Abstract
Atrial fibrillation (AF) is a multifaceted and highly variable disease that is often difficult to manage within the traditional health-care model. The conventional model of regular or pre-scheduled appointments with physicians or allied health professionals is poorly suited to the unpredictable and often urgent clinical needs of patients with AF. Mobile health (mHealth) has the potential to dramatically transform the delivery and quality of AF care. In this brief review, we summarize the current limitations and evidence gaps in treating patients with AF. We then describe the current mHealth landscape, changes in telehealth coverage and reimbursement, and recent technological advances of smartphones, mobile applications, and connected wearable devices. We also describe important barriers and challenges, such as clinical management of large volumes of data, application of predictive analytics/machine learning, and the need for high-quality randomized clinical trials.
Keywords: Atrial fibrillation, Mobile health, Digital health, Smartphone, Mobile apps, Wearables
1. Introduction: evidence gaps and unmet needs in care of AF
Atrial fibrillation (AF) is a common and important disease that is challenging and expensive to manage. There are unique facets to the treatment of AF: treatment is directed both at preventing complications (stroke, heart failure) and improving quality of life, and decision-making is complicated due to frequent competing comorbidities and potential harms of the therapies themselves. AF may be self-limited, occurring a single time after cardiac surgery, for example, or follow a more chronic and relapsing pattern more consistent with other chronic heart disease such as heart failure. Furthermore, care of AF is delivered across a wide range of health-care settings and providers, leading to high variation and heterogeneity in disease assessment, treatment, and follow-up [1].
Complicating these issues is the often unpredictable and sporadic nature of AF, associated symptoms, and disease progression. In lone paroxysmal AF, patients are typically symptomatic only when AF occurs, which much like earthquakes, is still only predictable moments before onset. Yet, the typical paradigm of care in most health-care systems is prescheduled, infrequent visits to the clinician. Unless AF is prolonged and symptoms are sustained, these visits often have no relationship to when the patient has AF or has AF symptoms. Patients with paroxysmal AF frequently seek emergency care because the tools and care infrastructure to support to manage episodes in an ambulatory setting are lacking, although specialized AF centers may be better resourced. Furthermore, because the short office visit provides a very limited snapshot into the chronicity of the patient’s AF and treatment, patients are often asked to maintain logbooks with vital signs and symptoms. However, reliance on symptoms may fail to account for periods of silent AF, which may augment stroke risk and also predispose to cardiomyopathy from untreated tachycardia. In cases of persistent or permanent AF, symptoms and heart rate control may fluctuate, often without obvious correlation to environmental or other factors.
Ambulatory ECG monitoring, ranging from 24 h to 14 days, may be sporadically ordered to provide extended “snapshots” of AF, but these still represent a small portion of the denominator of the patient’s experience, and health-care insurers often reject coverage of repeat or sequential ambulatory ECG monitoring. After AF ablation, there remains no consensus on intensity of arrhythmia surveillance, leading to a large practice variation as a result. In patients on non-vitamin K oral anticoagulants, assessment of adherence is difficult and not standardized (unlike for warfarin), leading to decreased medication adherence [2, 3]. In sum, these unique aspects of AF disease and care structure illustrate how the shortcomings of traditionally health-care delivery have led to large evidence gaps in decision-making and in optimizing treatment.
2. Mobile health: emergence and importance
Over the last 10 years, mobile telecommunication technology has spurred rapid innovation and growth globally across many sectors. While health care has traditionally been a late adopter of process innovation, the provisions of health-care reform from the Obama administration has served as a major catalyst. The mandatory implementation of electronic health records, introduction of quality- and value-based incentives for hospital reimbursement, and new reimbursements for non-face-to-face management of chronic care conditions have established new incentives to deliver care outside of the brick-and-mortar health-care establishments [4].
In parallel, there has been rapid uptake of mobile and smartphone technologies. Nearly two thirds (64 %) of Americans owned a smartphone in 2015, up from 58 % in the previous year [5]. Eighty percent of Internet users search for health information online, while more than half seek information on a specific disease or treatment [6].
