ABSTRACT
Heterodimers of glycoproteins H (gH) and L (gL) comprise a basal element of the viral membrane fusion machinery conserved across herpesviruses. In human cytomegalovirus (HCMV), the glycoprotein UL116 assembles onto gH at a position similar to that occupied by gL, forming a heterodimer that is incorporated into virions. Here, we show that UL116 promotes the expression of gH/gL complexes and is required for the efficient production of infectious cell-free virions. UL116-null mutants show a 10-fold defect in production of infectious cell-free virions from infected fibroblasts and epithelial cells. This defect is accompanied by reduced expression of two disulfide-linked gH/gL complexes that play crucial roles in viral entry: the heterotrimer of gH/gL with glycoprotein O (gO) and the pentameric complex of gH/gL with UL128, UL130, and UL131. Kifunensine, a mannosidase inhibitor that interferes with endoplasmic reticulum (ER)-associated degradation (ERAD) of terminally misfolded glycoproteins, restored levels of gH, gL, and gO in UL116-null-infected cells, indicating that constituents of HCMV gH complexes are unstable in the absence of UL116. Further, we find that gH/UL116 complexes are abundant in virions, since a major gH species not covalently linked to other glycoproteins, which has long been observed in the literature, is detected from wild-type but not UL116-null virions. Interestingly, UL116 coimmunoprecipitates with UL148, a viral ER-resident glycoprotein that attenuates ERAD of gO, and we observe elevated levels of UL116 in UL148-null virions. Collectively, our findings argue that UL116 is a chaperone for gH that supports the assembly, maturation, and incorporation of gH/gL complexes into virions.
IMPORTANCE HCMV is a betaherpesvirus that causes dangerous opportunistic infections in immunocompromised patients as well as in the immune-naive fetus and preterm infants. The potential of the virus to enter new host cells is governed in large part by two alternative viral glycoprotein H (gH)/glycoprotein L (gL) complexes that play important roles in entry: gH/gL/gO and gH/gL/UL128-131. A recently identified virion gH complex, comprised of gH bound to UL116, adds a new layer of complexity to the mechanisms that contribute to HCMV infectivity. Here, we show that UL116 promotes the expression of gH/gL complexes and that UL116 interacts with the viral ER-resident glycoprotein UL148, a factor that supports the expression of gH/gL/gO. Overall, our results suggest that UL116 is a chaperone for gH. These findings have important implications for understanding HCMV cell tropism as well as for the development of vaccines against the virus.
KEYWORDS: ERAD, bioassembly, cytomegalovirus, glycoproteins, herpesviruses, human herpesviruses, tropism, virion structure
INTRODUCTION
The core viral cell entry machinery that drives membrane fusion events during herpesvirus infection is comprised of glycoprotein B (gB) and a heterodimer of glycoprotein H/glycoprotein L (gH/gL). gB is widely posited to be the proximal viral fusogen, which undergoes a substantial conformational rearrangement during fusion of virus and target cell membranes, while gH/gL regulates the fusogenic activity of gB. Herpes simplex viruses 1 and 2 and other alphaherpesviruses express gH/gL lacking covalently bound accessory proteins. However, several different beta- and gammaherpesviruses, such as human cytomegalovirus (HCMV), Epstein-Barr virus (EBV), and human herpesviruses 6A/6B and 7, express alternative gH/gL complexes that are derivatized with virus-encoded accessory glycoproteins. At least in certain cases, the accessory glycoproteins function as receptor-binding moieties. For example, the HCMV gH/gL/gO (Trimer) complex binds PDGFRα on the surface of target cells via physical interactions with gO (1–4). Meanwhile, the gH/gL/UL128/UL130/UL131 (Pentamer) complex binds neuropilin-2 (Nrp2) to drive entry into epithelial and endothelial cells, and the UL128-131 gene products form contacts with Nrp2 (5). The olfactory receptor OR14I1, a G-coupled protein receptor, and CD147 also are found to be required for Pentamer-dependent entry (6, 7), but the mechanistic details are less clear.
Although the identification of the two alternative HCMV gH/gL complexes has been pivotal to understanding how the virus enters the broad array of cell types that the virus infects, HCMV cell tropism may also be influenced by viral gene products that modulate the relative abundance in virions of the two gH/gL complexes. For example, UL148, a viral endoplasmic reticulum (ER)-resident glycoprotein, is required for high-level expression of trimer in HCMV strain TB40/E (8), and UL148-null mutants of TB40/E show enhanced tropism for epithelial cells. In addition, US16 has been found to be required for incorporation of Pentamer into the virion envelope (9).
In 2016, UL116, a glycoprotein encoded by gene directly adjacent to that encoding gL (UL115), was reported to form a complex with gH that is found in HCMV virions (10). UL116 assembles onto gH at a position similar to that occupied by gL. However, unlike gL, UL116 does not form a disulfide linkage to gH. Furthermore, coexpression of gH with UL116 is sufficient for export of the complex from the ER, while neither glycoprotein exports efficiently on its own. Therefore, UL116 represents yet another component that may impact HCMV tropism, either by interacting with hitherto unidentified host cell receptors in complex with gH or by affecting the composition of alternative gH complexes incorporated into virions.
During experiments with UL148-null mutant HCMVs, we observed discrepancies in the ratio of gH to gL in virion preparations (data not shown), leading us to hypothesize that UL148 affects the abundance of the gH/UL116 complex. Here, we present evidence that UL116 interacts with UL148 and that UL116-null mutants are globally defective for expression of gH/gL complexes. Our findings suggest that UL116 acts as a chaperone for gH during the assembly of gH/gL complexes. (During revision of this study, two papers addressing roles of UL116 were published whose results lend further support to our findings [11, 12].)
RESULTS
UL116-null viruses show a defect in production of infectious cell-free virions.
We disrupted UL116 in two different HCMV strains, AD169 and TB40/E, by replacing the 14th and 15th codons of the coding sequence with nonsense codons. TB40/E expresses both Pentamer (gH/gL/UL128-131) and Trimer (gH/gL/gO) and retains a complete UL148 gene. The AD169 derivative we used here, ADr131 (AD169), carries a repaired UL131 to rescue its expression of Pentamer. Strain AD169 does not encode a functional UL148 and also lacks approximately 14 other viral genes owing to deletions in the ULb′ region (13).
UL116-null viruses showed an ∼1-log defect in production of cell-free infectious progeny virions following infection of human fibroblasts at a multiplicity of infection (MOI) of 1 50% tissue culture infective dose (TCID50) per cell (Fig. 1). During infection of ARPE-19 epithelial cells, again at an MOI of 1 TCID50/cell (using titer values measured on fibroblasts), a similar ∼1-log defect in production of infectious cell-free virions was observed for the UL116-null AD169, while the defect for UL116-null TB40/E was even more severe, at roughly 2 logs. Because AD169 restored UL131 replicates more robustly in ARPE-19 than does TB40/E (8, 14), the more pronounced replication defect of UL116-null TB40/E most likely reflects additional rounds of replication owing to the fact that input MOI is set based on TCID50 values determined on fibroblasts.
FIG 1.
UL116-null mutant viruses exhibit a replication defect. Fibroblasts (HFF) (A) or epithelial cells (ARPE-19) (B) were infected at an MOI of 1 with strain TB40/E (TB_WT) or a UL116-null derivative (TB_116STOP). Supernatant titers from the indicated time points determined by TCID50. (C and D) Results of a similar experiment comparing WT versus UL116-null mutant strain AD169 repaired for UL131 (ADr131 versus ADr131_116STOP). (E) HFFs were infected at an MOI of 1 with strain TB40/E (TB_WT), a UL116-null derivative (TB_116STOP), or a rescue of UL116 (Rescue). (F) Cell-associated virus from fibroblasts infected at an MOI of 1 was collected at 5 dpi, and infectivity was quantified by TCID50 assay. (G) HFFs were infected at an MOI of 1 with strain TB40/E (TB_WT) or a UL116-null derivative (TB_116STOP). Cell-free genome copy numbers were quantified by qPCR assay.
A “rescuant” virus, in which a functional UL116 was restored to the UL116-null TB40/E mutant, replicated indistinguishably from wild-type (WT) parental TB40/E during infection of fibroblasts (Fig. 1E). This finding argues against the possibility that spurious mutations elsewhere in viral genome account for the defects observed for the UL116-null virus. Despite its defect in production of cell-free virions, the UL116-null TB40/E mutant produced cell-associated titers similar to those of the wild type (Fig. 1F). UL116-null viruses showed only a 3-fold defect in production of cell-free genomes following infection of human fibroblasts at an MOI of 1 TCID50 per cell (Fig. 1G). These findings may help explain why previous genome profiling studies failed to observe a replication defect for UL116-null mutant viruses (15, 16). Overall, these results indicate that UL116 is required for efficient production of infectious cell-free virions.
UL116-null viruses show reduced expression of gH/gL complexes.
