ABSTRACT
Foot-and-mouth disease virus (FMDV) is the pathogen of foot-and-mouth disease (FMD), which is a highly contagious disease in cloven-hoofed animals. To survive in the host, FMDV has evolved multiple strategies to antagonize host innate immune responses. In this study, we showed that the leader protease (Lpro) of FMDV, a papain-like proteinase, promoted viral replication by evading the antiviral interferon response through counteracting the 2′,5′-oligoadenylate synthetase (OAS)/RNase L system. Specifically, we observed that the titers of Lpro deletion virus were significantly lower than those of wild-type FMDV (FMDV-WT) in cultured cells. Our mechanistic studies demonstrated that Lpro interfered with the OAS/RNase L pathway by interacting with the N-terminal domain of swine RNase L (sRNase L). Remarkably, Lpro of FMDV exhibited species-specific binding to RNase L in that the interaction was observed only in swine cells, not human, monkey, or canine cells. Lastly, we presented evidence that by interacting with sRNase L, FMDV Lpro inhibited cellular apoptosis. Taken together, these results demonstrate a novel mechanism that Lpro utilizes to escape the OAS/RNase L-mediated antiviral defense pathway.
IMPORTANCE FMDV is a picornavirus that causes a significant disease in agricultural animals. FMDV has developed diverse strategies to escape the host interferon response. Here, we show that Lpro of FMDV antagonizes the OAS/RNase L pathway, an important interferon effector pathway, by interacting with the N-terminal domain of sRNase L. Interestingly, such a virus-host interaction is species-specific because the interaction is detected only in swine cells, not in human, monkey, or canine cells. Furthermore, Lpro inhibits apoptosis through interacting with sRNase L. This study demonstrates a novel mechanism by which FMDV has evolved to inhibit host innate immune responses.
KEYWORDS: FMDV, RNase L, Lpro, antagonistic mechanism., ISGs, foot-and-mouth disease virus
INTRODUCTION
Foot-and-mouth disease virus (FMDV) is a positive-sense single-strand picornavirus, and its infection can cause a highly contagious, debilitating disease in cloven-hoofed animals, including important livestock species such as cattle and swine. Taxonomically, FMDV belongs to the genus Aphthovirus in the Picornaviridae family (1, 2) and has seven serotypes: O, A, C, SAT1, SAT2, SAT3, and Asia1. The genome of FMDV contains a length of about 8,500 nucleotides. It has a single long open reading frame (ORF) which encodes a polyprotein that is subsequently processed into four mature structural proteins (VP1, VP2, VP3, and VP4) and 12 nonstructural proteins (leader protease [Lpro], 2A, 2B, 2C, 3A, 3B, 3C, 3D, 3AB or 3ABC, 2BC, and 3CD) (2). Among them, Lpro is a viral proteinase that self-cleaves from the nascent viral polyprotein precursor during FMDV infection and plays an important role in viral pathogenesis (3, 4). FMDV Lpro has two different forms, named Lab and Lb. Lab and Lb are translated by two AUGs which are separated by 84 nucleotides. The Lb region contains the papain-like protease activity domain, and both forms of Lpro exhibit the same enzymatic properties. Lpro is important for viral replication by inhibiting host antiviral activity through multiple mechanisms (5). First, Lpro blocks host cap-dependent mRNA translation through the cleavage of eIF4G that in turn reduces the expression of host antiviral proteins (6, 7). This mechanism has been well characterized. Second, Lpro disrupts the interferon (IFN) signaling pathway and inhibits production of type I and type III IFNs at the transcriptional level (8–10). Third, Lpro acts as a deubiquitinase and deISGylase to antagonize posttranslational modifications of innate immune signaling molecules such as ubiquitin and ISG15 (9, 11).
The type I IFNs, such as IFN-α/β, are important for defending cells against virus infection and thus are the key components of the innate immune response. IFN-α and IFN-β bind to their receptors on the cell surface and activate the type I IFN signaling pathway. The receptors connect with Janus kinase 1 (JAK1) and tyrosine kinase 2 (Tyk2) to phosphorylate the signal transducers and activators of transcription (STATs) STAT1 and STAT2. Phosphorylated STAT1 and STAT2 form a heterodimer, which further recruits IFN regulatory factor 9 (IRF9) to assemble the transcriptional activator complex, IFN-stimulated gene factor 3 (ISGF3) (12–14). ISGF3 then translocates into the nucleus and binds to an IFN-stimulated response element (ISRE) to induce more than 300 interferon-stimulated genes (ISGs) (15), such as ISG15, myxovirus resistance 1 (Mx1), 2′,5′-oligoadenylate synthetase (OAS), and double-stranded RNA-dependent protein kinase R (PKR). RNase L is the terminal component of the OAS/RNase L system that belongs to the innate immune systems. The type I IFNs induce the expression of OAS that is activated by double-stranded RNA (dsRNA) to polymerize ATP into 2′-5′ oligoadenylates (2-5A) (16, 17). 2-5A binds on the N-terminal ankyrin domain of RNase L to promote its dimerization and activation (18). The enzymatically active RNase L cleaves cellular and viral single-stranded RNA to decrease protein synthesis, inhibit viral replication, and promote cell apoptosis (19). On the other end of the spectrum, several viruses, including HIV-1 (20), HSV-1 (21), and vaccinia virus (22), have developed multiple mechanisms to antagonize the OAS/RNase L pathway. The viral evasion mechanisms that function by targeting the OAS/RNase L pathway are important for viral replication and propagation. However, whether FMDV inhibits the OAS/RNase L pathway remains unclear.
In this study, we observe an unusual function of FMDV Lpro in attenuating the OAS/RNase L pathway. We show that Lpro inhibits the OAS/RNase L pathway by interacting with the N-terminal domain of sRNase L. The Lb coding region of Lpro is required for inhibiting sRNase L activity. Furthermore, Lpro interacts with RNase L in a species-specific manner. Lastly, Lpro inhibits apoptosis through interacting with sRNase L. In summary, these data uncover a novel mechanism evolved by FMDV to antagonize host innate immune responses.
RESULTS
Lpro promotes FMDV replication by inhibiting the OAS/RNase L pathway.
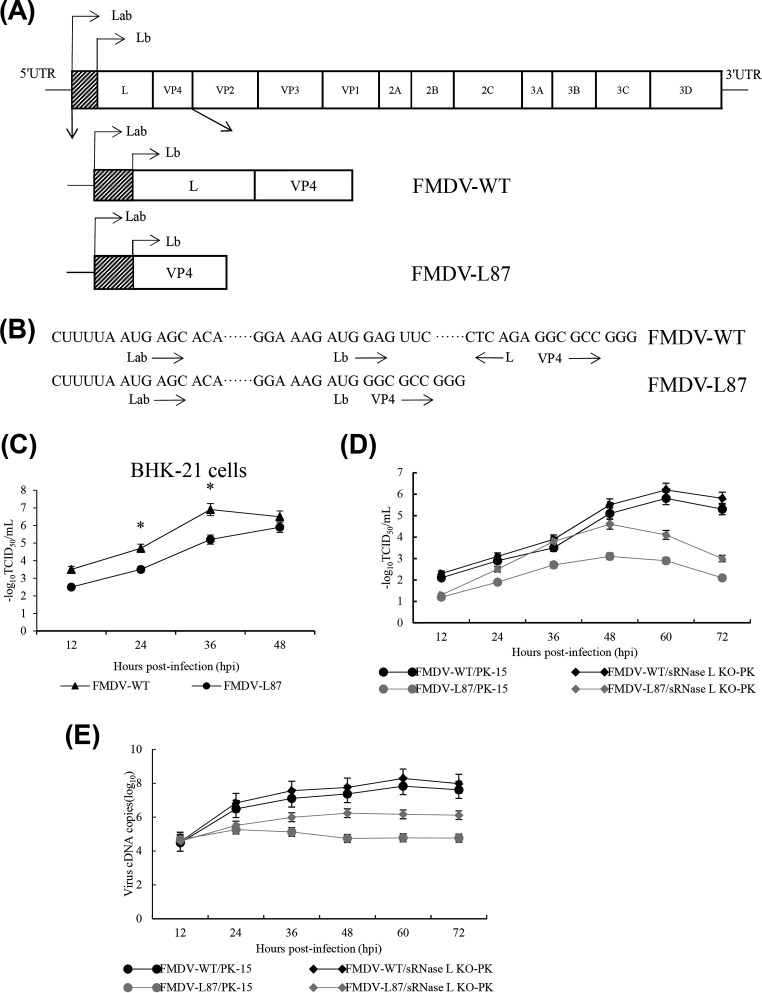
In order to determine whether Lpro is required for efficient FMDV replication in vitro, we generated the virus FMDV-L87 lacking Lb region using FMDV infectious clone and employed it to further study the Lpro function (Fig. 1A and B) (23). When we examined the viral titers, FMDV-WT grew much faster than did FMDV-L87 in BHK-21 cells (Fig. 1C). We further determined the titers and viral cDNA copies of FMDV-WT and FMDV-L87 in porcine kidney-15 (PK-15) and sRNase L knockout (KO)-PK cells. The titers and viral cDNA copies of FMDV-WT in PK-15 cells were similar to those in sRNase L KO-PK cells, whereas FMDV-L87 in PK-15 cells had lower titers and viral cDNA copies than those in sRNase L KO-PK cells (Fig. 1D and E), suggesting a role of Lb in inhibiting the function of sRNase L. These results indicate that Lpro promotes FMDV growth by inhibiting the function of the OAS/RNase L pathway.
