Supplemental Digital Content is available in the text.
Keywords: infant mortality, respiratory death, pediatric respiratory death, postmortem, Kenya
Abstract
Background:
In resource-limited settings, acute respiratory infections continue to be the leading cause of death in young children. We conducted postmortem investigations in children <5 years hospitalized with a clinical diagnosis of respiratory disease at Kenya’s largest referral hospital.
Methods:
We collected respiratory and other tissues postmortem to examine pathologic processes using histology, molecular and immunohistochemistry assays. Nasopharyngeal, trachea, bronchi and lung specimens were tested using 21-target respiratory pathogen real-time reverse transcription polymerase chain reaction assays deployed on Taqman Array Cards. Expert panels reviewed all findings to determine causes of death and associated pathogens.
Results:
From 2014 to 2015, we investigated 64 pediatric deaths (median age 7 months). Pneumonia was determined as cause of death in 70% (42/52) of cases where death was associated with an infectious disease process. The main etiologies of pneumonia deaths were respiratory syncytial virus (RSV) (n=7, 19%), Pneumocystis jirovecii (n=7, 19%), influenza A (n=5, 14%) and Streptococcus pneumoniae (n=5, 14%)—10% of cases had multi-pathogen involvement. Among the other 10 deaths associated with a nonpneumonia infectious process, 4 did not have an etiology assigned, the others were associated with miliary tuberculosis (2), cerebral thrombosis due to HIV (1), Enterobacteriaceae (1), rotavirus (1), and 1 case of respiratory infection with severe hypokalemia associated with RSV.
Conclusions:
In spite of well-established vaccination programs in Kenya, some deaths were still vaccine preventable. Accelerated development of RSV monoclonal antibodies and vaccines, introduction of seasonal influenza vaccination, and maintenance or improved uptake of existing vaccines can contribute to further reductions in childhood mortality.
Globally, acute respiratory illnesses are major causes of childhood morbidity and mortality, and sub-Saharan Africa bears the greatest burden.1,2 Included among the World Health Organization recommendations to control respiratory disease morbidity and mortality are the introduction of Haemophilus influenzae type B and pneumococcal conjugated vaccines and the implementation of the Integrated Management of Childhood Illness guidelines that foster a holistic approach to child health.3 These initiatives have been important in reducing childhood mortality during the past 10 years,2,4 and might have contributed to changes in respiratory pathogen distribution associated with pneumonia.5
Recent studies have identified respiratory viruses as important causes of pneumonia among hospitalized children, although their results reflected very few deaths.6,7 In a multi-site study done in the United States, respiratory viruses were associated with more than half of the pneumonia cases, with respiratory syncytial virus (RSV), human metapneumovirus, human rhinovirus, adenovirus and influenza the most frequently detected.6 While this observation might partly be a result of vaccines that target leading bacterial etiologies, resulting in decline of bacterial pneumonia,8,9 the development of molecular tests for respiratory viruses with improved sensitivity might play a role in the frequency of respiratory viruses being detected.10 Determination of a true distribution of pneumonia etiology in hospitalized patients is complicated by difficulties in obtaining adequate specimens from the lower respiratory tract, differing sensitivities of available assays, and assessment of the relevance of the finding as detection may not equate with disease.
Postmortem studies are the gold standard for determining cause of death, and, when combined with diagnostic assays,11 can provide a unique opportunity to investigate the etiology of fatal respiratory disease which can guide preventive care investments. Yet, these studies are challenging to conduct because families may be unwilling to consent to a full autopsy,12 and there is a lack of laboratory and histopathology capacity in lower-resourced countries. Here we describe the findings for the Pediatric Respiratory Etiology Surveillance Study13 conducted to investigate causes of death and the associated etiologies among children <5 years with respiratory illness admitted to a national referral hospital in Kenya.
MATERIALS AND METHODS
Setting and Design
From August 25, 2014 to December 31, 2015, we prospectively enrolled children 1-59 months of age who were admitted to the Kenyatta National Hospital (KNH) with respiratory illness and who died during hospitalization. The KNH, located in Nairobi, Kenya, is the country’s largest referral hospital, with 180-bed general pediatric ward, a 6-bed pediatric intensive care unit, and a 20-bed high dependency unit that serves critically ill patients of all ages.
We defined a case of respiratory illness as a child hospitalized with cough or difficulty in breathing, or one in which respiratory illness was recorded as the reason for admission. Trained grief counselors contacted parents/guardians of deceased children to offer counseling before seeking consent to perform postmortem examination. We collected sociodemographic and clinical data through interviews and from chart reviews. The KNH has a 24-hour mortuary where the bodies were kept refrigerated (4–8 °C) until postmortem examination. We performed minimally invasive tissue sampling techniques to obtain lung tissue specimens followed by conventional autopsy to collect multiple tissue samples. Tissues were examined by histopathology, and multiple laboratory techniques were applied to identify etiologies potentially associated with death. The protocol for this study was previously published13 and is summarized below.
