ABSTRACT
Rotavirus infection is highly prevalent in children, and the most severe effects are diarrhea and vomiting. It is well accepted that the enteric nervous system (ENS) is activated and plays an important role, but knowledge of how rotavirus activates nerves within ENS and to the vomiting center is lacking. Serotonin is released during rotavirus infection, and antagonists to the serotonin receptor subtype 3 (5-HT3 receptor) can attenuate rotavirus-induced diarrhea. In this study, we used a 5-HT3 receptor knockout (KO) mouse model to investigate the role of this receptor in rotavirus-induced diarrhea, motility, electrolyte secretion, inflammatory response, and vomiting reflex. The number of diarrhea days (P = 0.03) and the number of mice with diarrhea were lower in infected 5-HT3 receptor KO than wild-type pups. In vivo investigation of fluorescein isothiocyanate (FITC)-dextran transit time showed that intestinal motility was lower in the infected 5-HT3 receptor KO compared to wild-type mice (P = 0.0023). Ex vivo Ussing chamber measurements of potential difference across the intestinal epithelia showed no significant difference in electrolyte secretion between the two groups. Immediate early gene cFos expression level showed no difference in activation of the vomiting center in the brain. Cytokine analysis of the intestine indicated a low effect of inflammatory response in rotavirus-infected mice lacking the 5-HT3 receptor. Our findings indicate that the 5-HT3 receptor is involved in rotavirus-induced diarrhea via its effect on intestinal motility and that the vagus nerve signaling to the vomiting center occurs also in the absence of the 5-HT3 receptor.
IMPORTANCE The mechanisms underlying rotavirus-induced diarrhea and vomiting are not yet fully understood. To better understand rotavirus pathophysiology, characterization of nerve signaling within the ENS and through vagal efferent nerves to the brain, which have been shown to be of great importance to the disease, is necessary. Serotonin (5-HT), a mediator of both diarrhea and vomiting, has been shown to be released from enterochromaffin cells in response to rotavirus infection and the rotavirus enterotoxin NSP4. Here, we investigated the role of the serotonin receptor 5-HT3, which is known to be involved in the nerve signals that regulate gut motility, intestinal secretion, and signal transduction through the vagus nerve to the brain. We show that the 5-HT3 receptor is involved in rotavirus-induced diarrhea by promoting intestinal motility. The findings shed light on new treatment possibilities for rotavirus diarrhea.
KEYWORDS: 5-HT3 receptor, diarrhea, disease mechanisms, disease symptoms, motility, rotavirus, serotonin, vomiting
INTRODUCTION
Rotavirus infection causes severe diarrhea and vomiting and causes up to 200,000 child deaths each year that are caused by severe dehydration and organ failure associated with the infection (1, 2). Although diarrhea and vomiting are natural defense mechanisms, they sometimes end up doing more harm than favor in rotavirus-infected young children, who rapidly can become severely dehydrated because both vomiting and diarrhea contribute to loss of water and electrolytes. Oral rehydration solution is commonly used, but it could be unsuccessful on account of extensive vomiting and is often replaced by intravenous therapy.
In mice, rotavirus has been shown to activate the enteric nervous system (ENS) (3) and to infect enterochromaffin (EC) cells in the small intestine and stimulate them to secrete serotonin (5-hydroxytryptamine, or 5-HT) (4). In the gut, 5-HT is synthesized by both EC cells and neurons of the ENS, modulating a wide repertoire of physiological responses, including inflammatory responses (5, 6), intrinsic peristaltic and secretory reflexes (7), and activation of extrinsic sensory nerves that transmit information to the brain that leads to the sensation of nausea and discomfort to the central nervous system (CNS) (4, 8). 5-HT predominantly acts on 5-HT receptors, which are divided into seven subclasses (9), of which 5-HT3 and 5-HT4 are the most relevant to functional gastrointestinal diseases (10). In particular, the 5-HT3 receptor belongs to a family of ligand-gated ion channels that include nicotinic, glycine, and GABAA receptors (11). It is structurally and functionally distinct from the other six classes of 5-HT receptors, whose actions are mediated via G proteins (9).
There are five 5-HT3 receptor subunits (A to E), and all functional receptors require at least one A subunit (9, 12). In the periphery, the 5-HT3 receptors regulate gut motility, secretion, and peristalsis through the ENS and also transfer signals from the gastrointestinal tract to the CNS (4, 8, 13). The vomiting mechanism has been thoroughly studied through the effects of chemotherapy drugs (14). The studies indicate that EC cells leak 5-HT, which activates 5-HT3 receptors on vagal afferent nerves in the intestine and sends signals to the brain that induce the vomiting reflex (4, 12, 15). Additionally, enteric glia cells (EGCs) within the ENS, which have been shown to regulate motility, permeability, and inflammatory responses, also express 5-HT receptors (16–18). Most recently, EGCs and 5-HT were shown to be involved in the regulation of the epithelial barrier during rotavirus infection (19). Additionally, 5-HT3 receptor antagonists have been used to treat diarrhea in irritable bowel disease (20–22) and to treat nausea and vomiting caused by chemotherapy (12, 23, 24), radiation (24), and surgery (24). Yet the 5-HT receptor-related mechanisms involved in rotavirus-induced diarrhea and vomiting are not fully understood yet.
In this study, we investigated the specific role of the 5-HT3 receptor in rotavirus disease since this receptor is involved in nerve signaling within the ENS, and antagonists are effective to treat diarrhea and vomiting. By using a 5-HT3 receptor knockout (KO) mouse model, we found that this receptor is involved in rotavirus-induced intestinal motility but is not required for signaling to the vomiting center in the brain. This information provides new insight into the complex mechanisms of the pathophysiology of rotavirus infection and indicates that other targets than the 5-HT3 receptor may be suitable for symptom treatment. More studies of receptor signaling in the complex nerve system need to be performed to find good treatments for diarrhea, vomiting, and other sickness symptoms mediated by nerves.
