Abstract
Humans can reason about other minds, comprehend language and imagine. These abilities depend on association regions that exhibit evolutionary expansion and prolonged postnatal development. Precision maps within individuals reveal these expanded zones are populated by multiple specialized networks that each possess a spatially distributed motif but remain anatomically separated throughout the cortex for language, social and mnemonic / spatial functions. Rather than converge on multi-domain regions or hubs, these networks include distinct regions within rostral prefrontal and temporal association zones. To account for these observations, we propose the expansion-fractionation-specialization (EFS) hypothesis: evolutionary expansion of human association cortex may have allowed for an archetype distributed network to fractionate into multiple specialized networks. Human development may recapitulate fractionation and specialization when these abilities emerge.
Keywords: association cortex, precision neuroimaging, remembering, theory of mind, language
Introduction
Our abilities to re-experience past events, make inferences about others’ thoughts, and communicate through language are hallmarks of human cognition. Tasks targeting these functions have linked all three – autobiographical memory, social inference and language comprehension – to nearby or overlapping regions of association cortex [1–5]. Fueling further interest, these same distributed association zones are disproportionately expanded in the brains of large primates [6–8] and show prolonged postnatal development in humans [8–10] (see Figure 1]. A foundational question for the field concerns how networks supporting advanced cognitive abilities are organized in these expanded association zones.
Figure 1. Distributed regions of human association cortex exhibit disproportionate evolutionary and developmental expansion.
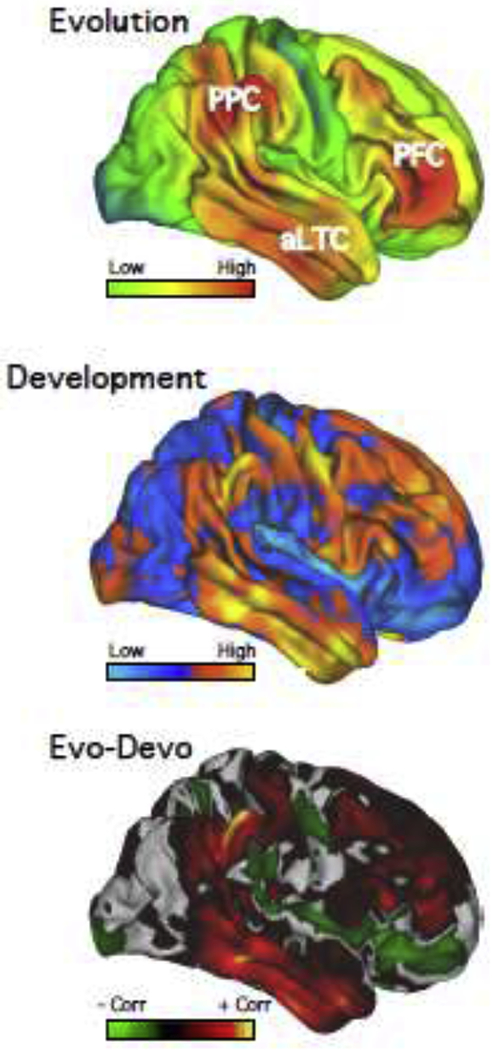
Estimates of cortical surface expansion (A) between macaque and humans and (B) between human infants and adults both exhibit disproportionate expansion across distributed association zones, including in prefrontal cortex (PFC), posterior parietal cortex (PPC) and anterior (rostral) lateral temporal cortex (aLTC). Similarities between evolutionary and developmental expansion are summarized in (C), where warm colors indicate positive correlations and cool colors negative correlations. Both evolutionary estimates and those from development highlight association zones distributed across posterior parietal, temporal, and prefrontal zones that are disproportionately larger in humans and late to develop. A central question in human neuroscience is how networks are organized in these expanded zones of association cortex in support of higher-level functions. Data adapted from [8].
A barrier to unravelling the organization of association networks has been that multiple networks often have component regions juxtaposed next to one another in the same zones, causing blurring between networks and sometimes the impression of convergence. Precision mapping within the individual provides insight into the detailed organization of networks by avoiding spatial blurring and allowing functional specialization to be examined within the idiosyncratic anatomy of a given individual [e.g., 3,11].
What has been discovered using precision mapping is a high degree of anatomical separation and functional specialization between networks, not just within a single zone of cortex, but across the entire distributed extents of multiple distinct higher-order networks. Each specialized network includes regions distributed across rostral prefrontal and temporal association cortical zones. The similar spatial motif – with network regions often side-by-side across cortex – raises the possibility that the multiple networks originated from a singular archetype that has fractionated and specialized to support advanced human cognitive abilities.
Distributed Regions of Human Association Cortex Exhibit Disproportionate Expansion
Comparing the human cortex to that of a monkey reveals a non-uniform expansion pattern, with disproportionately greater expansion in association zones that include prefrontal, posterior parietal, and rostral temporal cortex [6,8,12]. Figure 1A shows an estimate of the macaque-to-human expansion map [8]. Note how red regions, representing high relative expansion, appear widely distributed – not in a single zone, such as prefrontal cortex, but in multiple, separate zones.
What’s more, a similar pattern appears when examining human cerebral cortical expansion from infancy to adulthood (Figure 1B). Hill and colleagues [8] characterized human evolutionary and developmental expansion ratios and plotted correlations between these values (see also [9]). Regions showing markedly similar expansion were those distributed across association cortex, including zones in prefrontal cortex (‘PFC’ in Figure 1C], posterior parietal cortex (‘PPC’] and lateral temporal cortex (‘LTC’] [8].