Postmortem Procedures
Study pathologists conducted a postmortem evaluation within 5 days of death. We obtained 2 sets of lung specimens using sterile minimally invasive tissue sampling techniques. We placed 1 set of specimens in phosphate buffer solution sent to the Kenya Medical Research Institute (KEMRI) laboratory for molecular analyses. The remaining set was fixed in formalin and sent to the US Centers for Disease Control and Prevention in Atlanta for molecular, immunohistochemistry and histopathology analyses.13
During conventional autopsy, pathologists collected a nasopharyngeal swab, from which the tip was cut and placed in a cryovial containing viral transport media combined with a tracheal swab collected through a sterile transverse cut in the trachea below the thyroid cartilage and stored at −80 °C until molecular analysis could be performed.13
Pathologists also collected 4 pieces of trachea, 5 pieces of lung (1 from each lobe of both lungs) and 4 paired bronchial specimens (1 piece from each main bronchus) using sterile techniques. One piece of trachea, 1 piece of lung from each lobe and 1 pair of bronchial specimens were placed in tissue cassettes which were then placed separately in tissue jars containing phosphate buffer solution for molecular testing. The rest of the tissues along with 2 pieces of myocardium, 4 mediastinal lymph node specimens, 3 pieces each from liver, spleen and both kidneys, measuring approximately 2 × 2 centimeters, were separated in tissue cassettes in jars containing 10% formalin solution and fixed for 4–24 hours, and later stored in 70% ethanol. Additional tissue specimens were collected from organs with visible or suspected pathology. Ventricular blood was collected from the heart before dissection and dried blood spots were prepared by spotting whole blood on filter paper.13
Laboratory Analysis
Nasopharyngeal, trachea, bronchi and lung specimens were tested using 21-target respiratory pathogen real-time reverse transcription polymerase chain reaction assays deployed on Taqman Array Cards (TAC) (Thermo Fisher Scientific) at the KEMRI laboratory (Table, Supplemental Digital Content 1, http://links.lww.com/INF/E376).14 For each pathogen target, a TAC result was considered positive if the exponential fluorescence curve crossed the assigned cycle threshold at ≤35.0.13
We screened lung tissues for Mycobacterium tuberculosis at the KEMRI using GeneXpert (off-label application). Blood specimens on dried blood spots were tested for HIV at the Kenya AIDS Vaccine Initiative laboratory using the Roche Amplicor HIV-1 Monitor Test kit.15 Hematoxylin and eosin stains combined with clinical information and TAC results guided tests for further pathogens using histochemical stains, immunohistochemistry, or other molecular testing (eg, reverse transcription polymerase chain reaction targeting Streptococcus pneumoniae and H. influenzae serotyping genes).16,17
Determining Cause of Death
We followed the World Health Organization’s guidelines for medical certification in determining cause of death.18 Cause of death was broadly categorized into part I and part II. Part I includes the chain of events leading to death. Part Ia reflects the most recent or “immediate” condition that led to death whereas Part Ib, Ic, Id, etc. reflect sequential events leading to the immediate cause of death (ie, “intermediate” causes of death). The last diagnosis listed in part I reflects the “underlying cause” as the trigger of the chain of events leading to death. In some cases, only one cause of death for part I could be assigned, which was then considered as the underlying cause of death.19 Part II (comorbidities) comprised all other diseases and/or injuries that could have contributed to death, although they did not necessarily precipitate the cascade of events.
To determine cause of death we established a staged process. First, a Kenyan-based committee ascertained a probable cause of death based on data promptly available. Then 3 US pathologists reviewed histopathology findings and laboratory results to identified probable pathologic (histopathologic) diagnoses and suggested an etiology when possible. Finally, experts from both panels plus a medical epidemiologist and a microbiologist reviewed each case with all the data available and came to a consensus on the final causes of death (Figure, Supplemental Digital Content 2, http://links.lww.com/INF/E377). The committee assigned a possible etiologic agent to the cause of death (part I) if that was considered an infectious process. Pathogens detected via molecular methods were not considered associated with death if they were not known to be associated with the pathologic process assigned as cause of death; were interpreted as overgrowth during histopathology investigation; were found in nonsterile upper respiratory tract but not in the samples from the lower respiratory tract and could not be correlated with the histopathologic changes observed; or when reference laboratory could not confirm TAC findings through histopathology, immunohistochemistry and other laboratory tests (based on these inconsistent findings, these would be classified as inconclusive). The committee, when possible, also assigned an etiology or etiologies to part II of cause of death.
Data Analysis
We used proportions to describe categorical variables and median and interquartile ranges (IQRs) to describe continuous variables. Age was categorized into groups (<6, 6–11, 12–23, 24–48 and 49–59 months), level of consciousness was measured by the AVPU scale (an acronym from “alert, voice, pain, unresponsive”), and nutritional status was classified based on wasting and stunting assessed during autopsy.20
Ethical Considerations
The study protocol was approved by the Institutional Review Board at KEMRI (SSC no. 2692), with reliance from US Centers for Disease Control and Prevention’s Institutional Review Board (6599), and by the KNH/University of Nairobi (UoN).
