clinical use of cardiac-derived cells.
Analysis of various cardiac regions showed that there were no significant differences in the distribution of indium-111 oxine-labeled hCSCs between the two infusion techniques (Fig. 5). The overall retention of cells observed at 24 h (~4–5 %) was comparable to that observed in previous preclinical and clinical investigations using a variety of cell types [10, 11, 13, 16, 19, 21, 27–29]. We found a relatively high level of radioactivity in the apical portions of the right ventricular wall (Fig. 5). This observation can be accounted for by the fact that, in the pig, an accessory arterial branch originating from the distal LAD supplies the apical portions of the right ventricle [7, 22]. Radioactivity measurements of noncardiac organs indicated that the highest deposition of CSCs after intracoronary infusion is in the lungs followed by liver, kidneys, and spleen.
The specific protocol for the stop-flow technique (one 3-min occlusion without cell infusion followed by four 3-min occlusion/3-min reperfusion cycles) was chosen because it is the same protocol that was used in the SCIPIO trial [2]. The infusion of radiolabeled cells made it impossible to perform histologic analysis of the myocardium, because of the prolonged decay period of indium-111 oxine and attendant safety concerns. We were unable to ascertain whether remaining CSCs were adherent to walls of the microvasculature or had extravasated into the myocardium. To verify that the magnitude of damage produced by coronary occlusion/reperfusion was comparable between the two groups of pigs, we assessed LV function before and 30 days after infarction using echocardiography. Our measurements show that, 30 days after infarction, LV function did not differ significantly between the two groups, indicating a similar severity of the ischemic damage (Fig. 3).
The choice of the animal model was dictated by considerations related to clinical relevance. Clinically, the question of whether the risk of balloon inflation is justified arises most commonly in patients with chronic ischemic cardiomyopathy who have non-stented target coronary arteries. This issue is less relevant to patients with acute myocardial infarction, since in this setting the culprit vessel is usually stented during revascularization and the risk of dissection or injury associated with balloon inflation is therefore minimized. Accordingly, we decided to use a porcine model of old infarction and scarred myocardium, which mimics the clinical setting of chronic ischemic cardiomyopathy. Obviously, the effect of balloon inflation could not be studied in rodents. We could have used less complex and expensive porcine models (e.g., pigs without myocardial infarction or pigs with acute, rather than chronic, myocardial infarction); however, these models would not be relevant to the large cohort of patients with old stable myocardial infarcts (scars).
A variety of methods could have been used to assess cell retention at 24 h. The advantage of our methodology, based on quantification of residual radioactivity, is that it enabled us to assess the left ventricle as a whole. Alternative techniques such as PCR-based methods [10, 11] have the advantage of high sensitivity and precision in small samples, such as in murine hearts; however, they are not applicable to large tissue samples because the large amount of native DNA in such samples would cause a dilution in the targeted sequences of human genomic DNA of the residual hCSCs [10, 11]. This would lead to an underestimation of cell numbers. Additionally, PCR-based methods, as well as other methods such as fluorescence in situ hybridization of sex-mismatched donor–recipient pairs [25] and nuclear affinity labeling for cell tracking [13], enable quantification of cell numbers only in small myocardial samples assumed to be representative of the whole heart; these numbers must then be normalized to total myocardial weight to calculate global cardiac retention. Because cells may be distributed heterogeneously within the heart, as observed in the present study (Figs. 4, 5), these methodologies can potentially lead to inaccurate quantification due to sampling bias. We obviated these problems by measuring residual radioactivity in the entire heart.
In summary, using a clinically relevant porcine model of ischemic cardiomyopathy, we have demonstrated that the stop-flow technique does not result in superior hCSC retention 24 h after intracoronary infusion compared with non-occlusive hCSC infusion. Therefore, the increased procedural risks associated with balloon inflation do not appear to be warranted. These results have important practical implications for the design of future clinical trials in which hCSCs (or other stem/progenitor cells) are administered by the intracoronary route.
Acknowledgements
This study was funded by NIH grants P01-HL78825 and 1 UM1 HL-113530 and carried out in its entirety at the University of Louisville.