FIG 1.
The growth kinetics of FMDV-WT and FMDV-L87. (A) Diagram of the FMDV-WT genome and FMDV-L87 genome. The viral open reading frame is boxed, and the shaded box represents the 87 nucleotides between the two initiation codons of L gene. (B) Sequences of FMDV-WT and FMDV-L87 surrounding the two initiation codons for Lab and Lb. (C) BHK-21 cells were infected with FMDV-WT and FMDV-L87 at an MOI of 1. Culture supernatants were collected at indicated time intervals and the virus titers were analyzed at indicated time points. (D and E) PK-15 and sRNase L KO-PK cells were individually infected with FMDV-WT and FMDV-L87 at an MOI of 1. The culture supernatants were collected at indicated time intervals. One part of the culture supernatants was titrated for TCID50. The other part was used to extract viral RNAs for quantifying viral cDNA copies by a real-time RT-PCR assay. (D) The TCID50s of FMDV-WT and FMDV-L87 on PK-15 and sRNase L KO-PK cells were determined at indicated time intervals. (E) Viral cDNA copies of FMDV-WT and FMDV-L87 on PK-15 and sRNase L KO-PK cells were quantified at indicated time points. Data represent the means ± the standard deviations (error bars) of three experiments. *, P < 0.05.
Prevention of rRNA degradation by Lpro.
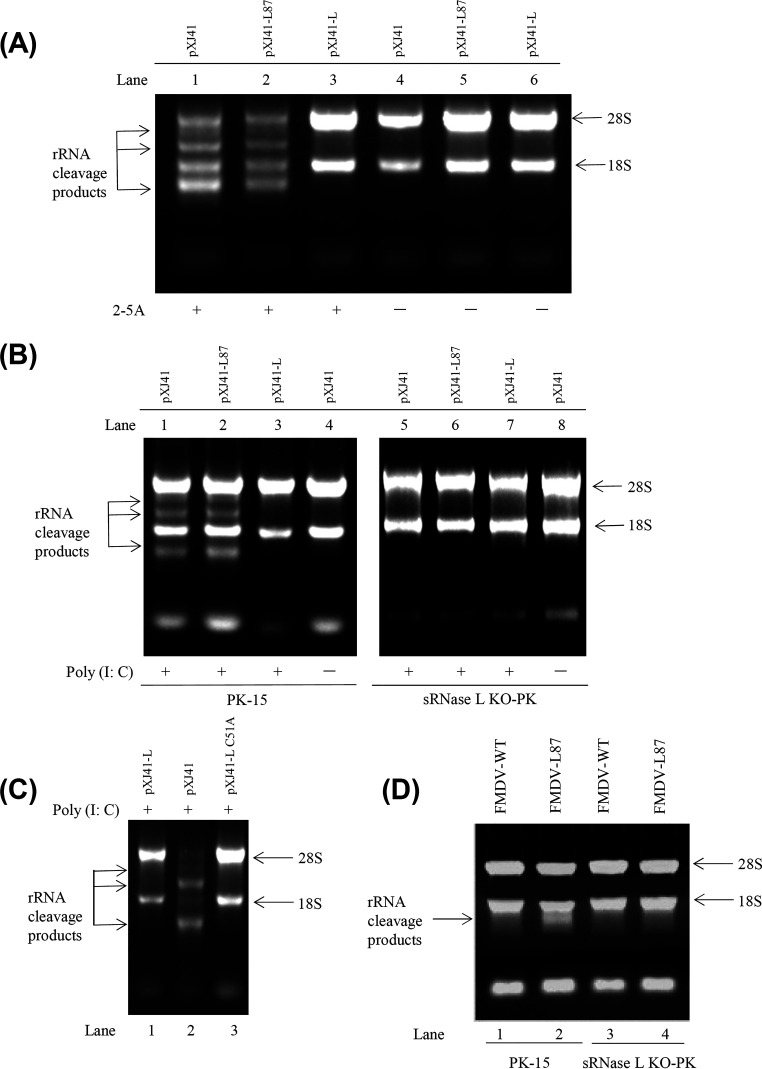
RNase L is an important component in the OAS/RNase L pathway. To identify the inhibitory step in the OAS/RNase L pathway by Lpro, PK-15 cells were transfected with pXJ41-L, pXJ41-L87, or pXJ41 and treated with 2-5A at 16 h posttransfection. Then, total RNA was extracted and RNA degradation was assessed on 1% agarose gel. The 2-5A-induced RNA degradation was observed in the pXJ41- and pXJ41-L87-transfected cells (Fig. 2A, lanes 1 and 2) but not in pXJ41-L-transfected cells and untreated mock control cells (Fig. 2A, lanes 3 to 6). These results suggest that FMDV Lpro is able to inhibit the activation of sRNase L by 2-5A and that inhibition of the OAS/RNase L pathway by Lpro occurs downstream of the OAS catalytic activity.
FIG 2.
Lpro prevents RNA degradation. A total of 1 × 106 of PK-15 and sRNase L KO-PK cells were seeded in 12-well plates and grown to 80% confluence. Cells were transfected with 1 μg of pXJ41, pXJ41-L, pXJ41-L C51A, or pXJ41-L87 for 24 h and then stimulated with 2.5 μg/ml poly(I:C) for 7 h or 2.5 μM 2-5A for 5 h. Total RNAs were extracted and analyzed for RNA degradation by 1% agarose gel electrophoresis. (A) RNA degradation in PK-15 cells induced by 2-5A. (B) RNA degradation in PK-15 or sRNase L KO-PK cells induced by poly(I:C). (C) RNA degradation in PK-15 cells treated by poly(I:C). (D) PK-15 and sRNase L KO-PK cells were individually infected with FMDV-WT or FMDV-L87 at an MOI of 1. At 12 h postinfection, total RNAs were extracted and analyzed for RNA degradation. Data are from three independent experiments with similar results.
To determine whether the RNA degradation was specifically caused by sRNase L, we compared the RNA degradation in PK-15 and sRNase L KO-PK cells after transfection with pXJ41-L, pXJ41-L87, or pXJ41. As expected, RNA degradation was detected in PK-15 cells transfected with pXJ41 and pXJ41-L87 (Fig. 2B, lanes 1 and 2) but not in sRNase L KO-PK cells (Fig. 2B, lanes 5 and 6). Furthermore, neither the pXJ41-L-transfected PK-15 nor sRNase L KO-PK cells showed detectable RNA degradation (Fig. 2B, lanes 3 and 7). The control samples of the PK-15 and sRNase L KO-PK cells transfected with pXJ41 but untreated with poly(I:C) failed to trigger RNA degradation (Fig. 2B, lanes 4 and 8). Meanwhile, we generated pXJ41-L C51A plasmid, a papain-like protease inactive mutant of FMDV Lpro, to explore whether papain-like protease activity of FMDV Lpro played a critical role in inhibition of the OAS/RNase L pathway. PK-15 cells were transfected with pXJ41-L, pXJ41-L C51A, or pXJ41 and then treated with poly(I:C). rRNA degradation was detected only in pXJ41-transfected cells but not in pXJ41-L- and pXJ41-L C51A-transfected cells. It was suggested that rRNA degradation regulated by RNase L was not dependent on papain-like protease activity of FMDV Lpro (Fig. 2C). In addition, the PK-15 and sRNase L KO-PK cells were infected with FMDV-WT or FMDV-L87 at an MOI of 1, and at 12 h postinfection, total RNA was extracted. rRNA cleavage products were detected in FMDV-L87-infected PK-15 cells (Fig. 2D, lane 2). However, FMDV-L87-infected sRNase L KO-PK cells and FMDV-WT-infected PK-15 and sRNase L KO-PK cells failed to trigger RNA degradation (Fig. 2D, lanes 1, 3, and 4). These data show that sRNase L can trigger RNA degradation and that FMDV Lpro inhibits sRNase L activity.
FMDV Lpro interaction with sRNase L.
