Abstract
Background
Fabry disease (FD) is a rare lysosomal storage disease, caused by mutations in the gene encoding the enzyme α-galactosidase A (α-Gal A). Cardiac involvement is one of the main causes of death and it is characterized by progressive concentric left ventricular hypertrophy (LVH), which in most cases is symmetric. Mild thickening of the left-sided valves is seen in as many as a quarter of patients. Severe aortic stenosis is an extremely rare disorder in FD.
Case summary
In this report, we describe the case of a 57-year-old male, who was diagnosed with a cardiac variant of FD 10 years ago. Since the patient had severe LVH, he was started on enzyme replacement therapy when he was 47 years old with an intravenous infusion of 0.2 mg/kg of agalsidase alpha every 14 days. The patient remained stable and asymptomatic for 9 years, until he presented with dyspnoea in New York Heart Association functional class II–III and severe aortic stenosis (aortic valve area: 0.97 cm2) together with severe systolic dysfunction [ejection fraction (EF): 29%]. Because of the patient’s comorbidities and high surgical risk, he underwent successful transfemoral transcatheter aortic valve implantation (TAVI). At 2 months following TAVI, the patient was asymptomatic and, in spite of his Fabry cardiomyopathy, the EF had increased to 45%.
Discussion
To our knowledge, this is the first case in the literature to demonstrate a rapid progression of aortic stenosis with severe impairment of left ventricular function and worsening in functional class in a patient with FD, who following TAVI improved his EF, with disappearance of symptoms and ventricular arrhythmias.
Keywords: Fabry disease, Transcatheter aortic valve implantation (TAVI), Severe aortic stenosis, Left ventricular dysfunction, CoreValve Evolute R prosthesis, Case report
Learning points
Fabry disease (FD) is characterized by progressive concentric left ventricular hypertrophy and leads to myocardial fibrosis and the end-stage phase of the disease with left ventricular systolic dysfunction (Fabry cardiomyopathy).
Early treatment with enzyme replacement therapy may result in remarkable improvement of cardiac symptoms as long as the disease process has not reached an advanced stage.
Cardiac involvement is one of the main causes of death in patients with advanced FD.
Although the presence of valve disease is common, severe valve disease is an extremely rare disorder in FD.
Introduction
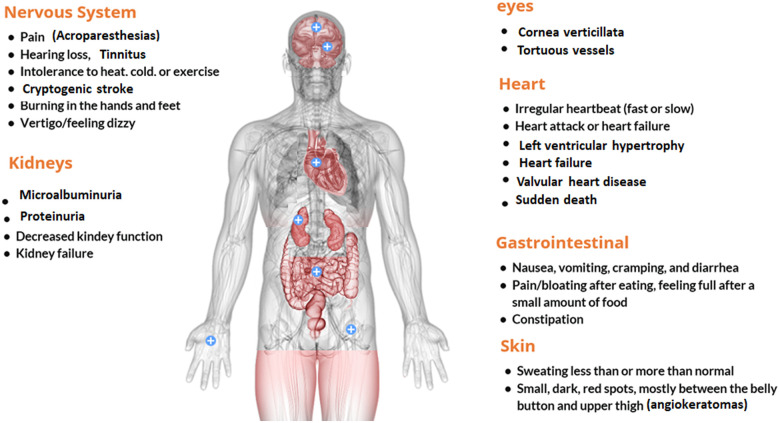
Fabry disease (FD) is an X-linked rare lysosomal storage disease, caused by mutations in the gene encoding the enzyme α-galactosidase A, which results in a deficit in enzyme activity. The consequence is a progressive accumulation of glycosphingolipids (Gb3), inside the lysosomes of various organs. These deposits cause the characteristics signs and symptoms (Figure 1): angiokeratomas, acroparesthesias, anhidrosis, cold intolerance, cornea verticillata, gastrointestinal manifestations, kidney failure, stroke, and left ventricular hypertrophy (LVH). Death in early adulthood in affected persons may be due to progressive cardiac, renal, and cerebral involvement.