RESULTS
Study Population
During August 2014 through December 2015, we identified 200 children <5 years of age with respiratory illness who died during hospitalization (Figure, Supplemental Digital Content 3, http://links.lww.com/INF/E378). We offered grief counseling to 134 (67%) of parents/guardians that we were able to contact; 113 (84%) of these were counseled, and 65 (58%) of those counseled consented to postmortem investigation of their child. We performed 64 autopsies.
The median age of children included in the postmortem investigation was 7 months (range 1–48 months), and approximately half was female. Based on clinical assessment at admission, 8 (13%) were born prematurely (<37 weeks of gestation) and 8 (13%) had a congenital disorder. At the time of autopsy, 40 (63%) children were moderately to severely wasted and 16 (25%) were moderately to severely stunted (Table 1). A total of 31 (48%) children died within the first 24 hours of admission. The median time interval from illness onset to admission was 10 days (IQR 6–21 days). The median length of hospital stay was 2 days (IQR 1–4.5 days), and the median time interval from death to autopsy was 3 days (range 0–5 days).
TABLE 1.
Characteristics of Deceased Children Enrolled for Postmortem Investigation—KNH, Nairobi, Kenya, August 2014 to December 2015.
| Characteristics | n/N* (%)† |
|---|---|
| Demographics | |
| Age in months, median (range‡) | 7 (1–48) |
| <6 months | 24/64 (38) |
| 6–11 months | 23/64 (36) |
| 12–23 months | 11/64 (17) |
| 24–48 months | 6/64 (9) |
| Male | 31/64 (48) |
| Comorbidity based on admission assessment | |
| Congenital heart disease | 7/64 (11) |
| Congenital lung disease | 1/64 (2) |
| HIV infection | 5/64 (8) |
| Neurologic/neuromuscular disorders | 6/64 (2) |
| Prematurity (born <37 weeks) | 8/64 (13) |
| Symptoms and signs on admission (clinical report) | |
| Reported or measured fever (temperature ≥38 °C) | 48/61 (79) |
| Cough | 52/61 (85) |
| Difficulty breathing | 50/61 (82) |
| Tachypnea§ | 45/51 (88) |
| Diarrhea | 17/61 (28) |
| Vomiting | 13/61 (21) |
| Convulsion | 10/61 (16) |
| Lethargy | 29/61 (48) |
| Level of consciousness | |
| Alert | 38/60 (63) |
| Response to verbal stimuli | 3/60 (5) |
| Response to pain stimuli | 13/60 (22) |
| Unconscious | 6/60 (10) |
| Nutritional status at postmortem | |
| Moderate to severe wasting¶ | 40/64 (63) |
| Moderate to severe stunting∥ | 16/64 (25) |
| Care seeking | |
| Sought healthcare before admission | 37/61 (61) |
| Severity measures | |
| Hypoxia (oxygen saturation ≤90%) | 15/47 (36) |
| Died within 24 hours of admission | 31/64 (48) |
| Recommendation for ICU admission | 7/61 (11) |
| Admitted to ICU | 1/7 (14) |
| Timelines (in days) | |
| Time of illness onset to admission (all patients), median (IQR) | 10 (6–21) |
| Among those who sought health care before admission | 14 (7–22) |
| Among those who did not seek health care before admission | 9 (5–15) |
| Length of hospital stay, median (IQR) | 2 (1–5) |
| Time of death to postmortem investigation, median (range) | 3 (0–5) |
Denominator varies based on data availability.
Unless specified as median (range) or (IQR) in row.
IQR: 4–12 months.
Tachypnea is defined as a respiratory rate of ≥60 breaths per minute for children <2 months of age, ≥50 for children 2–11 months and ≥40 for children 12–59 months.
Moderate to severe wasting: weight vs. height WHO z scores <−2.
Moderate to severe stunting: height vs. weight WHO z scores <−2.
ICU indicates intensive care unit.
Detection of Pathogens
From all the tissues tested, 37 different pathogens were identified (Fig. 1; Laboratory results, Supplemental Digital Content 4, http://links.lww.com/INF/E379). A median of 5 pathogens was detected per child (range 1–12). The most frequent bacterial pathogens detected were Klebsiella pneumoniae, 42/64 (66%); Pseudomonas aeruginosa, 38/64 (59%); and S. pneumoniae, 30/64 (47%). The most frequent viral pathogens detected were enterovirus/rhinovirus, 29/64 (45%); RSV, 17/64 (27%); and adenovirus, 17/64 (27%).
FIGURE 1.
