Abstract
Exposure to and perceptions of stress have been associated with altered systemic inflammation, but the intermediate processes by which stress links to inflammation are not fully understood. Diurnal cortisol slopes were examined as a pathway by which self-reported psychosocial stress is associated with inflammation [i.e., C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, E-Selectin, and Intercellular Adhesion Molecule-1 (ICAM-1)] in a large sample of adults (i.e., the Midlife in the US study; n = 914; 55.9% female; aged 34–84 years). Structural equation modeling indicated that perceived psychological stress was associated with flattened diurnal cortisol slopes and flatter diurnal cortisol slopes were, in turn, associated with heightened inflammation in these cross-sectional analyses (index of indirect pathway, ω = 0.003, 95% CI [0.001, 0.004], ωSTD = 0.027; with covariates, ω = 0.001, [0.0002, 0.002], ωSTD = 0.011). A similar indirect effect was evident for self-reported traumatic life events (ω = 0.007, [0.004, 0.012], ωSTD = 0.030); however, inclusion of covariates (i.e., age, gender, race, ethnicity, body mass index, and other factors associated with physical health) accounted for this finding (ω = 0.001, [−0.001, 0.004], ωSTD = 0.005). These results support an allostatic load model of psychosomatic health, in which cortisol (along with other stress-responsive signaling molecules) is a necessary component for understanding links between stress exposure, perceived stress, and immune functioning.
Keywords: stress, diurnal cortisol, inflammation, allostatic load
Psychosocial stress is a critical risk factor for worsened physical health and for the onset of multiple physical illnesses, such as cardiovascular disease, respiratory conditions, rheumatoid arthritis, and even some forms of cancer (Everson-Rose & Lewis, 2005; Glaser & Kiecolt-Glaser, 2005; Huyser & Parker, 1998; Kiecolt-Glaser et al., 1995; Powell et al., 2013; Steptoe & Kivimäki, 2012; Wright et al., 1998). For example, one review estimated that chronic psychosocial stress was associated with a 40%−60% increase in the occurrence of coronary heart disease (Steptoe & Kivimäki, 2012). One physiological mechanism through which psychosocial stress may affect physical health is systemic inflammation, a critical part of the immune system’s response to injury and illness (Cohen et al., 2016; Hänsel et al., 2010; Liu et al., 2017).
Accumulating evidence has suggested an association between psychosocial stress and systemic inflammation, which is commonly indexed by inflammatory cytokines (e.g., interleukin-6 [IL-6]) and acute phase proteins (e.g., C-reactive protein [CRP], fibrinogen; Hughes et al., 2017; Johnson et al., 2013). Elevated systemic inflammation, in turn, has been linked to a large number of negative health outcomes, including cardiovascular disease, Type-2 diabetes, some cancers, rheumatoid arthritis, and Alzheimer’s disease (Ershler & Keller, 2000; Liu et al., 2017; V. H. Perry, 2004). The intermediate processes through which psychosocial stress is related to inflammation, however, have not been thoroughly examined. The purpose of the current study was to examine alterations in hypothalamic-pituitary-adrenal (HPA) axis activity indexed by diurnal cortisol slopes as a potential pathway linking psychosocial stress to inflammation.
Psychosocial stressors have been commonly linked to elevated inflammation (Cohen et al., 2012, 2016; Ershler & Keller, 2000; Gouin et al., 2012; Kiecolt-Glaser et al., 2011; Slopen et al., 2010). For example, a meta-analysis reported that exposure to traumatic life events, such as childhood adversity and violence, was consistently associated with increased levels of IL-6 and CRP, though no association was found with fibrinogen (Tursich et al., 2014). In addition to exposure to traumatic life events, perceived stress, the event-independent perception of one’s ability to effectively deal with recent life stressors (Cohen et al., 1983), has also been found to predict systemic inflammation (Cohen et al., 2016). For example, perceived stress has been associated with elevated CRP (Das, 2016; McDade et al., 2006). However, evidence in this area is inconclusive as only weak associations between perceived stress and CRP (McDade et al., 2013) or IL-6 have been reported (Christian et al., 2009; Hostinar et al., 2015; O’Donovan et al., 2009), and some studies have reported null associations between perceived stress and IL-6 (Gassen et al., 2018; Grosse et al., 2016).
The association between chronic psychosocial stress and inflammation is well illustrated in the allostatic load (AL) model, which proposes that the wear and tear of the body caused by repeated exposure to stress is regulated by an interconnected network of mediators, including stress hormones and immune markers. Allostatic load specifically refers to the physiological deterioration of the body, which results from chronic and repeated experiences of allostasis, the transient and adaptive physiological response mounted by the organism in the face of stressors (McEwen & Stellar, 1993). In the AL model, the activation of various interconnected physiological systems associated with stress are beneficial to survival in the short term, but can be detrimental to health when sustained (McEwen, 2000). In a cumulative fashion, stress can lead to physiological dysregulation in multiple systems, including the immune system. Presumably, this occurs through the chronic over-activation of neuroendocrine stress-responses systems, particularly the HPA axis (Juster et al., 2010), which in turn can alter immunity as described below.
Stress-related alterations in the activity of the HPA axis represent an intermediate pathway through which psychosocial stress ultimately modulates inflammation. The stage model of stress and disease proposed by Cohen et al. (2016) highlights the HPA axis as a crucial intermediary for the effects of life stress and perceived stress on disease-related physiological systems, such as the immune system, and ultimately the onset or worsening of disease. The HPA axis is one endocrine component of the stress-response system, and its function is commonly assessed by cortisol secretion (Golden et al., 2011). In the AL model, cortisol and certain cytokines are considered primary mediators while metabolic, cardiovascular, and immune responses to the over/under production of primary mediators are considered secondary outcomes (i.e., a prodromal phase of disease). Disease endpoints, indicative of allostatic overload, are considered tertiary outcomes.