RESULTS
Rotavirus-induced diarrhea is partly regulated by 5-HT3 receptors.
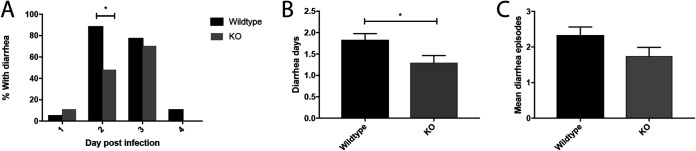
In the first set of experiments, we investigated whether the absence of the 5-HT3 receptor provides protection against diarrhea during rotavirus infection. Five- to 7-day-old mice were orally infected as previously described (4, 25) with the epizootic diarrhea of infant mice (EDIM) rotavirus, and diarrhea response was recorded twice a day starting at 24 h postinfection (hpi) and up to 120 hpi. On the second day of infection, fewer 5-HT3 receptor KO mice than wild-type mice had diarrhea (P = 0.012; Fig. 1A). Moreover, the mean number of diarrhea days was also lower (P = 0.03) in the KO mice than in the wild-type mice (Fig. 1B), as was the mean number of total diarrhea episodes (P = 0.07) (Fig. 1C). These findings indicate that the 5-HT3 receptors are partly contributing to rotavirus-induced diarrhea.
FIG 1.
Severity of diarrhea in rotavirus-infected wild-type and 5-HT3 receptor KO mice. (A) Percentage of KO pups and wild-type pups with diarrhea on day 2 (*, P = 0.012), which is the time point when the most severe diarrhea occurs in infant mice. (B) Number of mean diarrhea days in KO and wild-type mice (*, P = 0.03). (C) The mean number of diarrhea episodes in wild-type and KO pups show that there were fewer diarrhea episodes in the KO pups (P = 0.07). Wild-type mice, n = 18; KO mice, n = 27. Data are expressed as mean with SEM. (A) Fisher’s exact test; (B and C) Mann-Whitney test.
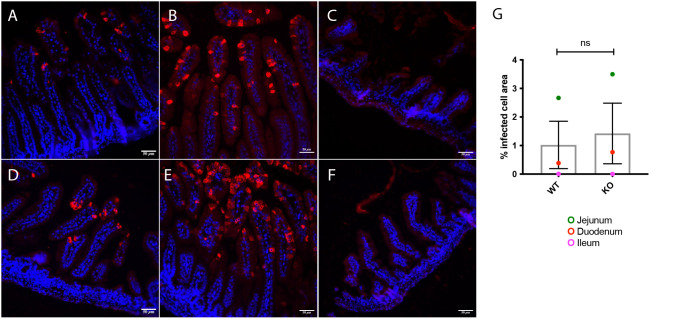
Mice were genotyped for the 5-HT3A receptor according to Jackson Laboratory protocol after diarrhea recording. Infection was confirmed by immunofluorescence localization of rotavirus-infected cells in the duodenum, jejunum, and ileum. No significant difference in infected cell area could be observed between wild-type and KO mice (Fig. 2). Therefore, the reduced level of diarrhea in KO mice is not explained by lower degree of infection.
FIG 2.
Immunofluorescence staining showing rotavirus infection in the small intestine of wild-type and 5-HT3 receptor KO mice. The images depict rotavirus infection in the duodenum (A and D), jejunum (B and E), and ileum (C and F) of wild-type (A to C) and 5-HT3 receptor KO (D to F) pups at 16 hpi. The intestine samples were stained with a primary antibody against viral protein 6 (an in-house guinea pig antibody) followed by goat anti-guinea-pig Alexa594 (red, virus). Nuclear staining with DAPI (blue). (G) Rotavirus fluorescence quantification was performed on a surface area with minimum of seven intestinal villi from each segment of wild-type and 5-HT3 receptor KO mice. The graph presents the percentage of rotavirus fluorescence correlated with the total surface area. ns, no significance; n = 7 villi.
Rotavirus-induced gut motility is dependent on the 5-HT3 receptor.
Rotavirus infection has previously been shown to increase gut motility in mice via activation of the ENS (25). To determine the role of 5-HT3 receptors in gut motility, wild-type (n = 7) and 5-HT3 receptor KO infant mice (n = 6) were infected and motility determined based on the transit time of 4 kDa fluorescein isothiocyanate (FITC)-dextran along the gastrointestinal tract at 48 hpi as described previously (25). As reported before (25), rotavirus-infected wild-type mice displayed greater motility than noninfected, but interestingly, infected 5-HT3 receptor KO mice had less motility (P = 0.0023) than infected wild-type mice, although 5-HT3 receptor KO displayed high basal motility (Fig. 3). This indicates that the 5-HT3 receptor contributes to gut motility during rotavirus infection.
FIG 3.
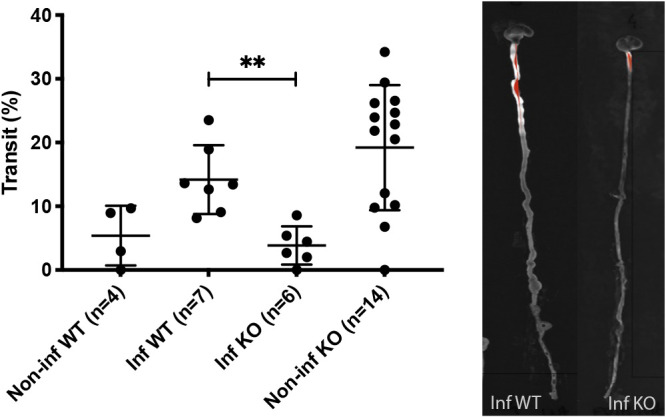
Rotavirus-induced intestinal motility in wild-type and 5-HT3 receptor KO mice. The percentage transit of a 4-kDa FITC-dextran probe through the intestinal tract (from the pylorus to the rectum) (left) was visualized by a UV spectrophotometer as a fluorescence-dependent white color (right). The infected wild-type mice showed higher percentage of transit than infected 5-HT3 receptor KO mice. This indicate that increased motility due to rotavirus infection is partly dependent of the presence of the 5-HT3 receptor. Data are expressed as median with interquartile range. **, P = 0.0023; Mann-Whitney test.