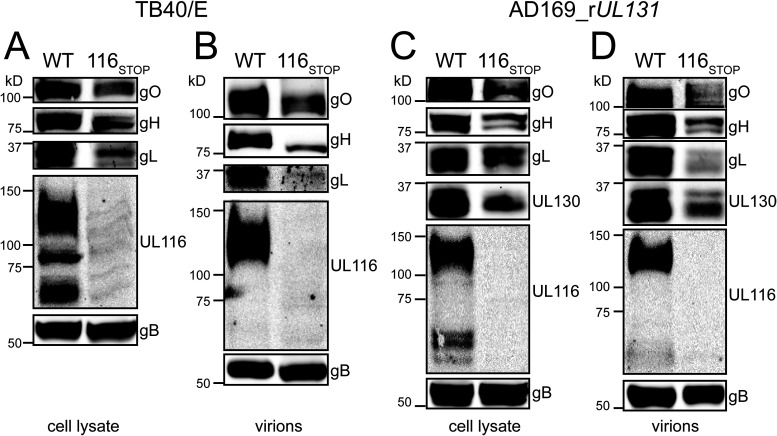
Since UL116 is a constituent of a gH complex found in HCMV virions, we next evaluated the relative expression of the two gH/gL complexes and of gH/UL116 in infected cell lysates and in virions. After collecting virions by ultracentrifugation through a sorbitol cushion, we lysed virions in nonionic detergent to extract envelope glycoproteins, normalizing loading such that gB levels were roughly equivalent across samples during Western blot analyses. As expected, bands immunoreactive to an anti-UL116 monoclonal antibody were absent from UL116-null infected cells and from UL116-null virions (Fig. 2). Interestingly, UL116-null mutants also showed reduced expression of gH and of other representative viral glycoproteins that participate in multiprotein complexes with gH, i.e., gO, gL, and UL130, and these effects were seen both in lysates of infected cells and in virions (Fig. 2). From these results, we conclude that UL116-null mutants show reduced expression of gH and of components of gH/gL complexes (Fig. 2).
FIG 2.
UL116-null viruses poorly express gH/gL complexes. (A) Lysates of HFF infected at an MOI of 1 with the indicated strain TB40/E-derived viruses were collected at 4 dpi, resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for gO, gH, gL, UL116, and gB. (B) Virions were collected at 6 dpi and concentrated by ultracentrifugation. Levels of gB were normalized across samples, and then expression of gO, gH, gL, and UL116 was compared by Western blotting. (C) Lysates of ARPE-19 cells infected at an MOI of 1 with the indicated strain AD169_rUL131-derived viruses were collected at 4 dpi and immunoblotted for gO, gH, gL, UL130, UL116, and gB. (D) Virions were collected at 6 dpi and concentrated by ultracentrifugation. Levels of gB were normalized across samples, and then expression of gO, gH, gL, UL130, and UL116 was compared by Western blotting.
An inhibitor of ERAD normalizes levels of gH/gL complex in HCMV mutants disrupted for UL116.
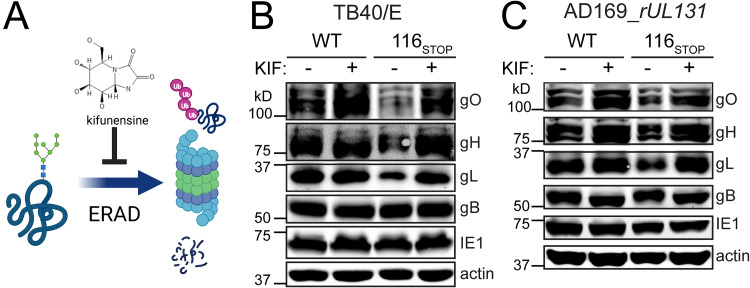
There are no known upstream splice junctions on the gL coding strand that might trivially explain a global reduction in gH/gL complexes in our UL116-null mutant viruses (17). Nonetheless, it was important to exclude the possibility that the nonsense codons we inserted in UL116 cause reduced levels of UL115 (gL) mRNA. Because this region of the viral genome is transcriptionally complex, with several neighboring genes sharing a coterminal polyadenylation signal with gL, we opted to instead ask whether gL is posttranslationally unstable in the absence of UL116. Kifunensine (KIF) is a potent and specific pharmacological inhibitor of alpha-mannosidase activities that play key roles in targeting terminally misfolded glycoproteins for ER-associated degradation (ERAD) (18). We reasoned that if loss of UL116 posttranslationally destabilizes the assembly of gH/gL complexes, then gH, gL, and gO levels should be rescued by KIF treatment (Fig. 3A), whereas if the gL (UL115) transcript were somehow being subjected to nonsense-mediated decay or otherwise poorly transcribed due to the incorporation of nonsense codons in UL116, KIF would fail to show any effect.
FIG 3.
Pharmacological inhibition of ERAD reverses defects in expression of gH, gL, and gO during UL116-null infection. (A) Kifunensine (KIF) is an alkaloid that inhibits mannosidase I, an enzyme involved in glycoprotein quality control. KIF plays roles in maturation of N-glycans and is also necessary for trimming back high-mannose N-linked glycans of terminally misfolded glycoproteins to target them for ER-associated degradation (ERAD). Fibroblasts (HFFT) were infected at an MOI of 1 with strain TB40/E (WT) or a UL116-null derivative (116STOP) (B) or AD169_rUL131 (WT) or a UL116-null derivative (116STOP) (C). Cells were treated with KIF at 2.5 μM (final concentration) or 0.1% carrier alone (water) at 48 hpi. At 72 hpi, expression of gO, gH, gL, gB, IE1, and actin was analyzed by Western blotting.
We observed that KIF treatment dramatically increased levels of gH, gL, and gO in UL116-null mutant viruses, both in strain TB40/E and in AD169 (Fig. 3), while failing to affect levels of gB or IE1. KIF treatment did slightly increase the mobility of gB and gL, consistent with one of its known activities: preventing conversion of high-mannose N-glycans to more complex, higher-molecular-weight species. We did see that KIF increased levels of gO and gH in WT AD169 and of gO in WT TB40/E, but the strongest, clearest effects were seen for UL116-null mutants. It is striking to note that KIF treatment normalized levels of these glycoproteins between wild-type and UL116-null settings. Overall, these results are consistent with a model in which gH, gL, and gO are being translated at equivalent levels in the absence of UL116 but that the proteins are being targeted for ERAD to a greater degree in the UL116-null setting. From these results, we conclude that differences in posttranslational stability of gH, gL, and gO likely account for the defects seen during UL116-null infection.
To assess if UL116 is sufficient to enhance gH/gL expression, we used adenovirus (Ad) vectors to express combinations of these proteins in both fibroblasts and epithelial cells. For these experiments, we expressed a soluble gH (sgH), lacking its C-terminal transmembrane anchor and cytoplasmic tail, from an Ad vector similar to one we used in previous studies (19). As expected (19), sgH was not secreted when expressed alone, but coexpression with gL resulted in accumulation of both proteins in the culture supernatants as apparent glycoforms of sgH/gL disulfide-linked heterodimers and homodimers of sgH/gL heterodimers (Fig. 4), as observed in previous studies (20, 21). When UL116 was expressed in place of gL, sgH was detected in the culture supernatants as apparent sgH monomers. Coexpression of all three glycoproteins led to an increase in the total amount of sgH in the supernatants, which predominately accumulated as homodimers of sgH/gL heterodimers. These results are consistent with the notion that UL116 stabilizes gH in the ER and thereby causes increased expression of gH/gL heterodimers, which, like gH/UL116 but not gH alone, are competent to traffic toward the Golgi membrane and beyond.
FIG 4.
UL116 promotes expression of gH/gL during ectopic coexpression of the proteins. ARPE-19 epithelial cells (A) or human fibroblasts (B) were infected with the indicated combinations of adenovirus (Ad) vectors expressing gL, UL116 fused to a myc tag, or a soluble gH (sgH) fused to a C-terminal polyhistidine tag. An equal total adenovirus MOI was maintained across conditions by including a control adenovirus vector expressing enhanced green fluorescent protein (eGFP). Soluble gH complexes were collected from culture supernatants using Ni-NTA agarose beads, resolved by reducing and nonreducing SDS-PAGE, and analyzed by Western blotting using antibodies specific for gH, gL, or UL116 (myc antibody), as indicated. β-ME, beta-mercaptoethanol.
UL148-null virions incorporate increased amounts of UL116.
The global decrease in the abundance of all gH-containing complexes that we observed for UL116-null mutants was reminiscent of UL148-null mutants, which likewise show decreased expression of gO and, hence, deceased levels of the gH/gL/gO Trimer in virions (8). Therefore, we directly compared the expression of gH/gL complexes in isogenic null mutants of UL116 and UL148 in the context of strain TB40/E. As expected, UL148-null virus-infected fibroblasts showed decreased expression of gO and gL relative to WT-infected cells (Fig. 5A). UL116-null infections likewise showed a decrease in expression of gO and gL, which was similar in magnitude to that observed for UL148-null infections.
FIG 5.