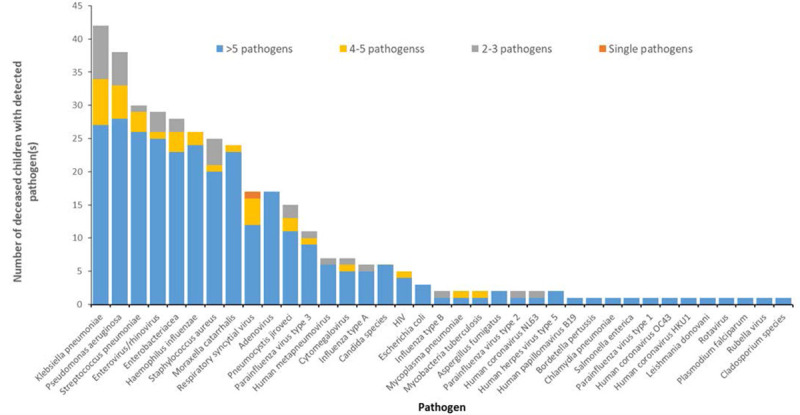
Frequency of detection of pathogens in all postmortem-collected tissue specimens from deceased children hospitalized with respiratory illness (N=64) — Nairobi, Kenya, August 2014–December 2015.
TAC assays conducted on tracheal, bronchial and lung tissues did not always detect the same pathogens consistently across the tissues of the respiratory tract (Figure, Supplemental Digital Content 5, http://links.lww.com/INF/E380). More pathogens were detected in tracheal and bronchial tissues (upper airways) than in the lung. On the other hand, B. pertussis, human coronavirus HKU1 and parainfluenza type 1, when detected, were consistently detected throughout the respiratory tract, and Pneumocystis jirovecii were mostly detected in the lung tissue.
Cause of Death
Cause of death could not be determined in 3 cases. The attending physician assigned severe pneumonia and meningitis as discharge diagnoses for 2 of these cases, but study pathologists could not identify anything to corroborate that during autopsy. No clinical data were available for the third case and the autopsy did not aid on cause of death deliberation. There were 61 cases with cause of death assigned, 52 associated with an infectious process, and 9 with noninfectious causes. Cause-of-death due to a noninfectious process were head injury (6), dilated cardiomyopathy (1), aspiration (1) and myocarditis (1).
Most children had pneumonia as cause of death (42/61; 70%) which was either defined as an intermediate cause (n=3) or underlying cause (n=39). The second most common underlying cause of death was head injury (n=6.10%), followed by gastroenteritis (n=5.8%), aspiration asphyxia (n=2.3%) and miliary tuberculosis (n=2.3%), detailed list of cases can be seen in Table, Supplemental Digital Content 6, http://links.lww.com/INF/E381, and Fig. 2. Immediate cause of death was determined in 36 cases; of those, 17 (47%) were due to acute respiratory distress syndrome resulting from pneumonia or sepsis. Other immediate causes of death included severe hypokalemia (n=3.8%), asphyxia (n=2.5%) and pulmonary edema (n=2.5%).
FIGURE 2.
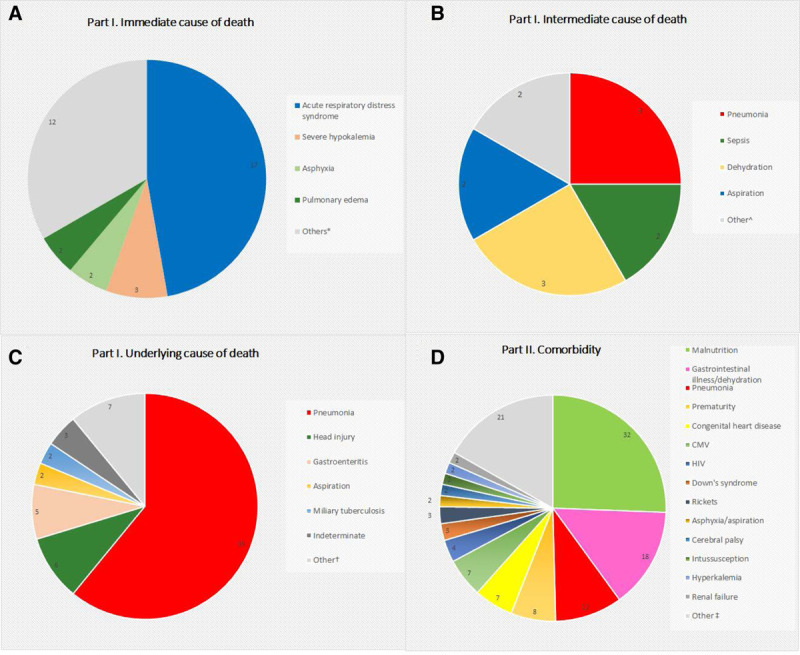
Causes of death among deceased children hospitalized with respiratory illness. Part I (A) immediate, (B) intermediate and (C) underlying; and part II (D) comorbidity—Nairobi, Kenya, August 2014 to December 2015. Other*: acute renal failure, aspiration, cerebral vascular accident, congestive cardiac failure, disseminated intravascular coagulation, hyperkalemia, hypovolemic shock, meningitis, meningoencephalitis, pulmonary hemorrhage, septicemia, and severe dehydration with electrolyte imbalance. Other^: acute respiratory distress syndrome, and multiple organ failure (sepsis). Other†: acute respiratory distress syndrome, dilated cardiomyopathy, HIV, malaria, myocarditis, respiratory infection (unspecified), and sepsis. Other‡: chronic liver disease (biliary cirrhosis), cleft lip and palate, congenital lung disease, congenital anomalies not specified, congenital rubella, congestive cardiac failure, developmental delay, congenital cytomegalovirus (CMV), failure to thrive, giant cell hepatitis, hydrocephalus, hypovolemic shock, interstitial nephritis, leishmaniasis, megacolon, myeloproliferative disorder (acute myeloid leukemia), obstructed inguinal hernia, pyelonephritis, respiratory tract infection, sickle cell disease, and HIV exposure.