Among smartphone owners, one fourth of them have limited or no other options for broadband access, making their smartphone their only means of accessing online information and services. Among Americans age 50 or more, 39 % used their smartphones to seek information on a health condition [5].
Mobile health (“mHealth”) is the delivery of health care via mobile communication devices [7]. Previously, mHealth referred almost exclusively to the use of cellular phones and simple interventions such as text messaging, which have shown promise in cardiovascular care [8]. More recently, mHealth has focused on “smart” mobile phones, which allow for complex user input and data display with touch screens. Smartphones often have their own sensors, such as accelerometers and cameras that can be exploited for physiological measurements and patient monitoring. Smartphones can be continuously connected to the Internet or a computer network (cellular transmission and Wi-Fi) and to wearables or sensor devices via Bluetooth. This continuous connectivity can allow for data transmission to and from cloud-based data repositories, such as a personal or electronic health record.
These factors have led to exponential growth of the digital and mHealth space. In 2014, total venture capital investment in digital health companies was US$4.3 billion, nearly equal to the total investment in the prior 3 years [9]. Digital medical devices and telemedicine were the third and fourth most funded categories, behind analytics and patient engagement. For AF, there was an abundance of new companies working on sensor technologies (electrocardiography, heart rate, blood pressure, and motion/exercise), remote care and personalized management, and patient engagement (drug adherence). Some of these applications extended to patients with implantable devices, aiming to improve clinician user experience of device interrogation and remote device management [10].
3. Solutions today
For AF, mHealth solutions extend across four broad categories: ECG or rhythm monitoring, heart rate monitoring, symptom and environmental annotation, and medication adherence.
3.1. Heart rate monitoring
Heart rate monitors have seen an explosion in available sensing devices, primarily due to the low cost of photoplethysmographic (PPG) sensor design. In response to a growing market in wellness and fitness tracking, the penetration of these devices has occurred primarily in a retail consumer space rather than in clinical use. PPG-based heart rate sensors approved as medical devices by the Food and Drug Administration (FDA) are widely available but have lagged in their innovation, possibly due to regulatory barriers. FDA-approved PPG heart rate monitors may have the capability to signal irregularity, but there remain no FDA-approved PPG or non-ECG sensor devices approved for AF detection or for arrhythmia discrimination.
Smartphones have also been used to directly measure heart rate without electrodes. In the Apple iPhone suite of devices, the placement of the camera (optical sensor) adjacent to the light emitting diode flash can facilitate transillumination of a finger and measurement of heart rate by detecting the change in detected light during pulse transit. There are no published randomized trials of the effect of ambulatory heart rate monitoring on clinical outcomes in AF.
3.2. ECG and rhythm monitoring
The landscape of ambulatory ECG monitoring devices has been extensively detailed by Mittal and Steinberg [11]. These devices are generally classified by their duration of monitoring (previously ranging from 24 h to 30 days or longer), the electrode style, and the presence or absence of mobile or real-time transmission capability. Since the publication of that paper, there have been some recent advances. A sampling of contemporary devices is shown in Fig. 1 that plots devices based on their duration of monitoring versus the expected setting of delivery of care. Traditional lead- or wire-based devices have been increasingly displaced by solutions with electrodes embedded in adhesive patches that can be worn up to 14 days [12, 13]. Unlike adhesive electrodes for lead-based systems, the water-resistant patches are not removed during the monitoring period leading to greater wear time, more analyzable time, and no lead reversal errors. The wireless coupling of these devices to smartphones via Bluetooth® provides some of them the capability for real-time telemetry, signal interpretation, and two-way communication. Similar chest-strap devices have been developed in the retail fitness and exercise performance markets [14].
Fig. 1.