These observations motivate examination of how these expanded and developmentally neotenous zones are organized.
Association Cortex Comprises Large-Scale, Distributed Networks Across Primate Species
Unlike early sensory systems, where areas have predominantly (but not exclusively) local connectivity to adjacent and nearby areas, higher-order association cortex is characterized by connectivity to association zones located in widely distributed positions throughout the cortex. Association regions in one zone of cortex (e.g., the inferior parietal lobule) will receive and send projections to zones of temporal, prefrontal, and midline association cortex. This anatomical motif has been well-characterized in macaques [13,14] and marmosets [15–17], and is also consistent with network estimates in the human [18–20]. Moreover, there is evidence for anatomical specialization even within prefrontal zones such that adjacent regions in parietal association cortex and adjacent regions in temporal association cortex will form parallel networks with distinct prefrontal regions [13,21; see also 14,22]. This anatomical organization is intriguing because it suggests a circuit motif that, via its multiple parallel instances, could support functional specialization.
Direct comparisons of human to monkey estimates of network organization reveal considerable homology, including for networks involving the rostral temporal and prefrontal association zones that are disproportionately expanded in humans [2,23–27]. Given that old world and new world primate lineages share a common ancestor about 45 million years ago, these homologies suggest the prototypical distributed association network was fully represented in a relatively small-brained primate ancestor many tens of millions of years ago. What is intriguing to consider is how disproportionate expansion of human association cortex might build upon this anatomical archetype and contribute to especially advanced cognitive abilities.
Multiple Parallel Distributed Networks Occupy Association Cortex
A barrier to fully unraveling details of network organization has been reliance on group-averaged estimates. Regions with in the evolutionarily-expanded association zones show marked variability between individuals as measured by functional connectivity MRI (fcMRI) [28–30] as well as through direct anatomical approaches [31–33]. Until recently, fcMRI estimates of whole-brain network organization (or ‘parcellations’) provided insight into general patterns [18–20], but blurred across individual variation. Broad swaths of association cortex at or near apex zones (i.e., far from sensorimotor hierarchies], for example, were commonly attributed to monolithic, multiple function networks (e.g., the canonical default network – DN] [24,34] or proposed to contain hubs of convergence [35].
Explorations of network organization leveraging within-individual approaches reveal finer-grained details [30,36–42]. Networks originally thought to support multiple functional domains or share regions of convergence (including ‘hubs’] have been revealed to possess anatomical separation and specialization. Such separation applies to many distinct networks, some hypothesized to contribute to multi-domain aspects of cognitive control and others supporting more specialized domains of information processing.
Here we focus on emerging evidence that reveals at least three distinct domain-specialized networks within the expanded association zones that differentially support language, social, and mnemonic / spatial functions [43,44]. While each of these three networks preserves the anatomical motif observed in other primates [13,26], ah exhibit parallel nodes, side-by-side but spatially distinct, across the distributed association regions (see Figure 2]. In the first decades of exploration of these networks, we and others believed that they converged on shared functional regions [18,35]; we have recently appreciated that much of the focus on convergence may arise from a technical artifact of between-individual averaging (spatial blurring] [e.g., 11,40].As precision estimation methods have improved, the multiple networks have been revealed to rely on largely – if not entirely – anatomically distinct adjacent regions contributing to specialized processing domains [37,43–45].1
Figure 2. Within an individual, parallel distributed networks differentially support remembering, theory-of-mind and language task contrasts.

(Top) Within an individual, DN-A, DN-B and LANG networks exhibit distributed regions that are side-by-side across association zones, including prefrontal cortex (PFC), posterior parietal cortex (PPC), anterior lateral temporal cortex (aLTC), posteromedial cortex (PMC) and medial prefrontal cortex (mPFC). (Bottom) Each of these networks exhibits preferential recruitment by task contrasts from distinct functional domains: DN-A for remembering, DN-B for theory of mind and LANG for language. This remembering task contrasted questions about personal past events to those targeting current feelings and beliefs (see [35,44]). This theory-of-mind task contrasted consideration of others’ emotional to physical pain (see [44,59,90]). The language task contrasted reading sentences comprising words to pronounceable nonwords (see [11,43]). Domain-preferential responses are a property of the entire distributed networks including rostral temporal and prefrontal regions. Data adapted from [43 (Subject 13), 44 (Subject 12)].
Two of the domain-specialized networks, termed DN-A and DN-B for convenience, were identified first as interdigitated within the expanded association zones, including lateral and medial PFC (lPFC and mPFC), PPC, LTC, and posteromedial cortex (PMC) [36]. Certain features of these two networks align with previously reported region-specific dissociations. For example, a more rostral region, along the temporoparietal junction (TPJ), is active during tasks targeting theory of mind, in contrast to an adjacent, caudal PPC region active during autobiographical memory retrieval tasks [1,34,46], What was unexpected is that the networks are anatomically separate throughout their entire distributed extents including tight interdigitation within midline zones that were previously difficult to parse [36; see 35].mPFC and PMC both feature juxtaposed but spatially separate regions of DN-A and DN-B [36–37,47]. In PMC in particular, DN-A features a reliable triad of regions that surround a region of DN-B, mirroring task differences observed in high-resolution individualized analyses [48,49]. Precision estimates of the two networks reveal that previously noted dissociations in local cortical zones are components of parallel distributed networks that are largely (or entirely) anatomically distinct throughout their cortical extents.