Comparison of gH/gL complexes between UL116-null and UL148-null mutants. (A) Cell lysates of fibroblasts infected at an MOI of 1 with the indicated HCMV strain TB40/E-derived viruses were collected at 4 dpi, and expression of gO, gH, gL, UL116, and gB was monitored by Western blotting. (B) Virions were isolated from infected cell supernatants (collected at 6 dpi) by ultracentrifugation. After normalizing for gB loading, the levels of gO, gH, gL, and UL116 in virions were compared by Western blotting. (C) Six-dpi virions were isolated by ultracentrifugation from infected cell supernatants of recombinant strain TB40/E carrying a myc tag at the C terminus of UL116. After normalizing for gB loading, the levels of myc (UL116), gO, gH, and gL in virions were compared by Western blotting.
The UL116-null mutant exhibited a defect in gH expression, which was not apparent in UL148-null settings (Fig. 5A). As expected, bands specifically immunoreactive to anti-UL116 monoclonal antibody (MAb) at ∼130 kDa and ∼60 kDa were absent from lysates of UL116-null mutant virus-infected cells, while the mature virion-associated ∼130-kDa UL116 glycoform (10) was absent from UL116-null virions (Fig. 5A and B). In side-by-side comparisons of virions loaded for equivalent gB levels, gH levels were only slightly decreased in UL148-null virions relative to the WT, while gO and gL showed a more striking decrease (Fig. 5B). Meanwhile, the ∼130-kDa UL116 species appeared to be present at slightly increased levels in UL148-null virions relative to the WT comparator.
Results of similar experiments making use of recombinant TB40/E viruses that encode a myc epitope tag at the C terminus of UL116 likewise indicated that UL148-null mutant virions show a modest yet appreciable increase in the incorporation of UL116 (Fig. 5C). UL116-null mutant virions showed poor expression of all three components of the Trimer, gO, gH, and gL, with an obvious decrease in gH abundance relative to the UL148-null and WT comparators (Fig. 5B).
From these results, we conclude that although UL116-null and UL148-null viruses each show decreased expression of gH/gL complexes, the UL148-null phenotype mainly affects the abundance in virions of gO and gL, as would be consistent with destabilized expression of the gH/gL/gO Trimer. Additionally, UL148-null mutants showed a modest-to-moderate increase in virion incorporation of gH/UL116, which would explain the compensatory effect on gH levels. In contrast, disruption of UL116 appears to destabilize the expression of all gH/gL complexes.
gH/UL116 accounts for the presence in virions of a major gH species not covalently bound to other glycoproteins.
Our results thus far suggest that UL116 strongly influences the abundance of gH/gL complexes in virions. Because UL148-null viruses produce virions that contain larger amounts of gH than would be expected given their poor incorporation of gL and gO, we hypothesized that the gH/UL116 complex is the source of the abundant virion gH species that is not covalently bound to other glycoproteins, which has long been observed in the literature (22, 23). To test this, we compared virions produced by parental WT TB40/E to those from the UL116-null derivative, TB_116STOP, and also compared virions produced by AD_r131 to its UL116-null derivative, AD_r131_116STOP, resolving virion lysates by nonreducing SDS-PAGE and then immunoblotting for gH and gL (Fig. 6).
FIG 6.
gH/UL116 accounts for the presence of an abundant virion gH species not covalently bound to other glycoproteins. Virions produced from fibroblasts infected with the indicated strain TB40/E-derived viruses were resolved by SDS-PAGE under nonreducing conditions and transferred to nitrocellulose membranes. Anti-gH MAb (AP-86) (A) or anti-gL (B) polyclonal sera were used for detection in Western blotting. Additionally, gB was detected by reducing SDS-PAGE to confirm comparable loading of virion material (shown below nonreducing anti-gH panels).
In virions of both strains, a prominent anti-gH immunoreactive band migrating with a relative mobility (Mr) of ∼85 kDa (slightly above the 75-kDa molecular weight marker) was readily detected from each parental WT virus but not from the UL116-null mutants. As this species was not detected by anti-gL serum, we interpret it to be the gH species not covalently bound to other glycoproteins long noted to be present in HCMV virions (22, 23). From extracts of WT TB40/E virions, we readily detected the gH/gL/gO Trimer migrating at just above the 250-kDa Mr marker and the gH band at Mr ∼85 kDa, which we interpret to be monomeric gH. However, in the UL116-null mutant virus, we detected lower levels of Trimer and a faint band at ∼135 kDa matching the expected size of gH/gL/UL128 and were entirely unable to detect any species matching the expected size of monomeric gH, suggesting that disruption of UL116 prevents the incorporation of gH species that are not covalently linked to other glycoproteins.
In AD_r131 virions, we observed all three of the major gH species reported by Wang and Shenk when they analyzed a similar AD169 derivative repaired for UL131 (23) (Fig. 6B), although the detection of the largest of these immunoreactive species, migrating just above 250 kDa, which corresponds to gH/gL/gO (Trimer), was detected only faintly by both anti-gH and anti-gL sera. The latter observation is consistent with observations from studies of strains AD169 and Merlin, such as AD_r131 (23) and Merlin repaired for UL128 (24), which suggest that the Trimer is inefficiently incorporated when Pentamer expression is high.
The immunoreactive band at Mr ∼135 kDa, interpreted to be gH/gL/UL128, was much more abundant in AD_r131 and its UL116-null mutant derivative than it was in TB40/E. This is consistent with findings from other groups, which suggest that TB40/E poorly expresses the Pentamer (1, 24). (The gH/gL/UL128 species is expected to lack UL130 and UL131, since these are not disulfide linked to gH/gL [23, 25, 26].) The third band detected by gH antibodies, which migrated at approximately 85 kDa, was readily detected from parental AD_r131 but not UL116-null virus. Again, we interpreted this band to be the gH species described in the two earlier studies to be comprised of gH monomers that are not disulfide linked to other glycoproteins (22, 23).
In the UL116-null derivative (116STOP), only the largest immunoreactive band, interpreted as the gH/gL/gO Trimer, and the immunoreactive band at ∼135 kDa, interpreted as gH/gL/UL128, were readily observed, with the Trimer band being only weakly detected, while the Mr ∼85-kDa band, interpreted to be gH lacking any disulfide-linked accessory proteins, was not detected. However, a faint anti-gH immunoreactive band at Mr 75 kDa did appear in the 116STOP condition (Fig. 6B, left). Since this band migrates more rapidly than authentic gH, we interpreted it to be nonspecific signal, although we cannot exclude the possibility that small amounts of hypoglycosylated gH were present in these UL116-null virion preparations.
Results from a duplicate set of samples probed with antiserum specific for gL further suggest that (i) the ∼85-kDa species is a gH band that lacks gL, (ii) the ∼135-kDa band is gH/gL/UL128, and (iii) the ∼250-kDa band is gH/gL/gO (Fig. 6). The only species the gL antisera robustly detected in virions of both AD_r131 and its UL116-null derivative was the ∼135-kDa band that we interpret as gH/gL/UL128. Notably, the gL antibody also detected gH/gL/gO complex from AD_r131 virions while failing to produce signal for the ∼85-kDa species robustly detected by gH antibody (Fig. 6A and B), which further supports our conclusion that the ∼85-kDa band is monomeric gH.
The species interpreted as gH/gL/UL128 appeared to be expressed in at least three glycoforms in UL116-null mutant AD_r131 virions, with only the slowest-migrating and presumably largest glycoform being present in virions from the parental AD_r131 virus. This may reflect slight differences in glycosylation that are influenced by UL116. Overall, from these results we conclude that UL116-null viruses produce virions that lack a prominent gH species found in WT virions. Further, the simplest interpretation of our data is that this species is gH/UL116.
Virions produced from UL116-null infections are poorly infectious.
We next sought to determine whether UL116-null virions would show differences in virion infectivity relative to the WT. For these studies, we compared WT strain TB40/E (TB_WT) to UL116-null (TB_116STOP) and UL148-null (TB_148STOP) derivatives. We chose to use this set of strain TB40/E viruses since TB_148STOP would provide a control in which tropism differences would be expected, and we did not yet know how TB_116STOP would behave. Preparations of cell-free virus from infected cell supernatants were measured by qPCR to determine the number of DNase I-resistant (presumably enveloped) HCMV genomes. Equivalent numbers of viral genomes then were measured for infectivity by TCID50 assay on fibroblasts and ARPE-19 epithelial cells. We found that UL116-null TB40/E virions were poorly infectious for both fibroblasts and epithelial cells. In contrast, TB_148STOP virions showed ∼2-fold reduced infectivity for fibroblasts but approximately 10-fold improved infectivity for ARPE-19 (Fig. 7), roughly in line with our previous findings (8). From these data, we conclude that virions released to the medium from UL116-null TB40/E-infected fibroblasts are poorly infectious on both fibroblasts and epithelial cells.
FIG 7.