Figure 1.
Signs and symptoms of Fabry disease.
Fabry disease is commonly misdiagnosed, so there is a delay before the correct diagnosis, with a period time from the onset of symptoms to the correct diagnosis of 13 years in men and 17 years in women.1
The majority of patients with FD (more than 60% over 40 years old) have cardiac involvement, and the probability and severity of finding cardiac involvement increase with age.2,3 Cardiac disease is mainly manifested as progressive concentric LVH4 that leads to myocardial fibrosis, the end-stage phase of the disease with left ventricular systolic dysfunction (Fabry cardiomyopathy) and is the main cause of death in patients with advanced FD.5 Early treatment with enzyme replacement therapy (ERT) may result in a remarkable improvement of cardiac symptoms as long as the disease process has not reached an advanced stage.6
These abnormalities are absent in males with the cardiac variant of the disease who typically present with a late-onset disorder that is primarily limited to the heart, and there is no involvement of the vascular endothelium. With advancing age, however, cardiac involvement progresses and leads to death.7
The buildup of Gb3 occurs on both left- and right-sided cardiac valves. Mild thickening of the left-sided valves is seen in as many as a quarter of patients.8 Although the presence of valve disease is common, few patients develop valve regurgitation or stenosis sufficient to warrant surgical intervention. Severe aortic stenosis is an extremely rare disorder in FD.9
To the best of our knowledge, we report the first case of rapid progression of aortic stenosis in a patient with FD treated with transcatheter aortic valve implantation (TAVI). We believe that in such cases, severe left ventricular systolic dysfunction should not be considered a contraindication for TAVI, even in the presence of Fabry cardiomyopathy under ERT treatment.
Timeline
|
Case presentation
A 57-year-old man was diagnosed in 2010 with a cardiac variant of FD. His alpha-galactosidase A activity in leucocytes was markedly reduced (0.31 µmol/l/h; normal value > 2.10 µmol/l/h) and a mutation was found in exon 3 of the alpha-galactosidase A gene (c.520 T>G).
The general physical examination was unremarkable. The patient had a normal renal function. His 12-lead electrocardiogram showed sinus rhythm, a normal PR segment, marked LVH, and right bundle branch block.
The transthoracic echocardiogram (TTE) showed severe symmetric LVH (septal and posterior wall thickness: 24 mm and 20 mm, respectively, left ventricular mass index: 386 gm2) and ejection fraction (EF) was 55%. The aortic valve was tricuspid, with mild sclerosis, aortic valve opening was normal and without systolic gradient (Video 1).
Cardiac magnetic resonance showed postero-lateral midwall fibrosis consistent with FD and infero-basal subendocardial fibrosis consistent with an inferior scar.
Coronary angiogram showed total occlusion in the mid-third of the right coronary artery and no other coronary lesions.
In 2010, when the patient was 47 years old, he was asymptomatic, but because he had Fabry cardiomyopathy, he started ERT with IV home infusions of agalsidase alpha, at 0.2 mg/kg every 14 days, without adverse effects. Global longitudinal strain (GLS) before treatment was reduced (−13.8%) and increased to −16.8%.
Six years later (2016), the TTE showed progression of hypertrophy with systolic dysfunction (EF: 40%), and mild calcified aortic valve stenosis: peak systolic gradient: 25 mmHg. The aortic valve area calculated by Doppler echocardiography using the continuity equation was 1.50 cm2.
In 2017, the TTE showed that the aortic stenosis had progressed and was moderate (peak systolic gradient: 34 mmHg, dimensionless index: 0.40, aortic valve area: 1.20 cm2), with greater left ventricular function impairment (EF: 35%).
The same year, the patient was admitted to the coronary care unit due to sustained ventricular tachycardia with an electric storm. An implantable cardioverter-defibrillator (ICD) was implanted, but the patient had two appropriate shocks. The 24-h Holter monitoring detected several episodes of non-sustained ventricular tachycardia. For this reason, radiofrequency catheter ablation was performed and successfully eliminated ventricular tachycardia.