Chloride secretion induced by rotavirus is not dependent on the 5-HT3 receptor.
Rotavirus-induced diarrhea results from a combination of electrolyte-driven water secretion, intestinal pathology, and increased motility. Next, we investigated whether the 5-HT3 receptor contributes to electrolyte secretion during infection. We used the Ussing chamber as an ex vivo method to determine electrolyte secretion (3, 19). As shown in Fig. 4, jejunal segments obtained from infected mice (at 48 hpi), irrespective of whether they were from wild-type (n = 4) or 5-HT3 receptor KO mice (n = 4), exhibited similar increased electrolyte secretion, as indicated by the potential difference (PD; measured in mV) across the intestinal epithelium. As shown in the figure, noninfected mice had significantly less electrolyte secretion than infected mice. This indicates that electrolyte secretion in the jejunal segments of rotavirus-infected mice may not be dependent on the 5-HT3 receptor.
FIG 4.
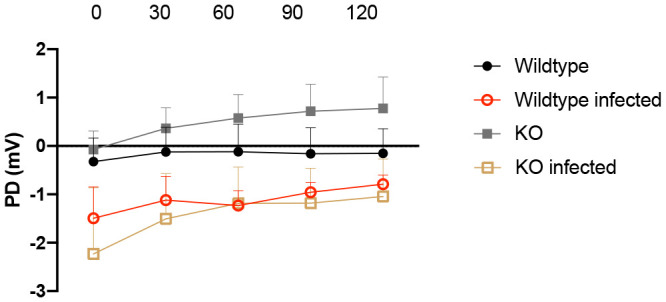
Role of the 5-HT3 receptor in intestinal secretion induced by rotavirus infection. Potential difference (PD) was measured in jejunal segments (4 mice per group) from wild-type and 5-HT3 receptor KO pups at 48 hpi. PD was measured every 30 min over 2 h in Ussing chambers. There was no significant difference between infected wild-type and 5-HT3 receptor KO mice. This suggests that the 5-HT3 receptor does not play a major role in rotavirus-induced electrolyte secretion. mV, millivolt. Data are expressed as mean with SEM. Two-way ANOVA test.
The 5-HT3 receptor modulates the IL-6 response to rotavirus infection.
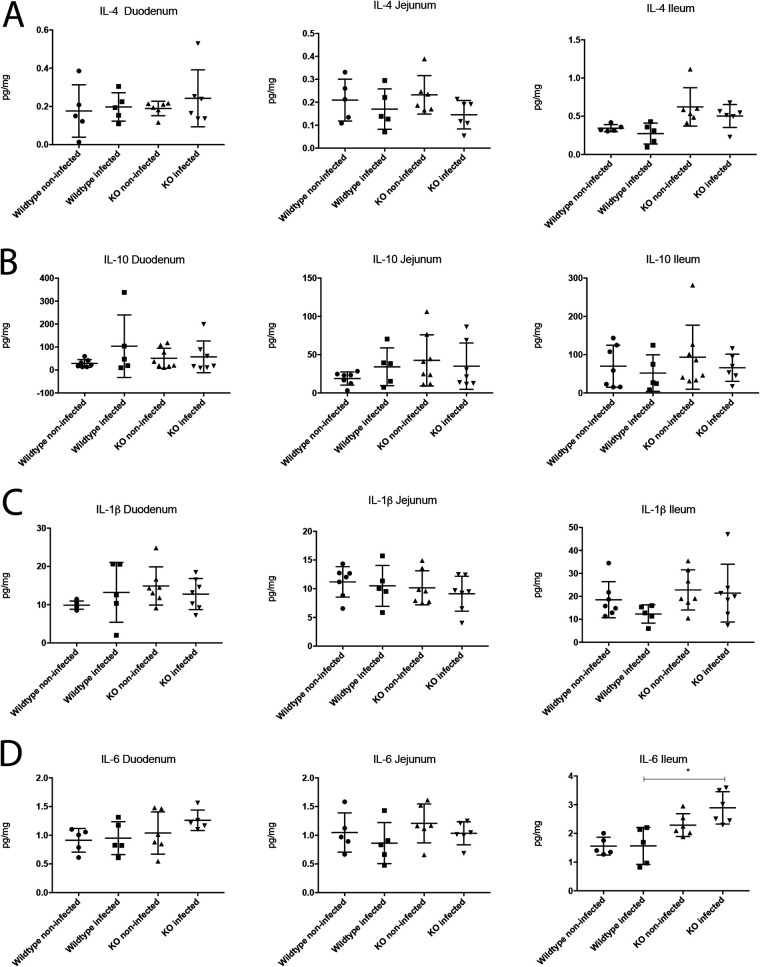
Since 5-HT is involved in inflammation (5, 26), we investigated whether the inflammatory responses to rotavirus infection were modulated by the 5-HT3 receptor by measuring the levels of pro- and anti-inflammatory cytokines. Previous studies have shown that the inflammatory response to rotavirus is low (27–29). We examined the cytokine responses between noninfected and infected, 5-HT3 receptor KO, and wild-type mice at 16 hpi. The levels of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and IL-6 and the anti-inflammatory cytokines IL-4 and IL-10 were measured in the duodenum, jejunum, and ileum using enzyme-linked immunosorbent assays (ELISAs). The TNF-α values were not detectable (data not shown). Only the IL-6 levels were found to differ in ileum between rotavirus-infected KO and wild-type mice (P = 0.0006) (Fig. 5A to C), but the noninfected KO mice also had higher IL-6 levels than the noninfected wild-type mice. There was no significant difference in IL-6 levels between noninfected and infected KO mice (P = 0.0649).