Chronic, uncontrollable stressors, particularly if perceived as threatening to one’s physical integrity, lead to alterations in HPA axis activity, which is commonly reflected by a relatively flat diurnal cortisol profile across the day (Miller et al., 2007; Zilioli et al., 2018). Indeed, both traumatic life events and perceived stress have been found to be associated with flattened diurnal cortisol profiles among adults (Dasgupta & Stout, 2014; Lovell et al., 2011; Sjörs et al., 2014; Young et al., 2019), though null findings have also been reported (Jobin et al., 2014; Zilioli et al., 2016).
Cortisol has potent immunosuppressive and anti-inflammatory actions. Hence, disruptions in diurnal cortisol secretion (e.g., a flattened diurnal slope) can affect inflammation and immunity (Elenkov & Chrousos, 2002). Alterations in cortisol secretion stemming from chronic stress are associated with heightened inflammation in several studies (Miller et al., 2002, 2009; Piazza et al., 2018). This elevated inflammation is seemingly due to chronic stress decreasing the number and sensitivity of immune cells’ glucocorticoid receptors, which decreases cortisol’s effectiveness at reducing or limiting inflammation (Cohen et al., 2012; Miller et al., 2009)1.
Flattened diurnal cortisol slopes have been consistently associated with inflammation, indexed by elevated activation of CD4+ T cells and low counts of natural killer cells, among clinical samples (for a review, see Adam et al., 2017). Only a few studies, however, have explicitly examined the effect of flattened diurnal cortisol slopes on systemic inflammation among general populations. In one population-based sample, a flattened diurnal cortisol slope was associated with elevated IL-6 and tumor necrosis factor-alpha (TNF-α; DeSantis et al., 2012). Although the relationships between 1) psychosocial stress and diurnal cortisol secretion, and 2) diurnal cortisol secretion and inflammation have been reported in previous studies, very few epidemiological studies have examined diurnal cortisol secretion as a mediator of the association between psychosocial stress and systemic inflammation.
The current study aimed to examine the mediating role of diurnal cortisol secretion on the cross-sectional associations between two indices of stress—perceived recent stress and traumatic life events—and systemic inflammation using the biomarker subsample from the second wave of Midlife in the United States (MIDUS 2). In a pre-registered hypothesis (https://osf.io/h3ke7), it was proposed that perceived stress [Hypothesis 1 (H1)] and traumatic life events (H2) would each be associated with a flattened diurnal slope, which, in turn, would be associated with elevated systemic inflammation.
Method
Participants and Procedures
The sample was drawn from the National Survey of Midlife in the United States (MIDUS). MIDUS is a multi-wave survey on health and well-being among adults. Data were collected via phone interviews and self-administered questionnaires in 1995–1996 (MIDUS 1) and 2004–2006 (MIDUS 2 and the Milwaukee African American project)2. The present study utilized participants from MIDUS 2 (N = 4,963) and two subprojects of MIDUS 2: The National Study of Daily Experiences (NSDE; N = 2,022) and the Biomarker Study (N = 1,255; Almeida et al., 2009; Love et al., 2010). The NSDE was conducted an average of 20.5 months (SD = 13.6) following the main MIDUS 2 assessment and included four days of saliva collection and eight days of phone interviews. Of the total NSDE sample, 1,736 (85.9%) individuals provided salivary cortisol samples. The Biomarker Study took place an average of 25 months (SD = 14) following the main MIDUS 2 questionnaires and required participants to travel to one of three MIDUS testing centers (University of California – Los Angeles, University of Wisconsin-Madison, or Georgetown University). At the testing center, individuals provided information about their medical history, completed an extensive physical examination, and underwent a fasting blood draw from which markers of inflammation were derived.
Eligibility criteria for the current study required that participants responded to self-reported stress questionnaires in the MIDUS 2 or Milwaukee project collection, provided saliva samples in the NSDE, responded to the Perceived Stress Scale (PSS), and provided a blood sample in the Biomarker Project. Therefore, the final study sample consisted of at most n = 914 adults (M = 55.4 years, SD = 11.6, range = 34–84 years; 18.3% people of color; 3.2% Hispanic/Latinx, 56.0% female). See Table 1 for means and correlations among variables for participants in this report. All participants provided informed consent and data collection was approved by the Institutional Review Board at the University of Wisconsin-Madison, UCLA, and Georgetown University.
Table 1.
Descriptive statistics and correlations among study variables.
| Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender (% female) | 56.0% | ||||||||||||
| 2. Race (% African American) | 13.3% | 0.066* | |||||||||||
| 3. Ethnicity (% Hispanic) | 3.2% | 0.035 | 0.146*** | ||||||||||
| 4. Age (years) | 55.43 (11.56) | −0.046 | −0.104** | −0.025 | |||||||||
| 5. BMI | 29.73 (6.56) | 0.004 | 0.206*** | 0.012 | −0.026 | ||||||||
| 6. Perceived Stress Scale | 21.88 (6.33) | 0.058 | 0.144*** | 0.004 | −0.186*** | 0.098** | |||||||
| 7. Traumatic Life Events | 3.56 (2.51) | −0.053 | 0.243*** | 0.093** | 0.014 | 0.154*** | 0.194*** | ||||||
| 8. CRP (mg/L) | 2.82 (4.51) | 0.132*** | 0.086** | −0.012 | 0.002 | 0.323*** | 0.074* | 0.060 | |||||
| 9. FGN (mg/dL)a | 342.7 (88.35) | 0.156*** | 0.169*** | 0.043 | 0.124*** | 0.310*** | 0.058 | 0.135*** | 0.478*** | ||||
| 10. IL-6 (pg/mL) | 2.90 (2.83) | 0.061 | 0.101** | −0.029 | 0.160*** | 0.236*** | 0.050 | 0.085* | 0.416*** | 0.361*** | |||
| 11. E-Selectin (ng/mL) | 40.5 (20.3) | −0.122*** | 0.098** | 0.007 | −0.072* | 0.219*** | 0.020 | 0.092** | 0.171*** | 0.101** | 0.132*** | ||
| 12. ICAM-1 (ng/mL) | 292.1 (109.2) | −0.007 | 0.005 | −0.027 | 0.121*** | 0.108** | 0.013 | 0.083* | 0.155*** | 0.187*** | 0.161*** | 0.135*** | |
| 13. Diurnal Cortisol Slopesb | −1.23 (0.46) | −0.003 | 0.304*** | −0.035 | 0.106** | 0.143*** | 0.121*** | 0.137*** | 0.118*** | 0.172*** | 0.191*** | 0.083* | 0.111*** |
Note:
FGN = fibrinogen
Diurnal cortisol slopes are reported in units of natural-log transformed nmol/L per hours since midnight ×10−1
p < .05
p < .01
p < .001
Measures
Stress
Perceived Stress.