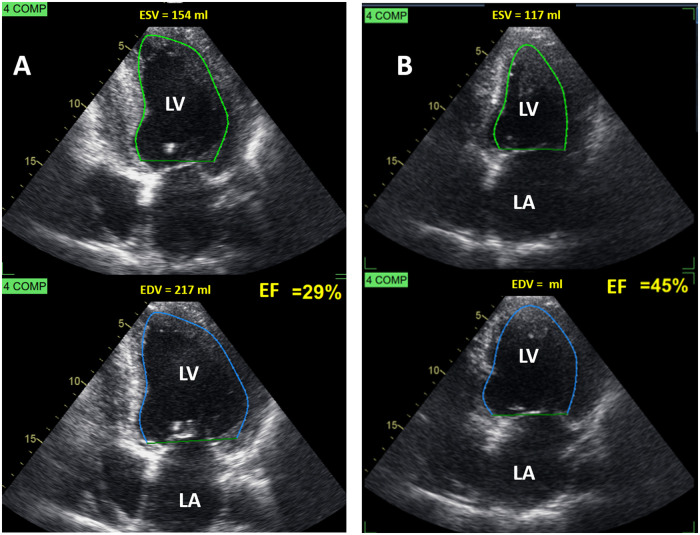
By October 2019, the patient complained of dyspnoea, New York Heart Association functional class II–III. TTE (Figure 2A and Video 2) showed severe systolic dysfunction [end-diastolic volume (EDV): 217 mL, end-systolic volume (ESV): 154 mL, EF: 29%, GLS: −2.1%]. There was thinning and akinesis of the posterior wall of the left ventricle, due to intramural fibrosis, characteristic of the end-stage Fabry cardiomyopathy. The aortic valve showed severe calcification and severe restriction in its systolic opening (Video 3). The patient had a rapid progression of his aortic stenosis to severe low-flow, low-gradient aortic stenosis10 due to low EF (stroke volume index: 28 mL/m2, peak velocity: 3 m/s, peak systolic gradient: 36 mmHg, mean gradient: 22 mmHg, dimensionless index: 0.20, aortic valve area: 0.97 cm2). Aortic valve calcium score by computed tomography was very high (3.828 Agatston units).
Figure 2.
Two-dimensional echocardiogram. Apical four-chamber view (A) 2019: before-transcatheter aortic valve implantation. Ejection fraction: 29% and (B) 2020: 2 months after-transcatheter aortic valve implantation. Ejection fraction: 45%. EDV, end-diastolic volume; ESV, end-systolic volume; LA, left atrium; LV, left ventricle.
To rule out that left ventricular systolic dysfunction was of ischaemic origin, the coronary angiography was repeated and was similar to the one performed 10 years ago.
Intervention is indicated in symptomatic patients with severe low-flow, low-gradient aortic stenosis (mean gradient < 40 mmHg) with reduced EF (Recommendation Class I, Level of Evidence C).8
The Heart Team assessed the case and concluded that the worsening of the systolic left ventricular function was due to the patient’s valve disease. Given the presence of comorbidities (coronary disease, severe ventricular arrhythmias, ICD, peripheral vascular disease, severe LVH, and severe systolic dysfunction) and an increased surgical risk (STS score: 5.7%), the decision was made to perform a percutaneous aortic valve implant (TAVI).
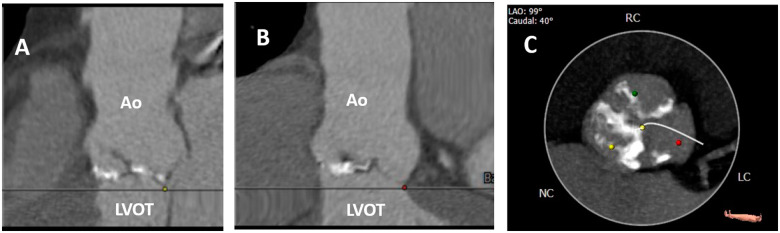
The patient’s computed tomography (Figure 3) showed a dilated aortic annulus. The perimeter and diameter of the aortic annulus were 97.2 mm and 31 mm, respectively, and the only TAVI prosthesis available for that perimeter and diameter was the CoreValve Evolute R # 34, which was implanted uneventfully under conscious sedation on October 29, 2019. The patient was discharged 24 h after the procedure, with a gradient of 12 mmHg and a mild degree leak.