FIG 5.
Role of the 5-HT3 receptor in modulating the immune response during rotavirus infection. The levels of pro- and anti-inflammatory cytokines were measured in segments of the small intestine. No difference was detected in the anti-inflammatory response of IL-4 (A) and IL-10 (B) nor the proinflammatory cytokines IL-1β (C) or TNF-α (below the detection limits). The levels of IL-6 (D), which can act as both a pro- and anti-inflammatory cytokine, were higher in the infected and noninfected 5-HT3 receptor KO mice, but infected KO mice had higher IL-6 levels than the infected wild-type pups (P = 0.0006). This difference was also observed between noninfected 5-HT3 receptor KO mice and wild-type mice. This indicates that the 5HT3 receptor may not be of importance for inflammatory response during rotavirus infection. n = 5 to 8 mice per group. Data are expressed as mean with SD. One-way ANOVA.
Rotavirus infection activates the vomiting center of the brain independent of the 5-HT3 receptor.
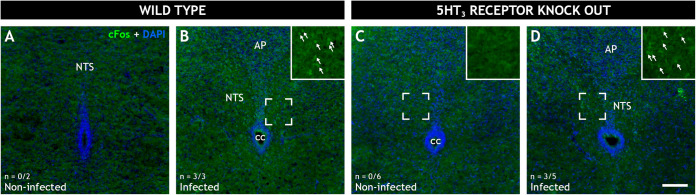
A major symptom of rotavirus infection is vomiting, and we have previously shown that rotavirus infection activates the nucleus of the solitary tract (NTS) and area postrema (AP), which are parts of the vomiting center in the brain (4). Since the vomiting reflex is attenuated by 5-HT3 receptor antagonists (12, 30), we wanted to investigate whether rotavirus signaling to the vomiting center was dependent on the 5-HT3 receptor. Nerve activation in the NTS and AP was investigated in rotavirus-infected and noninfected wild-type as well as 5-HT3 receptor KO mice pups at 16 hpi. Activation was assessed by immunofluorescence staining of brain sections with anti-cFos antibody that targets activated neurons (4, 31). Rotavirus infection led to nerve activation in the NTS (Fig. 6), but absence of the 5-HT3 receptor did not abolish or diminish this activation (Fig. 6D). These findings indicate that this particular ascending vagal signaling is not dependent on the 5-HT3 receptor.
FIG 6.
Role of the 5-HT3 receptor in rotavirus-induced cFos expression in the nucleus of the solitary tract of wild-type and 5HT3R receptor KO mice. Representative wide-field low-power fluorescence micrographs of coronal mouse brain sections from 5- to 7-day-old noninfected (A and C) and infected (B and D) wild-type (A and B) and 5HT3 receptor KO (C and D) pups. The sections were stained for the immediate early gene cFos (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue). The inserts (B, C, and D) present magnified views of the marked regions. cFos immunoreactive cell nuclei (arrows) can be observed in the nucleus of the solitary tract (NTS) of the infected mice (B and D) but not the noninfected mice (A and C). The micrograph indicates that there was no difference in cFos expression in the vomiting center of the brains of infected wild-type and 5-HT3 receptor KO-infected mice. This means that the receptor was not involved in the neural vomiting reflex mechanism. The number of animals (n) with cFos activity in the NTS is denoted in the bottom left corner. AP, area postrema; cc, central canal; NTS, nucleus of the solitary tract. Scale bar in panel D, 100 μm for panels A to D.
DISCUSSION
5-HT has been shown to play a role in the pathophysiological mechanisms of rotavirus infection (4, 19, 32–34). It has also been shown that rotavirus infection activates the ENS (3, 32) and increases intestinal motility in pups with rotavirus diarrhea (25). In the present study, we used a 5-HT3 receptor KO mouse model to specifically investigate the role of the 5-HT3 receptor in rotavirus-induced diarrhea, motility, inflammatory response, and the vomiting reflex via signaling to the brain.
We found that fewer KO than wild-type mice had diarrhea on the second day of infection, which previously has been reported to be the time point at which the most severe diarrhea occurs in infant mice (25). Additionally, the KO mice spent fewer days with diarrhea and had a lower number of diarrhea episodes during the entire observation period. Next, we investigated whether the severity of diarrhea was associated with intestinal motility, which we found to be reduced in infected mice lacking the 5-HT3 receptor compared to infected wild-type mice. We used our well-established FITC-dextran model (25) to study intestinal transit time through the gastrointestinal tract. Wild-type mice pups at 48 hpi, the time point of most severe diarrhea, had increased intestinal motility, as had previously been reported (25). We observed that the 5-HT3 receptor KO mice model has a higher basal intestinal motility which is decreased during rotavirus infection. An explanation of this phenomenon cannot currently be concluded. Nevertheless, infected KO had significantly less (P = 0.0023) motility than wild-type infected mice. The observations that mice that lack the 5-HT3 receptor had less diarrhea and reduced intestinal motility are consistent with previous reports that 5-HT3 receptor antagonists can attenuate rotavirus-induced diarrhea in mice (32) and children (34), indicating that the 5-HT3 receptor contributes to rotavirus-induced diarrhea by promoting intestinal motility.