Perceived stress was measured in the Biomarker Study through the Perceived Stress Scale (PSS; Cohen et al., 1983). The scale contained 10 items that indexed aspects of stress experienced within the past month (e.g., “In the last month, how often have you been upset because of something that happened unexpectedly?”). Responses were measured on a 5-point scale (1 = never to 5 = very often). Positively valenced items were reverse coded so that a higher score indicated greater perceived stress. The 10 items were summed for an overall score of perceived stress with mean substitution for individual missing items (α =0.87, 95% CI [0.86, 0.88]).
Traumatic Life Events.
Participants reported on whether major stressors such as personal assault, death of a close other, financial hardship, legal issues, or combat had occurred to them at any point throughout their lives. These stressors were captured in MIDUS 2 through a checklist of 27 life stress events derived from the standard life stressors measure (Turner et al., 1995). Participants responded to items such as, “Has a parent ever died?” or “Have you ever experienced combat?”. Responses were summed (Yes = 1; No = 0) so that a higher score reflected a greater frequency of life stress events.
Cortisol.
Saliva samples were collected during the NSDE for cortisol assay. Participants were asked to provide four saliva samples per day over four consecutive days using at-home saliva collection kits with detailed instructions (Almeida et al., 2009, 2020). Color-coded Salivettes with cotton swabs (Sarstedt, Nümbrecht, Germany) were used to collect saliva immediately after waking, 30 minutes after waking, before lunch, and before bed. Participants were instructed not to eat, drink, or brush their teeth prior to providing samples. Compliance was ensured with nightly telephone interviews, paper-and-pencil logs, and, in approximately 25% of the sample, by computerized timings from “smart boxes.”
Once all samples were collected, participants mailed the kit to the MIDUS Biocore Laboratory (University of Wisconsin, Madison, WI) where the kits were frozen at −60°C until they were shipped on dry ice to be assayed. Samples were processed at the Dresden LabService (Dresden, Germany) using immunoluminescence assay (IBL International, Hamburg, Germany) to analyze concentrations of free cortisol. Inter-assay and intra-assay coefficients of variance (CV) were below 5%.
Inflammation.
Similar to prior work (Hostinar et al., 2015), we focused on five markers of inflammation that were assessed during the Biomarker Study via fasting blood draws: Interleukin-6 (IL-6), C-Reactive Protein (CRP), E-Selectin, soluble Intercellular Adhesion Molecule-1 (ICAM-1), and fibrinogen. Blood was collected before breakfast and then placed in an ice bath until samples were centrifuged to extract plasma. Plasma was then stored at −70 °C until the samples were shipped on dry ice for assay.
IL-6 is a pro-inflammatory cytokine released as part of the innate immune response to antigens. This cytokine was processed in the MIDUS Biocore Laboratory (University of Wisconsin, Madison, WI) and was measured with a Quantikine high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). The intra-assay and inter-assay CVs were 3.7% and 15.7%, respectively.
The remaining inflammatory markers were assayed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). CRP is an acute-phase protein released from the liver following stimulation by rising IL-6 levels. CRP was measured with the BNII nephelometer utilizing particle enhanced immunonepholometric assay (Dade Behring Inc., Deerfield, IL).
Soluble E-Selectin is a cell adhesion molecule needed to recruit other inflammatory mediators and was measured with a high-sensitivity ELISA kit (Parameter human sE-Selectin Immunoassay; R&D Systems, Minneapolis, MN). Cases in which E-Selectin values fell below the range of detectable scores (i.e., concentrations less than 0.1 ng/mL) were adjusted to 0.09 ng/mL.
ICAM-1 is a ligand protein on leukocytes and was measured with an ELISA kit (R&D Systems, Minneapolis, MN). ICAM-1 values that fell below the range of detectable scores (i.e., less than 45 ng/mL) were adjusted to 44 ng/mL.
Fibrinogen plays a role in blood clotting and was measured by immunochemical reaction using a BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., Deerfield, IL). The inter-assay and intra-assay CVs were within acceptable ranges (<12%). Normalized (Z-score) and winsorized (absolute values ≥ 3 SD from the mean were converted to ±3 SD from the mean) concentrations of each inflammatory marker were mean averaged to create a composite score of inflammation (Hostinar et al., 2015).
Covariates.
The individual components of the biological pathways examined here have been linked with several factors. For example, age has been associated with a flattening of diurnal cortisol curves (Gaffey et al., 2016) and low-grade, chronic inflammation (Franceschi et al., 2007; Franceschi & Campisi, 2014). Gender is a critical factor in immune functioning (Marriott & Huet-Hudson, 2006) and has been inconsistently linked to stress-associated diurnal cortisol patterns (Liao et al., 2013). Obesity has been consistently linked to chronic low-grade inflammation (Cancello & Clément, 2006). Race and ethnicity have each been associated with cortisol and inflammation dysregulation due, in part, to structural and individually-experienced racism (e.g., Adam et al., 2015; Cuevas et al., 2020; Stepanikova et al., 2017).
Accordingly, analyses were statistically adjusted for age, gender3 (1 = female, 0 = male), race (1 = person of color, 0 = white), and ethnicity (1 = Hispanic/Latinx, 0 = not Hispanic/Latinx), which were self-reported in MIDUS 2 or the Milwaukee project. Body mass index (BMI) was calculated from measurements of participant height and weight in the Biomarker Study. Other covariates were related to physical health, including the sum of participants’ self-reported responses to a list of chronic medical conditions (1 = yes, have been diagnosed, 0 = no); and participants’ current use of medication (1 = taking any medication, 0 = not taking any medication). Lastly, the average waking time on days in which saliva samples were collected in the NSDE sampling period was recorded as hours from midnight and included as a covariate.