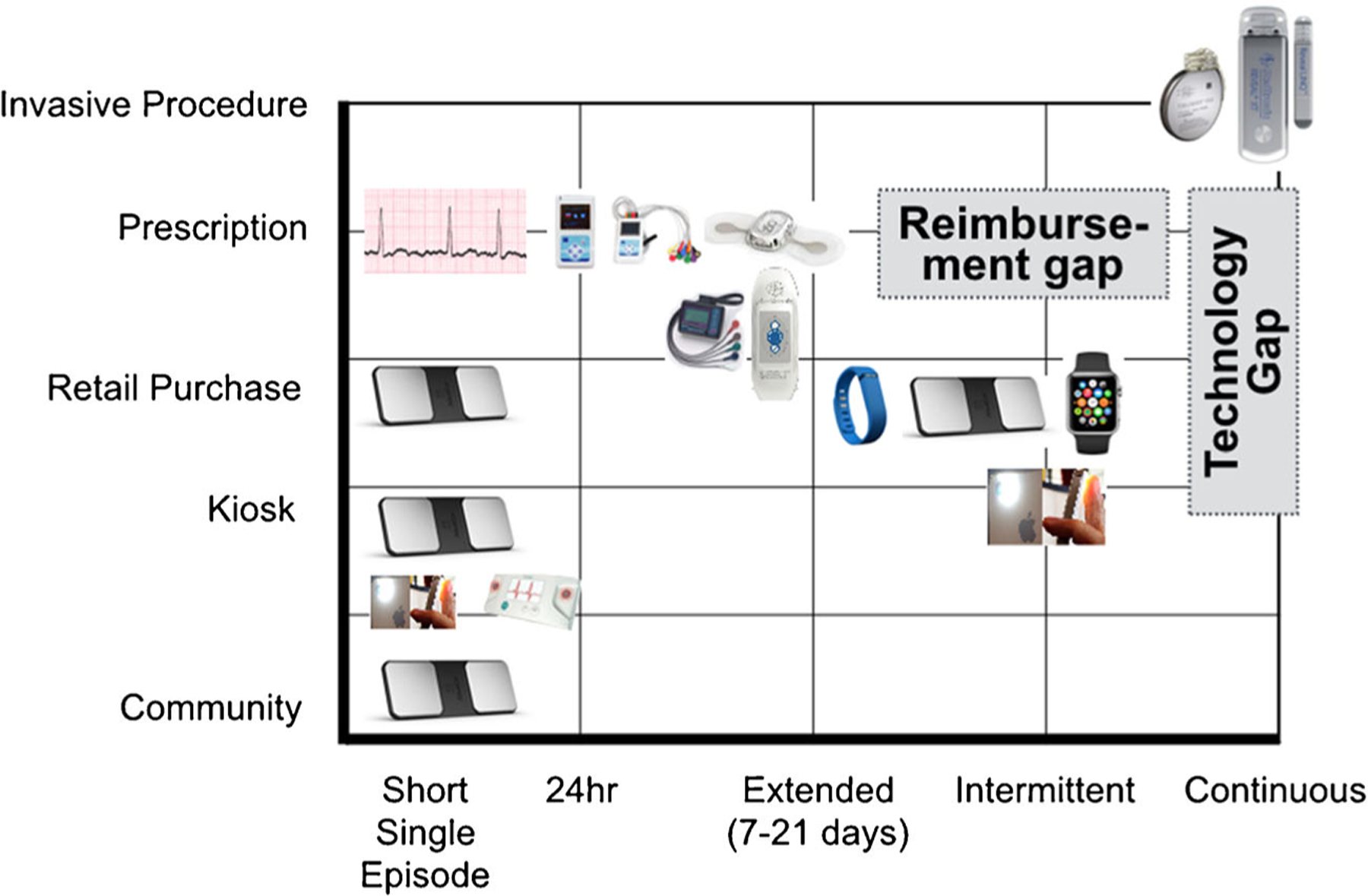
Landscape of atrial fibrillation monitoring devices
More recently, non-wearable solutions coupled to smartphones have emerged. These devices, including the AliveCor device (San Francisco, CA), allow the user (patient or clinician) to perform a “spot check” single lead ECG strip, usually of up to 30 s and sometimes longer [15]. The device may be used by clinicians as a point-of-care device to obtain a reimbursable rhythm strip in place of a 12-lead ECG. Patients may use these devices for ad hoc our routine evaluation of rhythm. The ECG data can be instantaneously transmitted for automated interpretation with the ability of the consumer to request a physician overread for a surcharge. Unfortunately, the device is not reimbursed by most health plans and patients usually must purchase them (Fig. 1).