Active chloride secretion occurs via neural reflexes within the ENS and can be initiated by 5-HT through both 5-HT3 and 5-HT4 receptor binding on intrinsic nerves projecting into the submucosal plexus, where interneurons activate vasomotor neurons to release acetylcholine and/or vasoactive intestinal peptide (VIP), both of which signal via crypt epithelial cells to stimulate ion and water secretion (35). Rotavirus intestinal secretion has been shown to be induced by enteric nerve signaling (3) and can be attenuated by VIP and 5-HT3 receptor antagonists (32). The 5-HT3 receptor has also been shown to be involved in secretory responses induced by cholera toxin (36), and administration of 5-HT to mice resulted in diarrhea within 30 min (4). In our study, we measured PD across the intestinal epithelium of infected wild-type and 5-HT3 receptor KO mice ex vivo in order to determine if the 5-HT3 receptor is crucial for electrolyte secretion. We observed no significant difference in PD across the intestinal epithelia between rotavirus-infected wild-type and 5-HT3 receptor KO mice. In our model, these results indicate that rotavirus-induced electrolyte secretion was not entirely dependent on the 5-HT3 receptor. However, we cannot exclude the possibility that released 5-HT may bind with higher affinity to the 5-HT4 receptor due to higher availability of unbound 5-HT.
It has previously been reported that infected 5-HT3 receptor KO mice exhibit increased intestinal permeability during rotavirus infection compared to infected wild-type mice (19). This implies the importance of the 5-HT3 receptor in the control of the epithelial barrier. Accordingly, increased permeability has been shown in inflammatory bowel dysfunction with 5-HT imbalance (26, 37) and in inflammatory bacterial gastrointestinal infections (38). Since 5-HT is involved in inflammation and an increase in permeability has been proposed to cause an inflammatory response (26), we investigated whether lack of the 5-HT3 receptor and increase in permeability may lead to an increase in inflammation by measuring the levels of pro- and anti-inflammatory cytokines. We found no major difference in cytokine response, except in ileum where the infected 5-HT3 receptor KO mice had higher IL-6 levels than infected wild-type mice (Fig. 5D). However, the fact that also noninfected KO mice had higher levels than noninfected wild type makes it difficult to draw any conclusions of its importance (Fig. 5D). Moreover, as IL-6 can act as both pro- and anti-inflammatory cytokine (39), and since other measured pro- and anti-inflammatory were unchanged, the 5HT3 receptor may not be of importance for inflammatory response during rotavirus infection.
In accordance with our previous findings (4), oral rotavirus infection in pups resulted in an increase in cFos in the NTS (Fig. 6), which is a brain area known to be part of the vomiting center (40). The cFos data presented here confirm previous findings (4) and strengthen the model wherein peripheral infections in the gut lead to altered brain activity. The data we present here are suggestive of the existence of gut-brain cross-talk independent of the 5-HT3 receptor.
Contrary to conditional KO models, the present model lacks the 5-HT3 receptor in all organs and tissues over the entire development. Yet no general phenotype alteration is observed (www.jax.org/strain/005251). Thus, it is plausible that certain 5HT3 receptor-dependent functions are preserved via other compensatory pathways. As 5-HT acts on multiple receptors with a variety of downstream signaling pathways, many redundant receptor candidates exist. Thus, similar to chemotherapy-induced emesis (41, 42), which has been well studied in the house musk shrew and Cryptotis parva, in animal models with vomiting response (14, 43), other substances apart from 5-HT may be involved in the vomiting response to rotavirus infection. Substance P and activation of neurokinin receptors have been shown to be part of the vomiting response (41, 42, 44).
The results from this study may have clinical implications for the treatment of rotavirus diarrhea and support the published human clinical data which show that 5-HT3 receptor antagonists may be useful as a treatment strategy in children with rotavirus diarrhea (34). Since diarrhea and vomiting from rotavirus illness contribute to severe dehydration in vulnerable groups such as in children and the elderly, treatments preventing fluid loss may be of great benefit. Currently, there is no treatment to reduce diarrhea in children. In adults, loperamide, an opioid receptor agonist that decreases the activity of the myenteric plexus, is widely used for traveler’s diarrhea, but the drug is not recommended for infants and small children (45). In contrast, 5-HT3-receptor antagonists such as ondansetron can be used even in infants and small children, as they have few and mild side effects. According to the U.S. Food and Drug Administration (FDA), ondansetron is safe for use in children from the age of 1 month (https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020007s043lbl.pdf). Apart from attenuating the vomiting response, this treatment also facilitates oral rehydration therapy (30, 46), as well as viral spread through aerosols after vomiting. Although further studies are needed to fully understand the signaling pathways involved in the vomiting response during rotavirus infection, our observations strengthen the role of 5-HT in rotavirus pathophysiology and indicate that 5-HT3 receptors contribute to rotavirus-induced intestinal motility.
To summarize, our present understanding of rotavirus pathophysiology strongly indicates the involvement of 5-HT and activation of the ENS as well as CNS to give rise to diarrhea and vomiting (3, 4, 19, 25, 34). This study gives further important insight into the signaling pathways to disease symptoms and realizes that the 5-HT3 receptor may be a possible target for treatment of rotavirus diarrhea.
MATERIALS AND METHODS
Animals.
Homozygous wild-type C57BL6J mice and C57BL6J mice that were heterozygous and homozygous for the 5-HT receptor-3A (5-HT3A) (B6.129X1-Htr3atm1Jul/J; Jackson Laboratories, CA, USA) were used and housed in standard cages with free access to food and water. Mice that are homozygous for the targeted mutation are viable, fertile, and normal in size and do not display any gross physical or behavioral abnormalities. Mutant mice have impaired response to pain.