The head injuries were identified as underlying cause of death during conventional autopsy and were not mentioned in the medical charts. Most of them were described in gross autopsy reports as acute hematoma that could indicate recent trauma. In 3 of these cases, the children died within 24 hours of hospitalization. The other 3 children were hospitalized from 9 to 35 days before death (Table, Supplemental Digital Content 7, http://links.lww.com/INF/E382). The finding of pneumonia in these 6 children was classified as comorbidity (part II) because it was not thought to be directly associated with cause of death.
In general, the most frequent comorbidities (part II) were malnutrition 32 (52%), gastrointestinal diseases 18 (30%) and pneumonia 12 (20%). These conditions might have contributed to death, although we could not assess this directly (Fig. 2 and Table, Supplemental Digital Content 6, http://links.lww.com/INF/E381).
Etiology Associated With Cause of Death
Among the 52/61 (85%) cases determined to be due to an infectious disease process, a specific etiologic pathogen was identified in 43/52 (82%) cases. In 10 (23%) of these 43 cases, >1 pathogen was considered to be involved in the cause of death. The expert committee did not attempt to give weight to specific pathogens when >1 pathogen was assigned to cause of death (Table 2).
TABLE 2.
Causes of Death (Part I) and Etiologies Associated With Infectious Causes Among Deceased Children Enrolled for Postmortem Investigation, KNH, Nairobi, Kenya, August 2014 to December 2015
| Cause of Death (Part I) | Case Number |
|---|---|
| Indeterminate | 3 |
| Noninfectious | 9 |
| Infectious | 52 |
| Indeterminate | 9 |
| RSV | 5 |
| Pneumocystis jirovecii | 4 |
| S. pneumoniae | 4 |
| Klebsiella pneumoniae | 3 |
| Influenza type A | 3 |
| H. influenzae not typeable | 2 |
| Escherichia coli | 2 |
| Human metapneumovirus | 2 |
| Mycobacterium tuberculosis | 2 |
| Enterobacteriaceae | 1 |
| Rotavirus | 1 |
| HIV | 1 |
| Plasmodium falciparum | 1 |
| Mycoplasma pneumoniae | 1 |
| Parainfluenza virus type 3 | 1 |
| RSV, Chlamydia pneumoniae | 1 |
| S. pneumoniae, Klebsiella pneumoniae | 1 |
| Parainfluenza virus type 1, H. influenzae not typeable | 1 |
| Pneumocystis jirovecii, human metapneumovirus | 1 |
| RSV, Pneumocystis jirovecii | 1 |
| Pneumocystis jirovecii, Bordetella pertussis | 1 |
| Aspergillus fumigatus, adenovirus, human metapneumovirus | 1 |
| Influenza type A, RSV | 1 |
| Salmonella enterica, influenza type A, parainfluenza virus type 3, human coronavirus HKU1 | 1 |
| Adenovirus, parainfluenza virus type 3 | 1 |
Of the 42 cases where pneumonia was associated with a part I cause of death, 51 pathogens were etiologically associated with the pneumonia in 37 cases, and 5 cases had no determined etiology (Table, Supplemental Digital Content 6, http://links.lww.com/INF/E381). The most common pathogens associated with pneumonia were RSV (n=7, 19%), P. jirovecii (n=7, 19%), influenza A (n=5, 14%) and S. pneumoniae (n=5, 14%) (Fig. 3). In the 5 deaths associated with S. pneumoniae, the serotype was identified in 4: serotype 1, serotype 19A, serotype 19F and serotype 22F/22A. Of the 7 deaths caused by P. jirovecii, 3 had co-detection with other pathogens (RSV, HMPV and pertussis) (Table 2).
FIGURE 3.