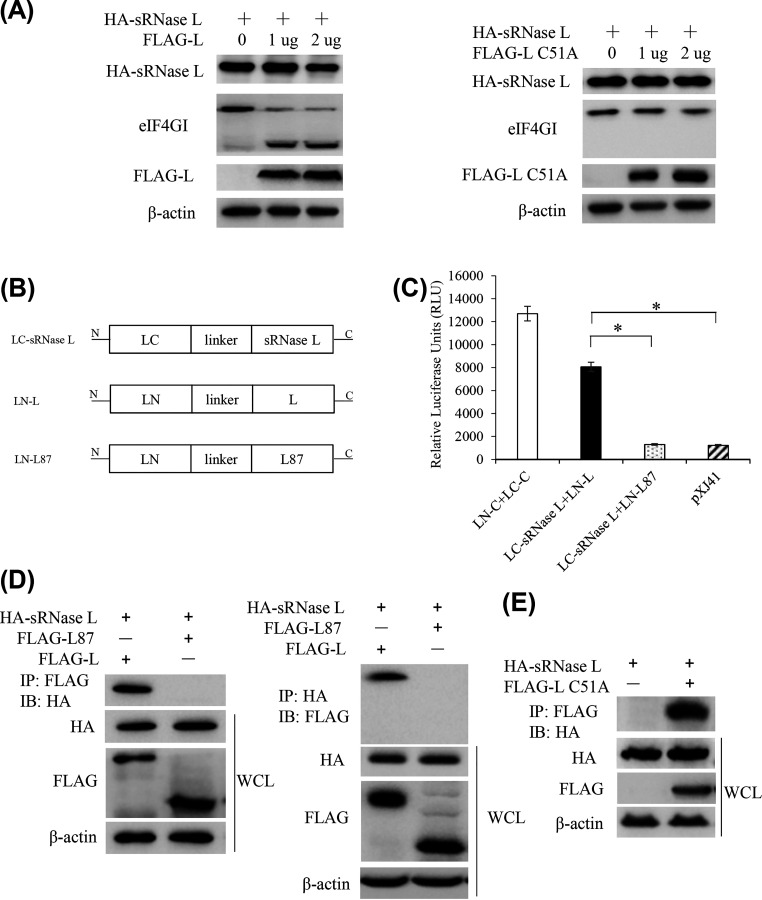
To determine whether FMDV Lpro affected sRNase L expression, HEK-293T cells were cotransfected with pXJ41-sRNase L and pXJ41-L or pXJ41-L C51A, and cell lysates were subjected to Western blotting. The expression level of sRNase L protein was slightly decreased with the increase of FMDV Lpro expression (Fig. 3A). To confirm the protease activity of FMDV Lpro, we compared the cleavage of eIF4G in pXJ41-L- or pXJ41-L C51A-transfected cells. The data showed that eIF4GI was cleaved by FMDV Lpro expression but not by FMDV Lpro C51A expression (Fig. 3A). We further generated three SLCA (split luciferase complementation assay) constructs (LN-L, LN-L87, and LC-sRNase L) (Fig. 3B). The pair of LN-C and LC-C that contain ZIKV C protein produced ∼12,400 relative luciferase units (RLU) and was used as a positive control, whereas pXJ41 was used as a negative control. The pair of LN-L and LC-sRNase L produced ∼8,100 RLU, which was significantly higher than the RLU of the LN-L87 and LC-sRNase L pair (Fig. 3C), suggesting that FMDV Lpro associates with sRNase L.
FIG 3.
FMDV Lpro interacts with sRNase L. (A) HEK-293T cells were seeded in 6-well plates and grown to 80% confluence. Cells were cotransfected with 1 μg of pXJ41-sRNase L (HA tag) and indicated dose of pXJ41-L (FLAG tag) or pXJ41-L C51A (FLAG tag) for 24 h followed by Western blotting. (B) Schematic representation of LC-sRNase L, LN-L, and LN-L87. (C) HEK-293T cells were seeded in 12-well plates and grown to 80% confluence. One μg of LC-sRNase L and 1 μg of LN-L or LN-L87 were cotransfected into cells. At 24 h posttransfection, cells were lysed and determined for recombination Renilla luciferase reporter activity by luciferase assay system. The data represent the means of three independent experiments, with each sample assayed in triplicate. Error bars indicate the standard deviations of three experiments. *, P < 0.05. (D) HEK-293T cells were seeded in 60-mm-diameter dishes and grown to 80% confluence. Cells were cotransfected with 3 μg of pXJ41-sRNase L (HA tag) and 3 μg of pXJ41-L (FLAG tag) or pXJ41-L87 (FLAG tag) for 24 h. Cell lysates were subjected to coimmunoprecipitation using indicated antibodies (IP: FLAG or IP: HA). Immunocomplexes were analyzed by Western blotting using indicated antibodies (WB: HA or WB: FLAG). Whole-cell lysis (WCL) was also subjected to Western blotting using anti-HA, anti-FLAG, or anti-β-actin antibody. (E) Three μg of pXJ41-sRNase L (HA tag) and 3 μg of pXJ41-L C51A (FLAG tag) were transfected into HEK293-293T cells for 24 h. Cell lysates were subjected to coimmunoprecipitation using anti-FLAG antibody. Immunocomplexes were analyzed by Western blotting using anti-HA antibody. Whole-cell lysis (WCL) was also subjected to Western blotting using anti-HA, anti-FLAG, or anti-β-actin antibody. Data are representative of three independent experiments with similar results.
To further confirm the interaction between sRNase L and FMDV Lpro, HEK-293T cells were cotransfected with pXJ41-sRNase L and pXJ41-L or pXJ41-L87. After 24 h posttransfection, cells were lysed and subjected to immunoprecipitation (IP) using anti-FLAG or anti-HA antibodies. IP with anti-FLAG antibody followed by immunoblot with anti-HA antibody showed that sRNase L was evident in cells cotransfected with pXJ41-sRNase L and pXJ41-L. However, the specific band of sRNase L was not detected in the control cells cotransfected with pXJ41-sRNase L and pXJ41-L87. IP with anti-HA antibody and blotting with anti-FLAG antibody showed the presence of FMDV Lpro in cells cotransfected with pXJ41-sRNase L and pXJ41-L, while the band of L87 was absent in the control cells cotransfected with pXJ41-sRNase L and pXJ41-L87 (Fig. 3D). In addition, the pXJ41-sRNase L and pXJ41-L C51A were cotransfected into HEK-293T cells. IP with anti-FLAG antibody and blotting with anti-HA antibody showed that sRNase L interacted with L C51A (Fig. 3E). These results suggest that FMDV Lpro interacts with sRNase L and that the interaction is independent of papain-like protease activity of Lpro.
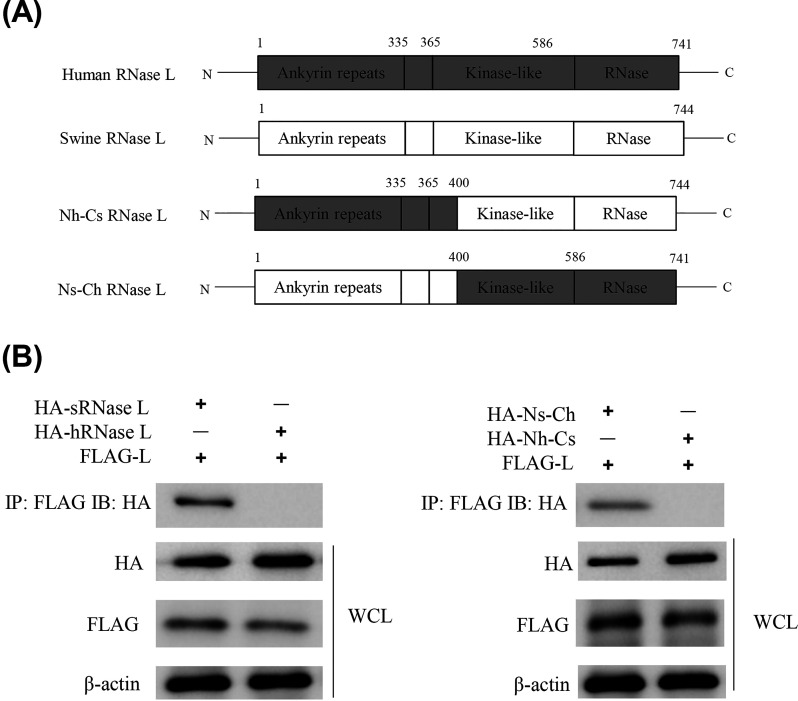
Interaction of FMDV Lpro with the N-terminal domain of sRNase L but not of human RNase L.
Based on the structure of human RNase L (hRNase L), we generated two more plasmids to encode chimeric hemagglutinin (HA)-tagged RNase L in addition to pXJ41-hRNase L and pXJ41-sRNase L (Fig. 4A). The pXJ41-Nh-Cs construct encodes the N-terminal 1 to 400 amino acids of hRNase L followed by the C-terminal 401 to 744 amino acids of sRNase L. The pXJ41-Ns-Ch construct encodes the N-terminal 1 to 400 amino acids of sRNase L followed by the C-terminal 401 to 741 amino acids of hRNase L. When FLAG-Lpro was immunoprecipitated, a signal corresponding to HA-sRNase L but not to HA-hRNase L was detected, suggesting the interaction of FMDV Lpro with sRNase L but not with hRNase L. Furthermore, FMDV Lpro was found to interact with Ns-Ch but not with Nh-Cs, which indicates that FMDV Lpro interacts with the N-terminal domain of sRNase L (Fig. 4B).
FIG 4.