The third, fully distinct network putatively labelled a language network (LANG) because of its anatomical positioning and lateralization, exhibits a similarly distributed pattern, as well as tight juxtaposition to DN-A and DN-B across association zones (Figure 2] [43]. This network includes regions in inferior frontal gyrus (IFG), posterior superior temporal cortex (pSTC) and the temporal pole (TP), included in clinical [50–52] and task-based fMRI studies [11] of language. Of historical note, this network was underappreciated in several well-referenced estimates of network organization that used group-averaging approaches (e.g., blurred, including within the DN, in [18–19]; but see [53–55]).
Evidence for these multiple separate but interdigitated networks has now been found within over two dozen unique individuals [36–37,43–44], including those extensively sampled (i.e., featuring more than 6 hours of fixation data each) and those scanned with high field strength (7T), reinforcing that these networks appear spatially separate, sometimes even within the same sulcus (see Fig. 9 in [37]). Parcellations from other within-individual data collection efforts (e.g., analyses of Human Connectome Project and Midnight Scan Club data) also show evidence for separate, interdigitated networks, particularly within subsets of individuals. For example, in one study, features of “Default A”, “Default B” and “Temporal Parietal” networks correspond to those in DN-A, DN-B and LANG (see Fig. 4 in [30]), and in another, midline solutions for the “Context” network relate to DN-A and “Default” to DN-B (particularly within MSC04, MSC05, MSC09, MSC10) and ‘Ventral Attention’ to LANG (across subjects; see Fig. 3 in [38]).
To reiterate, these networks occupy association zones that exhibit disproportionate expansion across human evolution and postnatal development. For example, compare the labelled zones in the parcellation estimate of a single individual in Figure 2 [43,44] to corresponding zones in the correlation map from Figure 1C [8]. That these networks feature a common parallel, distributed motif suggests the possibility that such an organization might result from fractionation of a less-differentiated proto-organization, perhaps early in development (discussed further below; see also [56]).
Parallel Distributed Association Networks Support Domain-Specialized Higher-Order Functions
Anatomically-separate networks within the expanded zones of association cortex participate in specialized functional domains. Recent evidence for network specialization has been obtained by identifying the networks within individuals and then exploring functional dissociations in task-based activation studies targeting (i) mnemonic / spatial, (ii) social and (iii) language functions [43,44]. Figure 2 summarizes a key set of direct dissociations in a single individual :DN-A is preferentially recruited for remembering, DN-B for social inference and LANG for language. Such evidence for specialization is striking, given the tight interdigitation of these networks, as well as prior group-based work suggesting potential convergence [1, but see 57,58]. Figure 2 shows data from one person, but task-based dissociations were independently replicated across almost all individuals tested to date [43,44]. Distinct network recruitment was also not limited to specific regions, but appeared across multiple zones [44], often including small distributed regions that might otherwise go unnoticed [43]. These findings collectively suggest that functional specialization – in relation to a task domain – is a property of the network as a whole, not just subregions.
For example, in a series of recent studies, we contrasted tasks involving remembering past and imagining future events, to a distinct set of task contrasts designed to probe theory of mind [44]. The theory-of-mind tasks were developed by Rebecca Saxe and colleagues to isolate regions recruited within-individuals when considering others’ mental states [59–60, see also 63,90]. We found evidence for a functional double dissociation, with DN-A preferentially recruited for remembering the past and imagining the future and DN-B for theory of mind. Evidence for functional dissociation was obtained for the network as a whole and across each of the five distributed regions labelled in Fig. 2 (with the strongest results in subsets of participants) [44]. Using another task contrast developed by Evelina Fedorenko and colleagues to isolate language-relevant regions (e.g., from nearby’ multiple demand’ regions) [61,62, see also 11], we explored recruitment of the LANG network. Task recruitment was highly selective for the LANG network as compared to the immediately adjacent association networks [43]. Of further interest, while the LANG network was left-lateralized in most individuals, one individual was right lateralized. The response to the language task contrast was specific and selective to the right-lateralized network regions in this individual, demonstrating that network organization predicts functional specialization even in instances of unusual organization.
Collectively, these results strengthen evidence that the three parallel networks – DN-A, DN-B and LANG – can be consistently identified as separate and functionally dissociated within individuals. While questions remain about network processes and the extent of specialization,1 these distributed networks occupy juxtaposed zones of association cortex and are differentially recruited by tasks from distinct, higher-order cognitive domains [43,44]. Probing specialized regions, or groups of regions, differentially supporting such functions is not novel [e.g., 1,3,34,57,63,64; see also 88]. What has newly emerged is that functional dissociation is present across the entire distributed networks for these domains, including rostral temporal and prefrontal associations regions, and with juxtapositions between networks mirrored in many locations throughout the cortex, as if a common originating proto-organization is fractionated and specialized to support diverse functions. In the next section we will speculate on how such specialization might emerge during development.