All of these contemporary devices have shown promise for large-scale population screening of undiagnosed AF. In the USA, modeling estimates have shown the population of undiagnosed AF to be approximately half a million patients with a societal health-care cost of US$3.1 billion [16]. The STROKESTOP study provided patients in Sweden age 75 or 76 a point-of-care rhythm strip device (Zenicor Medical Systems, Stockholm, Sweden) to be used over a 2-week period and found previously undiagnosed AF in 3 % of the 7173 participants [17]. In the SEARCH-AF study, a smartphone-coupled rhythm strip device (AliveCor, San Francisco, CA) was used to perform community-based screening at ten pharmacies in Australia age ≥65 and found newly diagnosed AF in 1 % of the 1010 participants [18]. The limitation of these devices in screening is the sampling error for detection of paroxysmal AF, which may indicate the substantially lower prevalence than what would be expected based on risk factors [19]. Population screening using patch-based adhesive 14-day monitors are underway ([20] mSTOPS study), and pilot data shows promise, with diagnostic yields of 5 % in high-risk samples [21].
For continuous, uninterrupted monitoring, the only current solutions are invasive and include implantable loop recorders (ILRs) (Medtronic Inc., Mounds View, MN; St. Jude Medical, St. Paul, MN). Their role in chronic AF management and in care pathways are still in evolution. Although they are more sensitive in detecting AF simply based on wear time alone, the impact on therapeutic strategy and clinical outcomes have not been tested in randomized trials. Several trials are underway to use ILRs to guide ad hoc non-vitamin K oral anticoagulant therapy [22–24]. Importantly, there still exists a major technology gap for continuous or uninterrupted rhythm monitoring that is non-invasive (Fig. 1).
Methods to classify AF and to discriminate arrhythmias using smartphone LED lights and cameras have been developed, with one irregularity quantification algorithm demonstrating high agreement with ECG rhythm strips [25]. Unfortunately, such technologies have not been validated in larger populations and their calibration or performance in patients with implanted pacemakers or with significant atrial or ventricular ectopy has not been well characterized. Still, improvements in PPG or optical sensors and detection algorithms could one day match the performance of ECG-based rhythm assessment. If so, then delivery of AF care would be expected to change substantially, as this would reflect a radical departure from relying on an ECG for ascertainment of AF.
3.3. Medication adherence
The emergence of the non-warfarin oral anticoagulants that do not require regular blood tests for monitoring has simultaneously resulted in a rise of adherence concerns.
Given the short half-life of these drugs compared to warfarin and the absence of routine or required monitoring for therapeutic effect, consistent and sustained medication adherence is crucial to maximally reduce risk of stroke. For example, with dabigatran, every 10 % reduction in medication adherence was associated with a 13 % increase in stroke and death.
Automated phone or text messaging has been described as a useful intervention in some health-care systems to improve cardiovascular medication adherence [8, 26]. Dozens of smartphone apps that allow patients to self-report and track medication adherence are available on iOS® and Android® platforms. One app allows users to share data with clinicians and family members to facilitate a support system approach to improving care (Care4Today™, Janssen Innovation, La Jolla, CA). The app will issue automated alerts to remind patients to take their medication(s) on time and if medications appear to have been skipped.
3.4. Patient-reported symptoms and environmental data collection
Correlation of AF occurrence to symptoms, patient behavior, or environmental triggers may be heterogeneous or difficult. The traditional way that patients have documented these parameters, often at the recommendation of their health-care professional, is to keep a logbook that documents blood pressure, heart rate, and any other comments. Similar logbooks are often encouraged during ambulatory ECG monitoring. However, in a study of 286 patients with 7-day ambulatory ECG monitoring with a detailed AF symptom assessment immediately following, there were no significant ECG predictors of AF symptom severity [27].