Statistical Analyses
Cortisol Slopes.
Cortisol concentrations were natural-log-transformed to correct a skewed distribution. To index cortisol’s diurnal slope, a multilevel model was specified with cortisol concentration regressed on time since midnight (in hours divided by 10 to scale the resulting slope values) with random linear slope and intercept per participant. The linear slope linking time of day to cortisol concentration was calculated and extracted via empirical Bayes estimation from all data available for each given participant (Zilioli et al., 2017).
Primary Analyses.
Primary analyses examined the indirect pathway linking self-reported stress (perceived stress and traumatic life events) to inflammation (composite mean of all biomarkers) via diurnal cortisol using structural equation modeling. All models were fit in R (v3.6.1) using lavaan (Rosseel, 2012). Model fit was determined by a χ2 test of model fit, as well as observation of TLI, CFI, and RMSEA4 using standard cutoffs (CFI ≥ 0.95; TLI ≥ 0.95; RMSEA ≤ 0.06; Hu & Bentler, 1999).
Estimates of the indirect effect were bootstrap bias-corrected (K = 1,000 resamples). All models were run with and without covariates loaded onto diurnal cortisol and the composite inflammation variable. Missing data were imputed via full-information maximum likelihood estimation. The extent to which a 95% confidence interval contained zero was taken as evidence of the robustness of the indirect association among self-reported stress, cortisol, and inflammation.
Exploratory Analyses.
In exploratory analyses, we examined the extent to which the hypothesized pattern in the primary analyses was evident for each individual biomarker of inflammation. The composite inflammation variable was replaced by separate paths for each biomarker of inflammation. The models were otherwise constructed as found in the primary analyses.
Sensitivity Analyses.
Sensitivity analyses were performed by re-running H1 and H2’s models after removing instances of cortisol concentration greater than 60 nmol/L and removing participants with average wake-up times before 4 AM or after 11 AM (for a similar approach, see Karlamangla et al., 2019). In a third sensitivity analysis, we removed participants from the models with CRP > 10 mg/L, which may be considered evidence of current infection5. We further examined the sensitivity of the results by applying all three filters simultaneously.
Results
Hypothesis H1: Perceived Stress
Primary analyses.
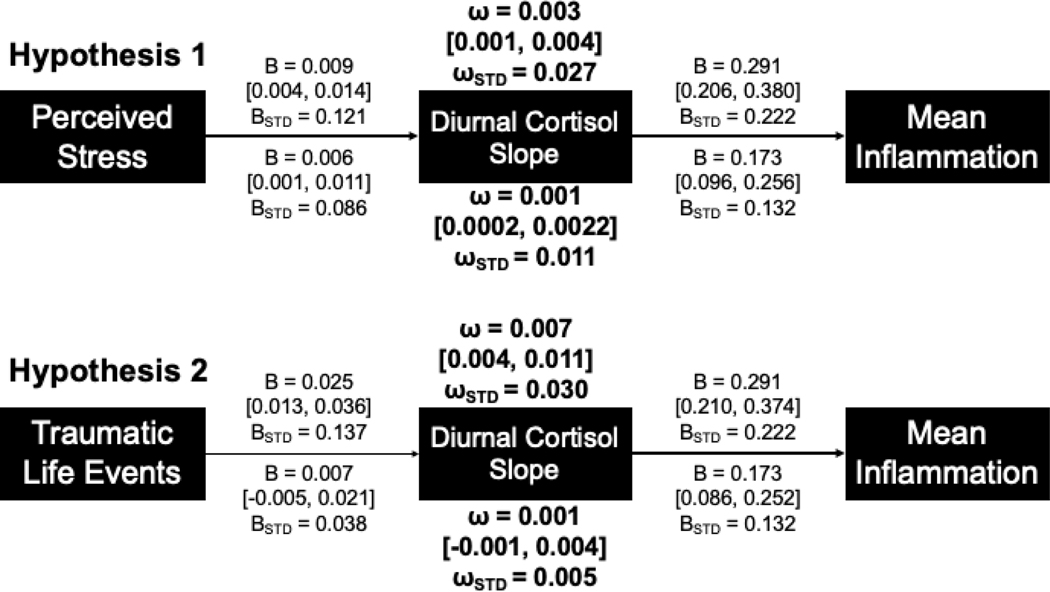
Fit indices for the H1 model were appropriate (χ(1)2= 0.418, p = 0.518, CFI = 1.000; TLI = 1.031; RMSEA = 0). In support of hypothesis H1, an indirect pathway was evident linking perceived stress and inflammation via cortisol slope (ω = 0.003, 95% CI [0.001, 0.004], ωSTD = 0.027; Figure 1). This model indicated that higher levels of perceived stress were associated with flatter diurnal cortisol slopes, which in turn were associated with elevated inflammation. A second model was run with race, ethnicity, gender, age, sum of self-reported chronic health conditions, use of medication, BMI, and average wakeup time added as covariates. Fit statistics from this second model were appropriate (χ(1)2= 0.050, p = 0.814, CFI = 1.000; TLI = 1.045; RMSEA = 0). Results from this model were similar to the indirect path observed in the initial model, though of slightly weaker magnitude (ω = 0.001, [0.0002, 0.002], ωSTD = 0.011).
Figure 1.
Representation of the primary models for hypotheses H1 and H2 with unstandardized and standardized coefficients (B, BSTD), indices of indirect pathways (ω, ωSTD), and 95% confidence intervals. Values listed above each model are results from the analyses without covariates; values below each model are from the analyses with covariates (regression paths on cortisol and inflammation and covariance paths on stress/traumatic life events not pictured for covariate variables). Secondary models were constructed similarly, but with mean inflammation replaced by separate paths for each of the inflammation biomarkers (see Table 2).
Exploratory analyses.