Proposed Role of Hierarchical Development in Network Organization
The expanded association zones of human cortex are populated by separate but intertwined networks. How might such an organization arise? The development of specialized functional areas and patches in visual extrastriate cortex provides insight into how distributed higher-order associations networks might fractionate and specialize [65]. Early in postnatal development, primate extrastriate regions (or ‘patches’ in monkeys) do not show the fully formed category-specific specialization (i.e., for faces or scenes) observed in adults [65–67]. Rather, extrastriate cortex is visually responsive and has a coarse retinotopic organization, featuring adjacent mapping of central-to-peripheral parts of the retina [68,71]. By one model, this proto-organization is hypothesized to scaffold further refinement of specialized zones that emerge via experience, with face-responsive patches forming within extrastriate zones aligned to the central portion of the retinotopic map and scene-responsive patches with in the peripheral portion [68–69]. The broad, early retinotopic organization may thus influence how extrastriate patches fractionate and specialize early in postnatal development [68]. Recently, evidence for biased connectivity (e.g., between central V1 and proposed face-selective cortical regions and peripheral V1 and scene-selective regions) was also observed in newborn human infants, further suggesting that connectivity may influence the development of network-specific functions, likely concurrently with top-down or other influences [70].2
Specialization of parallel networks within association cortex might similarly proceed hierarchically. An early proto-organization (perhaps reflecting broad, DN-like properties in apex zones) may fractionate and specialize through developmental processes that are biased by distinct features of network connectivity [44,56]. A distinguishing feature of DN-A, for example, is connectivity to posterior parahippocampal cortex (PHC) [36]. This feature, perhaps reflecting a hippocampal gradient of projections to association cortex, might bias network fractionation and specialization, with DN-A ultimately providing support for mnemonic and spatial processes [see also 56].3 Similarly, Silson and colleagues [49] proposed that the category-preferences in ventral temporal cortex may themselves impact specialization of such association regions as PMC. As another example, the association regions that form the canonical language network are not randomly positioned across the cortex. Prefrontal zones near what has historically been called ‘Broca’s area’ are near to the motor representations of the orofacial structures critical to speech. The temporal association zones are near to secondary auditory regions important to s peech perception [e.g., 43]. Anatomical adjacencies might reflect early developmental anchors that bias or constrain which specific association regions emerge as components of each higher-order network.
This proposal also allows for the networks, like extrastriate patches, to display both similar modes of processing and distinct specialization [66,72]. DN-A and DN-B, for example, may share a broad processing mode (i.e., for internally-constructed representations] and also specialization (i.e., preferentially supporting specific functional domains; see also [56]).
Proposing the Expansion-Fractionation-Specialization (EFS) Hypothesis
To account for the described network observations, we propose the Expansion-Fractionation-Specialization (EFS] hypothesis (see also [22,73]). The evolutionary expansion of homin in association cortex might have created an opportunity for network specialization by providing large zones of cortex that share in common a distributed anatomical-connectivity motif (Figure 3). Comparative analysis with monkey species suggests this core motif is at least 45 millions years old [15,26–27,74]. In modern humans this anatomical motif is expressed on a cerebral surface that is vastly expanded relative to the estimated primate ancestor. At birth, human association cortex may exhibit a proto-organization that reflects this ancient network motif but with poorly differentiated anatomical connectivity across the broad associations zones. Fractionation and specialization may then occur during early development, through competitive activity-dependent processes, producing finer-grained networks that, as a property, have close juxtapositions across the cortex [56]. Connectivity differences mayalsobias specialization, evidenced by the networks’ distinct features and differential recruitment for mnemonic / spatial, social and language domains [43,44]. In this way, fine-grained networks may develop in expanded association zones, with those farthest from sensorimotor hierarchies crucial to uniquely-flexible human functions [22,75].
Figure 3. The Expansion-Fractionation-Specialization (EFS) hypothesis.

(Left) The cerebral cortex of a primate common ancestor (i.e., shared between new and old world primate lineages over 45 million years ago) featured anatomically-connected distributed association regions. This ancient anatomical motif likely persists in its core form in modern humans. Association cortex exhibits disproportionate expansion across human evolution (Middle). This expansion may have provided the opportunity for the ancient prototype distributed network (Middle) to fractionate into multiple, specialized networks (Right). When we are imaging human adults using precision neuroimaging techniques, we may be visualizing the outcome of this process with domain-specialized networks populating portions of the expanded association zones, with distinct network regions juxtaposed throughout the many zones of association cortex.
A Note on the Relation Between Domain-Specialized Networks and Multiple-Domain Networks
While we have focused here on parallel networks that participate in domain-specialized functions, networks linked to domain-general cognitive and attentional control (e.g., ‘frontoparietal control’ network [23]) also occupy expanded association zones, including regions showing marked individual variability [28–29,76]. In this context, it is important to note that the presence of multiple specialized networks, with juxtaposed nodes within prefrontal cortex, challenges the notion that domain-general prefrontal regions exclusively control posterior domain-specialized regions. As far as we have been able to estimate, the association networks most specialized for domain-specific processing all include rostral (anterior) prefrontal and temporal association regions.4
By some views, this observation may be surprising given the expectation that anatomical integration might lead to more and more abstracted, domain-flexible representations in rostral portions of frontal cortex [e.g. see 89]. The collective recent results argue that this is not the case for all prefrontal regions. Rather, domain-specialized networks include prefrontal and temporal regions as anticipated by the seminal work of Patricia Goldman-Rakic [13]. And while there is also clear evidence for distinct domain-general networks that participate in cognitive control, these networks are anatomically distinct throughout the cerebral cortex including prefrontal cortex (see also [61]). Without the detailed knowledge of the exact locations of these functionally distinct regions, it would be easy to miss their full level of specialization and heterogeneity.
The detailed anatomical features identified in recent years via precision estimates of organization thus provide a view of specialization that includes considerably more modularity in certain zones of prefrontal cortex than might have been predicted, with domain-specialized prefrontal regions as components of widely-distributed specialized networks. Interactions between these and other networks are an open topic for future investigation. For example, domain-specialized networks maybe under the control of other networks. That is, rather than prefrontal regions acting as domain-general top-down control structures sending signals that bias processing in posterior regions, it may be that certain distributed networks control other distributed networks, with each network possessing its own anatomically-distinct prefrontal component.
Most broadly, it will be interesting for the field to revisit notions of modularity, domain specialization, and interactions with domain-general processing functions from a network perspective informed by precision anatomy [see also 76].