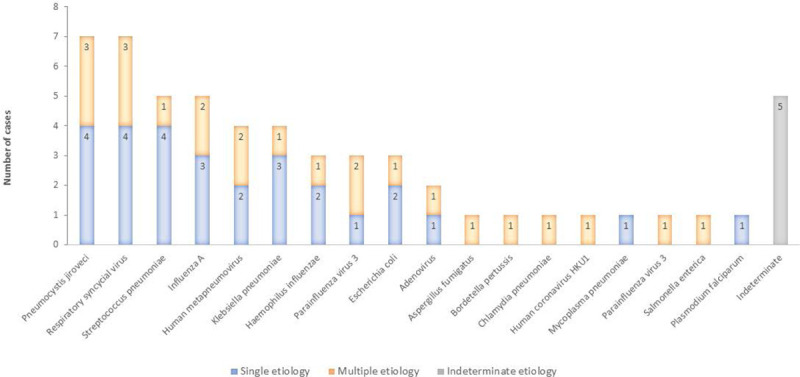
Distribution of pathogens associated with pneumonia specified as cause of death (part I) in deceased children hospitalized with respiratory illness—Nairobi, Kenya, August 2014 to December 2015.
Among the 10 cases of nonpneumonia infectious disease deaths, the panel could not assign an etiology to 4 cases, the others being 2 cases of miliary tuberculosis, 1 case of cerebral thrombosis due to HIV, 2 cases of gastroenteritis associated with Enterobacteriaceae and rotavirus each and 1 case was a respiratory infection with severe hypokalemia that was associated with RSV (although the autopsy and the histopathology did not indicate pneumonia) (Table 2). Table (Supplemental Digital Content 6, http://links.lww.com/INF/E381) lists pathogens potentially associated with comorbidities (part II), including 5 children with postmortem diagnosis of CMV and 4 with postmortem diagnosis of HIV.
DISCUSSION
We studied 64 children hospitalized with respiratory illness who died during hospitalization. Seventy percent of deaths were ascribed to pneumonia as the intermediate or underlying cause of death. Eighty-eight percent (37/42) of the pneumonia cases had an identified etiology, the main associated pathogens were RSV, P. jirovecii, influenza A virus and S. pneumoniae, either singly or with other pathogens. Overall, many children in our study were severely ill by the time they presented to hospital. In spite of well-established vaccination programs in Kenya, several of the cases were caused by vaccine-preventable pathogens.
Viruses were important contributors to cause of death. RSV has been described as the main cause of lower acute respiratory illness in children <5 years in LMICs, associated with high morbidity and mortality.21,22 RSV vaccines are in the horizon; however, access may be limited in LMICs with less resources and competing disease needs. Influenza has been well-documented to cause severe illness in children <5 years of age in Kenya and other African countries.23,24 In 2016, the Kenyan National Immunization Technical Advisory Group made provisional recommendations of influenza vaccine for 6–23 months old in Kenya, pending cost and implementation data.25 Studies aimed at understanding the benefit of adding new vaccines in LMICs’ immunization programs are important to advise policy makers and stakeholders on how to manage priorities.
We detected pneumococcal serotypes included in the ten-valent pneumococcal conjugate vaccine currently in use in Kenya’s routine immunization program as etiologic causes of death in children who were old enough to have completed the full 3-dose schedule. However, we could not verify their vaccination status. The impact of introduction of pneumococcal vaccine in reducing invasive pneumococcal disease in Kenya has been well-documented.26 The Expanded Program on Immunization, heavily supported by the Global Alliance for Vaccine and Immunization (GAVI),27 creates equal access to new and underused vaccines for children in the world’s poorest countries. Kenya is now considered a middle-income country and will be phasing out from GAVI support soon. The phasing out of GAVI could affect current Expanded Program on Immunization performance,28 especially as the country considers implementation of new vaccines (eg, influenza and HPV vaccines).25,29
Most of the detected pathogens were not determined to be an etiologic cause of death. Their detection may be a result of contamination, postmortem translocation from nonsterile sites, or molecular amplification of pathogens not directly responsible for the cause of death, reflecting past infection or “normal” microbiota.19,30 Many of the cases had some degree of malnutrition and 5 were HIV positive, each of which might have played a role in weakening response to infections and might partially explain the presence of multiple pathogens. In our study, P. jirovecii was a common etiology of pneumonia, the only etiologic agent assigned to cause of death in 4 of the 7 cases in which it was identified. P. jirovecii has been associated with interstitial pneumonia in malnourished infants as early as the 1940s,31 and it has been described as an important cause of severe pneumonia in hospitalized children in Mozambique, especially in infants.32
Our study is limited by sample size, and because studied respiratory deaths reflect a tertiary hospital in Kenya, that is, etiology of pneumonia may differ between those who survive or those who died in the community. It was also possible that detected pathogens deemed as noncontributor to the cause of death could have played a role, but we lacked enough evidence to support that. Similarly, we may have missed pathogens due to prolonged disease process, such as bacteria that may have not being detected due to antibiotic therapy. Nonetheless, we benefited from availability of medical history, series of respiratory samples and tissue specimens collected during full autopsy and a state-of-the-art laboratory support. We could not explore the impact of seasonality on pathogen distribution due to lack of consistent surveillance data over >1 calendar year. Moreover, despite our case definition, 10 children were likely hospitalized because of noninfectious processes, and the cause of death for 6 children was determined to be acute head injury (not detected at admission or in medical chart), limiting further interpretation of findings.