Our pre-registered exploratory analyses examined whether the indirect pathway linking higher stress to flatter cortisol slopes, and these slopes to elevated inflammation, was evident for each individual inflammatory marker rather than the composite mean. Fit statistics again indicated the model was fit appropriately (χ2(5) = 3.996, p = 0.550, CFI = 1.000, TLI = 1.011; RMSEA = 0). Results indicated that the pattern observed in H1 was evident for IL-6 and fibrinogen and was in the same direction but of weaker magnitude for CRP, ICAM-1 and E-selectin (Table 2).
Table 2.
Estimates of indirect pathways linking perceptions of recent stress (PSS), cortisol slopes, and individual biomarkers of inflammation from exploratory hypothesis H1.
| Cortisol to Inflammation Path | Estimate of Indirect Path (PSS to cortisol to inflammation) | ||||
|---|---|---|---|---|---|
| Estimate, B | 95%CI | Estimate, ω | 95%CI | Standardized Estimate (ωSTD) | |
| CRP | 0.176 | [0.007, 0.346] | 0.001 | [0.000, 0.003] | 0.006 |
| IL-6 | 0.220 | [0.122, 0.338] | 0.001 | [0.0002, 0.003] | 0.012 |
| Fibrinogen | 0.204 | [0.062, 0.357] | 0.001 | [0.0001, 0.003] | 0.008 |
| E-Selectin | 0.091 | [−0.049, 0.229] | 0.001 | [−0.0003, 0.002] | 0.004 |
| ICAM-1 | 0.134 | [0.000, 0.264] | 0.001 | [0.000, 0.002] | 0.006 |
Hypothesis H2: Traumatic Life Events
Primary analyses.
In Hypothesis H2, we replaced perceived stress with the sum of traumatic life events experienced. An initial model without covariates did not demonstrate appropriate fit indices (χ2(1)= 20.15, p < .001, CFI = 0.763, TLI = 0.288; RMSEA = 0.145) but the addition of covariates improved model fit indices somewhat (χ2(1)= 7.390, p = 0.007, CFI = 0.984, TLI = 0.695; RMSEA = 0.084). The indirect pathway linking life-course stress to inflammation via diurnal cortisol slopes was evident in the (poorly fitting) model without covariates (ω = 0.007, [0.004, 0.012], ωSTD = 0.030; Figure 1). However, the addition of covariates substantially reduced the strength of this indirect pathway such that the pathway was no longer distinguishable from zero (ω = 0.001, [−0.001, 0.004], ωSTD = 0.005).
Exploratory analyses.
As with H1, we explored the pathways linking life-course stress, cortisol, and individual inflammatory markers. Fit statistics indicated the exploratory model’s fit was appropriate (χ2(5) = 10.643, p = 0.059, CFI = 0.995, TLI = 0.937; RMSEA = 0.035). Although pathways from cortisol to each individual inflammatory marker were essentially unchanged from H1, the relatively weak association between traumatic life events and cortisol reduced the strength of the indirect pathways such that each of the pathways was indistinguishable from zero (Table 3).
Table 3.
Estimates of indirect pathways linking traumatic life events, cortisol slopes, and individual biomarkers of inflammation from exploratory hypothesis H2.
| Cortisol to Inflammation Path | Estimate of Indirect Path (traumatic life events to cortisol to inflammation) | ||||
|---|---|---|---|---|---|
| Estimate, B | 95%CI | Estimate, ω | 95%CI | Standardized Estimate (ωSTD) | |
| CRP | 0.176 | [0.008, 0.338] | 0.001 | [−0.001, 0.004] | 0.003 |
| IL-6 | 0.221 | [0.131, 0.333] | 0.002 | [−0.001, 0.005] | 0.005 |
| Fibrinogen | 0.204 | [0.060, 0.350] | 0.001 | [−0.001, 0.005] | 0.004 |
| E-Selectin | 0.091 | [−0.046, 0.222] | 0.001 | [−0.001, 0.003] | 0.002 |
| ICAM-1 | 0.132 | [−0.007, 0.273] | 0.001 | [−0.001, 0.004] | 0.003 |
Sensitivity Analyses
Sensitivity analyses (removing instances of cortisol concentration greater than 60 nmol/L, removing participants with average wake-up times before 4 AM or after 11 AM, and removing participants with CRP > 10 mg/L), performed individually and in concert, did not substantially alter the results of the models (Table 4).
Table 4.
Sensitivity analyses.
| Estimate, ω | 95%CI | Standardized Estimate, ωSTD | |
|---|---|---|---|
| Hypothesis H1: Perceived Stress | |||
| Primary Model | 0.0010 | [0.0002, 0.0022] | 0.011 |
| Removed Cortisol > 60 nmol/L | 0.0013 | [0.0003, 0.0025] | 0.010 |
| Mean Wake: 4 AM – 11 AM | 0.0011 | [0.0003, 0.0021] | 0.013 |
| Removed CRP > 10 mg/L | 0.0010 | [0.0003, 0.0021] | 0.013 |
| All filters | 0.0010 | [0.0002, 0.0022] | 0.011 |
| Hypothesis H2: Traumatic Life Events | |||
| Primary Model | 0.0012 | [−0.0009, 0.0036] | 0.005 |
| Removed Cortisol > 60 nmol/L | 0.0012 | [−0.0008, 0.0041] | 0.004 |
| Mean Wake: 4 AM – 11 AM | 0.0011 | [−0.0014, 0.0042] | 0.005 |
| Removed CRP > 10 mg/L | 0.0012 | [−0.0010, 0.0036] | 0.005 |
| All filters | 0.0012 | [−0.0009, 0.0036] | 0.005 |
Discussion
Psychosocial stress has been extensively linked to poor health outcomes. The present results add support to an AL model in which stress alterations in diurnal cortisol are associated with heightened systemic inflammation. In particular, higher levels of perceived stress and traumatic life events were associated with flatter diurnal cortisol slopes, which in turn were associated with increased levels of inflammatory biomarkers. After adding covariates, this pattern was still observed for perceived stress but not for traumatic life events. These associations were evident despite a study design in which measurements of self-reported stress levels and immune activity and measurements of cortisol activity occurred at different times.