Tropism assay. Fibroblasts were infected at an MOI of 1 with strain TB40/E (TB_WT), a UL148-null derivative (TB_148STOP), or a UL116-null derivative (TB_116STOP). Cell-free virions were quantified by qPCR. HFFs or ARPE-19 cells were infected in wells of 96-well plates at 50 genomes per cell. Cells were fixed and stained at 30 hpi using an IE1 monoclonal antibody to identify wells containing infected cells for determination of TCID50 for each viral genome. Relative infectivity was calculated by setting WT (TB_WT) infectivity on fibroblasts as 1.0 and expressing results for other conditions relative to that.
UL148 coimmunoprecipitates with UL116.
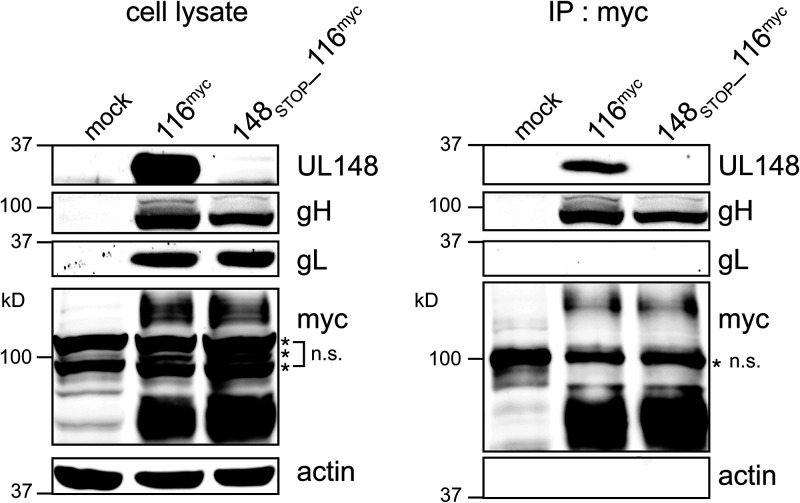
Because UL148 and UL116 each appear to positively influence virion incorporation of one or more constituents of HCMV gH/gL complexes, we sought to determine whether the two proteins might interact. Therefore, we generated WT and UL148-null strain TB40/E viruses that express a myc tag at the C terminus of UL116. We then carried out anti-myc immunoprecipitation (IP) to ask whether UL148 would coimmunoprecipitate from infected cells with UL116. Indeed, we observed a band strongly immunoreactive to UL148 antibodies was robustly detected from anti-myc immunoprecipitates of cells infected with TB_116myc but not TB_148STOP_116myc (Fig. 8). Notably, gL was not detected from anti-myc IPs of either virus infection setting, while gH was readily detected in both settings, as would be expected, since UL116 has been shown to form gL-free complex with gH (10).
FIG 8.
UL148 co-IPs from infected cells with UL116. Fibroblasts were infected at an MOI of 1 with HCMV strain TB40/E carrying a myc tag at the C terminus of UL116 (116myc) or a UL148-null mutant of the same virus (148STOP_116myc). Lysates collected at 4 dpi were subjected to immunoprecipitation (IP) using anti-myc antibody. Cell lysates (A) and IP eluates (B) were monitored by Western blotting for the indicated proteins. n.s., nonspecific band(s).
We next asked whether the putative protein-protein interaction between UL116 and UL148 could be detected from the setting of cells transiently expressing the viral glycoproteins. Therefore, we transfected HEK-293T cells with plasmid expression vectors for gH, myc-tagged UL116 (UL116myc), and HA-tagged UL148 (UL148HA), individually and in each combination of plasmids. Forty-eight hours later, we lysed cells and immunoprecipitated using anti-HA and anti-gH antibodies.
We found that gH and UL116 detectably immunoprecipitated with HA antibodies when constructs encoding either or both proteins were cotransfected with a plasmid expressing HA-tagged UL148 (Fig. 9). Meanwhile, HA-tagged UL148 was readily detected in anti-gH IPs of cells cotransfected with expression vectors for gH and UL148HA or gH, UL148HA and UL116myc but not when UL148HA was expressed on its own, which suggested that UL148 did not promiscuously or nonspecifically contaminate the anti-gH IPs. As expected, anti-gH antibodies robustly immunoprecipitated both gH and UL116myc when both proteins were present. Overall, these coimmunoprecipitation (co-IP) results from infected and transfected cell contexts together suggested to us that UL148 and UL116 are able to physically interact or participate together in a complex with other cellular and/or viral proteins.
FIG 9.
Reciprocal coimmunoprecipitation of gH, UL116, and UL148 from cells ectopically expressing the glycoproteins. HEK-293T cells in six-well cluster plates were transfected with 1 μg each plasmid expression vector encoding gH, UL116myc, or UL148HA; where appropriate, empty vector was added such that equivalent amounts (3 μg) of plasmid DNA was transfected across all conditions. Forty-eight hours posttransfection, cells were collected in lysis buffer and subjected to immunoprecipitation (IP) using anti-HA and anti-gH antibody. Cell lysates and IP eluates were monitored by Western blotting for the indicated proteins. WCL, whole-cell lysate.
DISCUSSION
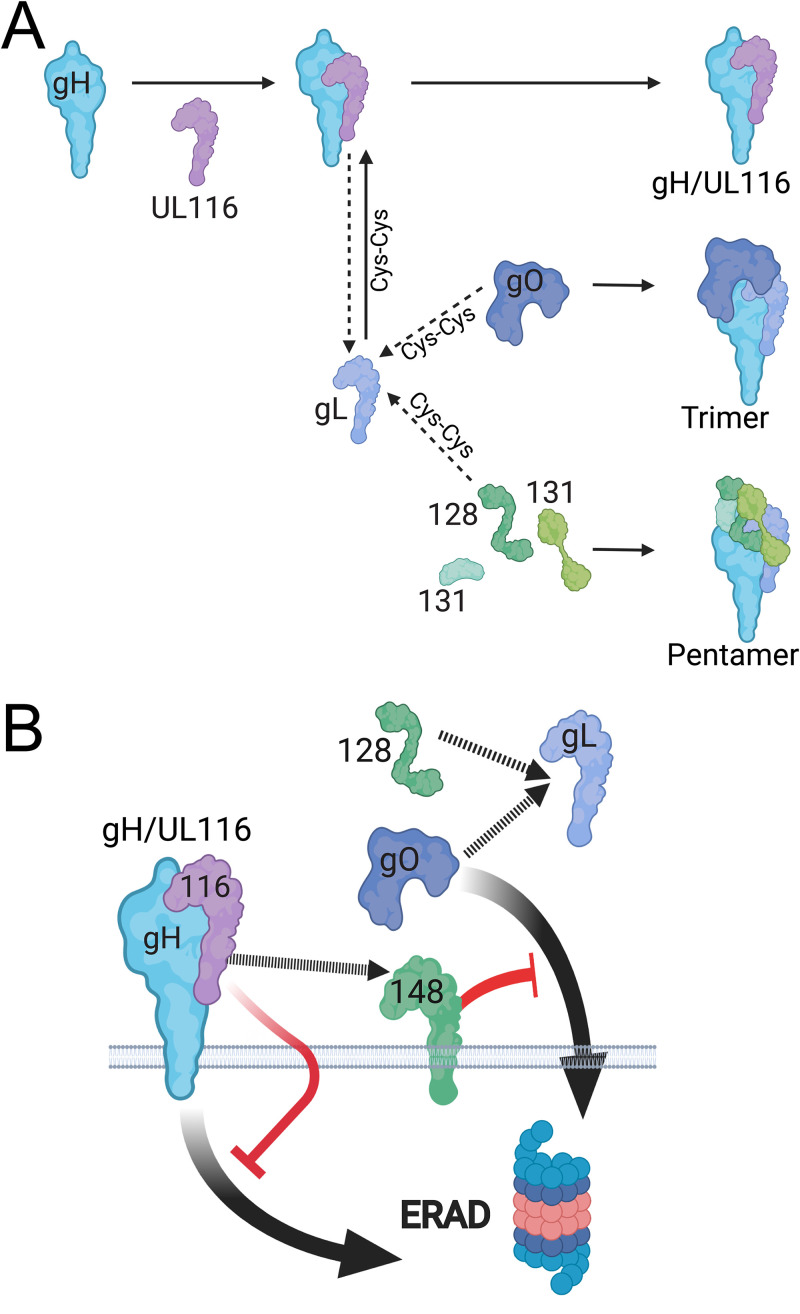
In this study, we have built upon our previous work that identified an HCMV virion gH complex that lacks gL and that instead contains UL116 (10). Importantly, unlike gL, UL116 does not form a disulfide link to gH. Despite our previous data indicating that UL116 and gL compete for assembly onto gH, our findings here show that UL116 is required for high-level expression of gH/gL complexes during infection. Therefore, we have demonstrated that UL116 plays a chaperone-like role for gH in HCMV. As illustrated in our model, we envision that UL116 stabilizes gH while gL forms a disulfide linkage to either UL128 or gO and that UL148 further stabilizes gO, which we have previously shown is uniquely susceptible to ER-associated degradation (ERAD) (Fig. 10) (27).
FIG 10.