While this simple method can sometimes reveal insights (e.g., worsening heart rate control at certain times per day), the limitation of such an approach is that it fails to account for additional data that may be useful as predictors, such as medication nonadherence, and as outcomes, such as activity or walk speed. Smartphone applications, or an integrated set of applications, could have a role here, by collecting data, actively or passively, on medication adherence, heart rate, rhythm, pulse transit time, blood pressure, symptom severity questions, walk speed (similar to 6-min walk), diet, caffeine and alcohol intake, sympathetic tone (galvanic skin response), sleep (including sleep apnea), and weight. Data exchange frameworks such as Apple HealthKit® and Google Fit® allow applications and connected devices to share data via common application programming interfaces.
Furthermore, machine learning, a statistical methodology that attempts to classify patterns of data without any prespecified null hypotheses, could be applied to attempt to generate insights in data associated with changes in AF symptom severity, exercise tolerance, mood, or other outcomes. While these hypothesis-generating analytics would not be prescriptive, they may help to provide insights to patients to motivate changes in behavior that could favorably affect AF or its symptoms. The smartphone app of the AliveCor smartphone-based ECG device (San Francisco, CA) allows for symptom annotation of rhythm strips (Fig. 2), but the current version (3.2) does not incorporate passive or external sensor data or provide analytical insights beyond data visualization. More AF-specific applications are expected to come to market with clinical trials expected to follow.
Fig. 2.
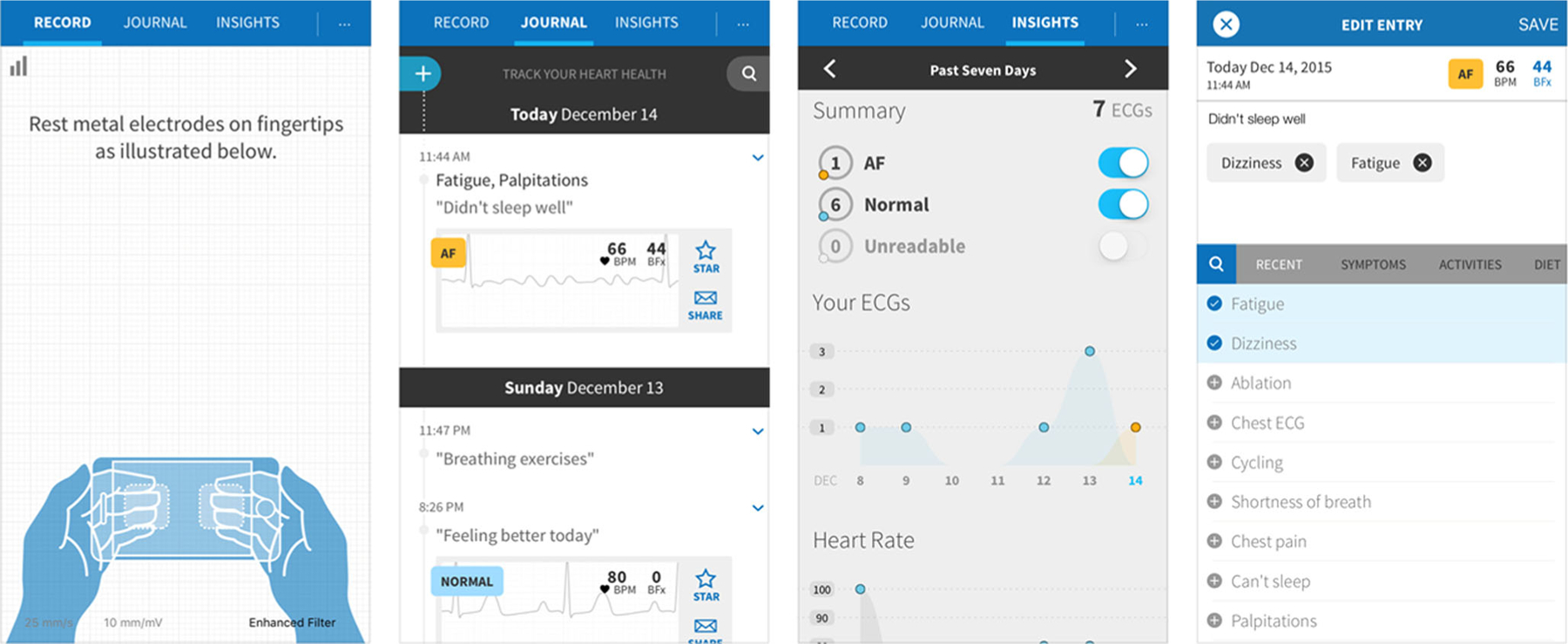
Application screenshots from the AliveCor iPhone mobile application (version 3.2)
3.4.1. Reimbursement
In order for mobile technologies to be incorporated into physician workflows, significant changes are required to reimbursement structures and patient-engagement platforms. The flood of patient data, even with optimized alarms and treatment algorithms, is likely to lead to heavier clinician workloads, which may lack adequate reimbursement incentives. In the current fee for service model, reducing clinical appointments and hospitalizations may not be in the best interest of physicians, who are financially dependent upon office visits and procedural volume. In addition, monitoring outpatient data also incurs potential liability risk or payment penalties for not meeting meaningful use criteria [28]. Fortunately, recent reimbursement legislation highlights that the culture of health care is advancing across multiple arenas. The majority of states have enacted laws regarding private payer reimbursement for remote patient care [29]. For example, Minnesota’s telemedicine parity law, which takes effect for health plans beginning in 2017, requires private payers to provide coverage in the same manner and at the same rate as in-person services [30]. However, the strongest incentive may be a new Medicare reimbursement code (CPT 99490), launched in 2015, to reimburse physicians for non-face-to-face care chronic care management (CCM) in patients with