FMDV Lpro interacts with the N-terminal domain of sRNase L. (A) Schematic representation of human, swine, and chimeric RNase L structure. (B) HEK-293T cells were seeded in 60-mm-diameter dishes and grown to 80% confluence. Cells were cotransfected with 3 μg of pXJ41-L (FLAG tag) and 3 μg of pXJ41-sRNase L (HA tag), pXJ41-hRNase L (HA tag), pXJ41-Nh-Cs (HA tag), or pXJ41-Ns-Ch (HA tag) for 24 h. Cell lysates were subjected to coimmunoprecipitation using anti-FLAG antibody. Immunocomplexes were analyzed by Western blotting using anti-HA antibody. Whole-cell lysis (WCL) was also subjected to Western blotting using anti-HA, anti-FLAG, or anti-β-actin antibody. Data are representative of three independent experiments with similar results.
Specific inhibition of sRNase L by FMDV Lpro.
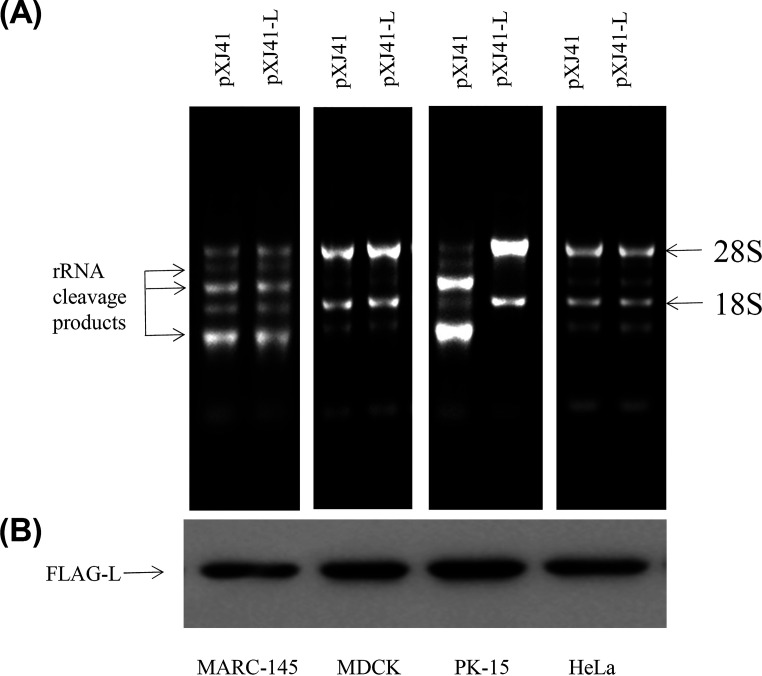
Monkey embryonic kidney epithelial cells (MARC-145), Madin-Darby canine kidney cells (MDCK), porcine kidney cells (PK-15), and human cervical cancer cells (HeLa) were individually transfected with pXJ41-L or pXJ41 to examine whether FMDV Lpro inhibited RNase L activity of other species. As shown in Fig. 5A, RNA degradation was detected in Lpro expressing cells and control cells of all species except PK-15 cells. The Lpro was expressed well in cells of different species, including PK-15 cells (Fig. 5B). These results show that FMDV Lpro inhibits activation of RNase L in a species-specific manner.
FIG 5.
FMDV Lpro inhibits activation of RNase L in a species-specific manner. (A) A total of 1 × 106 of monkey embryonic kidney epithelial cells (MARC-145), Madin-Darby canine kidney cells (MDCK), porcine kidney cells (PK-15), and human cervical cancer cells (HeLa) were seeded in 12-well plates and grown to 80% confluence. Cells were transfected with 1 μg of pXJ41 or pXJ41-L and subsequently transfected with 2-5A for 5 h to activate RNase L. RNA degradation was assessed by agarose gel electrophoresis. (B) Expression of Lpro in MARC-145, MDCK, PK-15, and HeLa cells was detected by Western blotting using anti-FLAG antibody.
Inhibition of sRNase L dimerization by Lpro.
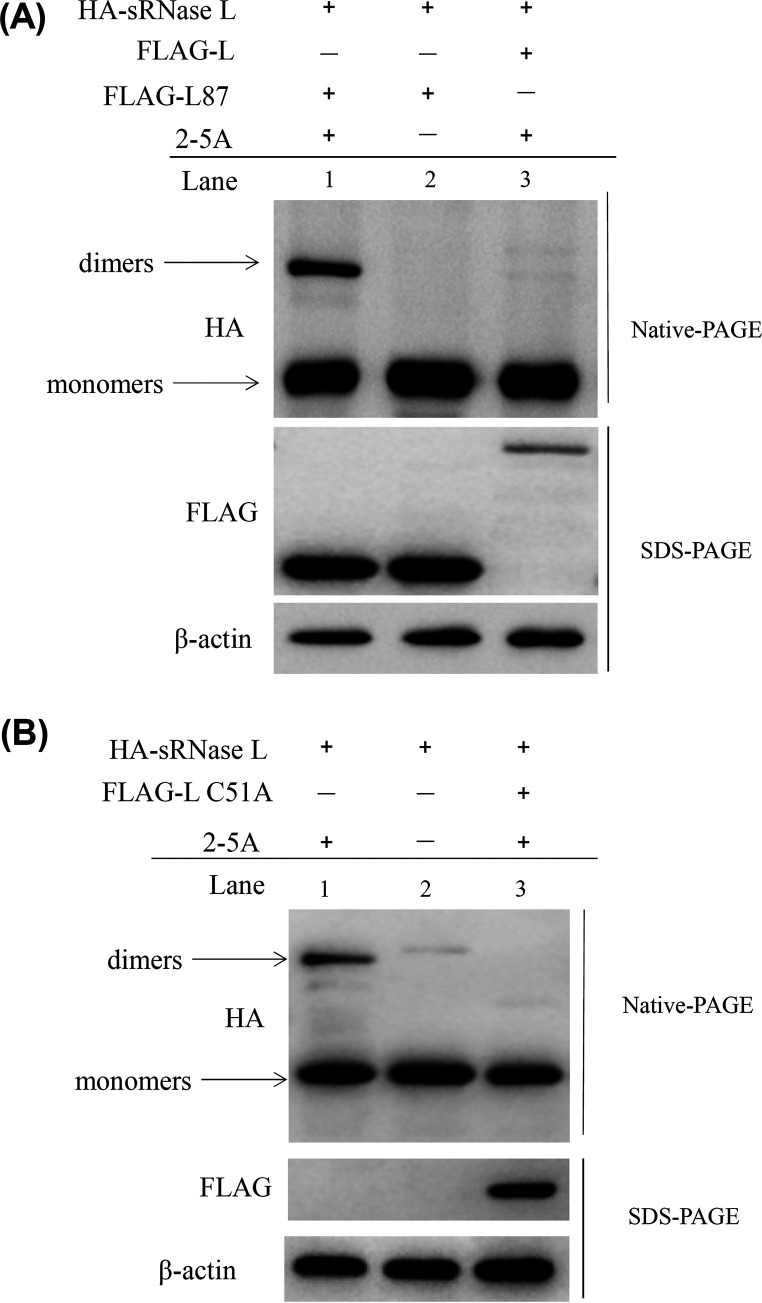
We next examined whether sRNase L dimerization was regulated by FMDV Lpro. The pXJ41-sRNase L along with pXJ41-L or pXJ41-L87 were cotransfected into HEK-293T cells. After treatment with 2-5A, cell lysates were prepared and subjected to IP using anti-HA antibody. The pXJ41-sRNase L- and pXJ41-L87-cotransfected cells without 2-5A treatment served as a control. As seen in Fig. 6, sRNase L dimerization was detected in pXJ41-L87-transfected cells (Fig. 6A, lane 1) but was absent in control cells and almost disappeared in pXJ41-L-transfected cells (Fig. 6A, lanes 2 and 3). In addition, the dimerization of sRNase L was not detected in pXJ41-sRNase L- and pXJ41-L C51A-cotransfected cells (Fig. 6B). These data show that 2-5A can induce sRNase L dimerization, while FMDV Lpro impairs this process through binding to sRNase L, and inhibition of sRNase L dimerization is independent of papain-like protease activity of FMDV Lpro.
FIG 6.
sRNase L dimerization is inhibited by FMDV Lpro. (A) Three μg of pXJ41-sRNase L (HA tag) and 3 μg of pXJ41-L (FLAG tag) or pXJ41-L87 (FLAG tag) were cotransfected into HEK-293T cells for 18 h. After being treated with 2-5A at a concentration of 5 μM for 5 h, cell lysates were prepared and analyzed by Western blotting. pXJ41-L87 (FLAG tag) transfected into HEK-293T cells without 2-5A treatment was used as a negative control. The upper panel shows sRNase L monomers and dimers using native-PAGE and Western blotting. The lower panel shows expression of Lpro or L87 by SDS-PAGE and Western blotting. (B) Three μg of pXJ41-sRNase L (HA tag) and 3 μg of pXJ41-L C51A (FLAG tag) were transfected into HEK-293T cells for 18 h. After treated with 5 μM 2-5A for 5 h, sRNase L dimerization was analyzed by native-PAGE and Western blotting. Expression of L C51A was analyzed by SDS-PAGE and Western blotting. β-actin served as a loading control. The data presented here are results representing data from three Western blotting experiments.
Inhibition of sRNase L induced apoptosis by FMDV Lpro.