The mice were bred to generate litters with mixed genotypes, namely, homozygous wild-type mice and mice that were heterozygous and homozygous for the 5-HT3 receptor. Only the homozygous wild-type, here referred to as just “wild type,” and homozygous 5-HT3A KO mice were used in the outcome analysis. Pregnant females were transferred to individual cages 1 week before the expected day of birth, and offspring remained with their mother during the experimental period. The animal experiments were approved by the animal ethics committee in Linköping, Sweden (approval nos. N141/15 and 55-15).
Rotavirus infection of mice.
Mice were infected with the EDIM rotavirus strain. They were orally administered 100 diarrhea doses (100 DD50) in 10 μl of 0.9% NaCl via a pipette as previously described (4, 25). Mock-infected control mice were administered 10 μl of 0.9% NaCl. Litters comprised of heterozygous, wild-type, and KO pups. During the diarrhea observation period, mice were marked with colored markers so that each mouse could be followed individually.
Definition of diarrhea.
Mice were examined twice a day for signs of diarrhea, which was defined as liquid yellow stools induced by gentle abdominal palpation, as described previously (3, 32). Individual mice were followed each day after inoculation, and the total number of days with diarrhea was recorded. The daily percentage of mice with diarrhea was calculated by dividing the number of mice with diarrhea by the total number of mice in the group.
Genotyping.
Genotyping was performed on ear biopsy samples of a few millimeters taken at the end of the experiment when the mice were sacrificed. Genomic DNA from ear biopsy samples was extracted using Bimake direct PCR kit (Bimake, Stockholm, Sweden) as per the manufacturer’s guidelines. Briefly, 100 μl of buffer L and 2 μl of Protease Plus were added into a 1.5-ml microcentrifuge containing each ear biopsy sample and subsequently incubated at 55°C for 30 min, followed by protease inactivation at 95°C for 5 min. Genotyping was done using PCR, and the 20-μl PCR contained 1× M-PCR Opti Mix (provided in the Bimak Direct PCR kit); 500 nM (each) oIMR3633, oIMR3634, and oIMR8162 primers; and 1 μl of the extracted mouse DNA. The PCR cycling conditions were provided by The Jackson Laboratory, which is the supplier of the mice. The PCR amplicons were subsequently separated by electrophoresis using 1.5% agarose gel to determine homozygous wild-type (based on detection of a single 400-bp amplicon) and heterozygous (detection of 400-bp and ∼210-bp amplicons) and homozygous (detection of a single ∼210-bp amplicon) mice for the 5-HT3 receptor.
Motility study.
Infected and noninfected infant (5- to 7-day-old), wild-type, and 5-HT3 receptor KO C57BL6 mice were orally administered 10 μl of 4-kDa FITC-dextran probe at a dose of 0.25 mg/pup (FD-4; Sigma) at 48 h after infection. FITC-dextran was dissolved in Milli-Q water, and the solutions were freshly prepared before each experiment. After 15 min, the pups were sacrificed, and the entire intestine, from the stomach to the rectum, was used for determination of transit time by UV light measurements in a ChemiDoc XRS system (Bio-Rad, Sweden). The front of the accumulating FITC-dextran was defined from the photo, and the software program Illustrator CS6 was used to measure the intestinal length and migration of the FITC-dextran probe. The percentage of intestinal transit was calculated as a measure of how far the FITC-dextran probe had passed through the entire length of the intestine, from the pylorus to the rectum (25).
Ussing chamber experiments.
We employed Ussing chambers to investigate PD across the epithelium in noninfected and rotavirus-infected wild-type and 5-HT3 receptor KO mouse pups. The small intestine was dissected, directly placed in oxygenated Krebs buffer, and transported to the laboratory. Segments of the villus epithelium from the jejunum were identified, dissected, and mounted in Ussing chambers (Harvard Apparatus Inc., Holliston, MA, USA) as previously described (47, 48). Mucosal compartments were filled with 1.5 ml of cold 10 mM mannitol in Krebs buffer, and the serosal compartments were filled with 1.5 ml of 10 mM glucose in Krebs buffer. The exposed surface area between the mucosal and serosal sides was set at 4.9 mm2. After the tissue was mounted, the chambers were kept at 37°C and continuously oxygenated in a 95% O2/5% CO2 atmosphere via gas flow circulation. Before the experiments were started, tissues were equilibrated for 30 min in the chambers to achieve transepithelial PD under steady-state conditions; at 10 min and 20 min, mannitol/glucose buffer (37°C) was replaced. Short-circuit current (Isc), transepithelial resistance (TER), and PD were monitored throughout the experiments to verify tissue viability. PD was analyzed as a measure of chloride secretion. For more details on the Ussing chamber experiments, please refer to reference 48.
Immunofluorescence analysis of the small intestine.
Rotavirus infection in mice was detected by immunofluorescence staining of tissue segments from the duodenum, jejunum, and ileum. The biopsy samples were embedded in paraffin, and 4-μM-thin sections were cut using a microtome, placed on glass slides (SuperFrost Plus, catalog no. J1800AMNZ; Fisher Scientific), and dried at 60°C for a minimum of 30 min. Before staining, paraffin was removed from tissue slides by treatment with Aqua dePar (catalog no. ADP1002M; BiocareMedical, CA, USA) at 80°C for 10 min. Antigen retrieval was performed in a pressure cooker at 121°C with the Rodent Decloaker solution (catalog no. RD913M; BiocareMedical). Slides were washed in running water and incubated in 1× Tris-buffered saline (TBS) for 10 min. Unspecific binding was blocked with Rodent Blocker (catalog no. RBM961; BiocareMedical) for 15 min, and an in-house polyclonal and guinea pig anti-VP6 primary antibody against rotavirus diluted 1:200 in 1× TBS were added and incubated for 2 h at room temperature (RT). After three washes with 1× TBS, tissue was incubated with Alexa 594 goat anti-guinea pig secondary antibody (code 106-585-003; Jackson ImmunoResearch) diluted to 1:200 in 1× TBS for 1 h at RT. Following three washes with 1× TBS, DAPI (4′,6-diamidino-2-phenylindole) nuclear staining was performed for 2 min, and the slides were washed four times and mounted with a cover glass using fluorescent mounting media (catalog no. P36980; Invitrogen). Immunofluorescence was detected with a Leica DM6000 B fluorescence microscope, and images were captured with a Leica camera DFC425 (Leica, Germany).