two or more chronic conditions, of which AF has been specifically targeted. The billing requirements include documentation of at least 20 min of clinical staff time (including non-physician and non-nursing staff) and a comprehensive care plan for management of chronic conditions. The average reimbursement is US$40–50 per patient directly to the practice as a professional fee. For a typical practice, this equates to more than US$75,000 of net annual revenue per full-time equivalent physician if only 50 % of eligible patients enroll [31]. Over 60 telehealth startups have launched since the implementation of this new reimbursement code.
4. Conclusion
Atrial fibrillation is a multifaceted and highly variable disease that is poorly managed within the traditional brick-and-mortar health-care model. Mobile health enables wide-reaching disease surveillance among entire patient panels and payer populations with real-time trends in patient rhythm, heart rate, symptoms, and medication adherence. New reimbursement models may encourage mobile health adoption and similarly stimulate greater innovation. The surging global penetration of smartphones and low-cost connected ECG and pulse waveform devices may enable worldwide populations at risk for AF to be screened efficiently and inexpensively. However, challenges remain, including clinical management of large volumes of data, limitations of predictive analytics and machine learning, and the need for high-quality randomized clinical trials. Despite these hurdles, mobile health has an extraordinary potential to improve the care of AF and rapidly redefine the framework for its management.
Footnotes
Compliance with ethical standards
Disclosures None
Publisher's Disclaimer: Disclaimer The content and opinions expressed are solely the responsibility of the authors and do not necessarily represent the views or policies of the Department of Veterans Affairs.
References
- 1.Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: the Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J 2013;165(1):93–101. e101. [DOI] [PubMed] [Google Scholar]
- 2.Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J 2014;167(6):810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shore S, Ho PM, Lambert-Kerzner A, Glorioso TJ, Carey EP, Cunningham F, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA 2015;313(14):1443–50. [DOI] [PubMed] [Google Scholar]
- 4.Turakhia MP, Ullal AJ. US health care policy and reform: implications for cardiac electrophysiology. J Interv Card Electrophysiol 2013;36(2):129–36. [DOI] [PubMed] [Google Scholar]
- 5.Pew Research Center, April, 2015, The smartphone difference http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/.
- 6.Fox S (February 1, 2011). Pew Research Center’s Internet & American life project—health topics http://pewinternet.org/Reports/2011/HealthTopics.aspx. [Google Scholar]
- 7.Carol E, Torgan PD(November 6, 2009.). The mHealth summit: local & global converge http://caroltorgan.com/mhealth-summit/. [Google Scholar]
- 8.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314(12):1255–63. [DOI] [PubMed] [Google Scholar]
- 9.Digital health funding: 2015 year in review. http://rockhealth.com/reports/digital-health-funding-2015-year-in-review/.