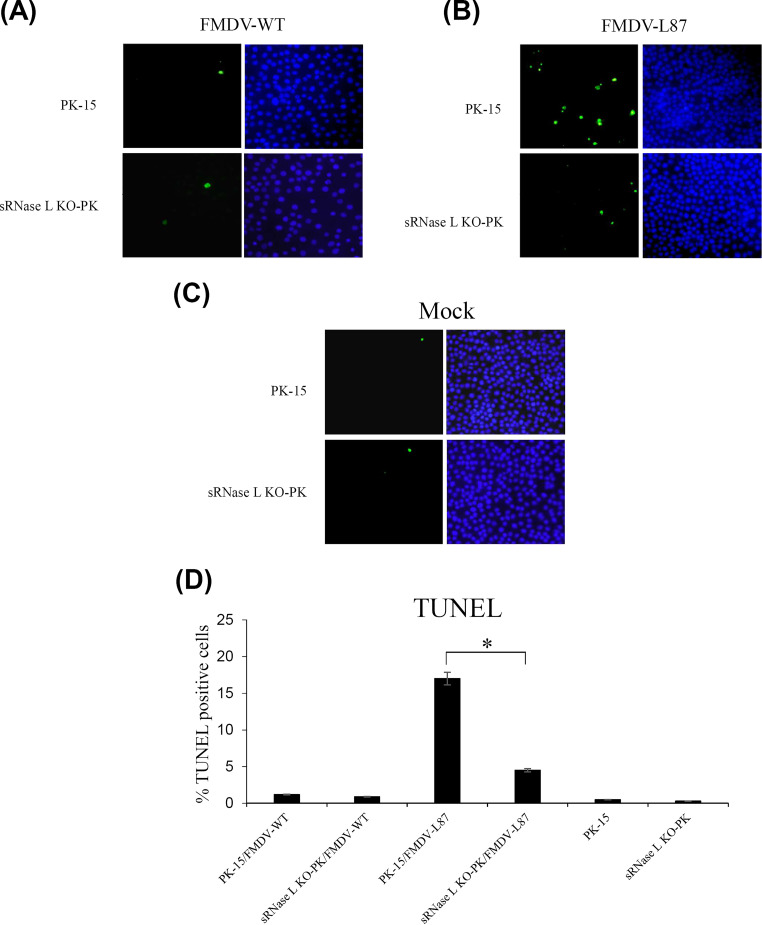
Previous studies have reported that RNase L initiates apoptosis through the mitochondrial pathway in virus-infected cells (24). We recently identified that sRNase L could induce apoptosis in PK-15 cells (25). In order to examine whether FMDV Lpro could inhibit the apoptosis induced by sRNase L, PK-15 and sRNase L KO-PK cells were grown on coverslips and infected individually with FMDV-WT or FMDV-L87 at an MOI of 1 for 12 h. The mock-infected cells were used as a control. The results of TUNEL staining showed that the numbers of apoptotic cells induced by FMDV-WT in PK-15 and sRNase L KO-PK cells were similar to that observed for the mock control cells (Fig. 7A, C, and D). In contrast, the number of apoptotic cells induced by FMDV-L87 in PK-15 cells was significantly higher than that observed in sRNase L KO-PK cells (Fig. 7B). The ratios of apoptotic cells in FMDV-WT-infected PK-15 and sRNase L KO-PK cells were 1.2% and 0.9%, and those in mock-infected PK-15 and sRNase L KO-PK cells were 0.5% and 0.3%, respectively. However, the relative numbers of apoptotic cells in FMDV-L87-infected PK-15 and sRNase L KO-PK cells reached 17% and 4.5%, respectively (Fig. 7D). The results show that FMDV Lpro inhibits cell apoptosis, which is induced by sRNase L.
FIG 7.
Apoptosis in FMDV-WT- and FMDV-L87-infected cells. (A to C) The nicked DNA in apoptotic cells induced by FMDV-WT (A), FMDV-L87 (B), or mock (C) was stained in green color by TUNEL assay and the nuclei were stained in blue color by DAPI. (D) The percentage of TUNEL positive cells. The number of cells representing apoptosis was determined by counting 100 cells in each random microscopic field. Each experiment was conducted in triplicate and repeated three times. Error bars indicate the standard deviations of three experiments. *, P < 0.05.
DISCUSSION
As an important nuclease for the innate immune system, the major function of RNase L is to cleave single-stranded RNAs, and as a result it inhibits viral propagation. Furthermore, RNase L regulates cellular mRNA stability and cell apoptosis (26). FMDV has developed various strategies to evade the host innate immune response through several viral proteins (27). In the present study, we first provide convincing evidence that FMDV Lpro inhibits antiviral activity of sRNase L through interacting with the N-terminal domain of sRNase L and that the Lb coding region of Lpro regulates the ability of Lpro to inhibit the activation of sRNase L.
Previous studies have demonstrated that FMDV Lpro restricts host innate immune responses by several mechanisms (27). For example, FMDV Lpro inhibits host antiviral activity through multiple mechanisms, including cleavage of eIF4G, G3BP1, and G3BP2, degradation of NF-κB, and suppression of IFN-β mRNA synthesis (8, 28, 29). Lpro decreases the expression of IRF3/7 to suppress dsRNA-induced IFN-β mRNA transcription (9). FMDV Lpro cleaves ubiquitin moieties from RIG-I, TBK1, TRAF3, and TRAF6 which belong to the type I IFN signaling pathway (30). The RNA helicase LGP2 is also cleaved by FMDV Lpro, which decreases the level of IFN-β mRNA and antiviral activity (31). In recent studies, MDA5, MAVS, and TBK1, which are important adaptors in the RIG-I-like receptor (RLR) pathway, are cleaved by FMDV Lpro to inhibit expression of type I IFN (32, 33). To determine whether FMDV Lpro could antagonize the OAS/RNase L pathway, we have explored this pathway by expressing FMDV Lpro in cells, generating Lb coding region deleted virus, and generating sRNase L knockout cell line. Our data suggest that the Lb coding region is necessary for Lpro to inhibit the OAS/RNase L pathway (Fig. 1). However, in FMDV-L87-infected sRNase L KO-PK cells, the viral titers could not reach the level of FMDV-WT. The reason may be that L87 loses some other functions of FMDV Lpro, such as cleavage of eIF4G, G3BP1, and G3BP2 and degradation of NF-κB (8, 28, 29), which can benefit virus replication.
Viruses may inhibit the OAS/RNase L pathway by multiple mechanisms, including degrading 2-5A (34, 35), binding to dsRNA to prevent activation of OAS (36), inhibiting 2-5A binding to RNase L (37), or inhibiting RNase L activation by viral RNA structure (38). Encephalomyocarditis virus (EMCV), Theiler’s murine encephalomyelitis virus (Theiler’s virus or TMEV), and poliovirus (PV) belong to the Picornaviridae family. EMCV induces expression of RNase L-specific protein inhibitor (RLI) to decrease 2-5A binding activity with RNase L (39). TMEV L* protein interacts with ankyrin repeats R1 and R2 of RNase L to inhibit 2-5A binding to RNase L and dimerization and oligomerization formation of RNase L (37, 40). RNA structure of poliovirus (PV) 3C protease open reading frame (ORF) inhibits the RNase L activity to protect PV mRNA (38). Though EMCV, TMEV, and PV belong to the Picornaviridae family, the inhibitory mechanisms of RNase L activity are different. Our observation that FMDV Lpro inhibited RNA degradation induced by 2-5A indicates that the inhibition of sRNase L acts downstream of the pathway (Fig. 2A), and there is interaction between FMDV Lpro and sRNase L, indeed (Fig. 3C and D). We demonstrate that FMDV Lpro interacts with the N-terminal domain of sRNase L (Fig. 4). hRNase L can form oligomers which have higher activity than that of dimers (18). However, structural studies indicate that sRNase L forms dimers induced by 2-5A, without evidence of further oligomerization beyond dimers (41). Our data show that FMDV Lpro binds to sRNase L to inhibit the dimerization of sRNase L (Fig. 6). These results suggest that Lpro may prevent the dimerization of sRNase L through inhibiting 2-5A binding to sRNase L. Moreover, Lpro inhibits the activation of sRNase L but does not inhibit the RNase L activation in human, monkey, or canine cells (Fig. 5), which suggests that Lpro inhibits RNase L activity in a species-specific manner.
FMDV Lpro not only inhibits antiviral activity of sRNase L but also affects apoptosis regulated by sRNase L. It should be noted that cellular apoptosis induced by RNase L is one defense strategy to limit viral replication (42). Many viruses have also developed other mechanisms to counteract apoptosis to benefit their replication fitness. For example, the coronavirus nonstructural protein 15 (nsp15) prevents activation of host dsRNA sensors, including MDA5, PKR, and the OAS/RNase L pathway, to inhibit early apoptosis of macrophages (43). In our study, FMDV Lpro also inhibits apoptosis by interacting with sRNase L to promote viral infection (Fig. 7). However, Theiler’s virus L* has no detectable influence on apoptosis in J774-1 cells by TUNEL staining and caspase 3/7 assays, even though Theiler’s virus and FMDV belong to the same Picornaviridae family and their L proteins have some similar features (37).