Model. (A) UL116 acts as a gH chaperone, stabilizing gH, while gL forms a disulfide bond (Cys-Cys) to either gO or UL128. gH/UL116 heterodimers dissociate to allow assembly onto gL linked to UL128 or gO, which assemble and mature into Pentamer and Trimer, respectively. When gL-UL128 or gL-gO assembles onto gH, gH/gL also form a disulfide bond that locks the gH/gL heterodimer together. A subset of gH/UL116 complexes transit beyond the ER to become incorporated into virions. (B) In the absence of UL116, increased amounts of gH are subjected to ER-associated degradation (ERAD). UL148, a viral ER-resident glycoprotein, has been found to bind to gH and stabilizes gO from being targeted to ERAD, represented by a cartoon schematic of a proteasome. Our evidence of a physical interaction between UL116 and UL148 suggest a functional relationship between the proteins.
We further show that the gH/UL116 complex is abundant in HCMV virions (Fig. 6). In fact, our results argue that the gH/UL116 complex accounts for the long-noted observance of gH monomers in Western blot analyses of virion lysates resolved under nonreducing SDS-PAGE conditions. In such gels, gH present in gH/gL/gO (Trimer) and gH/gL/UL128-131 (Pentamer) complexes migrate as much larger species due to covalent disulfide linkages between gH and gL and between gL and either gO or UL128 (23, 28). However, because UL116 does not form a disulfide link to gH, any gH/UL116 complexes in virions disassemble upon treatment with high concentrations of ionic detergents, such as SDS, causing the gH present in such complexes to migrate as an ∼86-kDa monomeric polypeptide species in SDS-PAGE.
Given its abundance in virions, whether gH/UL116 might play a role in viral entry remains an important unresolved question. Our data suggest that UL116-null viruses are poorly infectious, but the decreased expression of gH/gL complexes in such mutants is likely sufficient to explain this defect. Our finding of replication defects for UL116-null mutants contradict results from earlier genome profiling studies, which failed to note any substantial defects in viruses disrupted for UL116 (15, 16). However, given that these profiling studies evaluated hundreds of mutants at once and did not distinguish cell-free infectivity from cell-to-cell spread, the roughly ∼10-fold replication defect we observed in the yield of cell-free infectious virus for such viruses disrupted in UL116 may have gone unnoticed. Whether replication defects of UL116-null mutant viruses reflect roles in binding and/or entry of any unidentified cell surface molecule(s) requires further investigation.
Other possibilities must also be considered. For instance, gH/UL116 might function as a decoy for neutralizing antibodies or as a stabilizing factor that indirectly supports virion infectivity. Alternatively, the presence of gH/UL116 in virions may simply occur as a by-product of its role in attenuating ERAD of gH. Indeed, by acting as a chaperone for gH, some portion of gH/UL116 complexes might simply leak beyond the ER to the Golgi, after which it would further traffic to become incorporated into progeny virions.
We also found evidence for an interaction between UL116 and UL148, which both appear to have a strong potential to affect the maturation of gH complexes and their abundance in virions. This suggests that regulation of cell tropism is far more complex than previously appreciated. We have demonstrated that UL148 activates the integrated stress response to remodel the ER (29–31), which is where newly synthesized glycoproteins begin their journey to the cell surface or, for viral envelope glycoproteins, to trans-Golgi network (TGN)-derived vesicles in the juxtanuclear cytoplasmic assembly compartment where HCMV virions obtain their infectious envelopes. Although we previously reported that UL148 coimmunoprecipitates with gH, gL, UL131, and UL130 (8), subsequent studies in our laboratory indicate that of these, only the interaction with gH is repeatable; most likely, bands interpreted as UL130 or UL131 were spurious artifacts due to serum cross-reactivity (M. N. A. Siddiquey, C. C. Nguyen, and J. P. Kamil, unpublished data). Here, we observed that UL116 immunoprecipitates from infected cells contain UL148 and not gL, and these interactions could be validated in immunoprecipitation studies from transfected cells.
Together, our findings suggest interactions between UL148, gH, and UL116 play roles in stabilizing immature forms of gH from being degraded (Fig. 10), which in turn may have effects on levels of gL, gO, and the Pentamer-specific components UL128, UL130, and UL131. This model, illustrated in Fig. 10, is supported by our earlier work showing that gO is a constitutive ERAD substrate whose decay is attenuated by UL148 (27) as well as by our finding here that kifunensine, a chemical inhibitor of ERAD, causes a markedly more substantial increase in the steady-state levels of gL, gH, and gO during UL116-null than WT virus infections (Fig. 3).
UL116 assembles onto gH at a site similar to that occupied by gL, which is consistent with it playing a role as a chaperone for gH, especially since UL116 does not covalently link to gH. gL, on the other hand, forms covalent links both to gH and to either UL128 or gO (Fig. 10B). Hence, there may be good reason for UL116 to act as a placeholder to stabilize gH while gL forms disulfide linkages to either gO or UL128. Future work will no doubt be needed to test this model and to decipher precisely how UL148 stabilizes gO (8, 27) and how its reported interactions with UL116 (this study), gH (8), the ERAD adaptor SEL1L (27), and the immune costimulatory ligand CD58 (32) contribute to maturation of gH complexes. Regardless, because UL116-null mutants of strain AD169, which lacks UL148, are defective for expression of all gH complexes, the role of UL116 in stabilizing gH complexes does not appear to require UL148. Since UL116 appears to be more broadly conserved among betaherpesviruses than UL148 is, it no doubt will be interesting to determine whether evidence for gH chaperone functions can be found in other cytomegalovirus species.
MATERIALS AND METHODS
Cells.
Telomerase-immortalized human foreskin fibroblasts (HFFT) derived from primary HFFs have been described elsewhere (27). ARPE-19 retinal pigment epithelial cells were purchased from the ATCC (number CRL-2302), and human embryonic kidney 293T cells were purchased from Genhunter Corp. (Nashville, TN). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning) supplemented with 25 μg/ml gentamicin (Invitrogen), 10 μg/ml ciprofloxacin (Genhunter), and either 5% fetal bovine serum (FBS; number F2442; Sigma) or 5% newborn calf serum (NCS; number N4637; Sigma). For experiments with Ad vectors done in the Ryckman laboratory (Fig. 5), ARPE-19 were grown in DMEM-F12 medium (Gibco) supplemented with 10% FBS and penicillin-streptomycin, gentamicin, and amphotericin B.
Viruses.
Viruses were reconstituted from bacterial artificial chromosome (BAC)-cloned virus genomes by electroporation of purified BAC DNA into HFFT. WT HCMV strain TB40/E and its derivatives were reconstituted from TB40-BAC4 (33) or mutants thereof and were grown on HFFT until 100% cytopathic effect (CPE) was observed. For HCMV recombinants derived from AD169rv rUL131, a BAC clone of HCMV strain AD169 (34, 35) repaired for UL131 (8, 27), viruses were amplified at low MOI in ARPE-19 cells until 100% CPE was observed. Virus-containing culture supernatants were then subjected to centrifugation (1,000 × g) for 10 min to pellet cellular debris. Cell-associated virus was then released by Dounce homogenization of pelleted infected cells, clarified of cell debris by centrifugation (1000 × g, 10 min), combined with the cell-free medium, and then ultracentrifuged (85,000 × g, 1 h, 4°C) through a 7.5-ml 20% d-sorbitol cushion (25 mM Tris-HCl, pH 8.0, 1 mM MgCl2, 100 μg/ml bacitracin); resulting virus pellets were then resuspended in DMEM containing 20% NCS or, for analysis of virion glycoproteins, in Dulbecco's phosphate-buffered saline lacking magnesium or calcium (DPBS; Lonza America, Inc., Alpharetta, GA).
New recombinant HCMV BACs and plasmids for this study.
New recombinant viruses were constructed by en passant BAC recombineering (36, 37), as previously described (8, 30, 31, 38, 39). Briefly, for each recombinant virus, a primer pair (see below and Table 1) is used to PCR amplify an I-SceI-AphAI cassette from a BAC DNA template that contains the cassette. The cassette confers kanamycin resistance and is abutted by an I-Sce-I recognition site. The primers are designed to target homologous recombination into the targeted region of the BAC via bacteriophage lambda RecE/T and Gam (Red) recombinase activity and to generate ∼40-bp repeats on each side of the inserted cassette, which allow it to later be excised during a second recombination step. The PCR product is electroporated into GS1783 Escherichia coli (a gift of Gregory Smith, Northwestern University, Chicago, IL) carrying the BAC of interest to be modified. Kanamycin-resistant “integrate” colonies are isolated and then resolved in a second step, during which l-arabinose treatment is used to induce I-Sce-I homing endonuclease and a 41°C heat shock is used to induce lambda Red recombinase activity. This step causes removal of the I-Sce-I AphAI kanamycin resistance cassette and leaves behind only the mutation, insertion, or deletion of interest. All primers and synthetic DNAs for this study were synthesized by Integrated DNA Technologies (Coralville, IA) and are shown in Table 1.
TABLE 1.