Considerations for Future Research
Though we hypothesize that early networks may form from a proto-organization that fractionates and specializes, these ideas require testing. We predict that such fractionation may occur in the first years of life (i.e., coinciding with rapid surface area expansion [77]). Fractionation may occur by age 3, for example, given evidence that brain regions typically active during theory-of-mind tasks (e.g., overlapping with DN-B] respond preferentially to consideration of others’ thoughts by this age [78]. Infant work also shows evidence of long-distance connectivity even earlier in postnatal development (e.g., see Fig. 2 in [79]). Increasing efforts to examine infant cortical organization, prenatally or within days to weeks after birth [70,80–81] highlight exciting opportunities for future research.
A crucial and related question pertains to how disruptions to proposed network fractionation and specialization processes might have diverse clinical implications. Considerable research, for example, has explored whether differences in brain regions supporting social and language functions could inform understanding of autism spectrum disorder (ASD) [82–84]. As described, networks supporting social inference and language maybe tightly interwoven across the cortical mantle within neurotypical adults, raising the possibility that atypical fractionation and/or specialization contribute to the development of ASD symptoms. Exploring network trajectories during the first year of life, even prior to symptom emergence [85], could inform a potential role of network development.
Conclusions
We present recent evidence from precision mapping within individuals that parallel and distributed networks occupy regions of association cortex that exhibit disproportionate expansion during human evolution and development. Several of these networks appear to differentially support distinct higher-order functions including remembering, social inference and language [36,43,44]. To account for these findings, we propose the expansion-fractionation-specialization (EFS) hypothesis: the expansion of human association cortex might have allowed distributed association networks to fractionate and specialize, perhaps early in development, within zones farthest from (and least tethered to) sensorimotor hierarchies exhibiting links to advanced, sensory-independent fuctions [22,56].
Highlights.
Within-individual precision estimates reveal multiple juxtaposed distributed networks.
Adjacent networks share a spatial motif, potentially reflecting common origins.
Networks are differentially specialized for language, social and mnemonic/spatial functions.
We propose the expansion-fractionation-specialization (EFS) hypothesis.
Acknowledgements
We thank Rodrigo Braga and Evelina Fedorenko for comments, Ben Deen and Talia Konkle for helpful discussion, and David Van Essen and Tim Scott Coalson for sharing and assisting with the data featured in Figure 1. This work was supported by Kent and Liz Dauten, NIH grant P50MH106435 and Shared Instrumentation GrantS10OD020039. L.M.D. was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE1745303. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors – Randy L. Buckner and Lauren M. DiNicola – declare no conflicts of interest.
We review evidence for dissociable network recruitment across three broad domains, which suggests distinct specialization. But we do not yet understand the specific processes or full nature of the domains that are specialized. The task contrasts that have yielded dissociations to date provide clues about the networks’ distinct properties [43,44; see also 11,63], but there may be other ways to reveal the dissociations. The hard question of the computational nature of the specializations is thus unresolved. For this reason, we describe the roles of these networks broadly. For example, we label DN-A as participating in mnemonic / spatial processes because we are unsure yet how to fully characterize the functional domain of the network. Complex tasks, including episodic remembering and theory-of-mind decisions, call on multiple component processes.
By proposing that early, bottom-up connectivity differences might bias network specialization, we do not imply that specialization of extrastriate cortex emerges only from a retinotopic proto-organization. It could be a major, but not exclusive, developmental constraint. A retinotopic proto-organization, for example, leaves open why humans (and other primates) foveate faces [86, see also 87]. Bottom-up self-organization and top-down biases may together lead to strong developmental constraints that cause convergent anatomical and functional organization to emerge when expressed in the typical developmental environment (see also [91]).
There is a tricky, unresolved issue that concerns the functional neuroanatomy of the hippocampal formation along its anterior-to-posterior axis. DN-A shows strong coupling to posterior PHC [36] and can be linked to regions within the hippocampus proper at or near the subiculum [37]. However, MRI measures based on typically-used methods have poor signal properties in anterior hippocampal regions, including a large extent of entorhinal cortex. Thus, we do not know yet whether DN-B, or other networks, are coupled to distinct regions of the anterior hippocampal formation.
While our paper focusses on specialization between networks, one should not expect the multiple regions within a domain-specialized network to make the same computational contribution to the network’s overall functions. It is likely that anterior and posterior cortical regions possess their own local circuit motifs that are being used over and over across the multiple specialized networks in ways that are not revealed by our emphasis on domain specialization between the distributed networks.