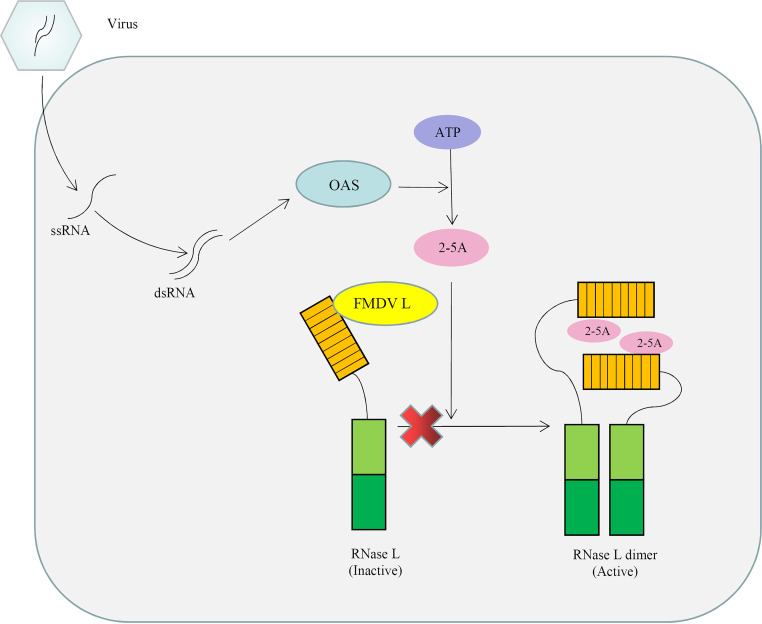
In summary, we elucidate a novel mechanism of FMDV Lpro-mediated inhibition of the OAS/RNase L pathway through interaction with the N-terminal domain of sRNase L (Fig. 8). In addition, results of our experiments reveal for the first time that FMDV Lpro inhibits apoptosis through interacting with sRNase L. The results of our studies provide novel insights into better understanding how the viral proteins of FMDV function as inhibitors of the OAS/RNase L pathway. Such information may be useful to design novel antiviral strategy for prevention and control of FMDV outbreaks in agricultural animals.
FIG 8.
The working model for the negative regulation of OAS/RNase L signaling pathway by FMDV Lpro. FMDV Lpro inhibits the OAS/RNase L pathway through interaction with the N-terminal domain of sRNase L, which occurs downstream of the OAS catalytic activity.
MATERIALS AND METHODS
Cell, virus, and chemicals.
Porcine kidney cells (PK-15), baby hamster kidney cells (BHK-21), Madin-Darby canine kidney cells (MDCK), monkey embryonic kidney epithelial cells (MARC-145), and HEK-293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, USA), 100 units of penicillin/ml, and 100 μg of streptomycin/ml. HeLa cells were cultured in minimum essential medium (MEM) α supplemented with 10% fetal bovine serum, 100 units of penicillin/ml, and 100 μg of streptomycin/ml. These cells were grown in a humidified incubator with 5% CO2 at 37°C. The sRNase L KO-PK cells with sRNase L knockout in PK-15 cells were generated in our lab by the CRISPR-Cas9 gene editing system and used previously to study the functions of sRNase L (25). FMDV type O, strain Tibet/CHA/99 (GenBank accession number AJ539138) was propagated, titrated on BHK-21 cells, and stored at −80°C until use. Poly(I:C) (Sigma-Aldrich, St. Louis, MO, USA), which is a surrogate for viral dsRNA, was used for transfection of cells to activate OAS. 2-5A was a small molecule to activate RNase L, which was kindly provided by Ling Zhao, Huazhong Agricultural University, Wuhan, China.
Plasmids.
The eukaryotic expression vector pXJ41 is a derivative of pXJ40 in which the polylinker region was modified (44, 45). The L87 gene lacked the Lb coding domain of FMDV L gene. The FMDV L gene and the L87 gene were amplified from the cDNA of FMDV type O, strain Tibet/CHA/99 using primers containing HindIII or Kpn I restriction sites and an N-terminal FLAG tag. The PCR fragments were cloned into pXJ41 and the resultant plasmids were designated pXJ41-L and pXJ41-L87. Specific mutation (C51A) was introduced to pXJ41-L using a QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) to obtain pXJ41-L C51A. hRNase L and sRNase L with a C-terminal HA tag were amplified from the cDNA of HeLa and PK-15 cells by reverse transcription using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The pXJ41-Nh-Cs and pXJ41-Ns-Ch plasmids containing a C-terminal HA tag were chimeric RNase L and constructed by gene splicing by overlap extension PCR (SOE PCR) to contain the N-terminal amino acids 1 to 400 of hRNase L with the C-terminal amino acids 401 to 744 of sRNase L and the N-terminal amino acids 1 to 400 of sRNase L with the C-terminal amino acids 401 to 741 of hRNase L. The 3D gene of FMDV was amplified and inserted into pXJ41 to obtain pXJ41-3D. Three SLCA constructs (LN-L, LN-L87, and LC-sRNase L) were generated to measure the interaction between FMDV Lpro and sRNase L as described elsewhere (46, 47). In brief, FMDV Lpro or FMDV L87 gene was individually cloned into SLCA with LN (aa 1 to 229) of Renilla luciferase at the N terminus. The sRNase L gene was cloned into SLCA with LC (aa 230 to 311) of Renilla luciferase at the N terminus. LN-C and LC-C plasmids were generated by cloning the Zika virus capsid-expressing cDNA gene into the N terminus of LN or LC. All of the expression plasmids were sequenced to confirm the correct tandem in-frame insertion of individual genes. The primers used in this study are listed in Table 1.
TABLE 1.
Primers used in this study
| Primer name | Sequence (5′-3′)a | Restriction site (tag) | Purpose |
|---|---|---|---|
| hRNase L-Fwd | GAGGAATTCCACCATGGAGAGCAGGGATCATAACA | EcoRI | hRNase L amplification |
| hRNase L-Rev | TATCTCGAGTTATCAAGCGTAATCAGGAACGTCGTAAGGGTAGCACCCAGGGCTGGC | XhoI (HA) | |
| sRNase L-Fwd | GAGGAATTCCACCATGGAGACCAAGCGCCATAACAAC | EcoRI | sRNase L amplification |
| sRNase L-Rev | GCTCGAGTTACTAAGCGTAATCAGGAACGTCGTAAGGGTAGGTCTGGCCATCAC | XhoI (HA) | |
| L-Fwd | GCGAAGCTTCCACCATGGATTACAAGGATGACGACGATAAGATGAGCACAACTGACTG | HindIII (FLAG) | FMDV L amplification |
| L-Rev | GCGGGTACCTATTATCTGAGTCGTTTCTGA | KpnI | |
| L87-Rev | GCGGGTACCTATTACATCTTTCCTTGTGCT | KpnI | FMDV L87 amplification |
| 3D-Fwd | GAGGAATTCATGGACTATGGAACT | EcoRI | FMDV 3D amplification for pXJ41-3D |
| 3D-Rev | TATGGATCCTGCATCACCGCACAC | BamHI | |
| qPCR-3D-F | TGGACTATGGAACTGGGTTT | FMDV 3D amplification for real-time PCR | |
| qPCR-3D-D | GCTTGGAATCTCAAAGAGG | ||
| LC-sRNase L-Fwd | CATCATCTCGAGGCCACCATGGAGACCAAGCGCCATAA | XhoI | LC-sRNase L amplification |
| LC-sRNase L-Rev | AGAAGAGCGGCCGCTCATTAGGTCTGGCCATCACCAGCTC | NotI | |
| LN-L-Fwd | CATCATCTCGAGGCCACCATGAGCACAACTGACTGTTT | XhoI | LN-L amplification |
| LN-L-Rev | AGAAGAGCGGCCGCTCATTATCTGAGTCGTTTCTGAACCT | NotI | |
| LN-L87-Rev | AGAAGAGCGGCCGCTCATTACATCTTTCCTTGTGCT | NotI | LN-L87 amplification |
| L C51A-F | AACAACCACGACAACGCCTGGCTGAACGCCATC | Site 51 of L mutagenesis | |
| L C51A-R | GATGGCGTTCAGCCAGGCGTTGTCGTGGTTGTT |
Restriction endonuclease sites are underlined. The FLAG or HA tag is in boldface. The mutated sites are in boldface and underlined.
Construction of infectious clones and rescue of FMDV.
The pSK-AmBC plasmid containing the full-length cDNA of FMDV type O, strain Tibet/CHA/99 was constructed and infectious virus was rescued as described before (48). The pSK-AmBC-L87 plasmid lacking the Lb coding region in the cDNA backbone of FMDV type O, strain Tibet/CHA/99 was generated from pSK-AmBC using SOE PCR. The plasmids pSK-AmBC and pSK-AmBC-L87 were linearized with Pac I, and the purified and linearized DNAs served as templates for in vitro transcription using an mMESSAGE mMACHINE T7 Ultra kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA pellet was resuspended in water and used for transfection of BHK-21 cells. Supernatants from cells at 48 h posttransfection were serially passaged on PK-15 cells (five passages, each for 2 days). The viruses FMDV-WT and FMDV-L87 were harvested.