Oligonucleotides and synthetic DNAs used in this study
| Name | Sequence (5′ to 3′) |
|---|---|
| d131_128_Fw | ATGATGTCTCATAATAAAGCTTTCTTTCTCAGTCTGCAACTAGGGATAACAGGGTAATCGATTT |
| d131_128_Rv | TCGTTCACTTTGCCGCAGCGTATTCGCCCGTCAGCTTCGAGCCAGTGTTACAACCAATTAACC |
| UL116_MYC_Fw | ACTGGCTCCTTACCGTCACACTCTCATCGTGCCGCAGACTGAGCAGAAGCTCATCTCTGAAGAGGATCTGTGATGTAGGGATAACAGGGTAATCGATTT |
| UL116_MYC_Rv | GGTGAGAAAGAGAAGCCGCAATCCGGGCGGCGGCACATCACAGATCCTCTTCAGAGATGAGCTTCTGCTCAGTCTGCCAGTGTTACAACCAATTAACC |
| UL116stop_Kan_Fw | CCTGCCCACCTCATGAAGCGGCGGCGGCGATGGCGGGGCTAGTAGCTTTTCCTGGCCCTGTAGGGATAACAGGGTAATCGATTT |
| UL116stop_Kan_Rv | CGCTTCACACAGTAAGCAAAAGCACAGGGCCAGGAAAAGCTACTAGCCCCGCCATCGCCGCCGCGCCAGTGTTACAACCAATTAACC |
| UL116-REV_Kan_Fw | CCTGCCCACCTCATGAAGCGGCGGCGGCGATGGCGGGGCTGGCTCCTTTTCCTGGCCCTGTAGGGATAACAGGGTAATCGATTT |
| UL116-REV_Kan_Rv | CGCTTCACACAGTAAGCAAAAGCACAGGGCCAGGAAAAGGAGCCAGCCCCGCCATCGCCGCCGCGCCAGTGTTACAACCAATTAACC |
| UL116_TB_opt_MYC | TGTGGTGGAATTCTGCAGATACCATGAAAAGACGAAGGAGATGGAGAGGTTGGCTTCTTTTTCTCGCGTTGTGTTTCTGCCTTTTGTGTGAGGCTGTAGAAACTAACGCGACCACGGTAACATCTACCACCGCTGCTGCGGCCACAACAAATACGACCGTGGCCACGACTGGAACTACGACGACGAGTCCGAATGTAACGTCCACTACATCCAATACGGTGATTACCCCCACGACAGTCTCTAGCGTCTCCAATCTGACATCAAGTGCGACTTCCATTCCCATATCTACTTCTACCGTTTCTGGGACTAGAAACACCCGAAACAATAACACTACTACCATCGGAACTAATGTTACCTCACCATCCCCATCAGTTAGTATTCTTACCACCGTCACTCCAGCGGCCACGTCTACAACATCCAACAACGGTGATGTGACGTCCGACTATACACCGACCTTCGACCTTGAGAACATCACAACCACGAGAGCGCCAACTAGACCCCCTGCCCAAGACCTGTGCAGTCATAACCTCTCAATTATTTTGTACGAGGAAGAGTCCCAGTCCAGTGTAGATATTGCGGTGGACGAGGAGGAGCCCGAGCTTGAAGACGACGACGAATATGACGAATTGTGGTTCCCACTTTATTTTGAAGCTGAATGCAATTTGAATTACACTTTGCAGTACGTCAACCACAGCTGTGACTATTCAGTACGACAGTCCTCCGTATCTTTCCCCCCTTGGAGAGACATAGACAGTGTTACGTTCGTCCCTCGAAACTTGTCCAATTGCTCTGCACATGGTCTCGCCGTGATAGTAGCGGGAAACCAGACCTGGTACGTCAATCCTTTTTCACTTGCCCACCTTCTGGACGCCATCTACAATGTACTCGGTATAGAAGACTTGTCAGCGAACTTTCGGAGACAGTTGGCCCCCTACCGCCACACGCTGATCGTCCCACAAACAGGTGGGAGCGGCGGCTCAGAGCAAAAGTTGATCAGCGAAGAGGATCTCTGATCCAGCACAGTGGCGGCCG |
| TB_gHopt_Fw | GTGTGGTGGAATTCTGCAGATACCATGAGACCCGGGCTCCCATTC |
| TB_gHopt_Rv | CGGCCGCCACTGTGCTGGATTCAGCAAGTTTTCAGCATTCGATAGAGC |
| UL69 Fw | CGTCCAGTTCGTCGTCAATAA |
| UL69 Rv | CCTACGACTTTCGGTTCTTCTC |
To construct TB_116myc, a TB40-BAC4 derivative in which a Myc epitope tag was inserted at the C terminus of UL116, the primer pair UL116_myc_Fw and UL116_myc_Rv was used to carry out en passant procedures on TB40-BAC4. To construct TB_116STOP, in which tandem stop codons were inserted at UL116 amino acid positions 10 and 11 in the context of TB40-BAC4, the primer pair UL116stop_Kan_Fw and UL116stop_Kan_Rv was used. A rescue virus was constructed from TB_116STOP using the primer pair UL116-rescue_Kan_Fw and UL116-rescue_Kan_Rv. TB_148STOP_116myc, a strain TB40/E derivative null for UL148 and also encoding a myc tag at the C terminus of UL116, was constructed from TB_148STOP (27), using UL116stop_Kan_Fw and UL116stop_Kan_Rv primers (Table 1). Similarly, an AD169_rUL131-derived virus, AD169_rUL131_UL116STOP, was constructed using the primer pair UL116stop_Kan_Fw and UL116stop_Kan_Rv. For all new viruses, Sanger sequencing of modified regions was carried out by Genewiz, Inc. (Piscataway, NJ), to confirm the presence of intended changes without spurious mutations.
The plasmid pEF1_ UL116_GGS_Myc was constructed by inserting a synthetic double-stranded DNA gBlock encoding a codon-optimized (Homo sapiens codon bias) UL116 open reading frame from strain TB40/E, fused to a Gly-Ser-Gly linker and a Myc epitope tag into the EcoRV site of pEF1 V5 His C (Invitrogen) using NEB HiFi assembly mix (New England Biolabs, Ipswitch, MA). All primers and synthetic DNAs for this study are shown in Table 1.
Replication-defective Ad vectors.
The nonreplicating (E1) Ad vector expressing a soluble gH protein, in which the transmembrane and cytoplasmic UL75 (gH) domains were replaced by a hexahistidine (6×His) tag sequence, was derived using a previously characterized AdMax shuttle vector expressing the full-length UL75 gH protein from HCMV strain TR (24). Briefly, a DNA fragment encompassing the downstream half of the codon-optimized UL75 sequence was PCR amplified from the AdMax TR gH pDC316(io) shuttle plasmid (24) using primers sDCgH_PstI and asDCsgH6xHIS, resulting in an amplicon in which the 25 C-terminal amino acids of gH were replaced by a 6×His tag. The amplicon was then ligated into the AdMax TR gH pDC316(io) using PstI-HindIII restriction sites. AdUL116myc was generated by subcloning the UL116_GGS_Myc sequence from pEF1_UL116_GGS_Myc plasmid to the AdMax pDC316(io) shuttle plasmid using the primers sDC116TBco and asDC116cocmyc (Table 1). AdUL115gL and AdGFP were described previously (24, 40). All Ad vectors were propagated and titers determined by TCID50 on HEK293IQ cells.
Human ARPE-19 epithelial cells or fibroblast cells (as indicated) were infected with each Ad vector at 3 TCID50/cell, with the total Ad multiplicity standardized by the addition of Ad eGFP. Ad-infected cultures were maintained in DMEM-F12 or DMEM with 2% FBS for 7 days. Supernatants were then harvested, adjusted to pH 8.0 using 1 M Tris-HCl, pH 8.0, and incubated at room temperature for 1 h with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen). Beads were washed twice with PBS, and bound complexes were eluted at room temperature for 20 min using 20 mM Tris-buffered saline (TBS) (pH 6.8) with 2% SDS, 10% glycerol, and bromophenol blue. Samples were resolved by nonreducing 6% SDS-PAGE, transferred to polyvinylidene difluoride membranes in 25 mM Tris, 192 mM glycine, and 10% methanol, pH 8.3, and probed with anti-gH (AP86, mouse monoclonal) or anti-gL (rabbit polyclonal) antibodies and detected using anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase (Sigma-Aldrich) and Pierce ECL-Western blotting substrate (Thermo Fisher Scientific). Chemiluminescence was detected using a Bio-Rad ChemiDoc MP imaging system.
Virus titration.
Infectivity of virus stocks and samples was determined by the TCID50 assay on hTERT immortalized fibroblasts, as previously described (27, 29, 30). Briefly, serial dilutions of virus were used to infect multiple wells of a 96-well plate. At 12 days postinfection (dpi), wells were scored as positive or negative for CPE, and TCID50 values were calculated according to the Spearman-Kärber method, as described previously (30).
Virus growth kinetics.