References
- [1].Andrews-Hanna JR, Saxe R, Yarkoni T: Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage 2014,91:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Binder JR, Desai RH, Graves WW, Conant LL: Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex 2009, 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fedorenko E, Kanwisher N: Neuroimaging of language: Why hasn’t a clearer picture emerged? Lang Linguist Compass 2009, 3:839–865. [Google Scholar]
- [4].Svoboda E, McKinnon MC, Levine B: The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, Sallet J, Kanske P: Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull 2020. DOI: 10.1037/bul0000303. [DOI] [PubMed] [Google Scholar]
- [6].Chaplin TA, Yu H-H, Soares JGM, Gattass R, Rosa MGP: A conserved pattern of differential expansion of cortical areas in simian primates. J Neurosci 2013, 33:15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krubitzer L: The magnificent compromise: cortical field evolution in mammals. Neuron 2007, 56:201–208. [DOI] [PubMed] [Google Scholar]
- [8].Hill J, Inder T,Neil J, Dierker D, Harwell J, Van Essen D: Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA 2010, 107:13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, Rosa MGP, Walhovd KB : Organizing principles of human cortical development – Thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cereb Cortex 2016, 26:257–267. [DOI] [PubMed] [Google Scholar]
- [10].Gogtay N, Giedd JN, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen L S, Toga AW, Rapoport JL, Thompson PM: Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004, 101:8164–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fedorenko E, Hsieh PJ, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N: New method for fMRI investigations of language: Defining ROIs functionally in individual subjects. J Neurophysiol 2010, 104:1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu T, Nenning K-H, Schwartz E, Hong S-J, Vogelstein JT, Goulas A, Fair DA, Schroeder CE, Margulies DS, Smallwood J, Milham MP, Langs G: Cross-species functional alignment reveals evolutionary hierarchy within the connectome. Neuroimage 2020, 223:117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goldman-Rakic PS: Topography of cognition: Parallel distributed networks in primate association cortex. Ann Rev Neurosci 1988, 11:137–156. [DOI] [PubMed] [Google Scholar]
- [14].Mesulam MM: Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 1990, 28:597–613. [DOI] [PubMed] [Google Scholar]
- [15].BurmanK J, Reser DH, Yu H-H, Rosa MGP: Cortical input to the frontal pole of the marmoset monkey. Cereb Cortex 2011, 21:1712–1737. [DOI] [PubMed] [Google Scholar]
- [16].Reser DH, Burman KJ, Yu H-H, Chaplin TA, Richardson KE, Worthy KH, Rosa MGP: Contrasting patterns of cortical input to architectural subdivisions of the area 8 complex: A retrograde tracing study in marmoset monkeys. Cereb Cortex2013, 23:1901–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ: Forebrain connectivity of the prefrontal cortex in the marmoset monkey ( Callitrhix jacchus): An anterograde and retrograde tract-tracing study. J Comp Neurol , 2007, 502:86–112. [DOI] [PubMed] [Google Scholar]
- [18].Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL: The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011, 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE: Functional network organization of the human brain. Neuron 2011, 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doucet G, Naveau M,Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio-Mazoyer N, Mazoyer B, Mellet E, Joliot M: Brain activity at rest: A multi-scale hierarchical functional organization. J Neurophysiol 2011, 105:2753–63. [DOI] [PubMed] [Google Scholar]
- [21].Selemon LD, Goldman-Rakic PS: Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the Rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 1988, 8:4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buckner RL, Krienen FM: The evolution of distributed association networks in the human brain. Trends Cogn Sci 2013, 17:648–665. [DOI] [PubMed] [Google Scholar]
- [23].Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL: Evidence fora frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 2008, 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buckner RL, Andrews-Hanna JR, Schacter DL: The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 2008, 1124:1–38. [DOI] [PubMed] [Google Scholar]
- [25].Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M: Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Nat Acad Sci 2009, 106:20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[26].Buckner RL, Margulies DS: Macroscale cortical organization and a default-like apex transmodal network in the marmoset monkey. Nat Commun 2019, 10:1976. [DOI] [PMC free article] [PubMed] [Google Scholar]; Exploring marmoset anatomical data, the authors found evidence for distributed networks, including an apex association network that is a candidate default network homologue. This adds to evidence that the distributed network motif observed in human association cortex maybe shared with a distant common primate ancestor (see also [15–17]).
- [27].Ghahremani M, Hutchison RM, Menon RS, Everling S: Frontoparietal functional connectivity in the common marmoset. Cereb Cortex 2017, 27:3890–3905. [DOI] [PubMed] [Google Scholar]
- [28].Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H: Individual variability in functional connectivity architecture of the human brain. Neuron 2013, 77:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[29].Mansour S, Tian Y, Yeo BTT, Cropley V, Zalesky A: High-resolution connectomics fingerprints: Mapping neural identity and behavior. Neuroimage 2021, 229:117695. [DOI] [PubMed] [Google Scholar]; The authors used multiple techniques – including structural, functional and diffusion-weighted MRI – to probe for individual-specific features. In particular, they found evidence for unique properties within association zones.
- **[30].Kong R, Li J, Orban C, Sabuncu MR, Liu H, Schaefer A, Sun N, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BTT: Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion. Cereb Cortex 2019, 29:2533–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors compared multiple approaches for within-individual network estimation, including their multi-session hierarchical Bayesian model (MS-HBM], Results showed the power of their model and the potential role of network topography in behavioral prediction.
- [31].Rajkowska G, Goldman-Rakic PS: Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 1995, 5:323–337. [DOI] [PubMed] [Google Scholar]
- [32].Amunts K, Schleicher A, Burge lU, Mohlberg H, Uylings HBM, Zilles K: Broca’s region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 1999, 412:319–341. [DOI] [PubMed] [Google Scholar]
- [33].Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K: The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage 2006, 33:430–448. [DOI] [PubMed] [Google Scholar]
- [34].Spreng RN, Grady CL: Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind and their relationship to the default mode network. J Cogn Neurosci 2010, 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- [35].Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL: Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[36].Braga RM, Buckner RL: Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 2017, 95:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; Examining network estimates within 4 extensively-scanned individuals, the authors found fully distinct, parallel networks within the bounds of the canonical default network (DN -A and DN-B], These and additional nearby networks exhibited adjacent regions across distributed association zones. This work and [38], characterizing network estimates within the Midnight Scan Club, demonstrated how within-individual estimates can reveal novel network details.