Western blotting.
HEK-293T cells were lysed in lysis buffer supplemented with phenylmethylsulfonyl fluoride (PMSF; Beyotime, China). The lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Briefly, the cell lysates were resolved in a polyacrylamide gel. Separated proteins were then transferred onto a nitrocellulose (NC) membrane. The membranes were blocked with 5% nonfat milk and probed with anti-FLAG antibody (Sigma-Aldrich), anti-HA antibody (Sigma-Aldrich), anti-eIF4GI antibody (Proteintech, Rosemont, IL, USA), or anti-β-actin (Boster, Wuhan, China). Specific proteins were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Boster, Wuhan, China). The membranes were developed using SuperSignal West Pico Chemiluminescent Substrate according to the manufacturer’s instructions (Pierce, Rockford, IL, USA). Digital signal acquisition and analysis were conducted using the Quantity One program, version 4.6 (Bio-Rad).
Coimmunoprecipitation assay.
For IP, HEK-293T cells were washed with cold phosphate-buffered saline (PBS) and harvested in lysis buffer supplemented with PMSF. The lysates were centrifuged at 4°C and the supernatants were collected and reacted with anti-FLAG or anti-HA antibody by gentle agitation for 2 h at 4°C. The lysates were incubated with protein A/G beads (Beyotime, China) for another 2 h at 4°C. The beads were then washed 5 times with lysis buffer without PMSF. Immunoprecipitated proteins were resolved by SDS-PAGE and subjected to Western blotting. The IP samples with anti-FLAG antibody were probed with anti-HA antibody for Western blotting and the IP samples with anti-HA antibody were probed with anti-FLAG antibody.
Dimerization assay.
HEK-293T cells were seeded in 60-mm-diameter dishes and grown to 80% confluence. Three μg of pXJ41-sRNase L plasmid was transfected into cells along with 3 μg of pXJ41-L, pXJ41-L C51A, or pXJ41-L87. Eighteen h later, the cells were transfected with 5 μM 2-5A. At 5 h posttransfection, the cells were washed with cold PBS and harvested. The samples were analyzed by SDS-PAGE or native polyacrylamide gel electrophoresis (native-PAGE) as described previously (49). The sRNase L monomers and dimers were detected with anti-HA antibody using native-PAGE and Western blotting. The expression of Lpro, L87, or L C51A was detected with anti-FLAG antibody by SDS-PAGE and Western blotting.
Growth kinetics.
BHK-21, PK-15, or sRNase L KO-PK cells were seeded in 12-well plates and grown to 80% confluence and then infected with FMDV-WT or FMDV-L87 at an MOI of 1. After 1 h of virus adsorption, the cell monolayers were washed three times and fresh medium was added. At 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h postinfection, supernatants were collected. One part of the supernatant was subjected to microtitration infectivity assay on PK-15 cells, and the titers were calculated for 50% tissue culture infective dose (TCID50) using the Reed-Muench method (50). The other part of the supernatant was analyzed by real-time PCR using the primers for FMDV 3D gene (Table 1).
Real-time PCR.
To determine the effect of FMDV Lpro on the function of sRNase L, PK-15 and sRNase L KO-PK cells were seeded in 12-well plates and grown to 80% confluence. Cells were infected with FMDV-WT and FMDV-L87. To evaluate the viral cDNA copies of FMDV, viral RNAs in the supernatants were extracted by TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. First-strand cDNA was synthesized using PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Tokyo, Japan), and 1 μl of cDNA was used for SYBR green PCR assay (TaKaRa) using real-time PCR primers (Table 1). For quantification, pXJ41-3D plasmid containing the 3D gene was 10-fold serially diluted and the standard curve was generated. The FMDV RNA from the sample was quantified using linear extrapolation of the CT value plotted against the standard curve.
TUNEL assay.
PK-15 and sRNase L KO-PK cells were grown on the coverslips overnight and infected with FMDV-WT or FMDV-L87 at an MOI of 1. Cells were washed three times with PBS and fixed with 4% paraformaldehyde in PBS for 1 h at 4°C. After washing three times with PBS, cells were permeabilized with 0.2% Triton X-100 for 10 min. After washing three times again, the coverslips were labeled using One Step TUNEL apoptosis assay kit according to the manufacturer’s protocol (Beyotime, China). The cells were treated with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 5 min and examined using the Olympus BX51 inverted fluorescence microscope. The number of cells positive for apoptosis was determined by counting 100 cells each in random microscopic field. Each experiment was conducted in triplicate and repeated three times.
RNA degradation assay.
Total cellular RNA was extracted using TRIzol reagent according to the manufacturer’s protocol. RNA degradation was assessed by 1% agarose gel electrophoresis in Tris-acetate-EDTA (TAE) buffer.
SLCA.
HEK-293T cells were cultured in 12-well plates overnight and cotransfected with LC-sRNase L and LN-L or LN-L87. LN-C and LC-C that contain ZIKV C protein were confirmed to be interactive between them and used as a positive control. pXJ41 empty vector was used as a negative control. At 24 h posttransfection, cells were lysed and the cell lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C. Renilla luciferase reporter activity was determined by SLCA using luciferase assay system (Promega, Madison, WI, USA) (46, 47).
Statistical analysis.
Data were compared and the differences were determined by one-way repeated measurement analysis of variance (ANOVA) and least significance difference (LSD). A P value of <0.05 was considered statistically significant (51).
Data availability statement.
The table and figures data used to support the findings of this study are included within the article.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2016YFD0501505), the National Natural Science Foundation of China (31672609), and Natural Science Foundation of Shandong Province (ZR2020KC005, ZR2020QC196).
We declare that there is no conflict of interest.
Contributor Information
Yijun Du, Email: duyijun0916@163.com.
Jing Qi, Email: qj-happy2008@163.com.
Julie K. Pfeiffer, University of Texas Southwestern Medical Center
REFERENCES
- 1.Fry EE, Stuart DI, Rowlands DJ. 2005. The structure of foot-and-mouth disease virus. Curr Top Microbiol Immunol 288:71–101. 10.1007/3-540-27109-0_4. [DOI] [PubMed] [Google Scholar]
- 2.Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin Microbiol Rev 17:465–493. 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conda-Sheridan M, Lee SS, Preslar AT, Stupp SI. 2014. Esterase-activated release of naproxen from supramolecular nanofibres. Chem Commun (Camb) 50:13757–13760. 10.1039/c4cc06340f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strebel K, Beck E. 1986. A second protease of foot-and-mouth disease virus. J Virol 58:893–899. 10.1128/JVI.58.3.893-899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberger J, Skern T. 2014. The leader proteinase of foot-and-mouth disease virus: structure-function relationships in a proteolytic virulence factor. Biol Chem 395:1179–1185. 10.1515/hsz-2014-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaney MA, Vakharia VN, Lloyd RE, Ehrenfeld E, Grubman MJ. 1988. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol 62:4407–4409. 10.1128/JVI.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradi A, Foeger N, Strong R, Svitkin YV, Sonenberg N, Skern T, Belsham GJ. 2004. Cleavage of eukaryotic translation initiation factor 4GII within foot-and-mouth disease virus-infected cells: identification of the protease cleavage site in vitro. J Virol 78:3271–3278. 10.1128/jvi.78.7.3271-3278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Los Santos T, Diaz-San Segundo F, Grubman MJ. 2007. Degradation of nuclear factor kappa B during foot-and-mouth disease virus infection. J Virol 81:12803–12815. 10.1128/JVI.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Fang L, Li P, Sun L, Fan J, Zhang Q, Luo R, Liu X, Li K, Chen H, Chen Z, Xiao S. 2011. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J Virol 85:3758–3766. 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Fang L, Liu L, Zhong H, Chen Q, Luo R, Liu X, Zhang Z, Chen H, Xiao S. 2011. Foot-and-mouth disease virus (FMDV) leader proteinase negatively regulates the porcine interferon-λ1 pathway. Mol Immunol 49:407–412. 10.1016/j.molimm.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Swatek KN, Aumayr M, Pruneda JN, Visser LJ, Berryman S, Kueck AF, Geurink PP, Ovaa H, van Kuppeveld FJM, Tuthill TJ, Skern T, Komander D. 2018. Irreversible inactivation of ISG15 by a viral leader protease enables alternative infection detection strategies. Proc Natl Acad Sci U S A 115:2371–2376. 10.1073/pnas.1710617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell JE, Jr, Kerr IM, Stark GR. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421. 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 13.Schindler C, Darnell JE. Jr, 1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–651. 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 14.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. 1998. How cells respond to interferons. Annu Rev Biochem 67:227–264. 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 15.Der SD, Zhou A, Williams BR, Silverman RH. 1998. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc Natl Acad Sci U S A 95:15623–15628. 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovanessian AG. 2007. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2’-5’oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev 18:351–361. 10.1016/j.cytogfr.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. 2011. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. J Interferon Cytokine Res 31:41–47. 10.1089/jir.2010.0107. [DOI] [PubMed] [Google Scholar]