Immunofluorescence quantification.
Representative photos of ×20 magnification, including a minimum of seven villi, were used for rotavirus fluorescence quantification by ImageJ. The percentage of rotavirus fluorescence was correlated with the total area that was measured and values given as percentage.
Protein extraction.
Tissue samples were weighed prior to tissue lysis. A protein extraction reagent (T-PER tissue protein extraction reagent; Thermo Fischer Scientific, USA) was mixed with a protease inhibitor (Roche cOmplete EDTA-free tablets, catalog no. 11697498001; Roche, Switzerland). Following this, 1 ml of extraction buffer was added to each tissue sample and homogenized using Qiagen TissueLyser II (Qiagen, Sweden). All samples were centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was collected and stored in a −20°C freezer until cytokine analysis by ELISA.
Enzyme-linked immunosorbent assay.
Cytokines in homogenized small intestinal tissues from infected and noninfected 5-HT3 receptor KO and wild-type mice were measured by commercial ELISA (Biosite, Sweden). TNF-α, IL-1β, IL-6, IL-4, and IL-10 were measured in accordance with the manufacturer’s instructions (Biosite, Sweden). Optical density was measured at an absorbance of 450 nm (Gemini XPS fluorescence microplate reader; Molecular Devices LLC, USA) (SoftMax Pro software; Molecular Devices LLC, USA).
Immunofluorescence analysis of mouse brain sections.
At 16 hpi, animals were sacrificed, and brains were resected and fixed in 4% formaldehyde solution (Histolab, Sweden) for 24 h and transferred into 15% sucrose solution for 1 week. Brains were embedded in cryoprotectant and kept frozen at −80°C until they were cryo-sectioned into 14-μm sections, mounted on SuperFrost Plus glass slides (Fisher Scientific, Sweden), and dried at 60°C for a minimum of 2 h. Slides were incubated in 1× TBS for 10 min, and unspecific binding was blocked with rodent blocker (BiocareMedical) for 15 min. Next, the tissues were incubated with primary rabbit anti-cFos antibody (Santa Cruz) diluted to 1/200 in 1× TBS and incubated for 2 h at RT. After three washes with 1× TBS, the tissue was incubated with Alexa 594 goat anti-rabbit secondary antibody diluted to 1:200 in 1× TBS for 1 h at RT. Following three washes with 1× TBS, DAPI nuclear staining was performed for 2 min, and the slides were washed four times and mounted with a cover glass using fluorescent mounting medium (Invitrogen). Immunofluorescence was detected with a Zeiss Axio Observer.Z1 microscope with a Plan-Neofluar 20×/0.4 Corr objective, and micrographs were acquired with a Zeiss AxioCam HRM Rev.3 charge-coupled-device (CCD)/low-dispersion (LD) camera.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism 8.3.0 and SPSS v26. For continuous variables, unpaired t test or ANOVA was used to analyze differences between two or more groups, respectively, if the variables followed normal distribution, as determined by the Shapiro-Wilks test. The experiment with PD was analyzed with two-way ANOVA. In cases of variables not normally distributed, the Mann-Whitney U test was used to analyze differences between two groups. The number of mice that experienced diarrhea 48 h after infection was compared between the wild-type and KO groups with the Fisher exact test with two-tailed significance. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the Swedish Research Council (grants 2014-02827, 2017-01479, and 2018-02862).
Contributor Information
Lennart Svensson, Email: lennart.t.svensson@liu.se.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network. 2016. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 62(Suppl 2):S96–S105. 10.1093/cid/civ1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA, Jr., Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr.. 2018. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172:958–965. 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491–495. 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 4.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O, Svensson L. 2011. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7:e1002115. 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloez-Tayarani I, Changeux JP. 2007. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol 81:599–606. 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Denna TH, Storkersen JN, Gerriets VA. 2019. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol Res 140:100–114. 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Sikander A, Rana SV, Prasad KK. 2009. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta 403:47–55. 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Gershon MD, Tack J. 2007. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132:397–414. 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lummis SC. 2012. 5-HT(3) receptors. J Biol Chem 287:40239–40245. 10.1074/jbc.R112.406496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiller R. 2002. Serotonergic modulating drugs for functional gastrointestinal diseases. Br J Clin Pharmacol 54:11–20. 10.1046/j.1365-2125.2002.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. 1991. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254:432–437. 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 12.Smith HS, Cox LR, Smith EJ. 2012. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann Palliat Med 1:115–120. 10.3978/j.issn.2224-5820.2012.07.07. [DOI] [PubMed] [Google Scholar]
- 13.Galligan JJ. 2002. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil 14:611–623. 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 14.De Jonghe BC, Horn CC. 2009. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296:R902-11. 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyers MB, Freeman AJ. 1992. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology 49:263–268. 10.1159/000227054. [DOI] [PubMed] [Google Scholar]
- 16.Grubisic V, Gulbransen BD. 2017. Enteric glia: the most alimentary of all glia. J Physiol 595:557–570. 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulbransen BD, Sharkey KA. 2012. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9:625–632. 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 18.Meir M, Flemming S, Burkard N, Wagner J, Germer CT, Schlegel N. 2016. The glial cell-line derived neurotrophic factor: a novel regulator of intestinal barrier function in health and disease. Am J Physiol Gastrointest Liver Physiol 310:G1118–G1123. 10.1152/ajpgi.00125.2016. [DOI] [PubMed] [Google Scholar]