Critically, links between stress and biomarkers of inflammation were weak or not evident without the inclusion of diurnal cortisol slopes as an intermediary. Hence, the present findings may be explained in part by putative AL mechanisms described in prior research. Specifically, stress has been linked to the downregulation of the sensitivity (Cohen et al., 2012; Miller et al., 2002) and the concentration of glucocorticoid receptors (Sapolsky et al., 1986). Downregulation of glucocorticoid receptors reduces the efficacy of cortisol as a stop signal in the HPA axis’ negative feedback loop, which may flatten diurnal cortisol curves (Jarcho et al., 2013). Downregulation of glucocorticoid receptors also reduces cortisol’s anti-inflammatory properties, potentially leading to greater inflammatory activity (Miller et al., 2014).
Alternative and complementary explanations are evident in the broader AL literature, including stress-induced increases in sympathetic activity and reductions in parasympathetic activity, which both may serve to increase pro-inflammatory activity in a context dependent manner (Pongratz & Straub, 2014; Williams et al., 2019); stress-linked increases in gut permeability, which result in increased exposure to endotoxin and pro-inflammatory signaling (de Punder & Pruimboom, 2015; Knight et al., 2020); and behavioral responses to stress, including consumption of high-fat diets, alcohol, and tobacco (Khan et al., 2019; Kiecolt-Glaser, 2010; Raposa et al., 2014). Future work may begin to unravel these interconnected systems by studying stress-linked physiology and behavior more broadly and comprehensively in experimental and naturalistic settings.
Indirect pathways linking stress, cortisol, and inflammation were evident and robust for a measure of recent perceived stress. These pathways were not as robust for traumatic life events, particularly when controlling for relevant demographic and health covariates. Part of the explanation for these differences may be explained by the more diffuse nature of the traumatic life events measure. Such a broad concept – i.e., capturing lifetime racial and ethnic disparities, childhood adversity, familial loss, and other stressors – may work through multiple routes to impact stress physiology and health, and not through alterations in diurnal cortisol activity per se. Further, the possible intersectionality of these traumatic life events – that is, the compounding effect of being a member of multiple marginalized groups and/or having experienced multiple adversities as a result of holding multiple social identities – may synergistically dysregulate physiological responses to stress in a manner not readily evident in these analyses (Crenshaw, 1989; Perry et al., 2013; Seng et al., 2012). Yet, some prior work has shown that traumatic life events are linked to alterations in cortisol activity (Doom et al., 2018; Tursich et al., 2014). Future work must consider these associations more carefully, perhaps through cohort designs that track individuals across the life-course in order to examine acute and longer-term changes in stress and health physiology.
Several aspects of the present analyses may limit the potential impact of the findings. In particular, the cross-sectional study design prevents any inference of causality. Measuring stress, cortisol, and inflammation concurrently over longer and more intensive durations (e.g., a measurement burst design; Sliwinski, 2008) could improve understanding of the time-course and causal associations among these variables. The present report is also limited by a relatively short list of inflammatory biomarkers examined, focused on pro-inflammatory (and not anti-inflammatory) signaling in plasma. A broader approach, encompassing basal and stimulated, pro- and anti-inflammatory biomarkers would allow for more precise understandings of the immune pathways impacted by stress and their relation to HPA axis activity.
Lastly, the MIDUS sample is relatively homogenous in terms of race, and ethnicity. Consequently, these analyses do not readily speak to the multiple, intersectional factors linked to inequalities in stress and health. It will be critical to continue to examine these patterns in more representative samples and in specific subpopulations who may be at greater risk for stress-linked deficits in health and well-being. Such work will help provide targets on which to intervene to improve physical and mental resilience among marginalized and at-risk groups.
Perceived stress was associated with inflammation, but only via diurnal cortisol.
Stress linked with flatter cortisol slopes which linked with higher inflammation.
A similar pattern for traumatic life events was explained by covariates.
Results support an allostatic load model of stress and health.
Acknowledgements
Publicly available data was used for this research, provided by the longitudinal study titled “Midlife in the United States,” (MIDUS) managed by the Institute on Aging, University of Wisconsin. Since 1995 the MIDUS study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network; National Institute on Aging (P01-AG020166); National institute on Aging (U19-AG051426). Biomarker data collection was further supported by the NIH National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program as follows: UL1TR001409 (Georgetown); UL1TR001881 (UCLA); 1UL1RR025011 (UW).
Footnotes
Communication between the HPA axis and inflammation is bidirectional. For example, IL-6 stimulates secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland and cortisol from the adrenal glands (Späth-Schwalbe et al., 1994).
A third wave of self-report data, MIDUS 3, was collected in 2013–2014 but is not examined here.
Here, we use “gender” throughout this report to fully encompass the psychosocial and biological differences between men and women (Darnall & Suarez, 2009).
The SEM package, lavaan, sets RMSEA to zero when the χ2 test value is less than the test’s degrees of freedom.