For determination of virus growth kinetics, fibroblasts (HFFT) and epithelial cells (ARPE-19) were seeded in a 24-well plate at 1.5 × 105 cells per well. Cells were then infected at the indicated MOI in 0.5 ml of medium per well. Inocula were removed after 20 h, the cells were washed two times with 1 ml of DPBS, and 0.5 ml of fresh DMEM–5% NCS was added to each well. Cell-free supernatants were collected at the indicated times postinfection and stored at –80°C until analysis. For determination of cell-associated virus titers at 5 dpi, cells were washed twice using 1 ml DPBS per wash, collected by scraping into 0.5 ml DMEM–5% NCS, and then stored at –80°C until analysis. After thawing, infected cell material was subjected to Dounce homogenization and subsequently centrifuged (1,000 × g, 10 min) to pellet cell debris.
Quantification of viral genome copies.
Two hundred microliters of 120-h postinfection (hpi) cell-free supernatant containing TB40/E virus (TB_WT) or a UL116-null derivative (TB_116STOP)-purified virus preparation was treated at 37°C for 30 min, using 20 μl of RQ1 RNase-free DNase I (M610A; Promega) according to the manufacturer’s instructions to degrade any viral DNA nonspecifically bound to the virion surfaces. DNase I digestion was stopped by adding 20 μl of RQ1 stop solution at 65°C for 10 min. Virions were lysed and DNA extracted using a PureLink viral RNA/DNA minikit according to the manufacturer’s instructions. The viral genomes in 1 μl of the resuspended DNA material were quantified in triplicated real-time qPCRs by detecting UL69 copies, including a standard curve for absolute quantification.
Kifunensine treatment.
Where indicated, kifunensine (KIF) (APeXBio Technology, LLC, Houston, TX) was applied at a final concentration of 2.5 μM at 48 hpi (at an MOI of 1) for 24 h prior to collecting infected cell lysates at 72 hpi. Since the KIF stock solution was prepared at 2.5 mM in water, water (vehicle alone) was added to 0.1% in control treatments.
Immunoprecipitation.
For experiments with infected cells, a total of 1 × 107 fibroblasts (HFFs) were infected at an MOI of 1 TCID50 per cell with TB_116myc and TB_148STOP_116myc viruses. At 96 hpi, cells were washed once in DPBS and then lysed in lysis buffer (50 mM HEPES [pH 7.5], 1% Triton X-100, 400 mM NaCl, 0.5% sodium deoxycholate, 10% glycerol, supplemented with 1× protease inhibitor cocktail [APeXBio]), and 250 μl of lysate was incubated with 2 μl of mouse Myc-Tag antibody clone 9B11 (2276S; Cell Signaling Technology) for 4 h with rotation at 4°C. Twenty-five microliters of a 50% slurry of protein G magnetic beads (LSKMAGG10; Millipore Sigma) preequilibrated in lysis buffer was then added to each IP reaction, and reactions were allowed to rotate overnight at 4°C. The next day, the protein G magnetic beads were washed three times in lysis buffer and eluted by heating (50°C, 10 min) in 2× Laemmli buffer (4% SDS, 20% glycerol, 0.004% bromphenol blue, 0.125 M Tris-HCl, pH 6.8) lacking reducing agent.
To obtain starting material for IP experiments from transfected cells, 3 μg of mammalian expression vector plasmids based on pEF1α V5 His C (Invitrogen) encoding codon-optimized gH, UL116myc, or UL148HA was transfected using 9 μl of TransIT-2020 reagent (number MIR 5404; Mirus, Inc.) into 6 × 105 HEK-293T cells that had been seeded into wells of a six-well cluster plate. As needed, transfections were supplemented with empty pEF1α V5 His C vector to maintain equivalent amounts of DNA per transfection reaction. Plasmid transfection reagent complexes were prepared and applied to cells according to the manufacturer’s instructions. At 48 h posttransfection, cells were washed in DPBS and subsequently lysed in 200 μl of lysis buffer (50 mM HEPES [pH 7.5], 400 mM NaCl, 0.5% sodium deoxycholate containing 1× protease inhibitor cocktail [APeXBio]). Ninety microliters of cell lysate was rotated at 4°C together with 10 μl of mouse anti-gH clone 14-4b antibody (41) for 4 h. Next, 25 μl of preequilibrated protein G magnetic beads (as 50% slurry in lysis buffer) was added to each reaction mixture and rotated overnight at 4°C. Magnetic beads were then washed three times in lysis buffer, and bound proteins were eluted as described above for infected cell IPs. For anti-HA IPs, 25 μl of anti-HA magnetic bead slurry (number 8837; Pierce), equilibrated in lysis buffer, was directly added to 90 μl lysate and incubated overnight before washing and processing for elution as described above.
Tropism studies.
Fibroblasts (HFFTs) were infected at an MOI of 1 TCID50 per cell with TB_WT, TB_148STOP, and TB_116STOP viruses. Duplicate aliquots of cell supernatants containing cell-virus were collected at 144 hpi. One aliquot was stored at –80°C for later infectivity analyses, and the other aliquot was treated with DNase I (RQ1 DNase; Promega) prior to purification of viral DNA using a PureLink viral RNA/DNA minikit (ThermoFisher), as described elsewhere (42). Viral genome copies eluted from the PureLink kit were then quantified using SYBR green-based real-time quantitative PCR (qPCR) assay using a pair of qPCR primers directed at intron A of the viral major immediate-early locus: iP1 qPCR Fw and iP1 qPCR Rv (Table 1). The PCR efficiency of this primer pair was determined to be 99.4%. Fibroblasts (HFFT) and epithelial cells (ARPE-19) in 96-well plate format were then infected on the basis of viral genomes, using a 10-fold serial dilution series of virus genome equivalents. Each dilution of virus, starting with 1 × 106 viral genomes per well and ending at 0.1 genomes per well, was applied to a row of eight wells of a 96-well cluster plate, with wells having been seeded at a density of 2 × 104 cells per well. At 30 hpi, cells were fixed in ice-cold methanol and stained using IE1 monoclonal antibody (MAb) clone 1B12, as described previously (43). To identify wells positive for virus, the aminoethyl carbazole (AEC) substrate kit (Thermo Fisher) was used to detect IE1-positive nuclei. Briefly, in this assay AEC staining detects IE1 MAb-stained nuclei (a hallmark of HCMV-infected cells) by virtue of peroxidase activity from horseradish peroxidase-conjugated streptavidin bound to biotinylated secondary antibodies, which in turn are used to detect mouse anti-IE1 MAb 1B12. Infectivity was determined in units of TCID50 per viral genome in three biological replicates per experiment, and data were normalized across different conditions by setting TB_WT infectivity on fibroblasts to 1.0.
Western blots.
Mouse MAb specific for UL116 (clone H4) was provided by GSK Vaccines (Rockville, MD). Mouse anti-gH MAb clone AP86 (44) was a generous gift from William J. Britt (University of Alabama, Birmingham). A custom-generated anti-gL polyclonal antiserum was raised in rabbits by immunization with synthetic peptide matching the C-terminal 21 amino acids of gL (Cys-KQTRVNLPAHSRYGPQAVDAR; gL residues 258 to 278) linked to keyhole limpet antigen via the appended cysteine residue at the N terminus (Pacific Immunology, Ramona, CA). The peptide sequence used was identical to that used in a previous study to generate gL antisera (19). Western blot analyses of virion glycoproteins were carried out as described previously (8).
ACKNOWLEDGMENTS
This project was supported by NIH grants R01-AI116851 (to J.P.K), P30-GM110703 (LSUHS Center for Molecular and Tumor Virology), and R01-AI097274 (B.J.R.) as well as an American Heart Association grant to E.P.S. (17POST33350043). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
We thank William J. Britt, Thomas E. Shenk, Klaus Osterrieder, B. Karsten Tischer, Gregory A. Smith, Christian Sinzger, and Ulrich Koszinowski for generously sharing reagents.
Performed research: M.N.A.S., except for Fig. 4, which was done by Q.Y. Designed research: M.N.A.S., M.M., and J.P.K., except for Fig. 5, which was designed by E.P.S. and B.J.R. Interpreted data: M.N.A.S., E.P.S., B.J.R., M.M., and J.P.K. Contributed new reagents: UL116 monoclonal antibody, D.A., G.V., D.Y., D.M., and M.M. Contributed new adenovirus vectors: J.-M.L. and B.J.R. Developed all other new reagents: M.N.A.S. and J.P.K. Obtained funding: J.P.K. and B.J.R. Wrote the manuscript: J.P.K. and B.J.R., with input from all authors.
D.Y. and D.M. are employed by the GSK group of companies. D.Y. and D.M. report ownership of GSK shares and/or restricted GSK shares. M.M. is an employee of the University of Naples Federico II with a consultancy contract with GlaxoSmithKline Biologicals SA. All authors had full access to the data and approved the manuscript before it was submitted by the corresponding author.
Contributor Information
Jeremy P. Kamil, Email: jkamil@lsuhsc.edu.