- [37].Braga RM, Van Dijk KRA, Polimeni JR, Eldaief MC, Buckner RL: Parallel distributed networks resolved at high resolution reveal close juxtaposition of distinct regions J Neurophysiol 2019, 121:1513–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF: Precision functional mapping of individual human brains. Neuron 2017, 95:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huth AG, de Heer WA, Griffiths TL, Theunissen FE, Gallant JL: Natural speech reveals the semantic maps that tile human cerebral cortex. Nature 2016, 532:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[40].Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen M-Y, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, Schlaggar BL, Mumford JA, Poldrack RA, Petersen SE: Functional system and areal organization of a highly sampled individual human brain. Neuron 2015, 87:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]; For this landmark study (as part of the My Connectome project] a single individual underwent repeated scanning, and data from 84 sessions allowed for detailed estimation of network organization. This work illustrated the feasibility of extensive neuroimaging sampling as well as the power of a within-individual approach for identifying fine-grained and individual-specific network features.
- [41].Michalka SW, Kong L, Rosen ML, Shinn-Cunningham BG, Somers DC: Short-term memory for space and time flexibly recruit complementary sensory-biased frontal lobe attention networks. Neuron 2015, 87:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xue A, Kong R, Yang Q, Eldaief MC, Angeli P, DiNicola LM, Braga RM, Buckner RL, Yeo BTT: The detailed organization of the human cerebellum estimated by intrinsic functional connectivity within the individual. J Neurophysiol 2020. DOI: 10.1152/jn.00561.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Braga RM, DiNicola LM, Becker HC, Buckner RL: Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. J Neurophysiol 2020, 124:1415–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[44].DiNicola LM, Braga RM, Buckner RL: Parallel distributed networks dissociate episodic and social functions within the individual. J Neurophysiol 2020, 123:1144–1179. [Erratum in J. Neurophysiol 2020,123:307.] [DOI] [PMC free article] [PubMed] [Google Scholar]; Examining network functions within 18 individuals, the authors found a double dissociation, with DN-A preferentially recruited for episodic projection tasks and DN-B for those requiring theory of mind. This was true not only in previously-observed regions [1] but across the full extent of the networks. With [43], results provide evidence that parallel networks, with adjacent regions across distributed zones, maybe specialized to support distinct higher-order domains.
- [45].Gordon EM, Laumann TO, Marek S, Raut RV, Gratton C, Newbold DJ, Greene DJ, Coalson RS, Snyder AZ, Schlaggar BL, Petersen SE, Dosenbach NUF, Nelson SM: Default-mode network streams for coupling to language and control systems. Proc Natl Acad Sci USA 2020, 117:17308–17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].DuPre E, Luh WM, Spreng RN: Multi-echo fMRI replication sample of autobiographical memory, prospection and theory of mind reasoning tasks. Sci Data 2016, 3:160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Toro-Serey C, Tobyne SM, McGuire JT: Spectral partitioning identifies individual heterogeneity in the functional network topography of ventral and anterior medial prefrontal cortex. Neuroimage 2019, 205:116305. [DOI] [PubMed] [Google Scholar]
- **[48].Peer M, Salomon R, Goldberg I, Blanke O, Azry S: Brain system for mental orientation in space, time, and person. Proc Nat Acad Sci USA 2015, 112:11072–11077. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors found differential activity patterns with in high-resolution, individual task analyses of posteromedial cortex during judgments about the physical closeness of locations vs. personal closeness of people. Observed distinctions aligned with those later shown between DN-A and DN-B [e.g., 36], within a region previously considered difficult to parse [35; see also 49].
- [49].Silson EH, Steel A, Kidder A, Gilmore AW, Baker CI: Distinct subdivisions of human medial parietal cortex support recollection of people and places. eLife 2019, 8:e47391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Geschwind N: The organization of language and the brain. Science 1970, 170:940–944. [DOI] [PubMed] [Google Scholar]
- [51].Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF: Voxel-based lesion-symptom mapping. Nat Neurosci 2003, 6:448–450. [DOI] [PubMed] [Google Scholar]
- [52].Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ: Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 2013, 136:691–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee MH, Hacker CD, Snyder AZ, Corbetta M, Zhang D, Leuthardt EC, Shimony JS: Clustering of resting state networks. PLoS One 2012, 7:e40370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, Corbetta M: Resting state network estimation in individual subjects. Neuroimage 2013, 82:616–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ji JL, Spronk M, Kulkarni K, Repovs G, Anticevic A, Cole MW: Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage 2019, 185:35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buckner RL, DiNicola LM: The brain’s default network: Updated anatomy, physiology, and evolving insights. Nat Rev Neurosci 2019, 20:593–608. [DOI] [PubMed] [Google Scholar]
- [57].Kurczek J, Wechsler E, Ahuja S, Jensen U, Cohen NJ,Trane lD, Duff M: Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychologia 2015, 73:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rosenbaum RS, Stuss DT, Levine B, Tulving E: Theory of mind is independent of episodic memory. Science 2007, 318:1257. [DOI] [PubMed] [Google Scholar]
- [59].Bruneau EG, Pluta A, Saxe R: Distinct roles of the ‘Shared Pain’ and ‘Theory of Mind’ networks in processing others’ emotional suffering. Neuropsychologia 2012, 50:219–231. [DOI] [PubMed] [Google Scholar]
- [60].Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R: fMRI item analysis in a theory of mind task. Neuroimage 2011, 55:705–712. [DOI] [PubMed] [Google Scholar]
- **[61].Fedorenko E, Duncan J, Kanwisher N: Language-selective and domain-general regions lie side by side within Broca’s area. Current Biology 2012, 22:2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using within-individual task analyses (innovated by these authors [3,11,63,66]), distinct, juxtaposed sub regions were identified at or near Broca’s area that exhibited differential recruitment for language and other (e.g., math, working memory] tasks. This demonstrated that tightly adjacent regions within association zones can differentially support domain-specialized or domain-general functions.