Abbreviations
- SCIPIO
Cardiac stem cell infusion in patients with ischemic cardiomyopathy trial
- CSC
Cardiac stem cell
- RAA
Right atrial appendage
- LV
Left ventricular
- EF
Ejection fraction
- PBS
Phosphate buffered saline
- FGF
Fibroblast growth factor
- LAD
Left anterior descending artery
- CD
Cluster of differentiation
Footnotes
Conflict of interest Authors have no disclosures and no conflicts of interest.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): initial results of a randomised phase 1 trial. Lancet 378:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J (2013) Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R (2012) Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126:S54–S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A (2013) Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart 99:225–232 [DOI] [PubMed] [Google Scholar]
- 6.Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM (2007) Dynamic tracking during intracoronary injection of 18f-fdg-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med Off Publ Soc Nucl Med 48:1708–1714 [DOI] [PubMed] [Google Scholar]
- 7.Gomez FA, Ballesteros LE (2014) Morphologic expression of the left coronary artery in pigs An approach in relation to human heart. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular 29:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175 [DOI] [PubMed] [Google Scholar]
- 9.Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R (2009) Coronary microembolization: from bedside to bench and back to bedside. Circulation 120:1822–1836 [DOI] [PubMed] [Google Scholar]
- 10.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R (2014) C-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 9:e96725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R (2013) A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keith MC, Tang XL, Tokita Y, Li QH, Ghafghazi S, Moore Iv J, Hong KU, Elmore B, Amraotkar A, Ganzel BL, Grubb KJ, Flaherty MP, Hunt G, Vajravelu B, Wysoczynski M, Bolli R (2015) Safety of intracoronary infusion of 20 million c-kit positive human cardiac stem cells in pigs. PLoS One 10:e0124227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiker M, Suzuki G, Iyer VS, Canty JM Jr, Lee T (2008) Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 17:911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH (2011) 2011 accf/aha/scai guideline for percutaneous coronary intervention: a report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation 124:e574–e651 [DOI] [PubMed] [Google Scholar]
- 15.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW (2007) Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 50:1761–1767 [DOI] [PubMed] [Google Scholar]
- 16.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marban L, Marban E (2013) Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 128:2764–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM (2008) Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29:1807–1818 [DOI] [PubMed] [Google Scholar]
- 18.Meluzin J, Vlasin M, Groch L, Mayer J, Kren L, Rauser P, Tichy B, Hornacek I, Sitar J, Palsa S, Klabusay M, Koristek Z, Doubek M, Pospisilova S, Lexmaulova L, Dusek L (2009) Intracoronary delivery of bone marrow cells to the acutely infarcted myocardium. Optimization of the delivery technique. Cardiology 112:98–106 [DOI] [PubMed] [Google Scholar]
- 19.Musialek P, Tekieli L, Kostkiewicz M, Majka M, Szot W, Walter Z, Zebzda A, Pieniazek P, Kadzielski A, Banys RP, Olszowska M, Pasowicz M, Zmudka K, Tracz W (2011) Randomized transcoronary delivery of cd34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of (9)(9)(m)tc-labeled cells activity. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 18:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak B, Weber C, Schober A, Zeiffer U, Liehn EA, von Hundelshausen P, Reinartz P, Schaefer WM, Buell U (2007) Indium-111 oxine labelling affects the cellular integrity of haematopoietic progenitor cells. Eur J Nucl Med Mol Imaging 34:715–721 [DOI] [PubMed] [Google Scholar]
- 21.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD (2012) Cardiovascular cell therapy research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the focus-cctrn trial. JAMA 307:1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahni D, Kaur GD, Jit H, Jit I (2008) Anatomy & distribution of coronary arteries in pig in comparison with man. Indian J Med Res 127:564–570 [PubMed] [Google Scholar]
- 23.Sanganalmath SK, Bolli R (2013) Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113:810–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I et al. (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2:358–367 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki G, Weil BR, Leiker MM, Ribbeck AE, Young RF, Cimato TR, Canty JM Jr (2014) Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PLoS One 9:e113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tossios P, Krausgrill B, Schmidt M, Fischer T, Halbach M, Fries JW, Fahnenstich S, Frommolt P, Heppelmann I, Schmidt A, Schomacker K, Fischer JH, Bloch W, Mehlhorn U, Schwinger RH, Muller-Ehmsen J (2008) Role of balloon occlusion for mononuclear bone marrow cell deposition after intracoronary injection in pigs with reperfused myocardial infarction. Eur Heart J 29:1911–1921 [DOI] [PubMed] [Google Scholar]
- 27.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC (2013) Effects of intracoronary cd34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res 112:165–173 [DOI] [PubMed] [Google Scholar]
- 28.Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC (2013) Comparison of transendocardial and intracoronary cd34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 128:S42–S49 [DOI] [PubMed] [Google Scholar]
- 29.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD (2004) Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363:783–784 [DOI] [PubMed] [Google Scholar]