- 10.Geneva Healthcare. Revolutionizing cardiac device data management http://www.genevahealthcare.com/. Accessed February 2, 2016.
- 11.Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. J Am Coll Cardiol 2011;58(17):1741–9. [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol 2013;112(4):520–4. [DOI] [PubMed] [Google Scholar]
- 13.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127(1):95 e11–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qardio—state of the heart technology https://www.getqardio.com/. Accessed March 10, 2016.
- 15.AliveCor love your heart http://www.alivecor.com/. Accessed March 9, 2016.
- 16.Turakhia MP, Shafrin J, Bognar K, Goldman DP, Mendys PM, Abdulsattar Y, et al. Economic burden of undiagnosed nonvalvular atrial fibrillation in the United States. Am J Cardiol 2015;116(5): 733–9. [DOI] [PubMed] [Google Scholar]
- 17.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, & Rosenqvist M (2015). Mass screening for untreated atrial fibrillation: The STROKESTOP Study. Circulation, doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 18.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111(6):1167–76. [DOI] [PubMed] [Google Scholar]
- 19.Chyou JY, Hunter TD, Mollenkopf SA, Turakhia MP, & Reynolds MR (2015). Individual and combined risk factors for incident atrial fibrillation and incident stroke: an analysis of 3 million at-risk US patients. J Am Heart Assoc, 4(7), doi: 10.1161/JAHA.114.001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinhubl Steven R., M., Mehta Rajesh R., R., Ebner Gail S., B., Ballesteros Marissa M., B., MBA, MPH, Waalena J, Steinberg Gregory, M., BCh, et al. (2016). Rationale and design of a home-based trial using wearable sensors to detect asymptomatic atrial fibrillation in a targeted population: the mHealth screening to prevent strokes (mSToPS) trial. American Heart Journal [DOI] [PubMed] [Google Scholar]
- 21.Turakhia MP, Ullal AJ, Hoang DD, Than CT, Miller JD, Friday KJ, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015;38(5):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein R, et al. (2015). Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation with Continuous Monitoring (REACT.COM) Pilot Study. J Cardiovasc Electrophysiol, doi: 10.1111/jce.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turakhia MP, & Estes NA 3rd (2015). Stroke risk stratification in atrial fibrillation: bridging the evidence gaps. J Cardiovasc Electrophysiol, doi: 10.1111/jce.12891. [DOI] [PubMed] [Google Scholar]
- 24.Keach JW, Bradley SM, Turakhia MP, Maddox TM. Early detection of occult atrial fibrillation and stroke prevention. Heart 2015;101(14):1097–102. [DOI] [PubMed] [Google Scholar]
- 25.McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, et al. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 2013;10(3):315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med 2014;174(2):186–93. [DOI] [PubMed] [Google Scholar]
- 27.Patel N, Chung EH, Mounsey JP, Schwartz JD, Pursell I, Gehi AK. Effectiveness of atrial fibrillation monitor characteristics to predict severity of symptoms of atrial fibrillation. Am J Cardiol 2014;113(10):1674–8. [DOI] [PubMed] [Google Scholar]
- 28.Lowes R (December 17, 2014). EHR meaningful use penalty will hit 257,000 clinicians in 2015 http://www.medscape.com/viewarticle/836824. [Google Scholar]
- 29.Hodge JG Jr, Weidenaar K, Baker-White A, Barraza L, Bauerly BC, Corbett A, et al. Legal innovations to advance a culture of health. J Law Med Ethics 2015;43(4):904–12. [DOI] [PubMed] [Google Scholar]
- 30.Revisor of Statutes, S. o. M. (2015). 62A.672 Coverage of telemedicine services https://www.revisor.leg.state.mn.us/statutes/?id=62A.672.
- 31.Basu S, Phillips RS, Bitton A, Song Z, Landon BE. Medicare chronic care management payments and financial returns to primary care practices: a modeling study. Ann Intern Med 2015;163(8):580–8. [DOI] [PubMed] [Google Scholar]