Richard M. Longnecker, Northwestern University
REFERENCES
- 1.Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M, Wildner S, Scrivano L, Adler B. 2017. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-alpha as a key for entry. PLoS Pathog 13:e1006281. 10.1371/journal.ppat.1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu K, Oberstein A, Wang W, Shenk T. 2018. Role of PDGF receptor-alpha during human cytomegalovirus entry into fibroblasts. Proc Natl Acad Sci U S A 10.1073/pnas.1806305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L. 2016. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol 1:16082. 10.1038/nmicrobiol.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegmann C, Hochdorfer D, Lieber D, Subramanian N, Stohr D, Laib Sampaio K, Sinzger C. 2017. A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells. PLoS Pathog 13:e1006273. 10.1371/journal.ppat.1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho H, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C. 2018. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174:1158–1171. 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 6.E X, Meraner P, Lu P, Perreira JM, Aker AM, McDougall WM, Zhuge R, Chan GC, Gerstein RM, Caposio P, Yurochko AD, Brass AL, Kowalik TF. 2019. OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc Natl Acad Sci U S A 116:7043–7052. 10.1073/pnas.1814850116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanarsdall AL, Pritchard SR, Wisner TW, Liu J, Jardetzky TS, Johnson DC. 2018. CD147 promotes entry of pentamer-expressing human cytomegalovirus into epithelial and endothelial cells. mBio 9:e00781-18. 10.1128/mBio.00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Nguyen CC, Ryckman BJ, Britt WJ, Kamil JP. 2015. A viral regulator of glycoprotein complexes contributes to human cytomegalovirus cell tropism. Proc Natl Acad Sci U S A 112:4471–4476. 10.1073/pnas.1419875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luganini A, Cavaletto N, Raimondo S, Geuna S, Gribaudo G. 2017. Loss of the human cytomegalovirus US16 protein abrogates virus entry into endothelial and epithelial cells by reducing the virion content of the pentamer. J Virol 91:e00205-17. 10.1128/JVI.00205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calo S, Cortese M, Ciferri C, Bruno L, Gerrein R, Benucci B, Monda G, Gentile M, Kessler T, Uematsu Y, Maione D, Lilja AE, Carfi A, Merola M. 2016. The human cytomegalovirus UL116 gene encodes an envelope glycoprotein forming a complex with gH independently from gL. J Virol 90:4926–4938. 10.1128/JVI.02517-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatault P, Jones IKA, Meyer C, Kreklywich C, Alexander T, Smith PP, Denton M, Powell J, Orloff SL, Streblow DN. 2021. Rat and human cytomegalovirus ORF116 encodes a virion envelope glycoprotein required for infectivity. Virology 557:23–33. 10.1016/j.virol.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezzani G, Amendola D, Yu D, Chandramouli S, Frigimelica E, Maione D, Merola M. 2021. The human cytomegalovirus UL116 glycoprotein is a chaperone to control gH-based complexes levels on virions. Front Microbiol 12:630121. 10.3389/fmicb.2021.630121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol 70:78–83. 10.1128/JVI.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. 2003. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A 100:14223–14228. 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu D, Silva MC, Shenk T. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A 100:12396–12401. 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatherer D, Seirafian S, Cunningham C, Holton M, Dargan DJ, Baluchova K, Hector RD, Galbraith J, Herzyk P, Wilkinson GW, Davison AJ. 2011. High-resolution human cytomegalovirus transcriptome. Proc Natl Acad Sci U S A 108:19755–19760. 10.1073/pnas.1115861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbein AD, Tropea JE, Mitchell M, Kaushal GP. 1990. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem 265:15599–15605. 10.1016/S0021-9258(18)55439-9. [DOI] [PubMed] [Google Scholar]
- 19.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 82:60–70. 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann I, Wen Y, Ciferri C, Schulze A, Fuhner V, Leong M, Gerber A, Gerrein R, Nandi A, Lilja AE, Carfi A, Laux H. 2015. Expression of the human cytomegalovirus pentamer complex for vaccine use in a CHO system. Biotechnol Bioeng 112:2505–2515. 10.1002/bit.25670. [DOI] [PubMed] [Google Scholar]
- 21.Fouts AE, Comps-Agrar L, Stengel KF, Ellerman D, Schoeffler AJ, Warming S, Eaton DL, Feierbach B. 2014. Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109. Proc Natl Acad Sci U S A 111:8209–8214. 10.1073/pnas.1404653111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Nelson JA, Britt WJ. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J Virol 71:3090–3097. 10.1128/JVI.71.4.3090-3097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Yu Q, Wechsler A, Ryckman BJ. 2013. Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope. J Virol 87:9680–9690. 10.1128/JVI.01167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandramouli S, Malito E, Nguyen T, Luisi K, Donnarumma D, Xing Y, Norais N, Yu D, Carfi A. 2017. Structural basis for potent antibody-mediated neutralization of human cytomegalovirus. Sci Immunol 2:eaan1457. 10.1126/sciimmunol.aan1457. [DOI] [PubMed] [Google Scholar]
- 26.Murrell I, Tomasec P, Wilkie GS, Dargan DJ, Davison AJ, Stanton RJ. 2013. Impact of sequence variation in the UL128 locus on production of human cytomegalovirus in fibroblast and epithelial cells. J Virol 87:10489–10500. 10.1128/JVI.01546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen CC, Siddiquey MNA, Zhang H, Li G, Kamil JP. 2018. Human cytomegalovirus tropism modulator UL148 interacts with SEL1L, a cellular factor that governs endoplasmic reticulum-associated degradation of the viral envelope glycoprotein gO. J Virol 92:e00688-18. 10.1128/JVI.00688-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. 2015. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiquey MNA, Zhang H, Nguyen CC, Domma AJ, Kamil JP. 2018. The human cytomegalovirus endoplasmic reticulum-resident glycoprotein UL148 activates the unfolded protein response. J Virol 92:e00896-18. 10.1128/JVI.00896-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Read C, Nguyen CC, Siddiquey MNA, Shang C, Hall CM, von Einem J, Kamil JP. 2019. The human cytomegalovirus nonstructural glycoprotein UL148 reorganizes the endoplasmic reticulum. mBio 10:e02110-19. 10.1128/mBio.02110-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen CC, Domma AJ, Zhang H, Kamil JP. 2019. Endoplasmic reticulum (ER) reorganization and intracellular retention of CD58 are functionally independent properties of the human cytomegalovirus ER-resident glycoprotein UL148. J Virol 94:e01435-19. 10.1128/JVI.01435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ECY, Pjechova M, Nightingale K, Vlahava VM, Patel M, Ruckova E, Forbes SK, Nobre L, Antrobus R, Roberts D, Fielding CA, Seirafian S, Davies J, Murrell I, Lau B, Wilkie GS, Suarez NM, Stanton RJ, Vojtesek B, Davison A, Lehner PJ, Weekes MP, Wilkinson GWG, Tomasec P. 2018. Suppression of costimulation by human cytomegalovirus promotes evasion of cellular immune defenses. Proc Natl Acad Sci U S A 115:4998–5003. 10.1073/pnas.1720950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinzger C, Hahn G, Digel M, Katona R, Sampaio KL, Messerle M, Hengel H, Koszinowski U, Brune W, Adler B. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J Gen Virol 89:359–368. 10.1099/vir.0.83286-0. [DOI] [PubMed] [Google Scholar]
- 34.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski UH. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol 74:7720–7729. 10.1128/JVI.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostermann E, Spohn M, Indenbirken D, Brune W. 2016. Complete genome sequence of a human cytomegalovirus strain AD169 bacterial artificial chromosome clone. Genome Announc 4:e00091-16. 10.1128/genomeA.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 37.Tischer BK, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197. 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Rak M, Nguyen CC, Umashankar M, Goodrum FD, Kamil JP. 2014. An epistatic relationship between the viral protein kinase UL97 and the UL133-UL138 latency locus during the human cytomegalovirus lytic cycle. J Virol 88:6047–6060. 10.1128/JVI.00447-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Li G, Schauflinger M, Nguyen CC, Hall ED, Yurochko AD, von Einem J, Kamil JP. 2013. The ULb' region of the human cytomegalovirus genome confers an increased requirement for the viral protein kinase UL97. J Virol 87:6359–6376. 10.1128/JVI.03477-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Zhou M, Stanton R, Kamil J, Ryckman BJ. 2018. Expression levels of glycoprotein O (gO) vary between strains of human cytomegalovirus, influencing the assembly of gH/gL complexes and virion infectivity. J Virol 92:e00606-18. 10.1128/JVI.00606-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Britt WJ, Vugler L, Butfiloski EJ, Stephens EB. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol 64:1079–1085. 10.1128/JVI.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou M, Lanchy JM, Ryckman BJ. 2015. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J Virol 89:8999–9009. 10.1128/JVI.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heider JA, Yu Y, Shenk T, Alwine JC. 2002. Characterization of a human cytomegalovirus with phosphorylation site mutations in the immediate-early 2 protein. J Virol 76:928–932. 10.1128/jvi.76.2.928-932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urban M, Britt W, Mach M. 1992. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J Virol 66:1303–1311. 10.1128/JVI.66.3.1303-1311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]