In conclusion, deciding which pathogens were associated with the illness leading to death was challenging, and in many instances, multiple pathogens may have played a role. Having histopathology results to substantiate findings from molecular diagnosis were helpful, but in future studies, control autopsies on children without respiratory illnesses could aid result interpretation. Public health interventions to reduce pediatric mortality worldwide need to address improvement of primary healthcare services. Accelerated development of RSV monoclonal antibodies and vaccines, introduction of influenza vaccination program, and strengthening of existing programs could contribute to further reductions in childhood mortality worldwide, but more studies on pediatric mortality are needed to substantiate interventions, especially in LMICs such as Kenya which might be limited by nonequitable access to vaccines and governments’ competing priorities.
ACKNOWLEDGMENTS
The authors acknowledge the significant contributions to the processes of case identification by the surveillance officers and pediatric nurses at the Kenyatta National Hospital (KNH). The authors thank the team of mortuary technicians for collecting and storing decedent’s bodies from the wards to the mortuary and for body preparation prior, during, and after postmortem examination and specimen collection. The authors specially thank the team of histopathology technicians at the KNH for preparing tissue slides for review by pathologists. In addition, they thank the entire team at the Infectious Disease Pathology Branch (IDPB), at the Centers for Control and Prevention (CDC) Atlanta for collective involvement in the receiving, processing, testing, and interpretation of specimens, and colleagues at the Mycotic Diseases Branch, Division of Global HIV and TB, Measles Mumps, Rubella and Herpesvirus Laboratory Branch, and Division of Bacterial Diseases-Streptococcus Laboratory for diagnostic testing. They would also like to thank the data team for creating study databases and maintaining data integrity, and Dr. Dean D. Erdman, virologist, and Mr. Brett Whitaker, virology technician with the Division of Viral Disease (CDC) who provided important contribution to the interpretation of the virologic results from the molecular diagnostic tool. Finally, special thanks to parents and guardians who consented to have their children’s bodies undergo postmortem examination.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supported by the Centers for Disease Control and Prevention (Cooperative agreement GH002133) and the US Department of Defense (DTRA/CBEP) (KE 03).
Since completion of this work, S.S.C. has joined Sanofi Pasteur, France and M.-A.W. has joined the Institute of Tropical Medicine in Antwerp, Belgium. The other authors have no conflicts of interest to disclose.
H.N.N., S.S.C., S.R.Z., D.J.R., C.L.F., E.R., B.F., M.A.W. and J.A.M. designed the study protocol. H.N.N. lead implementation, supervised data collection, analyzed the data and wrote the first draft of the article. S.S.C. provided leadership, supervised the implementation of the study, supported analysis and interpretation of findings. H.N.N. and S.S.C. contributed equally to the article writing. H.N.N., S.R.Z., D.J.R., C.L.F., E.R., B.F., M.A.W., J.A.M., S.S.C., M.K.K., J. Mathaiya, E.W., A.K.G., G.C.M., E.M.O., G.I., N.O., R.L., J. Maina, G.O.E., C.O.O., S.G., C.O., P.K. and M.B. contributed intellectually to the article writing, reviewed and approved final version.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Bryce J, Boschi-Pinto C, Shibuya K, et al. HO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Global Action Plan for Prevention and Control of Pneumonia (GAPP). 2009. Available at: https://apps.who.int/iris/bitstream/handle/10665/70101/WHO_FCH_CAH_NCH_09.04_eng.pdf?sequence=1. Accessed July 19, 2019. [DOI] [PMC free article] [PubMed]
- 4.Bhutta ZA, Das JK, Walker N, et al.; Lancet Diarrhoea and Pneumonia Interventions Study Group. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381:1417–1429. [DOI] [PubMed] [Google Scholar]
- 5.Gilani Z, Kwong YD, Levine OS, et al. A literature review and survey of childhood pneumonia etiology studies: 2000-2010. Clin Infect Dis. 2012; 54(suppl 2):S102–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Williams DJ, Arnold SR, et al.; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. Erratum in: Lancet. 2019;2019;394:394:757– 736.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–1186. [DOI] [PubMed] [Google Scholar]
- 9.MacNeil JR, Cohn AC, Farley M, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease–United States, 1989-2008. Clin Infect Dis. 2011;53:1230–1236. [DOI] [PubMed] [Google Scholar]