- [62].Blank I, Kanwisher N, Fedorenko E: A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J Neurophysiol 2014, 112:1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Saxe R, Kanwisher N: People thinking about thinking people: The role of the temporoparietal junction in “theory of mind”. Neuroimage 2003, 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- [64].Fedorenko E,Varley R: Language and thought are not the same thing: Evidence from neuroimaging and neurological patients. Ann NY Acad Sci 2016, 1369:132–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Arcaro MJ, Livingstone MS: A hierarchical, retinotopic proto-organization of the primate visual system at birth. eLife 2017, 6:e26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kanwisher N: Functional specificity in the human brain: a window into the functional architecture of the mind. Proc Natl Acad Sci 2010, 107:11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tsao DY, Livingstone MS: Mechanisms of face perception. Ann Rev Neurosci 2008, 31:411–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Arcaro MJ, Livingstone MS: Retinotopic organization of scene areas in macaque inferior temporal cortex. J Neurosci 2017,31:7373–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Srihasam K, Vincent JL, Livingstone MS: Novel domain formation reveals proto-architecture in inferotemporal cortex. Nat Neurosci 2014, 17:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[70].Kamps FS, Hendrix CL, Brennan PA, Dilks DD: Connectivity at the origins of domain specificity in the cortical face and place networks. Proc Natl Acad Sci 2020,117:6163–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scanning neonatal infants, the authors found evidence for differential functional connectivity between foveal V1 and a ‘proto’ face network and peripheral VI and a scene network as early as 27 days old. The authors proposed that early connectivity may influence functional development.
- [71].Livingstone MS, Arcaro MJ, Schade PF: Cortex is cortex: Ubiquitous principles drive face-domain development. Trends Con Sci 2019, 23:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lafer-Sousa R, Conway BR: Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat. Neurosci . 2013, 16:1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Krienen FM, Buckner RL: Human association cortex: Expanded, untethered, neotenous and plastic. In Evolution of Nervous Systems 2e vol. 4. Edited by Kaas J. Oxford:Elsevier; 2017:169–183. [Google Scholar]
- [74].RosaM GP, Soares JGM, Chaplin TA, Majka P, Bakola S, Phillips KA, Reser DH, Gattass R: Cortical afferents of area 10 in cebus monkeys: Implications for the evolution of the frontal lobe. Cereb Cortex 2019, 29:1473–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, Smallwood J: Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Nat Acad Sci 2016, 113:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marek S, Dosenbach NUF: The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci 2018, 20:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH, Shen D: Mapping region-specific language cortical surface expansion from birth to 2 years of age. Cereb Cortex 2013, 23:2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[78].Richardson H, Lisandrelli G, Riobueno-Naylor A, Saxe R: Development of the social brain from age three to twelve years. Nat Comm 2018, 9:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors scanned 122 children, aged 3–12 years, as they watched a movie featuring differences in characters’ physical and mental states. Regions typically active during theory of mind tasks could be distinguished from those active during physical pain by the age of three, with functional specialization increasing with age.
- [79].Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, Larkman DJ, Rees G, Edwards AD: Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA 2010, 107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Turk E, van den Heuvel MI, Benders MJ, de Heus R, Franx A, Manning JH, Hect JL, Hernandez-Andrade E, Hassan SS, Romero R, Kahn RS, Thomason ME, van den Heuvel MP: Functional connectome of the fetal brain. J Neurosci 2019, 39:9716–9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Howell BR, Styner MA, Gao W, Yap P-T, Wang L, Baluyot K, Yacoub E, Chen G, Potts T, Salzwedel A, Li G, Gilmore JH, Piven J, Smith JK, Shen D, Ugurbil K, Zhu H, Lin W, Elison JT: The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. Neuroimage 2019, 185:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lombardo MV, Pierce K, Eyler L, Barnes CC, Ahrens-Barbeau C, Solso S, Campbell K, Courchesne E: Different functional neural substrates for good and poor language outcomes in autism. Neuron 2015, 86:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Padmanabhan A, Lynch CJ, Shaer M, Menon V: The default mode network in autism. Biol Psychiatry Cogn Neuroscience Neuroimaging 2017, 2:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jouravlev O, Kell AJE, Mineroff Z, Haskins AJ, Ayyash D, Kanwisher N, Fedorenko E: Reduced language lateralization in Autism and Broader Autism Phenotype as assessed with robust individual-subjects analyses. Autism Research 2020, 13:1746–1761. [DOI] [PubMed] [Google Scholar]
- [85].Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS: A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 2010, 49:256–266. [PMC free article] [PubMed] [Google Scholar]
- [86].Powell LJ, Kosakowski HL, Saxe R: Social origins of cortical face areas. Trends Cogn Sci 2018, 22:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sugita Y: Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci USA 2008, 105:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fedorenko E, Thompson-Schill SL: Reworking the language network. Trends Cogn Sci 2014, 18:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Miller EK: The prefrontal cortex and cognitive control. Nat Rev Neurosci 2000, 1:59–65. [DOI] [PubMed] [Google Scholar]
- [90].Jacoby N, Bruneau E, Koster-Hale J, Saxe R: Localizing pain matrix and theory of mind networks with both verbal and non-verbal stimuli. Neuroimage 2016, 1:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Saygin ZM, Osher DE, Norton ES, Youssoufian DA, Beach SD, Feather J, Gaab N, Gabrieli JDE, Kanwisher N: Connectivity precedes function in the development of the visual word form area. Nat Neurosci 2016, 19:1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]