This sensitivity analysis was not pre-registered.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Levy DJ, Kemeny M, Brodish AB, Malanchuk O, Peck SC, Fuller-Rowell TE, & Eccles JS (2015). Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology, 62, 279–291. 10.1016/j.psyneuen.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, & King H. (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55(2), 219–237. 10.1080/19485560903382338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza J, Liu Y, & Zarit SH (2020). Use of saliva to better understand the daily experience of adulthood and aging. In Granger DA & Taylor M (Eds.), Salivary Bioscience (pp. 655–674). Springer, Cham. 10.1007/978-3-030-35784-9_27 [DOI] [Google Scholar]
- Cancello R, & Clément K. (2006). Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG: An International Journal of Obstetrics & Gynaecology, 113(10), 1141–1147. 10.1111/j.1471-0528.2006.01004.x [DOI] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, & Iams JD (2009). Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity, 23(6), 750–754. 10.1016/j.bbi.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gianaros PJ, & Manuck SB (2016). A stage model of stress and disease. Perspectives on Psychological Science, 11(4), 456–463. 10.1177/1745691616646305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 5995–5999. 10.1073/pnas.1118355109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Crenshaw K. (1989). Demarginalizing the intersection of race and sex: A black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics. University of Chicago Legal Forum, 1, 139–167. [Google Scholar]
- Cuevas AG, Ong AD, Carvalho K, Ho T, Chan SW (Celine), Allen JD, Chen R, Rodgers J, Biba U, & Williams DR (2020). Discrimination and systemic inflammation: A critical review and synthesis. Brain, Behavior, and Immunity, 89, 465–479. 10.1016/j.bbi.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, & Suarez EC (2009). Sex and gender in psychoneuroimmunology research: Past, present and future. Brain, Behavior, and Immunity, 23(5), 595–604. 10.1016/j.bbi.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. (2016). Psychosocial distress and inflammation: Which way does causality flow? Social Science and Medicine, 170, 1–8. 10.1016/j.socscimed.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Dasgupta N, & Stout JG (2014). Girls and Women in Science, Technology, Engineering, and Mathematics. Policy Insights from the Behavioral and Brain Sciences. 10.1177/2372732214549471 [DOI] [Google Scholar]
- de Punder K, & Pruimboom L. (2015). Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Frontiers in Immunology, 6. 10.3389/fimmu.2015.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, Seeman TE, & Shea S. (2012). Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology, 37(7), 1009–1018. 10.1016/j.psyneuen.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Cook SH, Sturza J, Kaciroti N, Gearhardt AN, Vazquez DM, Lumeng JC, & Miller AL (2018). Family conflict, chaos, and negative life events predict cortisol activity in low-income children. Developmental Psychobiology. 10.1002/dev.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, & Chrousos GP (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences, 966(1), 290–303. 10.1111/j.1749-6632.2002.tb04229.x [DOI] [PubMed] [Google Scholar]
- Ershler WB, & Keller ET (2000). Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine, 51, 245–270. 10.1146/annurev.med.51.1.245 [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, & Lewis TT (2005). Psychosocial factors and cardiovascular diseases. Annual Review of Public Health, 26(469), 500. 10.1146/annurev.publhealth.26.021304.144542 [DOI] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J. (2014). Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 69(S1), S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, & Salvioli S. (2007). Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development, 128(1), 92–105. 10.1016/j.mad.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Gaffey AE, Bergeman CS, Clark LA, & Wirth MM (2016). Aging and the HPA axis: Stress and resilience in older adults. Neuroscience and Biobehavioral Reviews, 68, 928–945. 10.1016/j.neubiorev.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen J, Prokosch ML, Makhanova A, Eimerbrink MJ, White JD, Proffitt Leyva RP, Peterman JL, Nicolas SC, Reynolds TA, Maner JK, McNulty JK, Eckel LA, Nikonova L, Brinkworth JF, Phillips MD, Mitchell JB, Boehm GW, & Hill SE (2018). Behavioral immune system activity predicts downregulation of chronic basal inflammation. PLOS ONE, 13(9), e0203961. 10.1371/journal.pone.0203961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, & Kiecolt-Glaser JK (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5(3), 243–251. 10.1038/nri1571 [DOI] [PubMed] [Google Scholar]
- Golden SH, Wand GS, Malhotra S, Kamel I, & Horton K. (2011). Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. European Journal of Epidemiology, 26, 511–525. 10.1007/s10654-011-9585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser J. (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31(2), 264–268. 10.1037/a0025536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Ambrée O, Jörgens S, Jawahar MC, Singhal G, Stacey D, Arolt V, & Baune BT (2016). Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology, 73, 24–31. 10.1016/j.psyneuen.2016.07.205 [DOI] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Cámara RJA, & von Känel R. (2010). Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience and Biobehavioral Reviews, 35(1), 115–121. 10.1016/j.neubiorev.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, & Miller GE (2015). Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology, 51(11), 1630–1644. 10.1037/dev0000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Hughes A, Kumari M, McMunn A, & Bartley M. (2017). Unemployment and inflammatory markers in England, Wales and Scotland, 1998–2012: Meta-analysis of results from 12 studies. Brain, Behavior, and Immunity, 64, 91–102. 10.1016/j.bbi.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Huyser B, & Parker JC (1998). Stress and rheumatoid arthritis: An integrative review. Arthritis and Rheumatism, 11(2), 135–145. 10.1002/art.1790110209 [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, & Burke HM (2013). Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biological Psychology, 93(1), 150–158. 10.1016/j.biopsycho.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin J, Wrosch C, & Scheier MF (2014). Associations between dispositional optimism and diurnal cortisol in a community sample: When stress is perceived as higher than normal. Health Psychology, 33(4), 382–391. 10.1037/a0032736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Abbasi A, & Master VA (2013). Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Molecular Diagnosis and Therapy, 17, 147–164. 10.1007/s40291-013-0026-7 [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Merkin SS, Almeida DM, Friedman EM, Mogle JA, & Seeman TE (2019). Early-life adversity and dysregulation of adult diurnal cortisol rhythm. The Journals of Gerontology: Series B, 74(1), 160–169. 10.1093/geronb/gby097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Lawyer G, McDonough S, Wang Q, Kassem NO, Kas-Petrus F, Ye D, Singh KP, Kassem NOF, & Rahman I. (2019). Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tobacco Control, 29, S102–S109. 10.1136/tobaccocontrol-2019-054958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK (2010). Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosomatic Medicine, 72(4), 365–369. 10.1097/PSY.0b013e3181dbf489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N, Malarkey WB, Beversdorf DQ, & Glaser R. (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73(1), 16–22. 10.1097/PSY.0b013e31820573b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Mercado AM, Malarkey WB, & Glaser R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196. 10.1016/S0140-6736(95)92899-5 [DOI] [PubMed] [Google Scholar]