- 10.Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(suppl 4):S284–S289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner GD, Bunthi C, Wonodi CB, et al. The role of postmortem studies in pneumonia etiology research. Clin Infect Dis. 2012;54(suppl 2):S165–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis C, Hill M, Arthurs OJ, et al. Factors affecting uptake of postmortem examination in the prenatal, perinatal and paediatric setting. BJOG. 2018;125:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njuguna HN, Zaki SR, Roberts DJ, et al. Determining the cause of death among children hospitalized with respiratory illness in Kenya: Protocol for Pediatric Respiratory Etiology Surveillance Study (PRESS). JMIR Res Protoc. 2019;8:e10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodani M, Yang G, Conklin LM, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zijenah LS, Humphrey J, Nathoo K, et al. Evaluation of the prototype Roche DNA amplification kit incorporating the new SSK145 and SKCC1B primers in detection of human immunodeficiency virus type 1 DNA in Zimbabwe. J Clin Microbiol. 1999;37:3569–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimenta FC, Roundtree A, Soysal A, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol. 2013;51:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO). WHO Manual. Laboratory Methods for Diagnosis of Meningitis Caused by Neisseria meningitides, Streptococcus pneumoniae, Haemophilus influenzae. 20112011. 2nd ed. 32–258Available at: https://apps.who.int/iris/bitstream/handle/10665/70765/WHO_IVB_11.09_eng.pdf;jsessionid=2E1E9E3166D51D51E6858B6C5C465DEE?sequence=1. Accessed June 17, 2020. [Google Scholar]
- 18.World Health Organization (WHO). Medical Certification of Cause of Death. 1979. Available at: https://apps.who.int/iris/bitstream/handle/10665/40557/9241560622.pdf;jsessionid=02A12F16565B8D67207E8753D3D93FC5?sequence=1. Accessed July 11, 2019.
- 19.Centers for Disease Control and Prevention (CDC). Medical and Coroners’ Handbook on Death Registration and Fatal Death Reporting. 2003. Available at: https://www.cdc.gov/nchs/data/misc/hb_me.pdf. Accessed January 25, 2020.
- 20.World Health Organization (WHO). Nutrition Landscape Information System (NLIS) Contry Profile Indicators Interpretation Guide. 2010. Available at: https://www.who.int/nutrition/nlis_interpretation_guide.pdf. Accessed January 22, 2020.
- 21.Emukule GO, Spreeuwenberg P, Chaves SS, et al. Estimating influenza and respiratory syncytial virus-associated mortality in Western Kenya using health and demographic surveillance system data, 2007-2013. PLoS One. 2017;12:e0180890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi T, McAllister DA, O’Brien KL, et al.; RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawa JA, Chaves SS, Nyawanda B, et al. National burden of hospitalized and non-hospitalized influenza-associated severe acute respiratory illness in Kenya, 2012-2014. Influenza Other Respir Viruses. 2018;12:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafond KE, Nair H, Rasooly MH, et al.; Global Respiratory Hospitalizations—Influenza Proportion Positive (GRIPP) Working Group. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982-2012: a systematic analysis. PLoS Med. 2016;13:e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawa J, Chaves SS, Ba Nguz A, et al.; Kenya National Immunization Technical Advisory Group (KENITAG). Developing a seasonal influenza vaccine recommendation in Kenya: process and challenges faced by the National Immunization Technical Advisory Group (NITAG). Vaccine. 2019;37:464–472. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M, Bigogo G, Kim L, et al. Impact of 10-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage and antibiotic susceptibility patterns among children aged <5 years and adults with HIV infection, Kenya 2009-2013. Clin Infect Dis. 2000; 70:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global Alliance for Vaccine and Immunisation (GAVI). 2019. Available at: https://www.devex.com/organizations/global-alliance-for-vaccines-and-immunisation-gavi-44118. Accessed August 30, 2019.
- 28.Ojal J, Griffiths U, Hammitt LL, et al. Sustaining pneumococcal vaccination after transitioning from GAVI support: a modelling and cost-effectiveness study in Kenya. Lancet Glob Health. 2019;7:e644–e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman AL, Oruko KO, Habel MA, et al. Preparing for human papillomavirus vaccine introduction in Kenya: implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya. BMC Public Health. 2014;14:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton JL, Saegeman V, Arribi A, et al.; ESGFOR Joint Working Group of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group of Forensic and Postmortem Microbiology and the European Society of Pathology. Postmortem microbiology sampling following death in hospital: an ESGFOR task force consensus statement. J Clin Pathol. 2019;72:329–336. [DOI] [PubMed] [Google Scholar]
- 31.VANEK J, JIROVEC O. [Parasitic pneumonia. Interstitial plasma cell pneumonia of premature, caused by pneumocystis Carinii]. Zentralbl Bakteriol Orig. 1952;158:120–127. [PubMed] [Google Scholar]
- 32.Lanaspa M, O’Callaghan-Gordo C, Machevo S, et al. High prevalence of Pneumocystis jirovecii pneumonia among Mozambican children <5 years of age admitted to hospital with clinical severe pneumonia. Clin Microbiol Infect. 2015;21:1018.e9–1018.e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