- 18.Dong B, Silverman RH. 1997. A bipartite model of 2–5A-dependent RNase L. J Biol Chem 272:22236–22242. 10.1074/jbc.272.35.22236. [DOI] [PubMed] [Google Scholar]
- 19.Malathi K, Dong B, Gale M, Jr, Silverman RH. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:816–819. 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinand C, Montavon C, Salehzada T, Silhol M, Lebleu B, Bisbal C. 1999. RNase L inhibitor is induced during human immunodeficiency virus type I infection and down regulates the 2-5A/RNase L pathway in human T cells. J Virol 73:290–296. 10.1128/JVI.73.1.290-296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sànchez R, Mohr I. 2007. Inhibition of cellular 2’-5’ oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J Virol 81:3455–3464. 10.1128/JVI.02520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Y, Condit RC, Vijaysri S, Jacobs B, Williams BR, Silverman RH. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J Virol 76:5251–5259. 10.1128/jvi.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccone ME, Rieder E, Mason PW, Grubman MJ. 1995. The foot-and-mouth disease virus leader proteinase gene is not required for viral replication. J Virol 69:5376–5382. 10.1128/JVI.69.9.5376-5382.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castelli JC, Hassel BA, Wood KA, Li XL, Amemiya K, Dalakas MC, Torrence PF, Youle RJ. 1997. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J Exp Med 186:967–972. 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui C, Jiang D, Wu X, Cong X, Li F, Shang Y, Wang J, Liu S, Shan H, Qi J, Du Y. 2019. CRISPR-Cas9 mediated RNase L knockout regulates cellular function of PK-15 cells and increases PRV replication. Biomed Res Int 2019:7398208. 10.1155/2019/7398208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezelle HJ, Malathi K, Hassel BA. 2016. The roles of RNase-L in antimicrobial immunity and the cytoskeleton-associated innate response. Int J Mol Sci 17:74. 10.3390/ijms17010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhu Z, Zhang M, Zheng H. 2015. Multifunctional roles of leader protein of foot-and-mouth disease viruses in suppressing host antiviral responses. Vet Res 46:1–13. 10.1186/s13567-015-0273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Weiss M, Grubman MJ, de los Santos T. 2010. Differential gene expression in bovine cells infected with wild type and leaderless foot-and-mouth disease virus. Virology 404:32–40. 10.1016/j.virol.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Visser LJ, Medina GN, Rabouw HH, de Groot RJ, Langereis MA, de los Santos T, van Kuppeveld FJM. 2018. Foot-and-mouth disease virus leader protease cleaves G3BP1 and G3BP2 and inhibits stress granule formation. J Virol 93:e00922-18. 10.1128/JVI.00922-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Fang L, Luo R, Ye R, Fang Y, Xie L, Chen H, Xiao S. 2010. Foot-and- mouth disease virus leader proteinase inhibits dsRNA-induced type I interferon transcription by decreasing interferon regulatory factor 3/7 in protein levels. Biochem Biophys Res Commun 399:72–78. 10.1016/j.bbrc.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Pulido MR, Sánchez-Aparicio MT, Martínez-Salas E, García-Sastre A, Sobrino F, Sáiz M. 2018. Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus. PLoS Pathog 14:e1007135. 10.1371/journal.ppat.1007135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visser LJ, Aloise C, Swatek KN, Medina GN, Olek KM, Rabouw HH, de Groot RJ, Langereis MA, Santos T, Komander D, Skern T, van Kuppeveld FJM. 2020. Dissecting distinct proteolytic activities of FMDV Lpro implicates cleavage and degradation of RLR signaling proteins, not its deISGylase/DUB activity, in type I interferon suppression. PLoS Pathog 16:e1008702. 10.1371/journal.ppat.1008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulido MR, Martínez-Salas E, Sobrino F, Sáiz M. 2020. MDA5 cleavage by the Leader protease of foot-and-mouth disease virus reveals its pleiotropic effect against the host antiviral response. Cell Death Dis 11:718. 10.1038/s41419-020-02931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornbrough JM, Jha BK, Yount B, Goldstein SA, Li Y, Elliott R, Sims AC, Baric RS, Silverman RH, Weiss SR. 2016. Middle east respiratory syndrome coronavirus NS4b protein inhibits host RNase L activation. mBio 7:e00258. 10.1128/mBio.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L, Jha BK, Wu A, Elliott R, Ziebuhr J, Gorbalenya AE, Silverman RH, Weiss SR. 2012. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe 11:607–616. 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min JY, Krug RM. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2’-5’ oligo (A) synthetase/RNase L pathway. Proc Natl Acad Sci U S A 103:7100–7105. 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorgeloos F, Jha BK, Silverman RH, Michiels T. 2013. Evasion of antiviral innate immunity by Theiler's virus L* protein through direct inhibition of RNase L. PLoS Pathog 9:e1003474. 10.1371/journal.ppat.1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han JQ, Townsend HL, Jha BK, Paranjape JM, Silverman RH, Barton DJ. 2007. A phylogenetically conserved RNA structure in the poliovirus open reading frame inhibits the antiviral endoribonuclease RNase L. J Virol 81:5561–5572. 10.1128/JVI.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinand C, Salehzada T, Silhol M, Lebleu B, Bisbal C. 1998. RNase L inhibitor (RLI) antisense constructions block partially the down regulation of the 2-5A/RNase L pathway in encephalomyocarditis-virus-(EMCV)-infected cells. Eur J Biochem 254:248–255. 10.1046/j.1432-1327.1998.2540248.x. [DOI] [PubMed] [Google Scholar]
- 40.Drappier M, Jha BK, Stone S, Elliott R, Zhang R, Vertommen D, Weiss SR, Silverman RH, Michiels T. 2018. A novel mechanism of RNase L inhibition: Theiler's virus L* protein prevents 2-5A from binding to RNase L. PLoS Pathog 14:e1006989. 10.1371/journal.ppat.1006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H, Zeqiraj E, Dong B, Jha BK, Duffy NM, Orlicky S, Thevakumaran N, Talukdar M, Pillon MC, Ceccarelli DF, Wan LC, Juang YC, Mao DY, Gaughan C, Brinton MA, Perelygin AA, Kourinov I, Guarné A, Silverman RH, Sicheri F. 2014. Dimeric structure of pseudokinase RNase L bound to 2-5A reveals a basis for interferon-induced antiviral activity. Mol Cell 53:221–234. 10.1016/j.molcel.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castelli JC, Hassel BA, Maran A, Paranjape J, Hewitt JA, Li XL, Hsu YT, Silverman RH, Youle RJ. 1998. The role of 2’-5’ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ 5:313–320. 10.1038/sj.cdd.4400352. [DOI] [PubMed] [Google Scholar]
- 43.Deng X, Hackbart M, Mettelman RC, O'Brien A, Mielech AM, Yi G, Kao CC, Baker SC. 2017. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc Natl Acad Sci U S A 114:E4251–E4260. 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao JH, Davidson I, Matthes H, Garnier JM, Chambon P. 1991. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell 65:551–568. 10.1016/0092-8674(91)90088-G. [DOI] [PubMed] [Google Scholar]
- 45.Du Y, Zuckermann FA, Yoo D. 2010. Myristoylation of the small envelope protein of porcine reproductive and respiratory syndrome virus is non-essential for virus infectivity but promotes its growth. Virus Res 147:294–299. 10.1016/j.virusres.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cissell KA, Rahimi Y, Shrestha S, Deo SK. 2009. Reassembly of a bioluminescent protein Renilla luciferase directed through DNA hybridization. Bioconjug Chem 20:15–19. 10.1021/bc8003099. [DOI] [PubMed] [Google Scholar]
- 47.Fujikawa Y, Kato N. 2007. Split luciferase complementation assay to study protein-protein interactions in Arabidopsis protoplasts. Plant J 52:185–195. 10.1111/j.1365-313X.2007.03214.x. [DOI] [PubMed] [Google Scholar]
- 48.Du Y, Bi J, Liu J, Liu X, Wu X, Jiang P, Yoo D, Zhang Y, Wu J, Wan R, Zhao X, Guo L, Sun W, Cong X, Chen L, Wang J. 2014. 3Cpro of foot-and-mouth disease virus antagonizes the interferon signaling pathway by blocking STAT1/STAT2 nuclear translocation. J Virol 88:4908–4920. 10.1128/JVI.03668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T. 2004. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J Biol Chem 279:9698–9702. 10.1074/jbc.M310616200. [DOI] [PubMed] [Google Scholar]
- 50.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. American J Epidemiology 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 51.Du Y, Lu Y, Wang X, Qi J, Liu J, Hu Y, Li F, Wu J, Guo L, Liu J, Tao H, Sun W, Chen L, Cong X, Ren S, Shi J, Li J, Wang J, Huang B, Wan R. 2014. Highly efficient expression of interleukin-2 under the control of rabbit β-globin intron II gene enhances protective immune responses of porcine reproductive and respiratory syndrome (PRRS) DNA vaccine in pigs. PLoS One 9:e90326. 10.1371/journal.pone.0090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The table and figures data used to support the findings of this study are included within the article.