- Knight EL, Majd M, Graham-Engeland JE, Smyth JM, Sliwinski MJ, & Engeland CG (2020). Gender differences in the link between depressive symptoms and ex vivo inflammatory responses are associated with markers of endotoxemia. Brain, Behavior, & Immunity - Health, 2, 100013. 10.1016/j.bbih.2019.100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Brunner EJ, & Kumari M. (2013). Is there an association between work stress and diurnal cortisol patterns? Findings from the whitehall II study. PLoS ONE, 8(12), e81020. 10.1371/journal.pone.0081020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Wang YX, & Jiang CL (2017). Inflammation: The common pathway of stress-related diseases. Frontiers in Human Neuroscience, 11, 316. 10.3389/fnhum.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the midus national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22(8), 1059–1080. 10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell B, Moss M, & Wetherell MA (2011). Perceived stress, common health complaints and diurnal patterns of cortisol secretion in young, otherwise healthy individuals. Hormones and Behavior, 60(3), 301–305. 10.1016/j.yhbeh.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Marriott I, & Huet-Hudson YM (2006). Sexual dimorphism in innate immune responses to infectious organisms. Immunologic Research, 34, 177–192. 10.1385/IR:34:3:177 [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, & Cacioppo JT (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosomatic Medicine, 68(3), 376–381. 10.1097/01.psy.0000221371.43607.64 [DOI] [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, & Kuzawa C. (2013). Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain, Behavior, and Immunity, 31, 23–30. 10.1016/j.bbi.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2000). Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology, 22, 108–124. 10.1016/S0893-133X(99)00129-3 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E. (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, & Kobor MS (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America, 106(34), 14716–14721. 10.1073/pnas.0902971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 24–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 21(6), 531–541. 10.1037/0278-6133.21.6.531 [DOI] [PubMed] [Google Scholar]
- Miller GE, Murphy MLM, Cashman R, Ma R, Ma J, Arevalo JMG, Kobor MS, & Cole SW (2014). Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, Behavior, and Immunity, 41, 191–199. 10.1016/j.bbi.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, & Epel E. (2009). Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain, Behavior, and Immunity, 23(4), 446–449. 10.1016/j.bbi.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BL, Harp KLH, & Oser CB (2013). Racial and Gender Discrimination in the Stress Process: Implications for African American Women’s Health and Well-Being. Sociological Perspectives, 56(1), 25–48. 10.1525/sop.2012.56.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH (2004). The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain, Behavior, and Immunity, 18(5), 407–413. 10.1016/j.bbi.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Piazza JR, Dmitrieva NO, Charles ST, Almeida DM, & Orona GA (2018). Diurnal cortisol profiles, inflammation, and functional limitations in aging: Findings from the MIDUS study. Health Psychology, 37(9), 839–849. 10.1037/hea0000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz G, & Straub RH (2014). The sympathetic nervous response in inflammation. Arthritis Research & Therapy, 16(6), 504. 10.1186/s13075-014-0504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Tarr AJ, & Sheridan JF (2013). Psychosocial stress and inflammation in cancer. Brain, Behavior, and Immunity, 30, S41–S47. 10.1016/j.bbi.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, & Brennan PA (2014). A developmental pathway from early life stress to inflammation. Psychological Science, 25(6), 1268–1274. 10.1177/0956797614530570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. (2012). lavaan : An R package for structural equation modeling. Journal of Statistical Software, 48(2). 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Sapolsky RM, Krey LC, & McEwen BS (1986). The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews, 7(3), 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Seng JS, Lopez WD, Sperlich M, Hamama L, & Reed Meldrum CD (2012). Marginalized identities, discrimination burden, and mental health: Empirical exploration of an interpersonal-level approach to modeling intersectionality. Social Science & Medicine, 75(12), 2437–2445. 10.1016/j.socscimed.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjörs A, Ljung T, & Jonsdottir IH (2014). Diurnal salivary cortisol in relation to perceived stress at home and at work in healthy men and women. Biological Psychology, 99, 193–197. 10.1016/j.biopsycho.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ (2008). Measurement-burst designs for social health research. Social and Personality Psychology Compass, 2(1), 245–261. 10.1111/j.1751-9004.2007.00043.x [DOI] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine, 72(7), 694–701. 10.1097/PSY.0b013e3181e9c16f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, & Oates GR (2017). Systemic Inflammation in Midlife: Race, Socioeconomic Status, and Perceived Discrimination. American Journal of Preventive Medicine, 52(1), S63–S76. 10.1016/j.amepre.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, & Kivimäki M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9, 360–370. 10.1038/nrcardio.2012.45 [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B, & Lloyd DA (1995). The epidemiology of social stress. American Sociological Review, 60(1), 104–125. 10.2307/2096348 [DOI] [Google Scholar]
- Tursich M, Neufeld RWJ, Frewen PA, Harricharan S, Kibler JL, Rhind SG, & Lanius RA (2014). Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Translational Psychiatry, 4, e413. 10.1038/tp.2014.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, & Thayer JF (2019). Heart rate variability and inflammation: A meta-analysis of human studies. Brain, Behavior, and Immunity, 80, 219–226. 10.1016/j.bbi.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Wright RJ, Rodriguez M, & Cohen S. (1998). Review of psychosocial stress and asthma: An integrated biopsychosocial approach. Thorax, 53(12), 1066–1074. 10.1136/thx.53.12.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ES, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, & Simpson JA (2019). The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: A prospective study. Psychological Science, 30(5), 739–747. 10.1177/0956797619833664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S, Imami L, & Slatcher RB (2017). Socioeconomic status, perceived control, diurnal cortisol, and physical symptoms: A moderated mediation model. Psychoneuroendocrinology, 75, 36–43. 10.1016/j.psyneuen.2016.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S, Imami L, Slatcher RB, Zilioli S, Imami L, & Slatcher RB (2018). Socioeconomic status and health-related biology: Links between socioeconomic disadvantage, psychological factors, and HPA activity in MIDUS. In Ryff CD & Krueger RF (Eds.), The Oxford Handbook of Integrative Health Science (pp. 431–442). Oxford University Press. 10.1093/oxfordhb/9780190676384.013.31 [DOI] [Google Scholar]
- Zilioli S, Slatcher RB, Chi P, Li X, Zhao J, & Zhao G. (2016). Childhood adversity, self-esteem, and diurnal cortisol profiles across the life span. Psychological Science, 27(9), 1249–1265. 10.1177/0956797616658287 [DOI] [PMC free article] [PubMed] [Google Scholar]