Figure 3.
Axial computed tomography performed pre-transcatheter aortic valve implantation in our patient with Fabry disease and severe aortic stenosis. (A and B) The aortic annulus is dilated (perimeter: 97.2 mm, diameter: 31 mm). (B) Severe aortic valve calcification. Ao, aortic root; LC, left coronary; LVOT, left ventricular outflow tract; NC, non-coronary; RC, right coronary.
At 2 months post-TAVI, the signs of heart failure disappeared, the patient was asymptomatic and his TTE (Figure 2B and Supplementary material online, Video S1) showed a peak systolic gradient of 12 mmHg, reverse remodelling (EDV: 211 mL, ESV: 117 mL), and increase in EF (45%, left ventricular global longitudinal strain: −4.6%).
At 14 months post-TAVI, the patient remains asymptomatic, and follow-up has proven uneventful. In addition, ventricular arrhythmia disappeared.
Discussion
ERT in FD improves cardiac function and morphology. Earlier initiation of ERT is desirable before irreversible cardiac changes such as fibrosis develop. ERT is less efficacious when started after the development of LVH and myocardial fibrosis. The presence of severe LVH and myocardial fibrosis in our patient indicates an advanced and irreversible stage of the disease that is not stopped by ERT. This explains the progression of LVH despite ERT.
Our patient exhibited LVH and right ventricular hypertrophy (RVH) with normal biventricular systolic function. RVH is common in patients with FD and correlates with the disease severity and LVH.11
In Fabry cardiomyopathy, deposition of Gb3 within the valve tissue explains the valve involvement. The infiltrative changes within the valvular fibres and cardiac valves exhibit fibrosis or retraction. Mild aortic and mitral regurgitation is frequently observed, especially in advanced stages of FD. This leads to valvular thickening and deformation, but valve abnormalities do not appear to be a clinical problem and rarely require surgical intervention.12
When severe aortic stenosis occurs in a patient with Fabry cardiomyopathy, it rapidly results in clinical and haemodynamic impairment, as observed in our patient.
Our hypothesis about the rapid progression of aortic stenosis is that it could have occurred due to severe calcification of the aortic valve or as a consequence of valve thickening due to FD or both processes that potentiated each other.
Rapid progression of aortic stenosis was defined as an increase in peak jet velocity >0.3 m/s/year or an increase in maximal instantaneous pressure gradient by ≥10 mm Hg per year or a decrease in aortic valve area ≥0.15 cm2/year.13 The rate of AS progression is faster when leaflet calcification is more marked. Our patient had severe calcification of the aortic valve on TTE. In addition, the high aortic valve calcification score determined by computed tomography (3828 Agatston units) indicated the presence of severe aortic stenosis.14 Progression rate is an individual and clinically unpredictable feature among patients with asymptomatic non-severe aortic stenosis. Rapid progressors patients have a two-fold higher risk for developing symptoms and clinical events, including mortality, compared with patients with slow progression.15 Decrease in Vmax at follow-up can occur due to severe systolic left ventricular dysfunction or at least moderate mitral or tricuspid regurgitation. This negative progression pattern, as shown in our patient, was associated with high rate of symptom development and poor outcomes.
Our patient was followed for 10 years, and in 3 years suffered a rapid progression from mild to severe low-flow, low-gradient aortic stenosis due to severe left ventricular systolic dysfunction.16
Although surgical replacement of the aortic valve is considered the gold standard of treatment, a TAVI may benefit patients like the one reported here, with increased surgical risk and comorbidities. An improvement in EF was reported after TAVI, compared to surgical replacement, as observed in our case, in which EF increased from 29% to 45%, and heart failure symptoms disappeared.