- 19.Hagbom M, De Faria FM, Winberg ME, Westerberg S, Nordgren J, Sharma S, Keita AV, Loitto V, Magnusson KE, Svensson L. 2020. Neurotrophic factors protect the intestinal barrier from rotavirus insult in mice. mBio 11:e02834-19. 10.1128/mBio.02834-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. 2000. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 355:1035–1040. 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 21.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. 2014. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63:1617–1625. 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskwa A, Boznanska P. 2007. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Wiad Lek 60:371–376. [PubMed] [Google Scholar]
- 23.Moreno J, Sahade M, del Giglio A. 2005. Low-dose granisetron for prophylaxis of acute chemotherapy-induced nausea and vomiting: a pilot study. Support Care Cancer 13:850–853. 10.1007/s00520-005-0817-4. [DOI] [PubMed] [Google Scholar]
- 24.Aapro M. 2004. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist 9:673–686. 10.1634/theoncologist.9-6-673. [DOI] [PubMed] [Google Scholar]
- 25.Istrate C, Hagbom M, Vikstrom E, Magnusson KE, Svensson L. 2014. Rotavirus infection increases intestinal motility but not permeability at the onset of diarrhea. J Virol 88:3161–3169. 10.1128/JVI.02927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis KG, Pothoulakis C. 2009. Serotonin has a critical role in the pathogenesis of experimental colitis. Gastroenterology 137:1562–1566. 10.1053/j.gastro.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramig RF. 2004. Pathogenesis of intestinal and systemic rotavirus infection. J Virol 78:10213–10220. 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg HB, Estes MK. 2009. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 136:1939–1951. 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris AP, Estes MK. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol 281:G303–G310. 10.1152/ajpgi.2001.281.2.G303. [DOI] [PubMed] [Google Scholar]
- 30.Levine DA. 2012. Oral ondansetron decreases vomiting, as well as the need for intravenous fluids and hospital admission, in children with acute gastroenteritis. Evid Based Med 17:112–113. 10.1136/ebmed.2011.100355. [DOI] [PubMed] [Google Scholar]
- 31.Hunt SP, Pini A, Evan G. 1987. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328:632–634. 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 32.Kordasti S, Sjovall H, Lundgren O, Svensson L. 2004. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 53:952–957. 10.1136/gut.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bialowas S, Hagbom M, Nordgren J, Karlsson T, Sharma S, Magnusson KE, Svensson L. 2016. Rotavirus and serotonin cross-talk in diarrhoea. PLoS One 11:e0159660. 10.1371/journal.pone.0159660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagbom M, Novak D, Ekstrom M, Khalid Y, Andersson M, Lindh M, Nordgren J, Svensson L. 2017. Ondansetron treatment reduces rotavirus symptoms-a randomized double-blinded placebo-controlled trial. PLoS One 12:e0186824. 10.1371/journal.pone.0186824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke HJ. 2000. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci 915:77–80. 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 36.Buchheit KH. 1989. Inhibition of cholera toxin-induced intestinal secretion by the 5-HT3 receptor antagonist ICS 205–930. Naunyn Schmiedebergs Arch Pharmacol 339:704–705. 10.1007/BF00168665. [DOI] [PubMed] [Google Scholar]
- 37.Spiller R, Major G. 2016. IBS and IBD - separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol 13:613–621. 10.1038/nrgastro.2016.141. [DOI] [PubMed] [Google Scholar]
- 38.Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. 2016. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7:e196. 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813:878–888. 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Andrews PLR, Hawthorn J. 1988. The neurophysiology of vomiting. Baillieres Clinical Gastroenterology 2:141–168. 10.1016/0950-3528(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 41.Navari RM. 2004. Inhibiting substance p pathway for prevention of chemotherapy-induced emesis: preclinical data, clinical trials of neurokinin-1 receptor antagonists. Support Cancer Ther 1:89–96. 10.3816/SCT.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 42.Girish C, Manikandan S. 2007. Aprepitant: a substance P antagonist for chemotherapy induced nausea and vomiting. Indian J Cancer 44:25–30. 10.4103/0019-509X.31164. [DOI] [PubMed] [Google Scholar]
- 43.Darmani NA. 1998. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neural Transm (Vienna) 105:1143–1154. 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- 44.Zhong W, Chebolu S, Darmani NA. 2019. Intracellular emetic signaling cascades by which the selective neurokinin type 1 receptor (NK1R) agonist GR73632 evokes vomiting in the least shrew (Cryptotis parva). Neurochem Int 122:106–119. 10.1016/j.neuint.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowie MD, Hill ID, Mann MD. 1995. Loperamide for treatment of acute diarrhoea in infants and young children. A double-blind placebo-controlled trial. S Afr Med J 85:885–887. [PubMed] [Google Scholar]
- 46.Fugetto F, Filice E, Biagi C, Pierantoni L, Gori D, Lanari M. 2020. Single-dose of ondansetron for vomiting in children and adolescents with acute gastroenteritis-an updated systematic review and meta-analysis. Eur J Pediatr 179:1007–1016. 10.1007/s00431-020-03653-0. [DOI] [PubMed] [Google Scholar]
- 47.Carlsson AH, Yakymenko O, Olivier I, Hakansson F, Postma E, Keita AV, Soderholm JD. 2013. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol 48:1136–1144. 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- 48.Keita AV, Gullberg E, Ericson AC, Salim SY, Wallon C, Kald A, Artursson P, Soderholm JD. 2006. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab Invest 86:504–516. 10.1038/labinvest.3700397. [DOI] [PubMed] [Google Scholar]