Conclusions
To the best of our knowledge, we report the first case of rapid progression of aortic stenosis in a patient with FD treated with TAVI. We believe that in such cases, severe left ventricular systolic dysfunction should not be considered a contraindication for TAVI, even in the presence of Fabry cardiomyopathy under ERT treatment.
Lead author biography

María Cristina Saccheri is the staff of Echocardiography Laboratory of the Hospital of the Government of the City of Buenos Aires "Dr. Cosme Argerich". Argentina. Full member of the Argentine Society of Cardiology Hypertrophic Cardiomyopathy Center and Research Institute. Hospital "Dr. Cosme Argerich", with more than 120 publications, 50 of which in Medline indexes Journals. Author of the chapter Hypertrophic Cardiomyopathy of the book New Techniques in Echocardiography, published in Spanish and Portuguese (2012).
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Hoffmann B, Mayatepek E.. Fabry disease-often seen, rarely diagnosed. Dtsch Arztebl Int 2009;106:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M. et al. ; FOS Investigators. Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet 2009;46:548–552. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey RP, Philip KJ, Schwarz ER.. Cardiac abnormalities in Anderson-Fabry disease and Fabry’s cardiomyopathy. Cardiovasc J Afr 2011;22:38–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Linhart A, Palecek T, Bultas J, Ferguson JJ, Hrudova J, Karetova D. et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J 2000;139:1101–1108. [DOI] [PubMed] [Google Scholar]
- 5.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, StöRk S. et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation 2009;119:524–529. [DOI] [PubMed] [Google Scholar]
- 6.Lidove O, West ML, Pintos-Morell G, Reisin R, Nicholls K, Figuera LE. et al. Effects of enzyme replacement therapy in Fabry disease—a comprehensive review of the medical literature. Genet Med 2010;12:668–679. [DOI] [PubMed] [Google Scholar]
- 7.Scheidt W. V, Eng CM, Fitzmaurice TF, Erdmann E, Hübner G, Olsen EGJ. et al. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med 1991;324:395–399. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard MN.The heart in Fabr’s disease. Cardiovasc Pathol 2011;20:8–14. [DOI] [PubMed] [Google Scholar]
- 9.Vlachou A, Gogakos AS, Cheva A, Drossos G.. Surgical treatment of severe aortic valve stenosis with concomitant Fabry disease. Cardiovasc Med 2019;22:w02066. [Google Scholar]
- 10.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 11.Graziani F, Laurito M, Pieroni M, Pennestrì F, Lanza GA, Coluccia V. et al. Right ventricular hypertrophy, systolic function, and disease severity in Anderson-Fabry Disease: an echocardiographic study. J Am Soc Echocardiogr 2017;30:282–291. [DOI] [PubMed] [Google Scholar]
- 12.Weidemann F, Strotmann JM, Niemann M, Herrmann S, Wilke M, Beer M. et al. Heart valve involvement in Fabry cardiomyopathy. Ultrasound Med Biol 2009;35:730–735. [DOI] [PubMed] [Google Scholar]
- 13.Benfari G, Nistri S, Marin F, Cerrito LF, Maritan L, Tafciu E. et al. Excess mortality associated with progression rate in asymptomatic aortic valve stenosis. J Am Soc Echocardiogr 2021;34:237–244. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura S, Izumi C, Nishiga M, Amano M, Imamura S, Onishi N. et al. Predictors of rapid progression and clinical outcome of asymptomatic severe aortic stenosis. Circ J 2016;80:1863–1869. [DOI] [PubMed] [Google Scholar]
- 15.Otto CM.Aortic stenosis progression: Doppler echocardiography shifted the paradigm. J Am Soc Echocardiogr 2021;34:245–247. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S. et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:254–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.